Highlights
-
•
Analysis of antigenicity and pathogenicity of QX-like IBVs were performed.
-
•
The results reveal a major differences between QX-like IBVs and other serotypes.
-
•
QX-like IBV SZ strain has lower pathogenicity in chickens compared with strain SD.
-
•
This finding provides important information for IBV prevention in poultry industry.
Keywords: Avian infectious bronchitis virus, Genotype, Serotype, Pathogenicity
Abstract
Avian coronavirus infectious bronchitis virus (IBV) causes considerable damage to the poultry industry worldwide and the proportion of QX-like genotype isolates have increased over time. Here, to better understand the antigenicity and pathogenicity of this genotype, we conducted sequence analyses, cross neutralization tests, and also examined the pathogenicity of two strains, SD and SZ. Sequence analyses revealed that SD and SZ isolates belong to the QX-like IBV genotype and share high homology in their full-length genomes. Cross neutralization tests showed high cross neutralization between SD and SZ, but distant relationships with other representative strains of the classical IBV serotypes. Virus infection experiments showed that SD caused high mortality with strong respiratory and renal pathogenicity in chickens, whereas SZ caused milder lesions by comparison. This study highlights the big discrepancy in antigenicity that exists between QX-like strains and other serotypes. Collectively, these findings provide important information about the epidemiology and pathogenicity of IBV, which may benefit the control of IB in the poultry industry.
1. Introduction
Infectious bronchitis (IB), an acute, highly contagious chicken viral disease caused by infections bronchitis virus (IBV), affects chickens of all ages, the most susceptible being chicks. IB poses a major economic threat to the poultry industry worldwide because of poor weight gain and lost feeding efficiency in broilers, and reduced egg numbers and quality in egg-laying birds (Jackwood, 2012, Xu et al., 2007, Yu et al., 2001). The typical symptoms of IB include coughing, sneezing, nasal discharge and tracheal rales (Cavanagh, 2007, Yan et al., 2016). Despite a predilection towards the respiratory tract, IBV also displays kidney and oviduct tropisms, thereby causing urogenital problems (Naqi et al., 2003, Zhong et al., 2016).
IBV is an enveloped virus with a single-stranded positive-sense non-segmented RNA genome of approximately 27.6-kb in length, and belongs to the Gammacoronavirus genus (Coronaviridae family, within Nidovirales) (Zhao et al., 2015). The IBV genome contains at least 10 open reading frames (ORFs) as follows: 5′-1a-1b-S1/S2-3a-3b-3c (E)-M-5a-5b-N-poly (A) −3′ (Xu et al., 2016). Approximately two-thirds of the genome encodes non-structural proteins (nsps) including the overlapping ORFs 1a and 1b, which are translated as the large polyprotein, 1ab. Protein 1ab is associated with RNA replication and transcription and can be further cleaved into 15 nsps (Zhao et al., 2014). The remaining 3′-end one-third of the genome encodes four major structural proteins: the spike (S) glycoprotein, the small envelope (E) protein, the membrane (M) glycoprotein, and the nucleocapsid (N) protein (Zhao et al., 2016). The S1 subunit of the spike protein is a major inducer of protective antibodies and is closely associated with viral tissue tropism and immune protection. The C-terminal portion of the S2 protein is intercalated in the virus envelope and assists S1 protein anchoring in the membrane (Li et al., 2012, Xu et al., 2016). The N protein is associated with the RNA genome and forms the ribonucleoprotein (Cavanagh, 2007).
The inaccuracy of the coronavirus RNA-dependent RNA polymerase and high frequency of genetic changes in IBV (e.g., gene insertion, mutation, deletion, and reconstruction), promote the emergence of new variant strains, genotypes and serotypes of IBV, and these are continuously reported (Cavanagh et al., 1986, Chen et al., 2017, Yan et al., 2016, Zhao. et al., 2016). IBV immunity is serotype-specific, and Mass-type vaccines (H120) can be ineffective against infection with different IBV isolates in China (Chen et al., 2017, Sun et al., 2011, Zhao et al., 2015). Therefore, the continuous testing of pathogenicity in new isolates, epidemic serotype determination, and typing new IBV strains remains crucial for better epidemiological understanding and control of IB in regions and/or countries.
