Abstract
Feline infectious peritonitis virus (FIPV) is a major pathogen of Felidae. Despite the extensive efforts taken in the past decades, development of the “ideal” live attenuated FIPV vaccine was not successful yet.
In the present study, we provide data of immunisation experiments with a recombinant FCoV pair differing only in the truncation (PBFIPV-DF-2) or completion (PBFIPV-DF-2-R3i) of their ORF3abc regions. In our previous in vivo studies, these viruses proved to show the characters of low virulent or avirulent FCoV phenotypes, respectively. Therefore, we hypothesised the ability of these viruses, as possible vaccine candidates, in conferring protection in specific pathogen free (SPF) Domestic Shorthair as well as in conventional purebred British Shorthair cats.
In SPF cats, after two oronasal and two intramuscular vaccinations with two weeks intervals, both vaccine candidates provided 100% protection against lethal homologous challenge with the highly virulent FIPV DF-2 strain. In contrast, the conventional purebred British Shorthair cats did not develop protection when they were immunised with the same vaccination regimes. In these groups 100% of the PBFIPV-DF-2-R3i immunised animals developed antibody-dependent enhancement (ADE). Prolonged survival was observed in 40% of the animals, while 60% showed fulminant disease course.
Genetic and more probably immunological differences between the SPF and non-SPF purebred kittens can explain the different outcome of the vaccination experiment. Our data highlight the diverse immune responses between SPF and conventional cats and suggest a decisive role of previous infection by heterologous causative agents in the outcome of the vaccination against FIP.
Keywords: Feline coronavirus, Feline infectious peritonitis, Vaccine, Protection
1. Introduction
Feline coronaviruses (FCoVs), members of the Alphacoronavirus genus within the Coronaviridae family are major pathogens of Felidae with worldwide distribution. Seroprevalence in cat populations can be as high as 90% (Pedersen, 2009). From the two serotypes of FCoV, type I is more predominant (80–95%) (Hohdatsu et al., 1992, Kummrow et al., 2005), while the less prevalent type II FCoV probably emerged via a double recombination process between type I FCoV and type II canine coronavirus (CCoV) (Herrewegh et al., 1998).
Both serotypes occur in two pathotypes: feline enteric coronavirus (FECV) replicates in the lower portion of the intestinal tract, spreads by faecal-oral route, and its clinical appearance is characterised by mild or unapparent enteritis (Pedersen et al., 1981, Herrewegh et al., 1997). In contrast, feline infectious peritonitis virus (FIPV) efficiently replicates in macrophages and monocytes, occurs sporadically but causes a highly lethal systemic granulomatous disease, feline infectious peritonitis (FIP), which can manifest in either wet or dry form (Addie and Jarrett, 1992, de Groot-Mijnes et al., 2005).
Although extensive efforts have been taken in the past decades, development of the “ideal” live attenuated FIPV vaccine replicating in the body without clinical signs, and inducing protective immunity against FIPV (Pedersen, 1989) has not been crowned with complete success yet. Vaccination with closely related heterologous live CoVs did not confer protection at all (Barlough et al., 1984, Barlough et al., 1985, Stoddart et al., 1988, Woods and Pedersen, 1979).
Similarly to inactivated and recombinant FCoV subunit vaccines (reviewed in Haijema et al., 2007), immunisation with FECV, low-virulence FIPV, or sublethal amounts of virulent FIPV elicited only partial protection (Pedersen and Black, 1983, Pedersen et al., 1984, Pedersen and Floyd, 1985) frequently leading to antibody enhancement of the disease (ADE) and the so-called early death syndrome.
Currently, a temperature-sensitive strain of FIPV is marketed worldwide with an ability to protect cats against FIPV (Gerber et al., 1990, Gerber, 1995) but its efficacy is uncertain (McArdle et al., 1995, Scott et al., 1995, Fehr et al., 1997). The most promising results were obtained with recombinant FIPV mutants lacking the ORF3abc or ORF7ab regions that provided 100% and 80% protection after a lethal homologous challenge (Haijema et al., 2004), respectively. However, no follow-up studies using these vaccine candidates have been published so far.
In the present study, we provide data of immunisation experiments with a recombinant FCoV pair differing only in the truncation (PBFIPV-DF-2) and intactness (PBFIPV-DF-2-R3i) of their ORF3abc regions (Bálint et al., 2012). In previous in vivo studies using specific pathogen free (SPF) cats, PBFIPV-DF-2 proved to be low virulent, shed only at limited titres in faeces, and was completely cleared by the immune system but elicited medium level immune response, while PBFIPV-DF-2-R3i showed active intestinal replication and faecal shedding, and it possessed completely avirulent phenotype (Bálint et al., 2013). Considering these advantageous characteristics of the two recombinant FCoVs, we evaluated their innocuity and efficacy as vaccine candidates in conferring protection in SPF as well as in conventional purebred British Shorthair cats.
