Abstract
Feline infectious peritonitis (FIP) cats show a decrease in peripheral blood lymphocyte counts, and a particularly marked decrease in T cells including CD4+ and CD8+ cells. In this study, we showed that lymphopenia observed in FIP cats was due to apoptosis, and that the ascitic fluid, plasma, and culture supernatant of peritoneal exudate cells (adherent cells with macrophage morphology, or PEC) from FIP cats readily induced apoptosis in specific pathogen-free cat peripheral blood mononuclear cells, particularly CD8+ cells. In addition, TNF-alpha released from macrophages and TNF-receptor (TNFR) 1 and TNFR2 mRNA expression in lymphocytes were closely involved in this apoptosis induction. In particular, in CD8+ cells cultured in the presence of the PEC culture supernatant, the expression levels of TNFR1 and TNFR2 mRNA were increased, indicating that CD8+ cells are more susceptible to apoptosis induction by TNF-alpha than other lymphocyte subsets, particularly B cells (CD21+ cells). The results of this study suggest that TNF-alpha, produced by virus-infected macrophages, is responsible for induction of apoptosis in uninfected T cells, primarily CD8+ T cells.
Keywords: FIP, Apoptosis, TNF-alpha, TNF-receptor, Lymphopenia, CD8+ cell
1. Introduction
Feline infectious peritonitis (FIP) is a virus-induced, chronic, progressive, usually fatal disease in domestic and wild felines. The causative agent of this disease is feline coronavirus (FCoV). FCoV is mainly composed of nucleocapsid (N) protein, transmembrane (M) protein, and peplomer spike (S) protein (Olsen, 1993), and is classified into types I and II according to S protein properties (Hohdatsu et al., 1991a, Motokawa et al., 1995, Motokawa et al., 1996). Each of these types consists of two viruses: FIP-causing FIP virus (FIPV) and non-FIP-causing feline enteric coronavirus (FECV). FIPV and FECV of the same type cannot be distinguished by their antigenicity or at the gene level, and differ only in their pathogenicity for cats.
In the early stage of experimental FIPV infection, general symptoms such as fever, anorexia, vomiting, and diarrhea occur. The development of pathological states after systemic viral dissemination is considered to depend largely on the immune state of the host, particularly the presence or absence of the induction of cell-mediated immunity (TH1 activity): its strong induction inhibits the development of FIP (Pedersen et al., 1984). In cats with overt FIP, the counts of peripheral blood T cells, including CD4+ cells and CD8+ cells, are markedly decreased (De Groot-Mijnes et al., 2005). The induction of humoral immunity (TH2 activity) is considered to be ineffective or rather to aggravate the condition. In the ascitic fluid cells of FIP cats, the production of IL-6, a cytokine involved in the induction of B cell differentiation, is increased (Goitsuka et al., 1990). Antibody production induces immune complexes formation and deposition and a type III hypersensitivity reaction. Recently, Kiss et al. (2004) suggested that immunity against FIP progress is associated with TNF-alpha and IFN-gamma response imbalance, with high TNF-alpha/low IFN-gamma mRNA response favouring disease and low TNF-alpha/high IFN-gamma mRNA responses being indicative of immunity.
The cell/tissue destruction in FIPV lesion is mainly due to tissue reaction with activation of type III (tissue destroyed by enzymes released by polymorphonuclear neutrophil leukocytes) or IV (cell-mediated cytotoxicity) hypersensitivity (Kipar et al., 1998, Pedersen, 1987). On the contrary, lymphocyte depletion and the presence of apoptotic cells were reported to be detected in the T-cell region of mesenteric lymph nodes and the spleen of FIP cats (Haagmans et al., 1996). Dean et al. (2003) reported that the FIPV antigen, TNF-alpha expression, and lymphocyte depletion were colocalized in the lymphoid tissues of FIP cats. Haagmans et al. (1996) reported that anti-human TNF-alpha did not inhibit apoptosis induced by ascitic fluid from FIPV-infected cats. Thus, the detailed mechanism of apoptosis induction in the lymphoid tissue of FIP cats remains unclear. As described above, FIP cats show a marked reduction in peripheral blood lymphocyte counts. Since FIPV does not replicate in peripheral blood lymphocytes, it is difficult to consider this lymphopenia to be due to direct destruction by virus infection of lymphocytes, leaving the causes unknown. Since the development of FIP is closely related to the immune function of the host, clarification of the mechanism of lymphopenia is important for elucidating the pathogenesis of FIP.
In this study, we showed that lymphopenia in FIP cats was due to apoptosis, and that TNF-alpha released from macrophages of FIP cats induced apoptosis in lymphocytes, particularly CD8+ T cells.
2. Materials and methods
2.1. Experimental animals
FIPV strain 79-1146 (104 TCID50/ml) was administered orally to 6–8-month-old, specific pathogen-free (SPF) cats. Nine cats that developed FIP symptoms (FIP cats), such as fever, weight loss, peritoneal or pleural effusion, dyspnea, ocular lesions, and neural symptoms, six cats infected but resistant to the development of disease (FIPV-infected non-FIP cats), and eight 6–8-month-old SPF cats as controls were used in this study.
