Abstract
Protection against clinical disease and prevention of the renal carrier state remain the key objectives of vaccination against leptospirosis in the dog. In the present paper, groups of dogs were vaccinated twice with a commercial bacterin (EURICAN® L) containing Leptospira interrogans serovars icterohaemorrhagiae and canicola and challenged with heterologous representatives of both serovars at 2 weeks (onset of immunity) or 14 months (duration of immunity) after the second vaccination. Control dogs were not vaccinated against leptospirosis and kept with the vaccinated dogs. The challenges, irrespective of the serovar, reliably produced clinical signs consistent with Leptospira infection in the control pups with up to 60% mortality. As expected clinical disease in the adult controls was less severe, but we were able to induce morbidity and mortality as well. Under these extreme challenge conditions, clinical signs in the vaccinated dogs were rare, and when observed, mild and transient in nature.
Following experimental infection, 100% of the control pups and 83% of the adult controls became renal carriers. Despite the heavy challenges, none of the 18 vaccinated puppies (onset of immunity studies) and only 2 out of the 16 vaccinated adult dogs (duration of immunity studies) developed a renal carrier state. These results show that a primary course of two doses of EURICAN® L provided quick onset and long-term protection against both clinical leptospirosis and the renal carrier stage. This vaccine should provide veterinarians with a powerful tool to prevent clinical disease in dogs and zoonotic transmission of leptospirosis to humans.
Keywords: Leptospira interrogans serovars canicola and icterohaemorrhagiae, Bacterin, Vaccine, Clinical leptospirosis, Renal carriage, Onset and duration of immunity
1. Introduction
Leptospirosis is an important zoonosis of worldwide distribution caused by infection with spirochaetes belonging to the pathogenic species of Leptospira. Infection typically results from direct or indirect contact with urine of infected animals. The clinical signs associated with Leptospira infection range from subclinical to acute disease characterized by anorexia, vomiting, lethargy, muscle pain, dehydration, jaundice, abdominal pain, diarrhoea, bloody urine, and death. Renal failure is the predominant finding in symptomatic dogs, with a small percentage also showing evidence of liver disease (Greene, 1998, review; Boutilier et al., 2003). Clinically recovered dogs frequently become asymptomatic renal carriers, and as such can be an important source of human leptospirosis (Center for Disease Control, 1972; Trevejo et al., 1998). Leptospira (L) interrogans serovars icterohaemorrhagiae and canicola are the two serovars traditionally associated with disease in dogs (Hartman, 1984, Trevejo et al., 1998), but new serovars play an increasingly important role (Scanziani et al., 2002, Ward et al., 2004, Moore et al., 2006, Geisen et al., 2007, Stokes et al., 2007). Hence, leptospirosis is now recognised as an important re-emerging disease in dogs (Bolin, 1996, Ward et al., 2002). While short-term clinical protection has been demonstrated experimentally in dogs after vaccination with several vaccines (Huhn et al., 1975, André-Fontaine et al., 2003, Klaasen et al., 2003, Schreiber et al., 2005a, Schreiber et al., 2005b), there is much debate on whether leptospirosis vaccines protect against the renal carrier state (André-Fontaine et al., 2003) or provide long-term immunity (Coyne et al., 2001). As far as we know, we were the first to demonstrate 10 months duration of immunity against L. interrogans serovar canicola provided by a classical bacterin (Tronel et al., 1999). Since then only one paper has been published demonstrating duration of immunity of 13 months for a commercial bacterin (Klaasen et al., 2003). In the current study, we confirm and extend our previous observations and demonstrate that two doses of EURICAN® 1 L provide both rapid onset and duration of protective immunity of at least 14 months against both serovars icterohaemorrhagiae and canicola. The vaccine was evaluated for protection against clinical disease and prevention of the renal carrier state.
2. Materials and methods
2.1. Experimental design
Four separate vaccination-challenge experiments, including 74 puppies, were performed to study onset and duration of immunity provided by EURICAN® L (referred to as studies 1–4, Table 1 ). Study 1 contained nine vaccinates and eight controls, study 2 nine vaccinates and ten controls, study 3 seven vaccinates and eight controls and study 4 nine vaccinates and ten controls (Table 1). Institutional Animal Care and Use Committee approvals were obtained before conducting the studies. In all studies, puppies were vaccinated twice subcutaneously, 4 weeks apart. Puppies were 8–9 weeks of age at the time of first vaccination. Dogs from studies 1 and 3 were challenged with L. interrogans serovar canicola at 2 weeks and 14 months, respectively, after the primary vaccination program of two doses. Dogs from studies 2 and 4 were challenged with L. interrogans serovar icterohaemorrhagiae at 2 weeks and 14 months, respectively, after the second vaccination of the primary vaccination course. Because it is difficult to induce clinical leptospirosis in adult dogs, two 2–4-month-old pups were added to studies 3 and 4 at the time of challenge to assess the severity of the infection. Following challenge, dogs were examined for the presence of clinical signs characteristic of leptospirosis. For leptospires isolation, blood and urine samples were collected at regular intervals, and a kidney and liver (study 4) sample were aseptically taken at necropsy. Blood was also sampled for serological, hematological, and biochemical analysis (study 4 only). At the end of the observation period or at death, dogs were necropsied, and organs were removed for histological examination.
Table 1.
Experimental design.
| Study | Designation | Group | # dogs | Challenge |
|
|---|---|---|---|---|---|
| Time after V2 | Serovar | ||||
| 1 | Onset of immunity | V | 9 | 2 weeks | Lc |
| C | 8 | ||||
| 2 | Onset of immunity | V | 9 | 2 weeks | Li |
| C | 10 | ||||
| 3 | Duration of immunity | V | 7 | 14 months | Lc |
| C | 8a | ||||
| 4 | Duration of immunity | V | 9 | 14 months | Li |
| C | 10a | ||||
Two control pups were added at the time of challenge. V = vaccinated, C = control, V2 = second vaccination, Lc = L. interrogans serovar canicola, Li = L. interrogans serovar icterohaemorrhagiae.