2. Materials and methods
2.1. Cells and viruses
Felis catus whole foetus 4 (FCWF-4) cells were used for virus propagation, titration and virus neutralisation tests. The cell line was maintained as monolayer culture in Dulbecco's Modified Eagle Medium (Sigma–Aldrich, Saint Louis, MO, USA) supplemented with 10% foetal bovine serum (FBS), 0.3 mg/ml glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.25 μg/ml amphotericin B, 1 mM sodium pyruvate and 1% non-essential amino acids (Sigma–Aldrich). The FIPV DF-2 strain was kindly provided by Berndt Klingeborn (SVA, Uppsala, Sweden). The whole genome of FIPV DF-2 was cloned into the pBeloBAC 11 low-copy vector that allows efficient intracellular production of the viral RNA from the cDNA by the cytomegalovirus (CMV) immediate-early promoter in order to gain the recombinant FCoV PBFIV-DF-2 (GenBank accession number: JQ408981.1). The originally truncated ORF3abc of this virus was replaced with the intact ORF3abc of a type I “FCoV-like” canine coronavirus (CCoV) reference strain Elmo/02 to construct PBFIPV-DF-2-R3i (GenBank accession number: JQ408980.1) (Bálint et al., 2012).
2.2. Animal experiments
SPF Domestic Shorthair IQHsdCpb kittens (Isoquimen SL, Barcelona, Spain) and conventional British Shorthair cats from a FCoV negative Hungarian cattery (regularly monitored for two generations) were used in the challenge experiments. The non-SPF cats were originated from three non-related queens and two toms. Kittens arrived at the facility at the age of 8–12 weeks. They were acclimated and used in the studies at the age of 14–18 weeks. The animals were kept in separate groups in a closed facility. Their FCoV negative status was checked with PCR and virus neutralisation tests. The absence of feline parvovirus (FPV), feline herpesvirus (FHV), feline calicivirus (FCV), feline immunodeficiency virus (FIV) and feline leukaemia virus (FELV) in the conventional cats was confirmed by PCR and/or ELISA tests. For study purposes, the same group structure was established both for SPF and conventional kittens such as two study groups (Group 1 and Group 2, each n = 5) and a control group (C-Group, n = 2) were formed. Kittens were inoculated oronasally (D0 and D14) and intramuscularly (D28 and D42) with 103 50% tissue culture infective doses (TCID50) of the recombinant viruses PFIPV-DF-2 (Group 1) and PFIPV-FD-2-R3i (Group 2), respectively. The vaccinated animals and unvaccinated controls were oronasally challenged (D56) with 103 TCID50 of the parent virus FIPV DF-2. The whole clinical observation period of time was altogether 8 months long. During this time kittens were clinically examined on a daily basis. Cats were scored for several clinical signs as described earlier (Haijema et al., 2004). Briefly, scoring was based on depression (inactivity for three consecutive days, 1 point), anorexia (not eating for three consecutive days, 1 point), and neurological disorders (swaggering, 1 point) on a daily basis, while fever (40.1 °C, 1 point), jaundice (yellow plasma, 1 point), weight loss (loss of 2.5% of body weight per week, 1 point), and lymphopenia (lymphocyte count of <0.5 × 109/l) was scored on weekly basis. Kittens showing signs of terminal FIP were euthanized in order to avoid unnecessary suffering, while healthy animals were exterminated at day 90 post-challenge (p.c.), followed by full post-mortem examination. All animal experiments were approved and supervised by the Ethical and Animal Welfare Committee of the National Food Chain Safety Office.
2.3. Pathology and histopathology
All animals that died or were euthanized during the experiment were subjected to pathological examination. Carcasses were dissected within two hours of the death. Samples taken from spleen, kidney, liver, lung, brain and intestine were fixed in 8% formaldehyde and embedded in paraffin wax. The blocks were sectioned at 4–6 μm and the sections were stained with haematoxylin-eosin and examined under light microscope.
2.4. Detection of virus shedding
To determine virus shedding, faecal and oropharyngeal swabs were taken at D3, D7, D10, D14, D21, D28, D35, D42, D49, D56, D62, D70 an D77 and placed in 500 μl of phosphate buffered saline (PBS 1×). After vortexing and 30 min incubation, the swabs were removed, and the extract was centrifuged at 1000 × g for 10 min to remove cell debris. The supernatant was collected and used for subsequent PCR.
Viral RNA was purified using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden Germany). To measure the copy numbers of the genome of the recombinant FCoV, a TaqMan assay targeting the 5′ end of the FIPV DF-2 genome was applied (Bálint et al., 2012).
2.5. Virus neutralisation assay
Serum samples were taken using Vacuette® tube (Greiner Bio-One, Germany) at the same days as faecal samples. For virus neutralisation (VN) assay, two-fold dilutions of heat-inactivated serum from kittens (50 μl) were incubated for 1 h at 37 °C with equal aliquots of FIPV DF-2 (50 μl of 103.5 TCID50/ml). The viruses were then added to FCWF-4 cells showing 70% confluency in a 96-well plate, and incubated for 48 h, until the development of cytopathic effect. Neutralising activity was determined by end-point dilution (Shiba et al., 2007).
3. Results
3.1. Immunisation of SPF cats with the recombinant FCoVs as vaccine candidates
To study whether PBFIPV-DF-2 and PBFIPV-DF-2-R3i inoculation would protect cats against a homologous FIPV challenge, vaccination and challenge experiments were performed. Group 1 and Group 2 cats were vaccinated oronasally twice with PFIPV-DF-2 and PBFIPV-DF-2-R3i (103 TCID50 at D0 and D14), respectively.
The in vivo characteristics of the recombinant viruses were similar to those of earlier experiments (Bálint et al., 2013). Cats in Group 1 showed only early mild clinical signs including transient fever from D3 to D8, anorexia and slight lymphopenia (Table 1 ), while cats in Group 2 showed neither any clinical signs typical of FIP nor diarrhoea (Table 1).