2.2. Cell cultures
Feline peripheral blood mononuclear cells (PBMC), CD4+ cells, CD8+ cells, CD21+ cells, peritoneal exudate cells (PEC), alveolar macrophages, and WEHI-164 murine sarcoma cells were maintained in RPMI 1640 growth medium supplemented with 10% FCS, antibiotics, 50 μM 2-mercaptoethanol, and 2 μg/ml of polybrene. WEHI-164 murine sarcoma cells (ATCC CRL1751) were obtained from the American Type Culture Collection.
2.3. Separation of PBMC
Heparinized blood (10 ml) was two-fold diluted with phosphate-buffered saline (PBS), and subjected to Ficoll-Hypaque density gradient centrifugation at 1700 rpm for 20 min. The PBMC layer was collected, washed twice with PBS, and resuspended with growth medium at 2 × 106 cells/ml.
2.4. Specimens from FIP cats and FIP-infected non-FIP cats
Blood collected from FIP cats and FIP-infected non-FIP cats using a heparinized syringe was centrifuged at 3000 rpm for 10 min, and the supernatant was used as a plasma sample. As for ascitic fluid samples, ascitic fluid was collected from FIP cats using a heparinized syringe and centrifuged at 3000 rpm for 10 min, and the supernatant was collected.
2.5. Recovery of PEC and alveolar macrophages
PEC were collected from the ascitic fluid of FIP cats. The cell pellet, obtained by centrifugation of the effusion collected from the FIP cats, was washed three times with Hank's balanced salt solution (HBSS), and suspended in growth medium at a density of 2 × 106 cells/ml. One milliliter of the cell suspension was placed into each well of a 24-well plate, and cultured at 37 °C for 2 h. The culture supernatant was discarded, the non-adherent cells were removed by washing with HBSS, and the remaining cells were used as PEC.
Feline alveolar macrophages were obtained by bronchoalveolar lavage with HBSS from coronavirus antibody-negative SPF cats, as previously described by Hohdatsu et al. (1991b).
2.6. Separation of CD8+, CD4+, and CD21+ cells
To separate CD8+ cells, PBMC (1 × 107 cells) were incubated with 500 μl of purified monoclonal antibody (MAb) OKT8 (ATCC CRL8014) IgG at 4 °C for 30 min. MAb OKT8 recognizes the alpha-chain of human CD8 antigen and cross-reacts with feline CD8+ cells (Hohdatsu et al., 1998, Pecorado et al., 1994). The cells were washed three times with PBS containing 2 mM EDTA and 0.5% BSA, 40 μl of microbeads coated with rat anti-mouse IgG2a+b (Miltenyi Biotec, Germany) were added to the cells, and the mixture was incubated at 4 °C for 15 min. After washing with PBS containing 2 mM EDTA and 0.5% BSA, the cells were fractionated into CD8+ cells and CD8+-depleted cells by a magnetic system using a MACS kit (Miltenyi Biotec, Germany). CD4+ and CD21+ cells were recovered using MAb fCD4 (Southern Biotechnology Associates, USA) or MAb CA2.1D6 IgG (Serotec Ltd., UK) as a primary antibody, and microbeads coated with rat anti-mouse IgG1 (Miltenyi Biotec, Germany) as a secondary antibody in the same manner as that for CD8+ cells.
The purity of the cell population was 92.0 ± 1.6% (mean ± S.D.) for CD8+ cells, 93.3 ± 3.8% for CD4+ cells and 96.9 ± 0.8% for CD21+ cells, respectively.
2.7. Apoptosis-inducing activities of FIP cat-derived specimens on peripheral CD4+, CD8+, and CD21+ cells
PBMC, CD4+ cells, CD8+ cells and CD21+ cells (2 × 106 cells) were cultured for 4 h in the presence of FIP cat-derived ascitic fluid (final concentration of 1:40), plasma (final concentration of 1:40), culture supernatant of PEC (final concentration of 1:2), and FIPV-infected non-FIP cats-derived plasma (final concentration of 1:40), and were examined for their apoptosis-inducing activities. Culture supernatants of PEC were prepared by culturing cells (2 × 106 cells/ml) recovered from ascitic fluids for 24 h. Apoptosis induction was detected by the TUNEL method using flow cytometric analysis.
2.8. Detection of apoptosis by TUNEL
A total of 2 × 106 cells were washed twice, resuspended in 200 μl of PBS, and fixed by adding an equal volume of 4% formaldehyde for 20 min at room temperature. The cells were washed once again with PBS, suspended in cell permeability buffer (0.5% saponin, 0.5% BSA, and 0.1% sodium azide in PBS), and incubated for 15 min in the dark. The fixed and permeabilized cells were washed twice with PBS containing 0.5% BSA and 0.1% sodium azide, and incubated with terminal deoxynucleotidyl transferase (TdT) and FITC-dideoxyuridine triphosphate (dUTP) at 37 °C for 1 h. Negative controls were incubated in the absence of TdT. After rinsing the cells with PBS containing 0.5% BSA and 0.1% sodium azide, the fluorescence intensity was measured using a FACS 440 cell sorter (Becton Dickinson, USA).