2.2. Vaccines
Routine production batches of EURICAN® L (Merial, Lyon, France), a whole cell, non-adjuvanted vaccine prepared from inactivated cultures of L. interrogans serovars icterohaemorrhagiae and canicola, were used. All batches complied with the potency requirements of monograph 0447 of the European Pharmacopoiea (2002). In studies 1 and 2, the vaccine was administered simultaneously, but at a separate site, with a vaccine containing a recombinant canine distemper virus and modified live canine adenovirus type 2, canine parvovirus, canine coronavirus, and canine parainfluenza type 2 virus. In studies 3 and 4, EURICAN® L was used as diluent to reconstitute a freeze-dried pellet containing a modified live canine distemper virus, canine adenovirus type 2, canine parvovirus and canine parainfluenza type 2 virus. This second combination vaccine is commercialized under the name EURICAN® DHPPi2L.
2.3. Animals
Seventy-four specific pathogen free (SPF) 8–16-week-old male and female beagle pups were purchased from Harlan Sprague Dawley (Indianapolis, USA or Zeist, The Netherlands) or from Ferme des Gouttes (Charles Rivers Laboratories, Inc., France). Dogs were barrier maintained and fed a high-quality commercial dry ration with unlimited access to water. Dogs were identified by a microchip implanted subcutaneously and/or by ear tattoo.
2.4. Challenge strains
L. interrogans serovar canicola, strain Moulton (National Veterinary Services Laboratory (NVSL), Ames, IA, USA) was used as challenge inoculum in studies 1 and 3. L. interrogans serovar icterohaemorrhagiae, strain CFI (NVSL) and strain 193 (Pasteur Institute, Paris, France) were used as challenge inocula in studies 2 and 4, respectively. The identity of all serovars was confirmed by the Pasteur Institute, Paris, France, using restriction fragment analysis.
2.5. Challenge protocol
After an initial culture in Ellinghausen–McCullough–Johnson–Harris (EMJH) medium, the strains were back-passaged twice (studies 3 and 4) or four times (studies 1 and 2) in hamsters to prevent loss of virulence through adaptation to culture conditions. Moribund hamsters were humanely euthanised and their livers and kidneys or spleens (study 4) were aseptically removed and homogenated in sterile saline. After sedimentation by centrifugation, the supernatant was diluted 1:10 in sterile saline and inoculated in the dogs of studies 1 and 2. Each dog received 8 mL of challenge suspension containing approximately 5 × 108 and 1 × 109 organisms of L. interrogans serovars icterohaemorrhagiae and canicola, respectively by the intraperitoneal route. In studies 3 and 4, after harvest, the challenge strains were passaged once in vitro (EMJH medium) to allow a more precise quantification of the bacterial suspension. Each dog received 11 mL (study 3) or 12 mL of challenge suspension with 0.5 mL instilled in the ventral conjunctival sac of each eye and the remainder administered intraperitoneally. The total challenge dose per dog was 2.1 × 109 and 5.6 × 109 organisms for L. interrogans serovars icterohaemorrhagiae and canicola, respectively.
2.6. Clinical examination
All animals were observed daily for 14 days (studies 1 and 2) or 35 days (studies 3 and 4) after challenge for signs consistent with leptospirosis, including, depression, anorexia, conjunctivitis, iritis, vomiting, diarrhoea, jaundice, petechiae, and signs of urinary disease (haematuria). Signs were scored by the use of a standardized protocol (Table 2 ). Rectal temperatures were taken and recorded daily for 14 days after challenge, and temperatures of 39.5 °C or more were considered as hyperthermia. Dogs from studies 3 and 4 were weighed once a week until the end of the study or death. A weight loss of more than 5% was considered significant. During the post-challenge clinical examination, any animals displaying serious and irreversible clinical signs that lead to suffering were humanely euthanised.
Table 2.
Clinical scoring protocol for canine leptospirosis.
| Clinical sign | Degree | Score |
|---|---|---|
| Conjunctivitis/iritis | Absent | 0 |
| Present | 1 | |
| General appearance | Normal | 0 |
| Apathy | 1 | |
| Depression | 2 | |
| Prostration | 3 | |
| Diarrhoea/vomiting | Absent | 0 |
| Mild | 1 | |
| Severe | 2 | |
| Anorexia | Absent | 0 |
| Present | 1 | |
| Jaundice | Absent | 0 |
| Present | 1 | |
| Haematuria | Absent | 0 |
| Present | 1 | |
2.7. Serology
Whole blood was collected at regular intervals before and after vaccination and challenge. Selected sera were tested for the presence of microscopic agglutination titres (MAT) by the College of Veterinary Medicine, Diagnostic Laboratory, University of Minnesota, St. Paul, USA (studies 1 and 2) or AFFSA, Laboratoire de Recherche Vétérinaire, Alfort, Paris, France (study 4). Sera were tested against L. interrogans serovars icterohaemorrhagiae and canicola using standardized procedures. Since serology has limited value for evaluating the efficacy of vaccines against leptospirosis, sera from study 3 were not tested. Antibody titres were expressed as the reciprocal of the highest serum dilution that induced at least 50% (studies 1 and 2) or 75% (study 4) agglutination. For the calculation of geometric mean titre (GMT), values under the lower limit of quantification (LLOQ) were replaced by LLOQ/2.
2.8. Haematology
EDTA blood samples were collected from dogs of studies 3 and 4 on at least 2 days before challenge and then daily for 7 days after challenge. Counts of platelets were performed using a MS-9 cell counter analyser (Melet Schloesing, France). Platelet counts were compared to reported standard values for dogs (2–9 × 1011 platelets/L (Kahn, 2005)).
2.9. Blood biochemistry
The following tests were only performed on dogs from study 4. Whole blood samples were collected before challenge and on days 4 and 6 after challenge. Sera were analyzed for urea nitrogen, creatinine, total bilirubin, serum glutamic oxalacetic transaminase (SGOT), and serum glutamine pyruvic transaminase (SGPT) by the Laboratoire Marcel Mérieux, Lyon, France. Urea nitrogen, creatinine, and total bilirubin were compared to normal values provided by the same laboratory. Because many pre-challenge values for SGOT and SGPT were outside the reported “normal” values, only large modifications of the baseline values were taken into account.