Table 1.
Total clinical scores of SPF cats after oronasal (D0 and D14) and parenteral (days 28 and 42) vaccination with PBFIPV (Group 1, n = 5) and PBFIPV-DF-2-R3i (Group 2, n = 5). The unvaccinated controls (C-Group, n = 2) were vaccinated with PBS.
| Virus and animal no. | Clinical score |
Total clinical score | Day of death post-vaccination | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fever | Depression | Anorexia | Jaundice | Neurological disorder | Weight loss | Lymphopenia | |||
| Group 1 | |||||||||
| 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 3 | – |
| 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 4 | – |
| 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | – |
| 4 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 4 | – |
| 5 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 | – |
| Group 2 | |||||||||
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C-Group | |||||||||
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
No oropharyngeal shedding of either PBFIPV-DF-2 or PBFIPV-DF-2-R3i was detected during the whole period of the immunisation experiments (data not shown). Shedding of PBFIPV-DF-2 in faeces of Group 1 was detected in four animals from D3 to D42 with very variable amounts close to the detection limit of the genomic quantitative RT-PCR (4.5 × 100–4 × 101 FCoV RNA copies per μl faecal extract) (Fig. 1 ). Group 2 cats began to shed PBFIPV-DF-2-R3i from D3, virus shedding peaked at D7 with 7.8 × 105 FCoV RNA copies per μl faecal extract, remained high until D14, then began to decrease until reaching 3.2 × 102 FCoV RNA copies per μl faecal extract at D49, and remained at this level until the end of the experiment (Fig. 1).
Fig. 1.
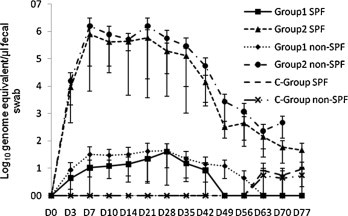
Faecal shedding of FCoV after oronasal (D0 and D14) and parenteral (D28 and D42) vaccination of SPF and conventional cats with PBFIPV-DF-2 (Group 1, n = 5), PBFIPV-DF-2-R3i (Group 2, n = 5) and PBS (C-Group, n = 5) followed by oronasal challenge (D56) with FIPV DF-2 (n = 20). The means of groups are given. Error bars represent standard deviations.
No neutralising activity was detected at D0 in any cat sera. In contrast, by D28, all the Group 1 cats had seroconverted and showed medium titres (1:160–1:640) of neutralising antibodies (Fig. 2 ). Group 2 cats showed variable results. Only three animals seroconverted by D28, and their VN titres remained at low levels (1:80–1:160) compared with those of the Group 1 cats (Fig. 2).
Fig. 2.
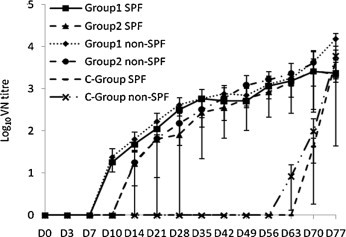
Induction of FCoV-neutralising antibodies after oronasal (D0 and D14) and parenteral (D28 and D42) vaccination of SPF and conventional cats with PBFIPV (Group 1, n = 5), PBFIPV-DF-2-R3i (Group 2, n = 5) and PBS (C-Group) followed by oronasal challenge (D56) with FIPV DF-2 (n = 20). The means of groups are given. Error bars represent standard deviations.
Since level of the humoral immune response after oronasal vaccination with the recombinant viruses was relatively low, two intramuscular vaccinations (103 TCID50 at D28 and D42) were applied. A control group (C-Group) was mock vaccinated with PBS. Similarly to the oronasal vaccinations, the intramuscularly inoculated kittens did not develop any signs FIP (Table 1). No faecal virus shedding of PBFIPV-DF-2 of Group 1 cats was detected, while faecal shedding of PBFIPV-DF-2-R3i from Group 2 kittens continued at low level (Fig. 1). Oropharyngeal shedding was not observed from any animal of the two vaccinated groups (data not shown). Neutralising antibody titres raised in all Group 1 animals after intramuscular vaccinations (from 1:160–1:640 to 1:640–1:2560, p = 0.067). Interestingly, one Group 2 animal did not seroconvert (the other's titres significantly raised from 1:80–1:160 to 1:320–1:1280, p = 0.023), and somewhat lower antibody values were obtained in four Group 2 animals compared to Group 1 (p = 0.51) (Fig. 2).
3.2. Challenge experiment on SPF cats
At D56, all kittens were challenged oronasally with 103 TCID50 of the virulent FIPV DF-2 strain, and were monitored for 6 months. The C-Group cats showed severe clinical signs of FIP starting on the second week (Table 2 ). The two kittens died at days 22 and 24 post-challenge (p.c.). The pathological and histopathological examinations revealed lesions characteristic of systemic, non-effusive FIP.
Table 2.