2.9. Apoptosis inhibitors
SB203580, Z-DEVD-FMK, and Z-IETD-FMK were used as apoptosis inhibitors. SB203580, a specific inhibitor of p38 mitogen-activated protein kinase (p38-MAPK), was purchased from Promega Corporation (USA). Z-DEVD-FMK (caspase-3 inhibitor) and Z-IETD-FMK (caspase-8 inhibitor) were purchased from Medical Biological Laboratories Corporation (Japan).
2.10. RNA isolation and cDNA preparation
Total cellular RNA was extracted from PBMC, PEC, and macrophages using a High Pure RNA Isolation Kit (Roche Diagnostics, Switzerland) according to the instructions of the manufacturer. RNA was dissolved in elution buffer.
Using total cellular RNA as a template, cDNA was synthesized using Ready-to-Go RT-PCR beads (GE Healthcare Life Sciences, USA). Reverse transcription was performed in a 50 μl final volume containing 0.5 μg oligo(dT)12–18 primers. The resulting solution was incubated at 42 °C for 1 h to synthesize cDNA.
2.11. Determination of levels of feline GAPDH, TNF-alpha, TNFR1, and TNFR2 mRNA expression
cDNA was amplified by PCR using specific primers for feline GAPDH, TNF-alpha, TNFR1, and TNFR2. The primer sequences are shown in Table 1 .
Table 1.
Sequences of PCR primers for feline GAPDH, TNF-alpha, and TNF receptors
| Orientation | Nucleotide sequence | Location | Length (bp) | Reference | |
|---|---|---|---|---|---|
| GAPDH | Forward | 5′-AATTCCACGGCACAGTCAAGG-3′ | 158–78 | 97 | Avery and Hoover (2004) |
| Reverse | 5′-CATTTGATGTTGGCGGGATC-3′ | 235–254 | |||
| TNF-alpha | Forward | 5′-TGGCCTGCAACTAATCAACC-3′ | 195–214 | 251 | Avery and Hoover (2004) |
| Reverse | 5′-GTGTGGAAGGACATCCTTGG-3′ | 426–445 | |||
| TNFR1 | Forward | 5′-CGAAGTGCCACAAAGGGACCTAC-3′ | 388–411 | 215 | Mizuno et al. (2001) |
| Reverse | 5′-TGGTTCTTCCTGCAGCCACACAC-3′ | 579–601 | |||
| TNFR2 | Forward | 5′-CTCAGGCAGCACCGCAGACGG-3′ | 1–21 | 244 | Mizuno et al. (2001) |
| Reverse | 5′-GCCGGAGGAGCTGGCATCCACG-3′ | 226–247 | |||
PCR was performed in a total volume of 50 μl. One microliter of sample cDNA was mixed with 10-fold concentrated reaction buffer (TaKaRa, Japan), 4 μl of deoxynucleotide mix (TaKaRa, Japan) containing 2.5 mM each, 2 μl of 20 μM primer mix, 0.25 μl of Ex Taq polymerase (1000 U; Takara, Japan), and 37.75 μl of distilled water. Using a PCR Thermal Cycler Dice (Takara, Japan), the DNA was amplified at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, primer annealing at 55 °C for 45 s, and synthesis at 72 °C for 45 s, with a final extension at 55 °C for 5 min. The PCR products were resolved by electrophoresis on 2% agarose gels. The gels were incubated with SYBR Green I Nucleic Acid Gel Stain (Roche Diagnostics, Switzerland), and bands were visualized using a UV transilluminator at 312 nm and photographed.
Band density was quantified under appropriate UV exposure by video densitometry using Scion Image software (Scion Corporation, USA).
To quantify TNF-alpha, TNFR1, and TNFR2 mRNA were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH.
2.12. Cytotoxic activity against TNF-alpha using WEHI-164 cells
WEHI-164 cells were grown to confluence in growth medium at 37 °C. The cells were detached with trypsin, washed in fresh medium, seeded into 96-well plates at a density of 105 cells/well in 100 μl of fresh medium, and allowed to adhere for 4 h at 37 °C. The medium was then aspirated, and 100 μl (10 μg/ml) of actinomycin D sulfate (Sigma–Aldrich, USA) were added to each well. Samples to be tested were diluted in medium, and 100 μl of each dilution was added to triplicate wells. The plates were incubated at 37 °C for 2 h. The culture supernatants were removed, and 100 μl of fresh medium were added to each well. After incubation at 37 °C for 12 h, 10 μl of WST-8 solution (WST-8 cell proliferation assay kit; Kishida Chemical Co. Ltd., Japan) were added, and the cells were returned to the incubator for 1 h. The absorbance of formazan produced was measured at 450 nm with a 96-well spectrophotometric plate reader, as described by the manufacturer. The percent cytotoxicity was calculated by the following formula: cytotoxicity (%) = (negative control OD – test sample OD/negative control OD) × 100.
2.13. Statistical analysis
Data were analyzed by Student's t-test. The data in Fig. 1 were also analyzed by the Mann–Whitney test. p-Values < 0.05 were considered to indicate a significant difference between compared groups.