2.10. Detection of leptospiraemia
Blood samples were collected on heparin tubes before challenge (day 2/day 0) and on days 1–7, and 10 after challenge. In study 4, an additional blood sample was taken on day 35. Blood samples were immediately inoculated in semisolid medium (1–3 drops of blood in 8 mL of medium (studies 1 and 2)) or in liquid EMJH medium (1 mL of blood in 9 mL medium (studies 3 and 4)) and transferred to the lab. Serial 10-fold dilutions (up to 10−3) were made in the same media and incubated at 30 °C. All the cultures were incubated for 6–9 weeks and observed weekly for the presence of leptospires using dark field microscopy.
2.11. Detection of leptospires in urine and organs
Urine samples were collected before challenge (day 2/day 0) and at 2, 3 and 5 weeks after challenge (studies 3 and 4) or by direct bladder tap at the time of euthanasia (studies 1 and 2). In studies 3 and 4, urine samples were collected either spontaneously after subcutaneous injection of the diuretic furosemide (DIMAZON®,2 Intervet, France) (0.5–1 mL/kg bodyweight) (females) or after probing with a urethra catheter (males). 5 Fluorouracil was added at a concentration of 100 μg/mL to the urine samples of study 4.
Samples from kidneys (all studies) and livers (study 4) were collected aseptically. Approximately 5–8 g of organ tissue was macerated into 10 mL of culture medium and vortexed. Tissue debris was allowed to settle, and serial 10-fold dilutions were made through 1:1000. Urine and organ cultures were made as described for the blood cultures.
2.12. Post-mortem examination
Immediately after euthanasia or death, the animals were necropsied and subjected to a macroscopic examination. Samples of kidneys and livers were fixed with 10% buffered formalin or frozen and processed for microscopic examination following standard procedures. Only organ samples from study 4 were submitted for microscopic examination. Histological sections were stained with haematoxylin–eosin (HE) and with Warthin–Starry silver stain for the detection of leptospires.
2.13. Analysis of the results
Statistical analyses were carried out using STATGRAPHICS® 3 software and SAS® release 12 software. The level of significance was set at P ≤ 0.05.
2.13.1. Clinical scores
The severity of clinical signs (sickness score) was compared among the vaccinated and control groups within one study by assigning the dogs to one of two disease categories: no or mild clinical disease and moderate-to-severe clinical disease. The sickness score was calculated by using the daily scores for each clinical sign on the basis of an algorithm, which gave a triple weighting to the scores for jaundice and haematuria. Thus, sickness score = 1× (daily score for conjunctivitis/iritis) + 1× (daily score for anorexia) + 1× (daily score for diarrhoea/vomiting) + 1× (daily score for general appearance) + 3× (daily score for jaundice) + 3× (daily score for haematuria). Each dog was classified according to the most severe daily score recorded during the after challenge observation period with a score of 0 for no disease, 1–2 for mild disease, 3–4 for moderate disease, and >4 for severe disease. Differences in the incidence of moderate-to-severe disease (scores ≥3) among groups were analyzed by the use of a Fischer's exact test.
2.13.2. Leptospiremia
Because no leptospiremia was found in the vaccinated pups from studies 1 and 2, no statistical analysis was performed.
A daily score between 0 and 3 was attributed to each animal from studies 3 and 4, according to the result of the blood culture (0 = negative, 1 = positive at dilution 10−1, 2 = positive at dilution 10−2, and 3 = positive at dilution 10−3). The duration of leptospiraemia and cumulative scores for the first 7 days post-challenge (area under the time–titre curve) were compared between vaccinated and control dogs using a one-sided Student's t-test (study 3) or Wilcoxon's test (study 4).
2.13.3. Renal carrier state
Any dog with at least one positive urine or kidney culture was defined as a renal carrier.
Differences in the incidence of renal carriers among groups were analyzed by the use of a Fischer's exact test.
2.13.4. Platelet counts
Platelet counts of vaccinated and adult control groups were compared using a Wilcoxon's test (study 4) or a mixed model with repeated measurements (study 3). SGOT and SGPT values of vaccinated and control groups were compared using a Wilcoxon's test and Chi-square test, respectively.
3. Results
3.1. Humoral responses to vaccination and challenge
Prior to vaccination, none of the dogs had detectable antibody titres against L. interrogans serovars icterohaemorrhagiae or canicola. All vaccinated dogs from studies 1 and 2 had detectable antibody titres on the day of challenge against L. interrogans serovar canicola (GMT = 549, range: 80–1280) and L. interrogans serovar icterohaemorrhagiae (GMT = 47, range 20–80). A booster effect was observed in one out of nine and eight out of nine dogs after L. interrogans serovars icterohaemorrhagiae and canicola challenge, respectively. High antibody titres were observed in the surviving controls after L. interrogans serovar icterohaemorrhagiae (GMT = 1280) and L. interrogans serovar canicola challenge (GMT = 3044, range 1280–10,240). After both challenges, antibody titres were higher on average in surviving controls than in vaccinates.
Four out of nine dogs from study 4 had detectable antibody titres against L. interrogans serovar icterohaemorrhagiae 4 weeks after the second vaccination (range: 100–200). Antibodies persisted until challenge in only one dog. A booster response was observed in all vaccinates after L. interrogans serovar icterohaemorrhagiae challenge. In the same study, seven out of nine dogs had detectable antibody titres against L. interrogans serovar canicola 4 weeks after the second vaccination (range: 200–400), and two out of nine animals still had low MAT antibody titres 5 days before challenge.
3.2. Clinical signs
The incidences of moderate to severe disease in vaccinated and control dogs from studies 1–4 are shown in Table 3 .
Table 3.