Total clinical scores of PBFIPV (n = 5) and PBFIPV-DF-2-R3i (n = 5) vaccinated SPF cats after challenge with FIPV DF-2 (n = 10) at D56.
| Virus and animal no. | Clinical score |
Total clinical score | Day of death post-challenge | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fever | Depression | Anorexia | Jaundice | Neurological disorder | Weight loss | Lymphopenia | |||
| Group 1 | |||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| Group 2 | |||||||||
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | – |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| C-Group | |||||||||
| 11 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 13 | 22 |
| 12 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 11 | 24 |
On the contrary, vaccination with both PBFIPV-DF-2 and PBFIPV-DF-2-R3i proved to be highly efficacious against lethal FIPV challenge. Group 1 and Group 2 cats remained healthy (Table 2) and survived for at least 6 months. The VN titres did not boost significantly after the challenge (Fig. 2). Pathological examinations confirmed that none of the vaccinated cats showed any lesion characteristic of FIP.
3.3. Immunisation and challenge experiments on conventional cats
To study the efficacy of the vaccine candidates in field conditions, the above experiment with the same protocols was repeated using conventional cats. Neither of the vaccinated kittens showed any signs of fatal FIP (Table 3 ). The Group 1 inoculated animals showed slightly stronger clinical signs than their SPF counterparts including lymphopenia, which is a characteristic sign of early FIPV infection, while only anorexia was observed in two of the Group 2 kittens (Table 3). Oropharyngeal virus shedding was not detected in any vaccinated cat (data not shown). Faecal virus shedding of conventional cats was approximately 0.5 log10 higher, more intense and of longer duration (14 days extended in PBFIPV inoculated animals) compared with that of the SPF animals (Fig. 1). Neutralising antibodies were induced in all animals showing approximately one log2 higher titres than was observed in the SPF animals (Fig. 2).
Table 3.
Total clinical scores of conventional cats after oronasal (D0 and D14) and parenteral (days 28 and 42) vaccination with PBFIPV (Group 1, n = 5) and PBFIPV-DF-2-R3i (Group 2, n = 5). The unvaccinated controls (C-Group, n = 2) were vaccinated with PBS.
| Virus and animal no. | Clinical score |
Total clinical score | Day of death post-vaccination | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fever | Depression | Anorexia | Jaundice | Neurological disorder | Weight loss | Lymphopenia | |||
| Group 1 | |||||||||
| 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 | – |
| 2 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 | – |
| 3 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 4 | – |
| 4 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 4 | – |
| 5 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 | – |
| Group 2 | |||||||||
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 7 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | – |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 9 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | – |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C-Group | |||||||||
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – |
After challenge, all the control cats showed clinical signs of FIP followed by death at day 19 and 21 p.c. similarly to the SPF animals with a slightly faster development of the disease and earlier death (Table 4 ). The pathological findings were characteristic of systemic, non-effusive FIP.
Table 4.
Total clinical scores of PBFIPV (Group 1, n = 5), PBFIPV-DF-2-R3i (Group 2, n = 5) and PBS (C-Group, n = 2) vaccinated conventional cats after challenge with FIPV DF-2 (n = 10) at D56.
| Virus and animal no. | Clinical score |
Total clinical score | Day of death post-challenge | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fever | Depression | Anorexia | Jaundice | Neurological disorder | Weight loss | Lymphopenia | |||
| Group 1 | |||||||||
| 1 | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 8 | 65 |
| 2 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 7 | 86 |
| 3 | 2 | 2 | 3 | 3 | 1 | 3 | 2 | 16 | 16 |
| 4 | 1 | 2 | 3 | 3 | 1 | 3 | 2 | 15 | 19 |
| 5 | 1 | 3 | 3 | 3 | 1 | 3 | 2 | 16 | 20 |
| Group 2 | |||||||||
| 6 | 2 | 3 | 3 | 3 | 1 | 3 | 2 | 17 | 14 |
| 7 | 1 | 3 | 2 | 1 | 1 | 3 | 2 | 13 | 18 |
| 8 | 2 | 3 | 3 | 3 | 1 | 3 | 2 | 17 | 15 |
| 9 | 2 | 3 | 3 | 3 | 1 | 3 | 2 | 17 | 17 |
| 10 | 1 | 2 | 3 | 1 | 1 | 3 | 2 | 13 | 19 |
| C-Group | |||||||||
| 11 | 2 | 2 | 2 | 3 | 1 | 3 | 2 | 15 | 19 |
| 12 | 2 | 2 | 2 | 3 | 1 | 2 | 2 | 14 | 21 |
Vaccination with PBFIPV-DF-2 showed variable results against lethal FIPV challenge. Two Group 1 cats (cat 1and cat 2) had slightly higher VN antibody titres (Fig. 3 ) and developed FIP later, from day 50–70 p.c, and died at days 65 and 86 p.c., respectively. Post-mortem examinations revealed characteristic FIP lesions similar to those observed in the fulminant course of the disease. Three cats of Group 1 developed FIP within a week (Table 4) followed by death between days 16–20 p.c. The fulminant course of the disease in the majority of the vaccinated animals suggested at least partial adverse effect of vaccination instead of protection.
Fig. 3.
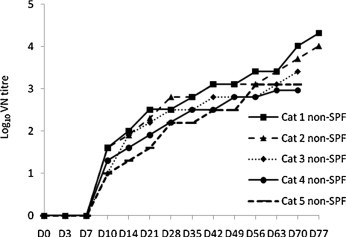
Induction of FCoV-neutralising antibodies after oronasal (D0 and D14) and parenteral (D28 and D42) vaccination of conventional cats with PBFIPV (Group 1, n = 5) followed by oronasal challenge (D56) with FIPV DF-2 (n = 5).