Fig. 1.
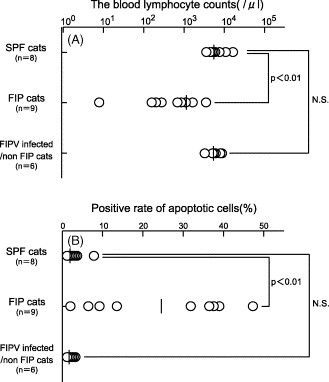
(A) The blood lymphocyte counts of FIP cats and FIP-infected non-FIP cats. (B) Detection of apoptotic cells in PBMC of FIP cats and FIP-infected non-FIP cats. PBMC were recovered from SPF cats, FIP cats, and FIP-infected non-FIP cats, and apoptotic cells were detected by TUNEL. Horizontal lines represent the median for each group. N.S. indicates not significant.
3. Results
3.1. Detection of apoptosis in PBMC of FIP cats and FIPV-infected non-FIP cats
Using PBMC of FIP cats and experimentally FIPV-infected non-FIP cats, apoptotic cells were detected by TUNEL, and the rates of apoptosis were compared with that in SPF cats. The blood lymphocyte counts at the time of blood sampling are shown in Fig. 1A. The blood lymphocyte counts in SPF cats, FIP cats and FIPV-infected non-FIP cats were 5177 ± 731 μl−1 (mean ± S.D.), 1100 ± 924 μl−1, and 4850 ± 1780 μl−1, respectively (Fig. 1A). The rate of apoptosis in the PBMC of FIP cats was significantly higher, at 23.4 ± 16.7% (mean ± S.D.), than that in non-FIP cats (0.9 ± 1.3%) and SPF cats (2.5 ± 2.8%) (Fig. 1B).
3.2. Apoptosis-inducing activities of FIP cat-derived specimens on peripheral CD4+, CD8+, and CD21+ cells
All FIP cat-derived specimens readily induced apoptosis in PBMC and their subsets of CD4+, CD8+, and CD21+ cells (Fig. 2 ), significantly more strongly in CD8+ than in CD21+ cells (p < 0.01 for ascitic fluid and PEC supernatant, p < 0.05 for plasma of FIP cats). A significant difference was noted in the apoptosis-inducing activity of PEC supernatant between CD4+ and CD8+ cells (p < 0.05).
Fig. 2.
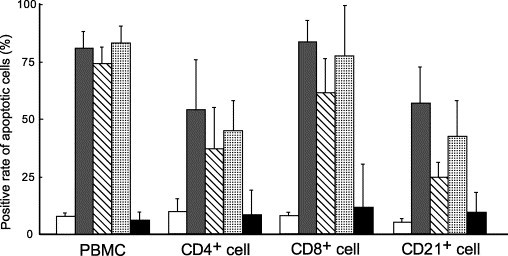
Apoptosis-inducing activities of specimens from FIP cats in peripheral blood CD4+, CD8+, and CD21+ cells. The PBMC (2 × 106) of SPF cats and their subsets of lymphocytes CD4+, CD8+, and CD21+ cells (2 × 106) were cultured at 37 °C for 4 h in the presence of the ascitic fluid, PEC culture supernatant, or plasma of FIP cats, and apoptotic cells were detected by TUNEL. CD4+, CD8+, and CD21+ cells were recovered with magnetic beads. Medium (white), ascitic fluid of FIP cats (gray), culture supernatant of PEC from FIP cats (oblique), plasma of FIP cats (dot), plasma of FIP-infected non-FIP cats (black).
We examined whether inhibitors of intracellular apoptotic signal transduction (caspase-3 inhibitor, caspase-8 inhibitor, and p38-MAPK inhibitor) inhibited the apoptosis-inducing activities of ascitic fluid and PEC culture supernatant in the PBMC of SPF cats. As a result, all these inhibitors inhibited apoptosis induction in a dose-dependent manner (Fig. 3 ).
Fig. 3.
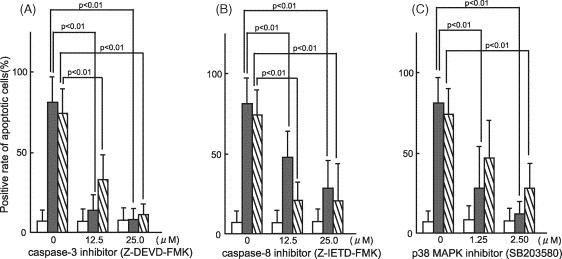
Effects of inhibitors of intracellular apoptotic signal transduction on apoptosis in PBMC. (A and B) The PBMC of SPF cats were incubated for 2 h with caspase-3 inhibitor (A) or caspase-8 inhibitor (B), and cultured at 37 °C in the presence of the ascitic fluid or culture supernatant of PEC from FIP cats. Four hours later, apoptotic cells were detected by TUNEL. (C) The PBMC of SPF cats were cultured at 37 °C for 1 h in the presence of the ascitic fluid or culture supernatant of PEC from FIP cats, and p38-MAPK inhibitor was added. After further culture for 3 h, apoptotic cells were detected by TUNEL. Medium (white), ascitic fluid of FIP cats (gray), culture supernatant of PEC from FIP cats (oblique).