Incidence of moderate to severe disease after challenge.
| Study | Group | Disease incidence (no. of dogs) |
P-value* | |
|---|---|---|---|---|
| No to mild | Moderate to severea | |||
| 1 | V | 9 | 0 | 0.00004 |
| C | 0 | 8 (4) | ||
| 2 | V | 9 | 0 | 0.0077 |
| C | 4 | 6 (6) | ||
| 3 | V | 7 | 0 | 0.0186 |
| C | 3 | 5 (1) | ||
| 4 | V | 9 | 0 | 0.124 |
| C | 7 | 3 (3) | ||
Abbreviations: V = vaccinated; C = control. Study 1 = onset of immunity L. interrogans serovar canicola; study 2 = onset of immunity L. interrogans serovar icterohaemorrhagiae; study 3 = duration of immunity L. interrogans serovar canicola; study 4 = duration of immunity L. interrogans serovar icterohaemorrhagiae.
In brackets the number of dogs that died or had to be euthanised after challenge.
Fisher's exact test.
All eight control pups from study 1 became ill after L. interrogans serovar canicola challenge; seven pups developed severe and one pup moderate disease. The affected pups were depressed and were frequently observed curled up in their food bowls. Some of these animals were vomiting, slightly dehydrated, and had haematuria. They also had foul smelling bloody diarrhoea. One pup developed jaundice on day 4 post-challenge. Four pups with severe clinical disease were humanely euthanised between day post-challenge (DPC) 5 and 6. In contrast, vaccinated pups showed no or only mild transient signs. Two vaccinated pups had slight conjunctivitis and one pup developed mild digestive signs lasting for 1 day. The incidence of moderate to severe disease was significantly lower in the vaccinated pups than in the control pups (P = 0.00004, Fisher's exact test).
All 10 control pups from study 2 developed clinical signs following L. interrogans serovar icterohaemorrhagiae challenge. Six control pups were humanely euthanised because of severe disease between DPC 4 and 7. Clinical signs were similar between dogs challenged with L. interrogans serovars icterohaemorrhagiae and canicola and included depression, anorexia, haemorrhagic diarrhoea, vomiting, icterus, and haematuria. Three control pups showed only mild clinical signs consisting of depression and mild diarrhoea, and one control pup showed no clinical signs. Only one vaccinated pup was depressed on DPC 2 and 8. The incidence of moderate to severe disease was significantly lower in the vaccinated pups than in the control pups (P = 0.0077, Fisher's exact test).
The two control puppies added at the time of challenge in study 3 died from severe leptospirosis on DPC 4 and 5, validating the challenge. As expected, clinical signs in the adult controls were less severe than in the control pups. Nevertheless, four control dogs developed moderate and one dog severe leptospirosis. The latter dog was humanely euthanised on DPC 7 after having shown characteristic signs of frank leptospirosis. Clinical signs in the moderately ill dogs included conjunctivitis, mild diarrhoea, dehydration, and weight loss. Five out of seven vaccinated dogs did not show any clinical signs during the observation period. One vaccinated dog showed conjunctivitis on DPC 18, and two dogs had mild diarrhoea for 2 consecutive days starting on DPC 23. The incidence of moderate to severe disease was significantly different between the vaccinated and control dogs (P = 0.0186, Fisher's exact test).
In study 4, one of the two control puppies added at the time of challenge died on DPC 6 and the other developed severe disease (sickness score of 6) but recovered. Unexpectedly, the challenge appeared to be very severe for the adult controls. Three out of 10 control dogs had to be humanely euthanised because of depression, diarrhoea, dehydration, and jaundice (one dog) on DPC 7 (two dogs) and 23 (one dog), respectively. Five controls had mild disease consisting of conjunctivitis, depression, and anorexia. Two controls had no disease. Clinical signs in the vaccinated dogs were mild (conjunctivitis in one dog) or absent. The incidence of moderate to severe disease was not significantly different between the vaccinated and control dogs (P = 0.124, Fisher's exact test). Due to the small number of dogs, the a posteriori power of the test was too low (0.15) to detect a significant difference. Twenty-one dogs per group would have been needed to detect the same difference (30%) with a probability equal to 80%.
3.3. Haematology
No thrombocytopenia was recorded after challenge in the vaccinated dogs from studies 3 and 4, except for one vaccinated dog on day 1 post-L. interrogans serovar icterohaemorrhagiae challenge. In contrast, 40% (study 4) to 75% (study 3) of the controls became thrombocytopenic after challenge. Over the 1–7 days post-challenge period, the platelet count was significantly lower in the controls than in the vaccinates after L. interrogans serovar canicola challenge (P = 0.0001, mixed model), and the difference was close to significance (P = 0.08, Wilcoxon's test) after L. interrogans serovar icterohaemorrhagiae challenge.
3.4. Biochemistry
Sharp increases in urea, creatinine, bilirubin, SGOT, or SGPT values were found after challenge in three adult controls from study 4 and in the two control puppies that were added to study 4 at the time of challenge. All these dogs developed severe clinical disease and all three adult dogs and one of the two puppies succumbed to the challenge. In contrast, none of the vaccinates had increased urea, creatinine, or bilirubin values. Large modifications of baseline values of SGPT were recorded in two vaccinated dogs.
3.5. Leptospiraemia
An overview of the results is provided in Fig. 1A (study 1) and B (study 2) and Table 4A (study 3) and 4B (study 4) . All blood samples from studies 1–4 were negative for leptospires before challenge. Leptospires could be isolated from the blood of all controls from studies 1 and 2 for at least 3 days following challenge. Leptospiraemia persisted for up to 6 and 10 days for L. interrogans serovars icterohaemorrhagiae and canicola, respectively. None of the vaccinated dogs from studies 1 and 2 developed leptospiraemia indicating that infection was not established in any of the vaccinated dogs.
Fig. 1.