In Group 2 all animals developed FIP within a week (Table 4) followed by death between days 14–19 p.c. The exclusive fulminant course of the disease also indicated clear adverse characteristics of this vaccine candidate. In all perished animals showing fulminant FIP, the VN titres elevated rapidly before death (Fig. 2).
4. Discussion
A number of scientific data indicate that FIP is a consequence of a recent interspecies jump of coronavirus, considering the fact that this disease has not been seen before 1950 (reviewed in Pedersen, 2009). The immunological aspects of FIP are complex and have not been fully elucidated yet. FIPV could negatively influence immune system intervening at several points. This virus variant is a highly virulent monocyte/macrophage pathogen causing systemic immune complex mediated infection (Vennema et al., 1998, Takano et al., 2008, Chang et al., 2010). These facts may explain why the acquired natural immunity in cats is rather limited against FIPV and also why no efficacious vaccine is available against FIPV despite the intensive efforts of academic and commercial researchers.
In our study the same vaccination regime was applied on SPF and conventional purebred British Shorthair cats using a recombinant FCoV pair differing only in the truncation (PBFIPV-DF-2) and intactness (PBFIPV-DF-2-R3i) of their ORF3abc region. The in vivo evaluation of this virus pair showed that these viruses possess low virulent and avirulent phenotype, respectively (Bálint et al., 2013).
The low and inconsistent level of faecal shedding observed in the present experiments following vaccination with the recombinant PFIPV-DF-2 is similar to that which was observed after the infection of ORF3abc truncated FCoVs (Chang et al., 2010, Pedersen et al., 2012). Seroconversion reached medium level after the two oronasal vaccinations, and it gradually elevated after the two intramuscular injections with the absence of antibody enhancement (ADE) leading to FIP. Strong antibody response was induced in each group after completion of the vaccination regime, however, high booster effect was not observed between the four individual inoculations. To reach higher antibody titres or to elicit more significant booster effect, modification of the vaccination protocol and further experiments might be required.
Vaccination with PFIPV-DF-2 caused low level lymphopenia that proved to be transient and was not followed by a second wave of significant lymphoid cell decrease characteristic to the course of fatal FIP. These, together with the lack of other adverse clinical signs are advantageous for the development of a safe vaccine.
After the vaccinations with PBFIPV-DF-2-R3i, no clinical signs were observed, which confirmed the avirulent phenotype and the FECV characteristics of this recombinant FCoV. Faecal virus shedding was higher and detected for a prolonged period compared with that of the PFIPV-DF-2. This phenomenon is in accordance with previous findings that the intact ORF3abc region is indispensable for enteric replication of FCoV (Chang et al., 2010, Pedersen et al., 2012, Bálint et al., 2013). High mutation frequency and recombination are common features of coronaviruses, which can lead to increased virulence of the attenuated strains used for vaccination. For this reason, the longer persistence and shedding of PBFIPV-DF-2-R3i after inoculation makes it a less favourable vaccine candidate than PFIPV-DF-2.
Low and inconsistent antibody response was observed subsequently after the two oronasal vaccinations with PBFIPV-DF-2-R3i, while the antibody level significantly increased after intramuscular inoculation to a level comparable to but clearly lower than that of the PBFIP-DF-2 vaccinated animals. The low and inconsistent initial antibody response may be the consequence of the intact ORF3abc, which directs FCoV replication to enterocytes and restricts it in macrophages (Bálint et al., 2012).
In the case of SPF cats, both recombinant FCoVs with truncated or complemented ORF3abc protected 100% of the vaccinated animals against a homologous challenge with the highly lethal FIPV DF-2 strain. The antibody titres in these animals did not increase significantly after the challenge, showing the absence of uncontrolled FIPV DF-2 replication.
Non-SPF and SPF cats reacted physiologically very similarly to the identical vaccination regime, although some of the clinical parameters (fever, depression, anorexia) of the non-SPF animals were somewhat different of those observed in SPF cats. Virus shedding and serological response levels were also slightly higher indicating more intensive virus replication and immune reaction.
However, the most striking difference between the two experiments was that the vaccine candidates provided far less or no protection in purebred cats against the challenge of the FIPV DF-2 strain. In most of the cases the challenge led to ADE and in all case to the development of severe symptoms of FIP in non-SPF animals, independently of the genetic characteristics of the applied vaccine candidates. ADE pathomechanism is not an exclusive feature of FIPV infection; it resembles to the ADE-based Dengue haemorrhagic shock syndrome, first described by Peiris and Porterfield (1979). The same phenomenon was also observed in the case of flaviviruses, alphaviruses, lentiviruses, influenzaviruses, enteroviruses and measles virus (Peiris and Porterfield, 1979, Porterfield, 1986, Takeda et al., 1988, Tamura et al., 1991, Chen et al., 2013, Iankov et al., 2013). It is suspected that ADE might be more severe by early FIPV challenge after vaccination. However, the lack of development of fatal FIP and ADE among SPF cats and the prolonged survival of two non-SPF animals after 14 days challenge suggest that other biological factors could have as strong effect on the development of FIP as early challenge.
Two PBFIPV-DF-2 vaccinated animals (originated from different litters but have common father) remained symptomless for weeks and their survival time was prolonged from the usual 3–4 weeks to 65–86 days after FIPV DF-2 challenge, which is rather unusual after infection. The course of their disease clearly differs from ADE and it resembles to that of latent or sequestered infection by FIPV, which can be reactivated by immunosuppressive agents, such as FeLV (Pedersen, 1987).