3.3. Determination of levels of TNF-alpha mRNA expression in PEC and PBMC of FIP cats and detection of TNF-alpha in their PEC culture supernatant and plasma
Since TNF-alpha is known as an apoptosis inducer, the levels of TNF-alpha mRNA expression were determined in the PEC of FIP cats, alveolar macrophages of SPF cats and FIPV-infected non-FIP cats, and PBMC of FIP cats, SPF cats and FIPV-infected non-FIP cats.
The level of TNF-alpha mRNA expression was markedly increased in the PEC of FIP cats (n = 7), and was significantly higher than that in the alveolar macrophages of SPF cats (n = 6) and FIPV-infected non-FIP cats (n = 3) (p < 0.01). Similar results were obtained for TNF-alpha mRNA expression in PBMC, and the expression level was significantly higher in the FIP cats than in the SPF cats and FIPV-infected non-FIP cats (Fig. 4A).
Fig. 4.
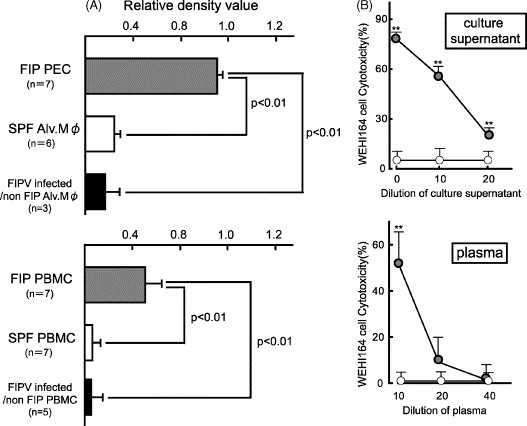
Determination of TNF-alpha mRNA expression in PEC and PBMC of FIP cats and TNF-alpha in their PEC culture supernatant and plasma. (A) The PEC of FIP cats, alveolar macrophages of SPF cats and FIPV-infected non-FIP cats, and PBMC of FIP cats, SPF cats, and FIPV-infected non-FIP cats were recovered, and TNF-alpha mRNA expression levels were measured. TNF-alpha mRNA was quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. (B) Using the culture supernatant of the PEC of FIP cats or macrophages of SPF cats, and the plasma of FIP or SPF cats, TNF-alpha was measured using the rate of cytotoxicity against WEHI-164 cells as an indicator. Specimens from FIP cats (gray circle), or SPF cats (white circle). **p < 0.01 vs. SPF cats.
We examined the plasma and culture supernatant of PEC from FIP cats for TNF-alpha using its cytotoxic activity against TNF-alpha-sensitive WEHI-164 cells. As a result, the plasma and culture supernatant of PEC from FIP cats exhibited cytotoxic activity against WEHI-164 cells in a dose-dependent manner, whereas alveolar macrophages and plasma SPF cats did not (Fig. 4B).
3.4. Analysis of TNFR1 and TNFR2 mRNA expression in PBMC of SPF cats
Since TNF-alpha binds to cell surface TNF-receptor (TNFR) 1 and TNFR2 to induce apoptosis and cell proliferation, we compared the levels of TNFR1 and TNFR2 mRNA expression in the PBMC of FIP cats, SPF cats and FIPV-infected non-FIP cats. As a result, the levels of TNFR1 and TNFR2 mRNA expression in PBMC were higher in FIP cats than in SPF cats and FIPV-infected non-FIP cats (Fig. 5 ).
Fig. 5.
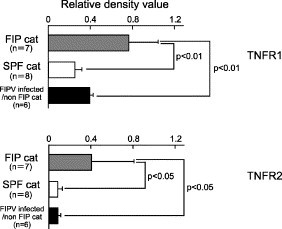
Analysis of TNF-receptor mRNA production in PBMC. The PBMC of SPF cats, FIP cats, or FIPV-infected non-FIP cats were recovered, and the levels of TNFR1 and TNFR2 mRNA expression were measured. TNFR1 and TNFR2 mRNA were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH.
3.5. In vitro effects of culture supernatant of PEC from FIP cats on the expression of TNFR1 and TNFR2 mRNA in PBMC
The PBMC of SPF cats were cultured in the presence of the culture supernatant of FIP cat-derived PEC, and TNFR1 and TNFR2 in PBMC were measured sequentially. As a result, both TNFR1 and TNFR2 mRNA increased from 1 h after culture, and decreased at 4 h (Fig. 6 ).
Fig. 6.
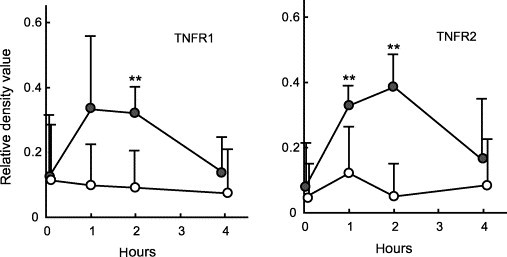
In vitro effects of the culture supernatant of PEC from FIP cats on the expression of TNFR1 and TNFR2 mRNA in PBMC. The PBMC of SPF cats (n = 5) were cultured in the presence of the culture supernatant of PEC from FIP cats, and the levels of TNFR1 and TNFR2 mRNA expression were measured 0, 1, 2, and 4 h after culture. The levels of TNFR1 and TNFR2 mRNA expression were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. The culture supernatant of PEC from FIP cats (gray circle), medium (white circle). **p < 0.01.