IBV was first isolated in the early 1930s in the USA. The Massachusetts serotype was believed to be the only serotype until a second (Connecticut) was identified in the mid-1950s (Keeler et al., 1998). However, in recent years, a large number of IBV serotypes, antigenic variants, and field strains have been isolated (Xu et al., 2016). A previous study has shown that the incidence of the QX-like genotype increased from 11.7% to nearly 70% in China over a 20-year period from 1994, whereas the vaccine-like genotype, including the common vaccine strains (such as Mass 41, H120, and Connecticut), declined from 50.4% to 4.4% (Zhao et al., 2016). Concurrently, the QX-like IBV-type was found to cause renal, respiratory and reproductive lesions in chicken flocks in Europe, Asia, Africa and the Middle East (Mo et al., 2013, Valastro et al., 2010, Worthington and Jones, 2006, Worthington et al., 2008). The emergence of many QX-like IBVs is a major problem in the poultry industry. We have previously isolated two wild IBV strains, called “SZ” and “SD”, and a primary phylogenetic analysis showed that they belong to the QX-like genotype. Therefore, we performed a series of experiments to investigate their molecular characteristics and identify the pathogenicity and antigenicity of QX-like IBVs for better understanding of them with the aim of improved disease control.
2. Materials and methods
2.1. Viruses
The SD IBV strain was isolated from chickens previously vaccinated with IBV Mass-type vaccines in 2013, and the birds showed obvious signs of respiratory (coughing, sneezing and tracheal rales) and renal disease. The SZ IBV strain was isolated in 2012 from IB-affected chickens in the Jiangsu Province of China, which exhibited signs of respiratory (coughing and sneezing). Other viruses, including two vaccine strains (M41 and 4/91), and two wild-type virulent strains (YN and GD), were used for virus cross neutralization tests in this study. The M41 and 4/91 strains belong to genotype Mass-type and 4/91-like, respectively. The YN strain (GenBank accession no: JF893452) is a YN-type originating from a H120 vaccinated broiler flock with a death rate of 30% in the Yunnan Province of China (Xu et al., 2016, Zhao et al., 2015). The GD strain was isolated from IB-affected chickens in the Guangdong Province of China, and is closely related to the TW-type according to phylogenetic analysis (Xu et al., 2016). All IBVs used in this study were propagated in 10-day-old embryonated specific-pathogen-free (SPF) chicken eggs via the allantoic route and the infectious allantoic fluid was collected at 40 h post-inoculation. The median embryo infectious doses (EID50) for these strains were calculated using the standard method (Reed and Muench, 1938).
2.2. Animals and ethics statement
SPF white leghorn chickens and eggs were purchased from the Beijing Merial Vital Laboratory Animal Technology Co., Ltd, China. The birds were maintained in isolators at China Agricultural University throughout the experiments and the animal rearing facilities were approved by the Beijing Administration Committee of Laboratory Animals under the auspices of the Beijing Association for Science and Technology (approval ID SYXK [Jing] 2013-0013). The study’s protocol was conducted according to the guidelines of animal welfare set by the World Organization for Animal Health, and approved by the Animal Welfare and Ethical Censor Committee at China Agricultural University (Permit number: 1605-05). The humane endpoint was used in the present study and euthanasia was performed in the sampling program or for animals exhibiting severe respiratory symptoms (abdominal breathing) and paralysis attributable to IB infection.
2.3. Viral genome sequencing
Viral RNA was extracted from the allantoic fluid using RNAprep Pure Tissue Kit (Tiangen Biotech, Beijing, China) following the manufacturer’s instructions. For the first cDNA strand, a mixture containing 1.0 μl random primers (500 μg/ml, Promega, Madison, WI) and 4.0 μl RNA template was incubated at 70 °C for 5 min, then 2.0 μl dNTPs (2.5 mM), 1.5 μl RNasin (50U/μl, Promega) and 0.5 μl M-MLV (10U/μl) were added and incubated at 37 °C for 60 min. The product was used in polymerase chain reaction (PCR) assays.