In our previous pilot vaccine trials we used different DNA and immune stimulating complex (ISCOM) vaccine candidates harbouring N, S and OFF7b genes/proteins in both SPF (n = 15) and conventional cats (n = 15). Applying FIPV DF-2 as a challenge strain, all the control and vaccinated animals perished within four weeks, with or without the signs of ADE (Farsang et al., unpublished result). Studies conducted by others are congruent with ours and indicate that virtually all cats develop FIP within four weeks after FIPV DF-2 challenge (reviewed in Pedersen, 2009). This is the first case when we observed long-term survival in our vaccination trials. It may be the consequence either of the slightly higher neutralising antibody titre of the two animals or it can be the result of the altered specificity of their immune response. The prolonged survival of 40% of the PBFIPV-DF-2 vaccinated cats makes PBFIPV-DF-2 a more promising candidate than PBFIPV-DF-2-R3i for further vaccine development.
Genetic and immunological differences between the SPF and non-SPF purebred kittens can explain the different outcome of the vaccination experiment. Although more frequent occurrence of FIP was reported from Australia in certain breeds including British Shorthair than in Domestic Shorthair cats (Norris et al., 2005) yet the significant influence of the genetic background of the animals is less likely because we were not able to find any survival neither among Domestic Shorthair cat controls in the present experiment nor among vaccinated Domestic Shorthair cats from our earlier ISCOM or DNA vaccination trials after FIPV DF-2 challenge.
Vaccination trials revealed that sub-neutralising level of spike protein-specific antibodies can lead to ADE by facilitating Fc receptor-mediated uptake of FCoV by macrophages/monocytes contributing to the development of early death syndrome (Weiss and Scott, 1981, Vennema et al., 1990, Corapi et al., 1992, Hohdatsu et al., 1998, Olsen et al., 1992). Vaccination of the British Shorthair cats with PBFIPV-DF-2 resulted in heterologous immunological response and survival times despite the common genetic background. These facts also suggest that immunity status has a more substantial role in the development of the disease course after vaccination than genetic background.
SPF kittens are bred in isolated circumstances, and they neither meet a range of pathogenic and non-pathogenic parasites, bacteria, fungi and viruses nor take up maternal antibodies in the colostrum against them, while non-SPF animals kept even under the best circumstances do encounter with such microorganisms. The conventional purebred British Shorthair cats developed higher VN antibody titres but these antibodies or their particular fraction might have led to the observed ADE.
Although no direct connection between the feline bacterial flora and virus neutralising titre was published so far, abundant scientific data confirm the direct and indirect influence of the microbiota composition on the outcome of pathogenic infections (Slifka et al., 2003, Teixeira et al., 2008, Wilks and Golovkina, 2012).
The presence of intestinal microbes directly facilitates the infection of reoviruses and polioviruses, both in vitro and in vivo (Kuss et al., 2011). The bacterial flora promotes maturation of secondary lymphoid organs in the intestine (Lee and Mazmanian, 2010) and can influence both gene expression in antigen-presenting cells and the way T cells respond to vaccines (Klaasen et al., 1993, Lamousé-Smith et al., 2011). Furthermore, interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus in vitro (Ivec et al., 2007). Since FIP is the result of type III or IV hypersensitivity reaction (Pedersen and Boyle, 1980, Paltrinieri et al., 1989), it is tempting to speculate that the mature immune system of non-SPF cats predisposes these animals to development of the disease.
5. Conclusion
In summary, vaccination experiments showed that hyperimmunisation of SPF cats with low virulent recombinant FCoVs conferred complete protection against lethal homologous challenge. However, partial or no protection was observed using the same vaccination protocol in non-SPF cats. These data highlight the diverse immune responses between SPF and conventional cats and suggest a decisive role of previous infection by heterologous causative agents in the outcome of the vaccination against FIP.
Conflict of interest statement
All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work.