In addition, the effects of the culture supernatant of PEC from FIP cats on the expression of TNFR1 and TNFR2 mRNA in CD4+, CD8+, and CD21+ cells separated from the PBMC of SPF cats were examined. When CD4+, CD8+, or CD21+ cells were cultured for 2 h in the presence of the supernatant of PEC, the levels of TNFR1 and TNFR2 mRNA expression increased in CD4+ and CD8+ cells, but remained unchanged in CD21+ cells (Fig. 7 ).
Fig. 7.
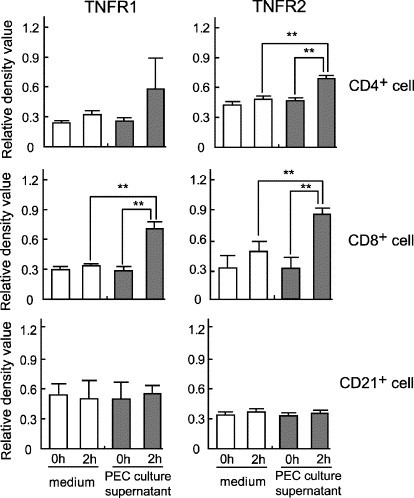
In vitro effects of the culture supernatant of macrophages from FIP cats on the expression of TNFR1 and TNFR2 mRNA in CD4+, CD8+, and CD21+ cells. CD4+, CD8+, and CD21+ cells were recovered from the PBMC of SPF cats using magnetic beads, and cultured in the presence of the culture supernatant of PEC from FIP cats. The levels of TNFR1 and TNFR2 mRNA expression were measured 0 and 2 h after culture. The levels of TNFR1 and TNFR2 mRNA expression were quantitatively analyzed in terms of the relative density value to the mRNA for the housekeeping gene GAPDH. **p < 0.01.
4. Discussion
Lymphocyte apoptosis has been detected in the lymph nodes and spleen of FIP cats (Haagmans et al., 1996, Kipar et al., 2001), but not in peripheral blood lymphocytes. This study appears to be the first to directly demonstrate that lymphopenia in FIP cats is due to apoptosis. Lymphocyte apoptosis in the lymph nodes and spleen of FIP cats may be associated with peripheral blood lymphocyte apoptosis; however, it has been reported that in SIV or influenza virus infection (Rosenberg et al., 1993, Tumpey et al., 2000), the rate of apoptosis in the spleen and lymph nodes is not correlated with that in peripheral blood lymphocytes. By comparing the rates of apoptosis in the spleen, lymph nodes, and peripheral blood lymphocytes, it should be further investigated whether apoptosis induction in lymphoid tissue is reflected in peripheral blood lymphocytes in FIPV infection.
The detailed mechanism of apoptosis induction in the lymphoid tissues of FIP cats is not clear. FIPV does not replicate in lymphocytes, suggesting that this apoptosis in lymphocytes is not directly induced by FIPV infection, but indirectly by other factors. In this study, we showed that: (1) the levels of TNF-alpha mRNA expression was increased in the PEC and PBMC of FIP cats, (2) the PEC culture supernatant and plasma of FIP cats showed cytotoxic activity against TNF-alpha-sensitive WEHI-164 cells, and (3) the ability of the ascitic fluid and PEC culture supernatant of FIP cats to induce apoptosis in the PBMC of SPF cats was inhibited by caspase-3, caspase-8, and p38-MAPK inhibitors, suggesting that apoptosis in the lymphocytes of FIP cats is mainly induced by TNF-alpha. These results support the report of Dean et al. (2003) that lymphocyte depletion and TNF-alpha expression were colocalized in the lymphoid tissues of FIP cats. However, Haagmans et al. (1996) reported that anti-human TNF-alpha did not inhibit apoptosis-induced by ascitic fluid from FIP-infected cats, which was inconsistent with our results and those of Dean et al. (2003). Although the cause of this discrepancy is not clear, experiments using anti-feline TNF-alpha antibodies are expected.
The ascitic fluid of FIP cats contains cells such as macrophages, lymphocytes, and neutrophils, all of which produce TNF-alpha. In this study, we used PEC containing adherent cells, obtained after removing non-adherent cells present in the ascitic fluid. These adherent cells had the morphology of macrophages, and the FIPV gene was detected in them by RT-PCR. The macrophage is one of the target cells of FIPV, and FIPV infection of macrophages is considered to be an important factor in the progression of FIP (Rottier et al., 2005, Stoddart and Scott, 1989). Thus, we speculate that FIPV-infected macrophages produce TNF-alpha, which induces apoptosis in lymphocytes, particularly CD8+ T cells. Further studies are needed to investigate the relationship of the macrophage tropism of FIPV with the production of TNF-alpha and with the induction of apoptosis in lymphocytes.