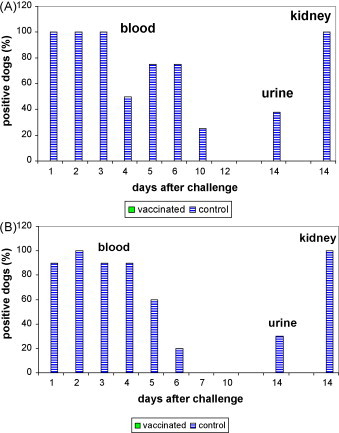
(A) Results of blood, urine and kidney cultures after challenge of puppies with L. interrogans serovar canicola. Puppies were challenged 2 weeks after a primary course of two doses of vaccine (study 1: onset of immunity). (B) Results of blood, urine and kidney cultures after challenge of puppies with L. interrogans serovar icterohaemorrhagiae. Puppies were challenged 2 weeks after a primary course of two doses of vaccine (study 2: onset of immunity).
Table 4A.
Results of blood, urine and kidney cultures after challenge of dogs with L. interrogans serovar canicola. Dogs were challenged 14 months after a primary course of two doses of vaccine (study 3: duration of immunity).
| Group | Dog no. | Days after challenge |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood |
Urine |
Kidney | |||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 10 | 0 | 16 | 21 | 35 | 35 | ||
| Vaccinated | 1 | − | +++ | +++ | +++ | − | − | − | − | − | − | − | − | − | − |
| 2 | − | +++ | +++ | +++ | +++ | + | − | − | − | − | ++ | + | − | − | |
| 3 | − | +++ | +++ | +++ | + | − | − | − | − | − | + | ++ | +++ | +++ | |
| 4 | − | +++ | +++ | +++ | + | − | − | − | − | c | − | c | − | − | |
| 5 | − | +++ | ++ | + | − | − | − | − | − | − | − | − | − | − | |
| 6 | − | +++ | ++ | + | − | − | − | − | − | − | − | − | − | − | |
| 7 | − | +++ | +++ | ++ | + | − | − | − | − | c | − | − | − | − | |
| Control (adults) | 8 | − | +++ | +++ | +++ | +++ | − | − | − | − | c | +++ | +++ | − | − |
| 9 | − | +++ | +++ | +++ | +++ | +++ | +++ | ++ | − | − | +++ | +++ | − | − | |
| 10 | − | +++ | +++ | +++ | +++ | ++ | − | ++ | − | c | +++ | + | +++ | +++ | |
| 11 | − | +++ | +++ | +++ | +++ | + | − | + | − | − | ++ | +++ | − | − | |
| 12 | − | +++ | +++ | +++ | +++ | +++ | +++ | ++ | − | − | +++ | c | +++ | − | |
| 13 | − | +++ | +++ | +++ | +++ | − | + | + | − | c | +++ | c | +++ | +++ | |
| 14 | − | +++ | +++ | +++ | +++ | +++ | − | − | − | c | − | c | − | − | |
| 15 | − | +++ | +++ | +++ | +++ | +++ | +++ | +++ | d | − | ++ | d | d | +++ | |
| Control (puppies) | 16 | − | +++ | +++ | +++ | d | d | d | d | d | c | d | d | d | +++ |
| 17 | − | +++ | +++ | +++ | +++ | d | d | d | d | − | d | d | d | c | |
+++, culture positive at dilution 1/1000; ++, culture positive at dilution 1/100; +, culture positive at dilution 1/10; −, culture negative; c = contaminated; d = died or euthanised.
All control puppies from studies 3 and 4 added at the time of challenge developed leptospiraemia. Leptospires could be isolated from all vaccinated and control dogs of study 3. The total amount of Leptospira isolated from the blood over the first 7 days after challenge was significantly lower in the vaccinated dogs than in the control dogs (P < 0.0001, one-sided Student's t-test). In addition, the duration of leptospiraemia was significantly shorter in the vaccinated dogs compared to the control dogs (P = 0.0002, Student's t-test).
All control dogs and seven out of nine vaccinated dogs from study 4 developed leptospiraemia. Both amount and duration of leptospiremia were significantly reduced in the vaccinated dogs compared to the control dogs (P = 0.0001 for both, Wilcoxon's test).
3.6. Isolation of leptospires from urine, kidney and livers
An overview of the results is given in Fig. 1A (study 1) and B (study 2) and Table 4A, Table 4B (study 4). All urine samples from studies 1–4 were negative for leptospires before challenge. Leptospires could be isolated from the kidneys of all control dogs and from the urine of 37.5% and 30% of the control dogs in studies 1 and 2, respectively. In contrast, none of the vaccinated dogs from studies 1 and 2 had any positive urine or kidney cultures at the time of euthanasia. The proportion of dogs with renal infection, characterized by the presence of leptospires in urine and/or kidneys, was significantly lower in the vaccinated dogs compared to the controls dogs in both studies (Table 5 ).
Table 4B.
Results of blood, urine and kidney cultures after challenge of dogs with L. interrogans serovar icterohaemorrhagiae. Dogs were challenged 14 months after a primary course of two doses of vaccine (study 4: duration of immunity).
| Group | Dog no. | Days after challenge |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood |
Urine |
Kidney |
||||||||||||||||
| −2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 10 | 35 | −2 | 14 | 21 | 35 | Day of death | 35 | Day of death | ||
| Vaccinated | 1 | − | +++ | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| 2 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | |||
| 3 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | |||
| 4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||
| 5 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |||
| 6 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | |||
| 7 | − | ++ | − | − | − | − | − | − | − | − | − | − | − | − | − | |||
| 8 | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | |||
| 9 | − | ++ | − | − | − | − | − | − | − | − | − | − | − | − | − | |||
| Control (adults) | 10 | − | +++ | +++ | +++ | − | − | − | d | d | d | − | d | d | d | NS | d | − |
| 11 | − | +++ | +++ | + | − | + | − | − | − | − | − | +++ | +++ | − | − | |||
| 12 | − | +++ | +++ | + | − | − | − | − | − | − | − | +++ | +++ | +++ | +++ | |||
| 13 | − | +++ | +++ | + | − | − | − | − | − | − | − | +++ | +++ | ++ | +++ | |||
| 14 | − | +++ | +++ | + | − | − | − | − | − | − | − | +++ | +++ | − | − | |||
| 15 | − | +++ | +++ | +++ | +++ | +++ | − | − | d | d | − | d | d | d | + | d | − | |
| 16 | − | +++ | +++ | + | − | − | − | − | − | − | − | +++ | +++ | ++ | ++ | |||
| 17 | − | +++ | +++ | +++ | − | − | − | − | − | − | − | +++ | +++ | − | +++ | |||
| 18 | − | +++ | − | + | − | − | − | − | − | − | − | − | − | − | − | |||
| 19 | − | +++ | +++ | ++ | − | − | − | − | − | − | − | +++ | +++ | d | + | d | + | |
| Control (puppies) | 20 | − | + | + | + | + | + | + | d | d | d | − | d | d | d | + | d | + |
| 21 | − | + | + | + | + | − | + | − | − | − | − | + | + | + | + | |||
+++, culture positive at dilution 1/1000; ++, culture positive at dilution 1/100; +, culture positive at dilution 1/10; −, culture negative; c = contaminated; d = died or euthanised; NS = no sample.