Acknowledgements
This work was supported by the Award of Excellence from the Swedish University of Agricultural Sciences, research grants from the AGRIA Animal Insurance Company (Agria Djurförsäkring) and The Swedish Kennel Club (Svenska Kennelklubben, SKK), OTKA, and NKTH (Mobilitás 08-C OTKA 81187), the János Bólyai Fellowship from the Hungarian Academy of Sciences (BO/00414/10).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Addie D., Jarrett O. A study of naturally occurring feline coronavirus infections in kittens. Vet. Rec. 1992;130:133–137. doi: 10.1136/vr.130.7.133. [DOI] [PubMed] [Google Scholar]
- Bálint Á., Farsang A., Zádori Z., Hornyák Á., Dencső L., Almazán F., Enjuanes L., Belák S. Molecular characterization of feline infectious peritonitis virus strain DF-2 and studies on the role of ORF3abc in viral cell tropism. J. Virol. 2012;86:6258–6267. doi: 10.1128/JVI.00189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint Á., Farsang A., Zádori Z., Belák S. Comparative in vivo analysis of recombinant type II feline coronaviruses with truncated and completed ORF3 region. Plos One. 2013 doi: 10.1371/journal.pone.0088758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlough J.E., Stoddart C.A., Sorresso G.P., Jacobson R.H., Scott F.V. Experimental inoculation of cats with canine coronavirus and subsequent challenge with feline infectious peritonitis virus. Lab. Anim. Sci. 1984;34:592–597. [PubMed] [Google Scholar]
- Barlough J.E., Johnson-Lussenburg C.M., Stoddart C.A., Jacobson R.H., Scott F.W. Experimental inoculation of cats with human coronavirus 229E and subsequent challenge with feline infectious peritonitis virus. Can. J. Comp. Med. 1985;49:303–307. [PMC free article] [PubMed] [Google Scholar]
- Chang H.W., de Groot R.J., Egberink H.F., Rottier P.J. Feline infectious peritonitis; insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J. Gen. Virol. 2010;91:415–420. doi: 10.1099/vir.0.016485-0. [DOI] [PubMed] [Google Scholar]
- Chen I.C., Wang S.M., Yu C.K., Liu C.C. Subneutralizing antibodies to enterovirus 71 induce antibody-dependent enhancement of infection in newborn mice. Med. Microbiol. Immunol. 2013 doi: 10.1007/s00430-013-0289-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Corapi W.V., Olsen C.W., Scott F.W. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J. Virol. 1992;66:6695–6705. doi: 10.1128/jvi.66.11.6695-6705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot-Mijnes J.D., van Dun J.M., van der Most R.G., de Groot R.J. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J.Virol. 2005;79:1036–1044. doi: 10.1128/JVI.79.2.1036-1044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr D., Holznagel E., Bolla S., Hauser B., Herrewegh A.A., Horzinek M.C., Lutz H. Placebo-controlled evaluation of a modified life virus vaccine against feline infectious peritonitis: safety and efficacy under field conditions. Vaccine. 1997;15:1101–1109. doi: 10.1016/S0264-410X(97)00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J.D., Ingersoll J.D., Gast A.M., Christianson K.K., Selzer N.L., Landon R.M., Pfeiffer N.E., Sharpee R.L., Beckenhauer W.H. Protection against feline infectious peritonitis by intranasal inoculation of a temperature-sensitive FIPV vaccine. Vaccine. 1990;8:536–542. doi: 10.1016/0264-410X(90)90004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J.D. Overview of the development of a modified live temperature-sensitive FIP virus vaccine. Feline Pract. 1995;23:62–66. [Google Scholar]
- Haijema B.J., Volders H., Rottier P.J. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis. J. Virol. 2004;78:3863–3871. doi: 10.1128/JVI.78.8.3863-3871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijema B.J., Rottier P.J.M., de Groot R.J. Coronaviruses. Caister Academic Press; UK: Norfolk. Caister Academic Press: 2007. Feline coronaviruses: a tale of two-faced types. [Google Scholar]
- Herrewegh A.A., Mahler M., Hedrich H.J., Haagmans B.L., Egberink H.F., Horzinek M.C., Rottier P.J., de Groot R.J. Persistence and evolution of feline coronavirus in a closed cat-breeding colony. Virology. 1997;234:349–363. doi: 10.1006/viro.1997.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., Smeenk I., Horzinek M.C., Rottier P.J., de Groot R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Ishizuka Y., Yamada H., Koyama H. The prevalence of types I and II feline coronavirus infections in cats. J. Vet. Med. Sci. 1992;54:557–562. doi: 10.1292/jvms.54.557. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Yamada M., Tominaga R., Makino K., Kida K., Koyama H. Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus. J. Vet. Med. Sci. 1998;60:49–55. doi: 10.1292/jvms.60.49. [DOI] [PubMed] [Google Scholar]
- Iankov I.D., Penheiter A.R., Griesmann G.E., Carlson S.K., Federspiel M.J., Galanis E. Neutralization capacity of measles virus H protein specific IgG determines the balance between antibody-enhanced infectivity and protection in microglial cells. Virus Res. 2013;172:15–23. doi: 10.1016/j.virusres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivec M., Botić T., Koren S., Jakobsen M., Weingartl H., Cencic A. Interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus. Antiviral Res. 2007;75:266–274. doi: 10.1016/j.antiviral.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Klaasen H.L., Van der Heijden P.J., Stok W., Poelma F.G., Koopman J.P., Van den Brink M.E., Bakker M.H., Eling W.M., Beynen A.C. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect. Immun. 1993;61:303–306. doi: 10.1128/iai.61.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummrow M., Meli M.L., Haessig M., Goenczi E., Poland A., Pedersen N.C., Hofmann-Lehmann R., Lutz H. Feline coronavirus serotypes 1 and 2: seroprevalence and association with disease in Switzerland. Clin. Diagn. Lab. Immunol. 2005;12:1209–1215. doi: 10.1128/CDLI.12.10.1209-1215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss S.K., Best G.T., Etheredge C.A., Pruijssers A.J., Frierson J.M., Hooper L.V., Dermody T.S., Pfeiffer J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamousé-Smith E.S., Tzeng A., Starnbach M.N. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS One. 2011;6:e27662. doi: 10.1371/journal.pone.0027662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Mazmanian S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle F., Tennant B., Bennett M., Kelly D.F., Gaskell C.J., Gaskell R.M. Independent evaluation of a modified live FIPV vaccine under experimental conditions (University of Liverpool experience) Feline Pract. 1995;23:67–71. [Google Scholar]