The 28 primer pairs that will be made available upon request were used to PCR-amplify the complete genomes of SD and SZ. For the PCRs, 0.5 μl of PrimeSTAR HS DNA Polymerase (Tamarac, Ohtsu, Japan), 2.0 μl of dNTP mixture and 20 pmol of each primer were added to 100 ng of template cDNA in a total reaction volume of 50 μl. The reaction conditions were as follows: initial denaturation at 98 °C for 5 min, followed by 30 cycles of denaturation at 98 °C for 10s, annealing for 15 s at primer-dependent temperatures, and extension at 72 °C for 2 min. The final extension was at 72 °C for 10 min. All the amplified PCR products were analyzed by 1% agarose gel electrophoresis. Each nucleotide was determined from at least three identical results generated from separate PCR products. Nucleotide sequencing was conducted by a commercial company (TsingKe Biological Technology, Beijing, China). The sequencing was performed using the Sanger dideoxy sequencing method with an ABI 3730XL automatic sequencing apparatus.
2.4. Sequence and phylogenetic analyses
Sequence assembly of the SZ and SD IBV strains was conducted using the SeqMan program in DNASTAR 5.0 Software (DNASTAR Inc., Madison, WI, USA). Nucleotide sequence editing, analysis, deduced amino acid sequences and alignments were performed using the CLUSTAL W multiple alignment algorithm in the MegAlign program of DNASTAR. The deduced amino acid sequences were aligned, and the phylogenetic tree of S1 gene was constructed using MEGA 5.05 software by the neighbor-joining (NJ) method. The results were validated by 1000 bootstrap replicates.
2.5. Pathogenicity tests
Sixty-six 3-week-old SPF chickens were randomly divided into 3 groups of 22 chickens each and housed in different isolators with positive pressure in air-conditioned rooms. Two separate groups were vaccinated with 200 μl of 105.0EID50 of IBV SD or SZ strains by combined intranasal and ocular routes. The control group was inoculated with sterile normal saline using the same method. All the birds were observed for 14 days, free drinking and eating during the experiment.
2.5.1. Clinical observations and sampling
All the chickens were monitored daily for the clinical signs attributable to IB infection, including cough, sneeze and tracheal rales. Two chicks from the groups were euthanized by cervical dislocation at days 3 and 5 post-inoculation (dpi), and gross lesions of the birds were recorded in detail. Tissue samples from the trachea, lungs, spleen, glandular stomach, kidneys, and bursa of Fabricius were collected for histology and tracheal samples were collected for trachea cilia movement analysis. The tissue samples of euthanized birds exhibiting the severity of IB signs during the clinical observation were collected in a timely manner.
2.5.2. Inhibition of ciliary activity
To evaluate tracheal ciliostasis, nine rings per bird from the upper, middle and lower part of the trachea of three rings each, were analyzed. The rings were placed in 96-well plates with Eagle’s culture medium containing 10% fetal bovine serum. They were then examined by inverted light microscopy at a magnification of 400× to determine the degree of integrity and preservation of ciliary movement in the tracheal cells. A score of 0 was given if the cilia in the complete tracheal section showed movement; a score of 1 was given if 75%–100% of the cilia in the tracheal section showed movement; a score of 2 was given if 50%–75% of the cilia in the tracheal section showed movement; a score of 3 was given if 25%–50% of the cilia in the tracheal section showed movement; and a score of 4 was given if <25% of the cilia in the tracheal section showed movement or there was no movement at all. The average ciliostasis score was calculated for each group.
2.5.3. Histopathology
Tissue samples from the trachea, lungs, spleen, glandular stomach, kidneys, and bursa of Fabricius were fixed in 10% neutral formalin for 48 h at room temperature. Fixed samples were processed routinely, embedded in paraffin wax, and cut into 5 μm sections. The sections were stained with hematoxylin and eosin and examined for microscopic lesions resulting from IBV infection by light microscopy.