Table 5.
Incidence of renal carrier state after challenge (any dog with at least one positive urine or kidney culture was defined as a renal carrier).
| Study | Group | Incidence of renal carries (no. of dogs) |
P-value* | |
|---|---|---|---|---|
| Absent | Present | |||
| 1 | V | 9 | 0 | 0.00005 |
| C | 0 | 8 | ||
| 2 | V | 9 | 0 | 0.00001 |
| C | 0 | 10 | ||
| 3 | V | 5 | 2 | 0.035 |
| C | 1 | 7 | ||
| 4 | V | 9 | 0 | 0.0006 |
| C | 2 | 8 | ||
Study 1 = onset of immunity study L. interrogans serovar canicola; study 2 = onset of immunity study L. interrogans serovar icterohaemorrhagiae; study 3 = duration of immunity study L. interrogans serovar canicola; study 4 = duration of immunity study L. interrogans serovar icterohaemorrhagiae. Abbreviations: V = vaccinated; C = control.
Fisher's exact test.
Seven out of eight adult control dogs from study 3 shed L. interrogans serovar canicola in the urine, and the kidneys of three control dogs were cultured positive. Leptospires could be isolated from the urine of two vaccinated dogs and from the kidney of one vaccinated dog. The incidence of renal carriers was significantly lower in the vaccinated dogs than in the control dogs (P = 0.035, Fisher's exact test, Table 5).
Urine could be collected from only nine adult control dogs of study 4. L. interrogans serovar icterohaemorrhagiae could be recovered from the urine of eight dogs and from the kidneys of five dogs, but not from the livers. None of the vaccinated dogs shed leptospires in the urine, and leptospires could not be isolated from any of the kidneys or livers at post-mortem examination. The incidence of renal carriage was significantly lower in the vaccinated dogs compared to the control dogs (P = 0.0006, Fisher's exact test, Table 5).
3.7. Necropsy and histopathology
3.7.1. Necropsy
The macroscopic lesions detected during necropsy were similar for all dogs from studies 1–4 that died or were humanely euthanised due to terminal illness. Gross findings included haemorrhages on the surface of the lungs and the abdominal cavity and the presence of red stained fluid in the pleural and abdominal cavity. The kidneys were enlarged and friable. When urine was present from these animals, it was tinged with blood up to severely haematuric. Typically, faecal material was liquid and tinged with blood having a fetid odour. In some dogs, the sclera, gingival, and subcutaneous tissues were jaundiced. Apart from some reactive mesenteric lymph nodes, control dogs that survived the experimental infection appeared normal on gross visual examination, as well as did all vaccinated dogs.
3.7.2. Microscopic examination
Prominent lesions in the kidneys of terminally ill control dogs included subacute to severe interstitial glomerulo-nephritis and tubular degeneration. Moderate to severe diffuse hepatic lesions were found in dogs with jaundice, mostly consisting of an acute degenerative hepatitis characterized by hepato-cellular dissociation and necrosis. Interestingly, in three surviving control dogs of study 4, there was evidence of subacute multi-focal interstitial nephritis compatible with leptospirosis infection. No specific lesions were found in the vaccinated dogs. Only the kidneys from dogs diagnosed with acute renal failure stained positive by Warthin–Starry silver indicating the presence of leptospires.
4. Discussion
A number of factors must be considered in the design and evaluation of efficacy trials for canine leptospirosis vaccines. These factors include the age of the dogs, recommended vaccination schedule, selection of challenge strain, and challenge method. The ultimate goal of vaccination against leptospirosis is to protect dogs against clinical disease, as well as against the establishment of a renal carrier state. The latter protection is especially important because carrier dogs can be a public health hazard when in close contact to humans (Center for Disease Control, 1972; Trevejo et al., 1998). Therefore, leptospirosis vaccines should be tested in models that reliably produce the series of clinical signs and renal colonization pattern that the vaccine is designed to prevent or reduce. Canine leptospirosis has been a difficult disease to reproduce under experimental conditions and usually requires the use of young puppies and a high challenge dose (Keenan et al., 1978). Furthermore, clinical signs may vary depending on the isolate (Greenlee et al., 2004), altered expression of bacterial proteins resulting from culture passage (Greenlee et al., 2004), and the timing of harvest after hamster passage (Minke, personal observation). Even when taking these factors into account, reported infection in control dogs often results in no evident (Klaasen et al., 2003) or subclinical disease (Broughton and Scarnell, 1985). In only a few studies has severe lethal disease been reported following experimental infection of dogs with L. interrogans serovar canicola (Schreiber et al., 2005a, Kerr and Marshall, 1974) or L. interrogans serovar icterohaemorrhagiae (Schreiber et al., 2005b, Kerr and Marshall, 1974). In our studies, puppies experimentally infected with L. interrogans serovars icterohaemorrhagiae and canicola developed a spectrum of disease that ranged from mild to lethal in severity. Renal, hepatic and hematological signs dominated the clinical presentation and supported the polysystemic nature of Leptospira infection. The overall mortality rate in control puppies was 60% and 58% for L. interrogans serovars icterohaemorrhagiae and canicola, respectively. Under these extreme challenge conditions, clinical signs in the vaccinated pups were rare, and when observed, mild and transient in nature. Clinical disease in adult dogs was less severe, but unexpectedly, we were able to induce morbidity and mortality in adult dogs as well, further demonstrating the severity of our challenge models. These results are in sharp contrast with those published by Klaasen et al. (2003), where no evident clinical symptoms associated with canine leptospirosis were observed in the adult control dogs. The reason for this difference is not clear but may be attributable to the choice of challenge strain and/or challenge dose. Hematological parameters and blood biochemistry were not intended to be a major criterion to assess the efficacy of the vaccine, but they supported the diagnosis of leptospirosis. Thrombocytopenia was the main hematological abnormality observed in control dogs after challenge, while vaccinated dogs were protected against thrombocytopenia. This hematological disorder is a common finding in canine leptospirosis (Greene, 1998) and has been reported after experimental challenge (Tronel et al., 1999, André-Fontaine et al., 2003, Klaasen et al., 2003, Schreiber et al., 2005a, Schreiber et al., 2005b). Blood biochemistry illustrated the alteration of hepatic and renal functions in control dogs. Significant changes in urea nitrogen, creatinine, bilirubin, SGOT, and SGPT were observed only in the control dogs with severe clinical signs. The increased levels of SGPT in two vaccinated dogs did not correlate with the clinical observations. It cannot be ruled out that the massive challenge induced transient liver damage in those dogs. At necropsy, macroscopic examination of the animals was consistent with clinical signs, and typical lesions of leptospirosis were observed in dogs succumbing to the challenge. In addition, microscopic analysis showed that even surviving controls had lesions of interstitial nephritis compatible with leptospirosis, whereas no specific lesions were observed in the vaccinated dogs.