- Norris J.M., Bosward K.L., White J.D., Baral R.M., Catt M.J., Malik R. Clinicopathological findings associated with feline infectious peritonitis in Sydney Australia: 42 cases (1990–002) Aust. Vet. J. 2005;83:666–673. doi: 10.1111/j.1751-0813.2005.tb13044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W., Corapi W.V., Ngichabe C.K., Baines J.D., Scott F.W. Monoclonal antibodies to the spike protein of feline infectious peritonitis virus mediate antibody-dependent enhancement of infection of feline macrophages. J. Virol. 1992;66:956–965. doi: 10.1128/jvi.66.2.956-965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrinieri S., Cammarata Parodi M., Cammarata G., Mambretti M. Type IV hypersensitivity in the pathogenesis of FIPV-induced lesions. Zentralblatt Vet. Med. B. 1989;45:151–159. doi: 10.1111/j.1439-0450.1998.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am. J. Vet. Res. 1980;41:868–876. [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981;42:368–377. [PubMed] [Google Scholar]
- Pedersen N.C., Black J.W. Attempted immunization of cats against feline infectious peritonitis, using avirulent live virus or sublethal amounts of virulent virus. Am. J. Vet. Res. 1983;44:229–234. [PubMed] [Google Scholar]
- Pedersen N.C., Evermann J.F., McKeirnan A.J., Ott R.L. Pathogenicity studies of feline coronavirus isolates 79-1146 and 79-1683. Am. J. Vet. Res. 1984;45:2580–2585. [PubMed] [Google Scholar]
- Pedersen N.C., Floyd K. Experimental studies with three new strains of feline infectious peritonitis virus: FIPV-UCD2 FIPV-UCD3, and FIPV-UCD4. Compend. Contin. Educ. Pract. Vet. 1985;7:1001–1011. [Google Scholar]
- Pedersen N.C. Virologic and immunologic aspects of feline infectious peritonitis virus infection. Adv. Exp. Med. Biol. 1987;218:529–550. doi: 10.1007/978-1-4684-1280-2_69. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C. Animal virus infections that defy vaccination: equine infectious anemia, caprine arthritis-encephalitis, maedi-visna, and feline infectious peritonitis. Adv. Vet. Sci. Comp. Med. 1989;3:413–428. doi: 10.1016/B978-0-12-039233-9.50017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009;11:225–258. doi: 10.1016/j.jfms.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Liu H., Scarlett J., Leutenegger C.M., Golovko L., Kennedy H., Kamal F.M. Feline infectious peritonitis: role of the feline coronavirus 3c gene in intestinal tropism and pathogenicity based upon isolates from resident and adopted shelter cats. Virus Res. 2012;165:17–28. doi: 10.1016/j.virusres.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Porterfield J.S. Antibody-mediated enhancement of flavivirus replication in macrophage-like cell lines. Nature. 1979;282:509–511. doi: 10.1038/282509a0. [DOI] [PubMed] [Google Scholar]
- Porterfield J.S. Antibody-dependent enhancement of viral infectivity. Adv. Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- Scott F.W., Corapi W.V., Olsen C.W. Independent evaluation of a modified live FIPV vaccine under experimental conditions (Cornell experience) Feline Pract. 1995;23:74–76. [Google Scholar]
- Shiba N., Maeda K., Kato H., Mochizuki M., Iwata H. Differentiation of feline coronavirus type I and II infections by virus neutralization test. Vet. Microbiol. 2007;124:348–352. doi: 10.1016/j.vetmic.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka M.K., Homann D., Tishon A., Pagarigan R., Oldstone M.B.A. Measles virus infection results in suppression of both innate and adaptive immune responses to secondary bacterial infection. J. Clin. Invest. 2003;111:805–810. doi: 10.1172/JCI13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart C.A., Barlough J.E., Baldwin C.A., Scott F.W. Attempted immunisation of cats against feline infectious peritonitis using canine coronavirus. Res. Vet. Sci. 1988;45:383–388. doi: 10.1016/S0034-5288(18)30970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Tuazon C.U., Ennis F.A. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988;242:580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- Takano T., Kawakami C., Yamada S., Satoh R., Hohdatsu T. Antibody-dependent enhancement occurs upon re-infection with the identical serotype virus in feline infectious peritonitis virus infection. J. Vet. Med. Sci. 2008;70:1315–1321. doi: 10.1292/jvms.70.1315. [DOI] [PubMed] [Google Scholar]
- Tamura M., Webster R.G., Ennis F.A. Antibodies to HA and NA augment uptake of influenza A viruses into cells via Fc receptor entry. Virology. 1991;182:211–219. doi: 10.1016/0042-6822(91)90664-w. [DOI] [PubMed] [Google Scholar]
- Teixeira L., Ferreira A., Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008;6:1000002e. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., de Groot R.J., Harbour D.A., Dalderup M., Gruffydd-Jones T., Horzinek M.C., Spaan W.J. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J. Virol. 1990;64:1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.C., Scott F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp. Immunol. Microbiol. Infect. Dis. 1981;4:175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks J., Golovkina T. Influence of microbiota on viral infections. PLoS Pathog. 2012;8:e1002681. doi: 10.1371/journal.ppat.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R.D., Pedersen N.C. Cross-protection studies between feline infectious peritonitis virus and porcine transmissible gastroenteritis viruses. Vet. Microbiol. 1979;4:11–16. [Google Scholar]