2.6. Antisera production and virus cross neutralization tests
Antisera against M41, 4/91, SD, SZ, GD, and YN IBV strains were prepared as previously described (Wang and Huang, 2000). To determine the antigenic relationship between the QX-like IBV SZ and other reference strains, cross neutralization tests, with a fixed concentration of virus and serial dilutions of serum, were performed according to a protocol previously described with slight modifications (Wang and Huang, 2000). Briefly, all sera were inactivated at 56 °C for 30 min, and then diluted ten-fold or serially two-fold and incubated with the same volume of 100 EID50 of the IBV strains at 37 °C for 1 h. The virus-serum mixtures were then inoculated into the allantoic cavity of 10-day-old SPF chicken embryos. Six days afterwards, the embryos were examined for the lesions typical of IBV infection (embryo dwarfing). The neutralizing titer of each serum sample against these viruses was determined and calculated by the Reed and Muench method (1938). A negative serum sample was always included in this study. The cross-neutralization R-values for the strains were calculated by the method described by Archetti and Horsfall (1950). Antigenic (serotype) difference between two given strains was defined as follows: an R value higher than 70% indicated antigenic identity between the two tested viruses, an R value between 33% and 70% indicated a minor subtype difference, an R value between 11% and 32% indicated a major subtype difference, and an R value below 11% indicated different serotypes.
2.7. Statistical analyses
Data were analyzed using an unpaired t-test in GraphPad Prism version 6.0 (GraphPad Software Inc., San Diego, California, USA) for Microsoft Windows to obtain a statistical analysis of the differences between SD and SZ groups in the pathogenicity test.
3. Results
3.1. IBV SD and SZ strains share the high sequence identity
The complete genomes of IBV strains SD and SZ were sequenced and the sequences were submitted to GenBank under the accession numbers KY421673 and KY421672, respectively. The sequence similarities of the structural protein genes between SD and SZ for S, E, M and N were 96.8%, 98.8%, 99.1%, and 94.5%, respectively. Further comparison of the two spike protein subunits between SD and SZ showed sequence similarities of 94.1% and 99.1% in the S1 and S2 subunits, respectively. The number of amino acid divergence in the structural proteins between SD and SZ strains were compared. Among them, there are 31 amino acid sites in the S1 protein that contain more different amino acids than the S2 (6 amino acid sites), E (1 amino acid sites), M (0 amino acid sites) and N (15 amino acid sites) protein.