The second objective of our studies was to determine whether EURICAN® L would protect dogs against the development of a renal carrier state. As in many instances, isolation results on kidney and urine samples were not concordant in our studies, we defined a renal carrier as a dog with at least one positive urine or kidney culture. Discrepancies between urine and kidney isolation results have also been reported in the literature and were attributed to the presence of specific inhibiting enzymes from kidney cells (Faine, 1998), high urine osmolarity and pH (Nervig and Garrett, 1979), and the fact that leptospires are shed intermittently (Nervig and Garrett, 1979). Overall we found that 100% of the control pups and 83% of the adult controls became renal carriers. Despite the heavy challenges, none of the 18 vaccinated puppies and only 2 out of the 16 vaccinated adult dogs developed a renal carrier state. It should be stressed that the challenge doses that we used were probably much higher than those observed in a natural infection, suggesting that the protection against renal carriage might be almost complete in the field. The literature has conflicting reports on the efficacy of leptospiral bacterins to protect against the renal carrier state. Much of the variability is likely the result of differences in the immunogenicity of the bacterins used, as was demonstrated by André-Fontaine et al. (2003). In that study, only one of the three commercial vaccines completely protected dogs against the establishment of a renal carrier state shortly after primo-vaccination in a challenge model that induced no mortality and severe clinical disease in only one out of the six control puppies. Culture appeared to more sensitive in our hands than silver staining to detect leptospires in the kidney, with the additional advantage that infectious material is detected, rather than fragments of the bacteria. We did not explore alternative detection methods like PCR or immuno-fluorescence.
Typical serological findings in the present studies were the relatively low and short-lived antibody responses against both serovars after vaccination. Several studies have reported low antibody responses after administration of leptospirosis inactivated vaccines (André-Fontaine et al., 2003, Schreiber et al., 2005b, Klaasen et al., 2003, Steger-Lieb et al., 1999). Furthermore, no correlation could be established between antibody titres after vaccination and protection against experimental infection. The absence of correlation has been classically described in other studies as well (Broughton and Scarnell, 1985, André-Fontaine et al., 2003, Klaasen et al., 2003, Schreiber et al., 2005a).
It is concluded that a primary course of two doses of EURICAN® L provided quick onset and long-term protection against both clinical leptospirosis and the renal carrier stage. This vaccine should provide veterinarians with a powerful tool to prevent clinical disease in dogs and zoonotic transmission of leptospirosis to humans.
Acknowledgements
The authors wish to thank Merial R&D Department for their help and Bob Nordgren for his critical reading of the manuscript.
Footnotes
EURICAN® is a registered trademark of Merial in France and elsewhere.
DIMAZON® is a registered trademark of Intervet Internationl B.V. in the United Kingdom and elsewhere.
STATGRAPHICS® is a registered trademark of Statistical Corporation in the United States of America; SAS® is a registered trademark of SAS Institute Inc. in the United States of America and elsewhere.
References
- André-Fontaine G., Branger C., Gray A.W., Klaasen H.L. Comparison of the efficacy of three commercial bacterins in preventing canine leptospirosis. Vet Rec. 2003;153(August (6)):165–169. doi: 10.1136/vr.153.6.165. [DOI] [PubMed] [Google Scholar]
- Bolin C.A. Diagnosis of leptospirosis: a reemerging disease of companion animals. Semin. Vet. Med. Surg. (Small Anim.) 1996;11(August (3)):166–171. doi: 10.1016/s1096-2867(96)80029-6. [DOI] [PubMed] [Google Scholar]
- Boutilier P., Carr A., Schulman R.L. Leptospirosis in dogs: a serologic survey and case series 1996 to 2001. Vet. Ther. 2003;4(2):178–187. (Summer) [PubMed] [Google Scholar]
- Broughton E.S., Scarnell J. Prevention of renal carriage of leptospirosis in dogs by vaccination. Vet. Rec. 1985;117(September (12)):307–311. doi: 10.1136/vr.117.12.307. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control, 1972. Leptospirosis surveillance. Annual Summary, p. 3.