TNF-alpha binds to TNFR to induce an apoptotic signal in cells (Herbein and O’Brien, 2000). Two types of TNFR are known to exist: TNFR1 has a death domain, and is directly involved in the induction of apoptosis (Herbein and O’Brien, 2000), whereas TNFR2 has no death domain, but assists in apoptosis-inducing signal transduction (Chan and Lenardo, 2000, Fotin-Mleczek et al., 2002). In this study, we showed that the levels of TNFR1 and TNFR2 mRNA expression were increased in the PBMC of FIP cats and the PBMC of SPF cats that had been cultured in the presence of the PEC culture supernatant, suggesting that the induction of apoptosis in the lymphocytes of FIP cats is closely associated with TNF-alpha. In particular, in CD8+ cells, the levels of TNFR1 and TNFR2 mRNA expression were increased, suggesting that CD8+ cells are more susceptible to apoptosis induction by TNF-alpha than other subsets of lymphocytes. The rate of apoptosis induction in CD21+ cells (B cells) by specimens from FIP cats was lower than those in CD8+ and CD4+ cells. Goitsuka et al. (1990) reported that IL-6 activity was increased in the ascitic fluid and PEC culture supernatant of FIP cats. IL-6 is known to be involved in the differentiation and proliferation of B cells and the inhibition of apoptosis (Dolcetti and Boiocchi, 1996, Liu et al., 1994, Moreno et al., 2001, Tumang et al., 2002). Therefore, the low rate of apoptosis induction in CD21+ cells suggests the influence of IL-6 contained in specimens from FIP cats. In CD21+ cells cultured in the presence of the PEC culture supernatant, the levels of TNFR1 and TNFR2 mRNA expression remained unchanged, also suggesting that IL-6 is involved in the rate of apoptosis induction by TNF-alpha. At present, it is not clear why the levels of TNFR mRNA expression increase in CD8+ and CD4+ cells cultured in the presence of the PEC culture supernatant. Further studies are needed to investigate the relationship between T-cell apoptosis induction and TNFR in FIP cats.
It is also possible that apoptosis-inducing factors other than TNF-alpha, such as Fas ligand and TRAIL, are involved in lymphocyte apoptosis in FIP cats, for which further investigation is necessary. Currently, we are investigating the relationship between lymphocyte apoptosis induction and the Fas ligand in FIP cats. The involvement of not only the death receptor pathway investigated but also the mitochondrial pathway should be investigated as an apoptosis induction pathway.
The above results suggest that, in FIP cats, TNF-alpha produced by FIPV-infected macrophages induces apoptosis in lymphocytes, particularly CD8+ cells, resulting in decreased cell-mediated immunity. Comparative analysis of mRNA expression levels of TNFR showed that CD8+ cells are particularly susceptible to apoptosis induction. The results of this study will aid understanding of the role of TNF-alpha in T-cell apoptosis and resultant lymphopenia in FIP diseased cats.
Acknowledgment
This work was supported by Ministry of Health, Labor, and Welfare Grant H16-Shinkoh-9.
References
- Avery P.R., Hoover E.A. Gamma interferon/interleukin 10 balance in tissue lymphocytes correlates with down modulation of mucosal feline immunodeficiency virus infection. J. Virol. 2004;78:4011–4019. doi: 10.1128/JVI.78.8.4011-4019.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan F.K., Lenardo M.J. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur. J. Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Dean G.A., Olivry T., Stanton C., Pedersen N.C. In vivo cytokine response to experimental feline infectious peritonitis virus infection. Vet. Microbiol. 2003;97:1–12. doi: 10.1016/j.vetmic.2003.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot-Mijnes J.D., Van Dun J.M., Van der Most R.G., De Groot R.J. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J. Virol. 2005;79:1036–1044. doi: 10.1128/JVI.79.2.1036-1044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti R., Boiocchi M. Cellular and molecular bases of B-cell clonal expansions. Clin. Exp. Rheumatol. 1996;14:3–13. [PubMed] [Google Scholar]
- Fotin-Mleczek M., Henkler F., Samel D., Reichwein M., Hausser A., Parmryd I., Scheurich P., Schmid J.A., Wajant H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J. Cell Sci. 2002;115:2757–2770. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- Goitsuka R., Ohashi T., Ono K., Yasukawa K., Koishibara Y., Fukui H., Ohsugi Y., Hasegawa A. IL-6 activity in feline infectious peritonitis. J. Immunol. 1990;144:2599–2603. [PubMed] [Google Scholar]