3.2. IBV SD and SZ strains belong to the QX-like genotype
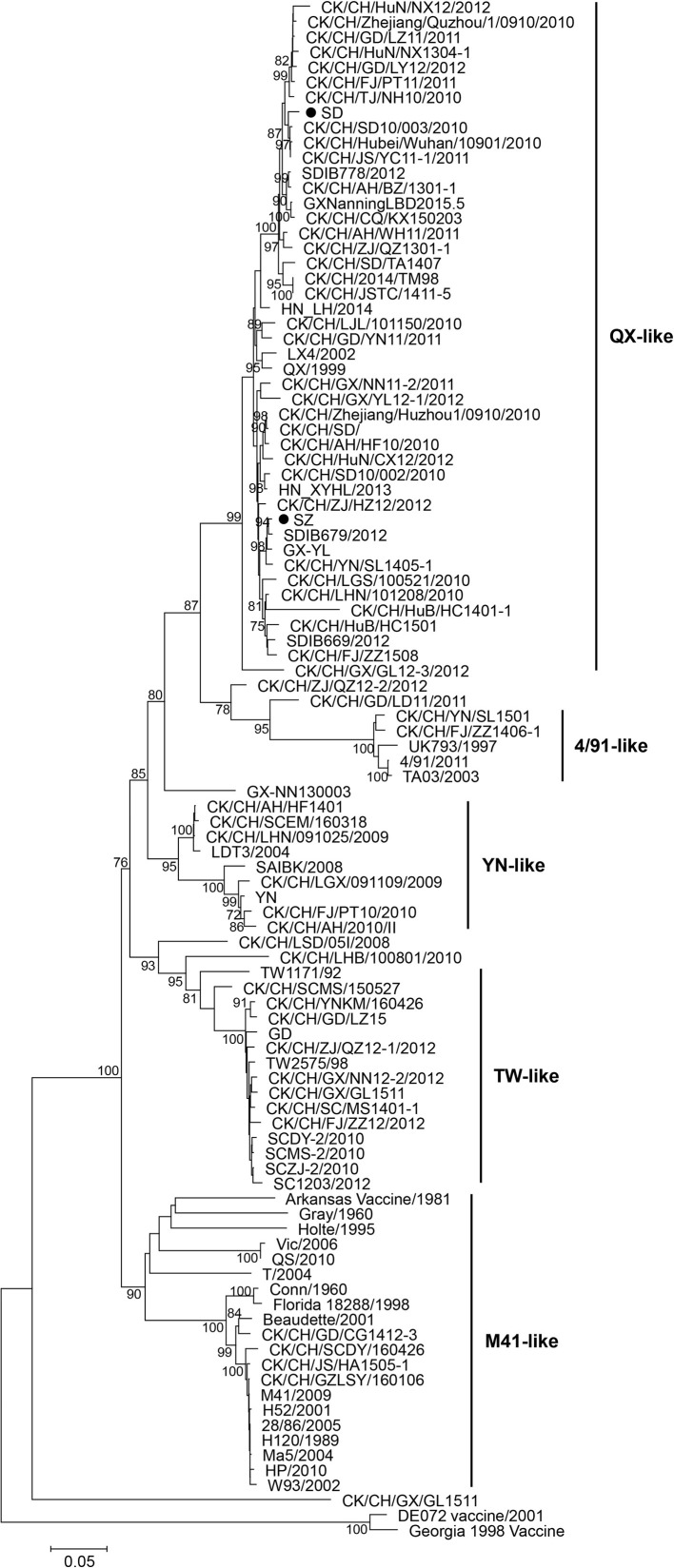
The phylogenetic tree of S1 gene revealed that the majority of the strains clustered into several distinct genotypes, including an M41-like group, a 4/91-like group, a YN-like group, a QX-like group, a TW-like group, and some smaller branches (Fig. 1 ). SD and SZ strains belong to the QX-like genotype, and are closely related to most of the prevalent isolates, but are distantly related to the most common vaccine strains in China, the M41-like strains.
Fig. 1.
Phylogenetic tree based on the nucleotide sequence of the S1 genes of IBVs. The tree was constructed with the neighbor-joining method using MEGA version 5.05. Bootstrap values (n = 1000 replicates) of <70% are not shown. The two IBV strains used in this study (SD and SZ) are each marked with a filled dot.
3.3. IBV SD and SZ strains exhibit different pathogenicity in SPF chickens
3.3.1. IBV SD strain causes high mortality
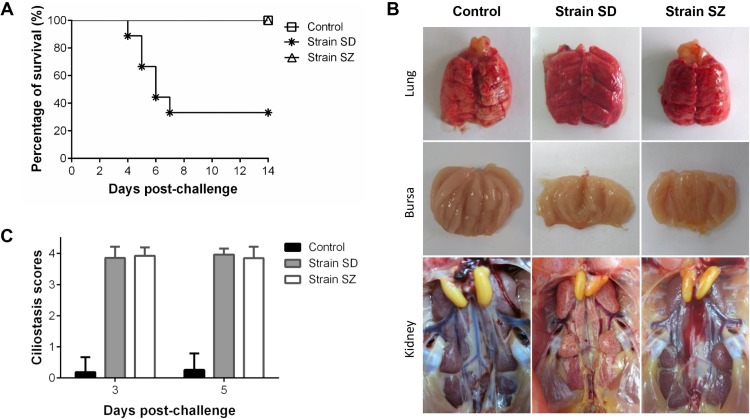
Chicks inoculated with the SD strain showed clinical signs as early as 3 dpi and twelve birds died during the experiment. The diseased chicks showed signs of coughing, sneezing, tracheal and bronchiolar rales, depression, and ruffled feathers. The birds in the SZ group showed no obvious signs. The percentage survival of the SD group was 33% during the 14-day observation period (Fig. 2 A), while the control group birds were alert and active in the experiment.
Fig. 2.
Percentage Survival (A), gross lesions (B) and trachea ciliostasis scores (C) of 3-week-old SPF chickens inoculated via the eye drop/intranasal (ED/IN) route with IBV SD and SZ strains at 105.0 EID50/bird. Eighteen birds were allocated to each group.
3.3.2. IBV SD strain causes severe gross lesions
In the SD group, obvious lesions were frequently detected in the respiratory tract, lungs, kidneys, and bursa of Fabricius at necropsy. The main clinical manifestations in the sampled chicks from the SD group were kidney swelling, urate deposition on the kidneys, and slight exudates of flaxen mucus on the bursa of Fabricius (Fig. 2B). In contrast and similar to the control group, no apparent pathological changes were observed in the SZ group throughout the observation period.