- Coyne M.J., Burr J.H.H., Yule T.D., Harding M.J., Tresnan D.B., McGavin D. Duration of immunity in dogs after vaccination or naturally acquired infection. Vet. Rec. 2001;149:509–515. doi: 10.1136/vr.149.17.509. [DOI] [PubMed] [Google Scholar]
- Faine S. Leptospirosis. In: Collier L., Ralows A., Sussman M., editors. Topley and Wilson's Microbiology and Microbial Infections. Edward Arnold; London: 1998. pp. 849–869. [Google Scholar]
- Geisen V., Stengel C., Brem S., Müller W., Greene C., Hartmann K. Canine leptospirosis infections—clinical signs and outcome with different suspected Leptospira serogroups (42 cases) J. Small Anim. Pract. 2007;48(June (6)):324–328. doi: 10.1111/j.1748-5827.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Greene C.E., editor. Infectious Disease of the Dog and Cat. 2nd ed. W.B. Saunders Co.; Philadelphia: 1998. pp. 273–281. [Google Scholar]
- Greenlee J.J., Bolin C.A., Alt D.P., Chevilla N.E., Andreasen C.B. Clinical and pathologic comparison of acute leptospirosis in dogs caused by two strains of Leptospira kirschneri serovar grippotyphosa. AJVR. 2004;65(8):1100–1107. doi: 10.2460/ajvr.2004.65.1100. [DOI] [PubMed] [Google Scholar]
- Hartman E.G. Epidemiological aspects of canine leptospirosis in the Netherlands. Zbl. Bakt. Hyg. 1984;258:350–359. doi: 10.1016/s0176-6724(84)80053-x. [DOI] [PubMed] [Google Scholar]
- Huhn R.G., Baldwin C.D., Cardella M.A. Immunity to leptospirosis: bacterins in dogs and hamsters. Am. J. Vet. Res. 1975;36(January (1)):71–74. [PubMed] [Google Scholar]
- Keenan K.P., Alexander A.D., Montgomery C.A. Pathogenesis of experimental leptospira interrogans serovar bataviae, infection in the dog: microbiological, clinical, hematologic and biochemical studies. Am. J. Vet. Res. 1978;39:449–454. [PubMed] [Google Scholar]
- Kerr D.R., Marshall V. Protection against the renal carrier state by a canine leptospirosis vaccine. Vet. Med. Small Anim. Clin. 1974:1157–1160. [PubMed] [Google Scholar]
- Klaasen H.L.B.M., Molkenboer M.J.C.H., Vrijenhoek M.P., Kaashoek M.J. Duration of immunity in dogs vaccinated against leptospirosis with a bivalent inactivated vaccine. Vet. Microbiol. 2003;95(August (1–2)):121–132. doi: 10.1016/s0378-1135(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Kahn C.M., editor. Merck Veterinary Manual. 9th ed. Merck & Co, Inc.; Whitehouse Station, NJ, USA: 2005. p. 2584. [Google Scholar]
- Monograph 0447 of the European Pharmacopoiea 2002, 1:2270.
- Moore G.E., Guptill L.F., Glickman N.W., Caldanaro R.J., Aucoin D., Glickman L.T. Canine leptospirosis, United States, 2002–2004. Emerg. Infect. Dis. 2006;12(March (3)):501–503. doi: 10.3201/eid1203.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervig R.M., Garrett L.A. Use of furosemide to obtain bovine urine samples for leptospiral isolation. Am. J. Vet. Res. 1979;40(August (8)):1197–1200. [PubMed] [Google Scholar]
- Scanziani E., Origgi F., Giusti A.M., Iacchia G., Vasino A., Pirovano G., Scarpa P., Tagliabue S. Serological survey of leptospiral infection in kennelled dogs in Italy. J. Small Anim. Pract. 2002;43(April (4)):154–157. doi: 10.1111/j.1748-5827.2002.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Schreiber P., Martin V., Najbar W., Sanquer A., Gueguen S., Lebreux B. Prevention of a severe disease by Leptospira vaccination with a multivalent vaccine. Rev. Med. Vet. 2005;156(8–9):427–432. doi: 10.1016/j.vetmic.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Schreiber P., Martin V., Najbar W., Sanquer A., Gueguen S., Lebreux B. Prevention of renal infection and urinary shedding in dogs by a Leptospira vaccination. Vet Microbiol. 2005;108(June (1–2)):113–118. doi: 10.1016/j.vetmic.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Steger-Lieb A., Gerber B., Nicolet J., Gaschen F. An old disease with a new face: canine leptospirosis does not loose its relevance. Schweiz. Arch. Tierheilkd. 1999;141(11):499–507. [PubMed] [Google Scholar]
- Stokes J.E., Kaneene J.B., Schall W.D., Kruger J.M., Miller R., Kaiser L., Bolin C.A. Prevalence of serum antibodies against six Leptospira serovars in healthy dogs. J. Am. Vet. Med. Assoc. 2007;230(June (11)):1657–1664. doi: 10.2460/javma.230.11.1657. [DOI] [PubMed] [Google Scholar]
- Trevejo R.T., Rigau-Pérez J.G., Ashford D.A., McClure E.M., Jarquín-González C., Amador J.J., de los Reyes J.O., Gonzalez A., Zaki S.R., Shieh W.J., McLean R.G., Nasci R.S., Weyant R.S., Bolin C.A., Bragg S.L., Perkins B.A., Spiegel R.A. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J. Infect. Dis. 1998;178(November (5)):1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- Tronel J.P., Bey R.F., Thevenon J., Minke J., Milward F. Efficacy of Leptodog vaccine in dogs demonstrated by experimental challenge: evaluation at short term and duration of immunity. Proceedings of the 24th World Small Animal Veterinary Congress Lyon, September 23–26, 1999; France; 1999. [Google Scholar]
- Ward M.P., Glickman L.T., Guptill L.E. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (1970–1998) J. Am. Vet. Med. Assoc. 2002;220(January (1)):53–58. doi: 10.2460/javma.2002.220.53. [DOI] [PubMed] [Google Scholar]
- Ward M.P., Guptill L.F., Prahl A., Wu C.C. Serovar-specific prevalence and risk factors for leptospirosis among dogs: 90 cases (1997–2002) J. Am. Vet. Med. Assoc. 2004;224(June (12)):1958–1963. doi: 10.2460/javma.2004.224.1958. [DOI] [PubMed] [Google Scholar]