- Haagmans B.L., Egberink H.F., Horzinek M.C. Apoptosis and T-cell depletion during feline infectious peritonitis. J. Virol. 1996;70:8977–8983. doi: 10.1128/jvi.70.12.8977-8983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein G., O’Brien W.A. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 2000;223:241–257. doi: 10.1177/153537020022300305. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Koyama H. Characterization of monoclonal antibodies against feline infectious peritonitis virus type II and antigenic relationship between feline, porcine, and canine coronaviruses. Arch. Virol. 1991;117:85–95. doi: 10.1007/BF01310494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Nakamura M., Ishizuka Y., Yamada H., Koyama H. A study on the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection in feline macrophages by monoclonal antibodies. Arch. Virol. 1991;120:207–217. doi: 10.1007/BF01310476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Okubo M., Koyama H. Feline CD8+ T cells non-cytolytic anti-feline immunodeficiency virus activity mediated by a soluble factor(s) J. Gen. Virol. 1998;79:2729–2735. doi: 10.1099/0022-1317-79-11-2729. [DOI] [PubMed] [Google Scholar]
- Kipar A., Bellmann S., Kremendahl J., Kohler K., Reinacher M. Cellular composition, coronavirus antigen expression and production of specific antibodies in lesions in feline infectious peritonitis. Vet. Immunol. Immunopathol. 1998;65:243–257. doi: 10.1016/S0165-2427(98)00158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar A., Kohler K., Leukert W., Reinacher M. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection. J. Comp. Pathol. 2001;125:182–191. doi: 10.1053/jcpa.2001.0501. [DOI] [PubMed] [Google Scholar]
- Kiss I., Poland A.M., Pedersen N.C. Disease outcome and cytokine responses in cats immunized with an avirulent feline infectious peritonitis virus (FIPV)-UCD1 and challenge-exposed with virulent FIPV-UCD8. J. Feline Med. Surg. 2004;6:89–97. doi: 10.1016/j.jfms.2003.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li H., de Tribolet N., Jaufeerally R., Hamou M.F., Van Meir E.G. IL-6 stimulates growth and inhibits constitutive, protein synthesis-independent apoptosis of murine B-cell hybridoma 7TD1. Cell. Immunol. 1994;155:428–435. doi: 10.1006/cimm.1994.1135. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Goto Y., Baba K., Masuda K., Ohno K., Tsujimoto H. TNF-alpha induced cell death in feline immunodeficiency virus-infected cells is mediated by the caspase cascade. Virology. 2001;287:446–455. doi: 10.1006/viro.2001.1042. [DOI] [PubMed] [Google Scholar]
- Moreno A., Villar M.L., Cámara C., Luque R., Cespón C., González-Porqué P., Roy G., López-Jiménez J., Bootello A., Santiago E.R. Interleukin-6 dimers produced by endothelial cells inhibit apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2001;97:242–249. doi: 10.1182/blood.v97.1.242. [DOI] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Aizawa C., Koyama H., Hashimoto H. Molecular cloning and sequence determination of the peplomer protein gene of feline infectious peritonitis virus type I. Arch. Virol. 1995;140:469–480. doi: 10.1007/BF01718424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa K., Hohdatsu T., Hashimoto H., Koyama H. Comparison of the amino acid sequence and phylogenetic analysis of the peplomer, integral membrane and nucleocapsid proteins of feline, canine and porcine coronaviruses. Microbiol. Immunol. 1996;40:425–433. doi: 10.1111/j.1348-0421.1996.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C.W. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 1993;36:1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorado M.R., Kawaguchi Y., Miyazawa T., Norimine J., Maeda K., Toyosaki T., Tohya Y., Kai C., Mikami T. Isolation, sequence and expression of a cDNA encoding the alpha-chain of the feline CD8. Immunology. 1994;81:127–131. [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. Virologic and immunologic aspects of feline infectious peritonitis virus infection. Adv. Exp. Med. Biol. 1987;218:529–550. doi: 10.1007/978-1-4684-1280-2_69. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Black J.W., Boyle J.F., Everman J.F., Mckeirnan A.J., Ott R.I. Pathogenic differences between various feline coronavirus isolates. Adv. Exp. Med. Biol. 1984;173:365–380. doi: 10.1007/978-1-4615-9373-7_36. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y.J., Zack P.M., White B.D., Papermaster S.F., Elkins W.R., Eddy G.A., Lewis M.G. Decline in the CD41 lymphocyte population in the blood of SIV-infected macaques is not reflected in lymph nodes. AIDS Res. Hum. Retrov. 1993;9:639–646. doi: 10.1089/aid.1993.9.639. [DOI] [PubMed] [Google Scholar]
- Rottier P.J., Nakamura K., Schellen P., Volders H., Haijema B.J. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 2005;79:14122–14130. doi: 10.1128/JVI.79.22.14122-14130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart C.A., Scott F.W. Intrinsic resistance of feline peritoneal macrophages to coronavirus infection correlates with in vivo virulence. J. Virol. 1989;63:436–440. doi: 10.1128/jvi.63.1.436-440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumang J.R., Hsia C.Y., Tian W., Bromberg J.F., Liou H.C. IL-6 rescues the hyporesponsiveness of c-Rel deficient B cells independent of Bcl-xL, Mcl-1, and Bcl-2. Cell. Immunol. 2002;217:47–57. doi: 10.1016/s0008-8749(02)00513-0. [DOI] [PubMed] [Google Scholar]
- Tumpey T.M., Lu X., Morken T., Zakim S.R., Katzm J. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J. Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