3.3.3. IBV SD and SZ strains cause severe tracheal ciliostasis
Inhibition of ciliary activity in the trachea was measured at 3 and 5 dpi. The SD and SZ groups showed a maximum average ciliostasis score of 4, while the average ciliostasis score in the control group was below 1 (Fig. 2C). No significant difference was observed for ciliostasis between SD and SZ inoculated groups, although the SZ strain induced no clinical manifestations.
3.3.4. IBV SD strain causes more serious histopathological changes
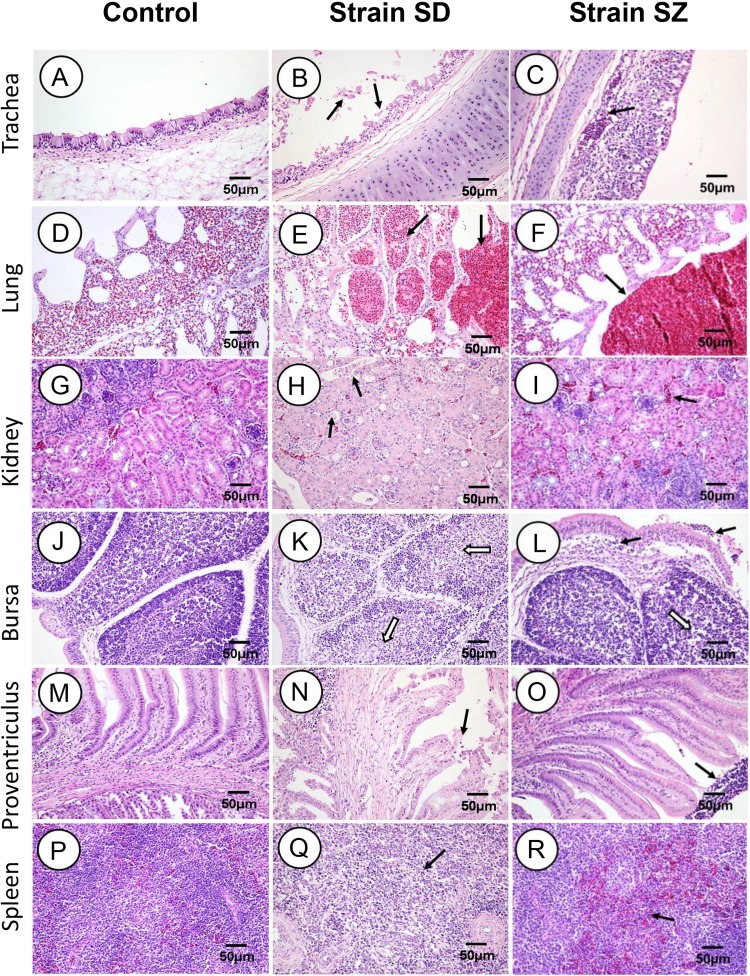
The pathological lesions in the organs collected from the inoculated chickens were further evaluated at 3 and 5 dpi. The representative pictures for 5 dpi are shown in Fig. 3 . Lesions in the SD infected group manifested themselves as follows. Severe desquamation and necrosis of the ciliated cells was seen in the trachea (Fig. 3B). Hemorrhages, congestion and lymphocytes were observed in the bronchial and air capillary lumina of the lungs (Fig. 3E). Epithelial necrosis and intense multifocal nephritis with lymphoplasmacytic infiltration occurred throughout the kidneys (Fig. 3H). Marked lymphocyte necrosis was detected in the bursa (Fig. 3K), and epithelial cell necrosis and detachment were seen in the proventriculus (Fig. 3N). Intense lymphocyte necrosis was observed in the spleen (Fig. 3Q). Chickens in the SZ group manifested the following lesions: Lymphocytes and tracheal mucosa lamina propria thickening occurred in the trachea (Fig. 3C). Congestion and erythrocytes were detected in the bronchi of the lungs (Fig. 3F). Slight congestion was scattered in the renal tubulointerstitia (Fig. 3I), and lymphoplasmacytic infiltration and lymphocyte necrosis in lymphoid follicles were detected in the bursa (Fig. 3L). Lymphocyte adherence to the surface of the mucosal in the proventriculus was seen (Fig. 3O), and a little lymphocyte necrosis and congestion was observed in the spleen (Fig. 3R). No IBV-related lesions were observed in tissue samples from the control group (Fig. 3A, D, G, J, M and P). Compared with the SD strain, SZ caused milder histopathological changes in the organs mentioned above.
Fig. 3.
Histopathologic changes detected at 5 days post-infection. B and C: Black arrows indicate degeneration and necrosis of the ciliated epithelial cells. E and F: Black arrows indicate hemorrhages and congestion in the pulmonary bronchus. H and I: Black arrows indicate degeneration and necrosis in the renal tubules. K and L: Black arrows indicate inflammatory cell infiltration in the Bursa mucous membrane surfaces; open arrows indicate lymphocyte necrosis in the entire lymphoid follicle. N and O: Black arrows indicate the loss of mucosa epithelial cells. Q and R: Black arrows indicate congestion and necrosis in the splenic lymphocytes.
3.4. Cross-neutralization assays reveal major serological differences among QX-like IBVs and other serotypes
The results of the two-way cross-neutralization assay are shown in Table 1 , including the neutralizing titers and R-values. The SZ QX-like strain, along with 4/91, M41, YN, SD and GD, which all represent different serotypes, showed different levels of cross-neutralization. Isolate SZ conferred poor cross neutralization to Mass-like M41 (R = 8.1%) or to YN-like YN (R = 6.9%), indicating that the strains belonged to different serotypes. The R-values for 4/91 (R = 14.6%) and GD (R = 16.7%) obtained in this study were below 32%, indicating that the strains belong to a different major subtype. Notably, the results showed that the antisera against SD used in this study could completely neutralize the SZ IBV isolate (R = 91.1%), a finding consistent with them belonging to the same serotype.
Table 1.
Crossing neutralizing test among QX-like IBV and other serotypes.
| Virus | Serum |
Antigen relatedness value (R) to SZ strain (%)b | |||||
|---|---|---|---|---|---|---|---|
| 4/91 | M41 | SZ | YN | GD | SD | ||
| SZ(QX-like) | 47.8a | 12.3 | 407.4 | 6.17 | 15.8 | 144.5 | 100 |
| SD(QX-like) | – | – | 331.1 | – | – | 141.3 | 91.1 |
| M41(M41-like) | – | 63.1 | 13.8 | – | – | – | 8.1 |
| 4/91(4/91-like) | 61.7 | – | 11.2 | – | – | – | 14.6 |
| YN(YN-like) | – | – | 70.8 | 223.9 | – | – | 6.9 |
| GD(TW-like) | – | – | 11.2 | – | 15.5 | – | 16.7 |
Titers were obtained in reciprocal β virus neutralization tests (diluted serum, constant virus).
The R values were calculated using the method described by Gravendyck et al. (1996). Criteria for classifying the antigenic relatedness: >0.70, antigenic identity; 0.33–0.70, minor subtype difference; 0.11–0.32, major subtype difference; <0.11, no relatedness (serotype difference).
4. Discussion
Recently, a large number of IBV genotypes and variants have been isolated in China, among which the QX-like genotype is widely prevalent there and inflicts large economic losses for farmers. The QX-like genotype was first isolated in 1998 in China, and it was considered to have a different genotype to all of the other IBVs (Li et al., 2012, Sun et al., 2011, Zhao et al., 2014). However, some studies have suggested that there is an uncertain correlation between its genotype and serotype (Chen et al., 2015). Therefore, accurate characterization of QX-like strains is an essential prerequisite for control measure implementation, and for understanding the epidemiology and evolution of IBVs. In this study, genotype, serotype and pathogenicity analyses of the QX-like strains SD and SZ were conducted.
The phylogenetic analyses revealed that the SD and SZ strains and many other field isolates grouped in the QX-like genotype cluster in the phylogenetic tree of S1 genes, and were distantly related to M41-like and 4/91-like strains, which are considered the main agents for disease outbreaks (Ji et al., 2011, Liu et al., 2009). SD and SZ share high sequence homology in all the structural protein genes including S1, which is known to be the main antigenic encoding gene in IBVs (Sun et al., 2011).
The same serotype for SD and SZ isolates was clearly established in this study through cross-neutralization testing, and high sequence similarities between SD and SZ were found in the spike protein gene (96.8%), S1 subunit gene (94.1%) and hypervariable region (HVR) 1 (94.3%). Consistent with previous reports is that the IBV serotype is associated with HVR 1 in the N-terminus (residues 45–114) of the S1 gene (Wang and Huang, 2000). The results of the cross-neutralization tests are in accordance with the phylogenetic analyses, which showed that the serotype of strains SD and SZ are obviously very different from those of the four selected serotypes (YN-like, TW-like, 4/91-like and Mass-like) investigated in this study.
The pathogenicity test indicated that inoculation of SPF chickens with the SD strain resulted in clinical signs consistent with an IB-like disease, and respiratory signs in the birds were most apparent. The mortality rate of the SD strain was higher (reaching 67%) than that reported previously (Gallardo et al., 2012) suggesting it is a highly pathogenic strain. The SD strain caused obviously swollen and pale kidneys, with ureters distended with urate. Lesions caused by the SD strain were similar to those described in previous reports, which have characterized it as a nephropathogenic IBV strain (Cong et al., 2013, Feng et al., 2012, Yan et al., 2016). However, we found that obvious clinical signs and lesions were not observed after the 3-week-old SPF chicks were inoculated with 105.0EID50 of SZ. Also, the SZ group, when compared with the SD group, presented with mild histopathological changes in the trachea, lungs, spleen, glandular stomach, kidneys, and bursa of Fabricius. These findings confirm the lower virulence of SZ compared with SD in 3-week-old-SPF chicks. But analysis of the degree of sequence homology among the strains showed that SD shared high identity with SZ strains. As we know, the pathogenicity of IBV is determined by multiple factors, which may involve its tissue tropism, replication efficacy and its ability to deal with the host’s immune system. The spike protein was shown to be an important virulence determination of IBV and some other protein genes have also been shown to exhibit obvious influence to IBV virulence (Armesto et al., 2009, Casais et al., 2003, Quinteros et al., 2015, Wickramasinghe et al., 2011). So the specific amino acids differences between them in the spike protein possibly contributed to the difference in the two viruses (SD and SZ). Alternatively, some of the distinct amino acid sites in the other structural proteins may have a positive influence on the pathogenicity of the two strains. There are 1 and 0 different amino acid sites in E and M proteins, respectively, suggesting that these proteins may not be directly linked with virulence, as has been suggested earlier (Neuman, 2011, Sapats and Wright, 1996). Clearly, more studies are needed to explore the function of these amino acids.
In summary, we have identified major differences between QX-like IBVs and other serotypes. The QX-like viruses are distinct from all other known IBV strains, not only genetically, but also serologically. Our study has also revealed that the QX-like IBV SZ strain has lower pathogenicity in chickens, and antisera against it could almost completely neutralize the IBV QX-like virulent strain SD, indicating that the SZ strain may be useful in vaccination programs under field conditions to reduce the economic losses caused by QX-like IBV infections.
Conflict of interest
None to declare.
Acknowledgment
This study was supported by the Beijing Agriculture Innovation Consortium of Poultry Research System (BAIC04-2017).
References
- Archetti I., Horsfall F.L. Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J. Exp. Med. 1950;92:441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armesto M., Cavanagh D., Britton P. The replicase gene of avian coronavirus infectious bronchitis virus is a determinant of pathogenicity. PLoS One. 2009;4(10):e7384. doi: 10.1371/journal.pone.0007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R., Dove B., Cavanagh D., Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 2003;77:9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Darbyshire J.H., Peters R.W. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J. Gen. Virol. 1986;67(Pt. 7):1435–1442. doi: 10.1099/0022-1317-67-7-1435. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Rec. 2007;38(2):281. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang T., Han Z., Liang S., Xu Y., Xu Q., Chen Y., Zhao Y., Shao Y., Li H., Wang K., Kong X., Liu S. Molecular and antigenic characteristics of Massachusetts genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2015;181(3–4):241–251. doi: 10.1016/j.vetmic.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Jiang L., Zhao W., Liu L., Zhao Y., Shao Y., Li H., Han Z., Liu S. Identification and molecular characterization of a novel serotype infectious bronchitis virus (GI-28) in China. Vet. Microbiol. 2017;198:108–115. doi: 10.1016/j.vetmic.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F., Liu X., Han Z., Shao Y., Kong X., Liu S. Transcriptome analysis of chicken kidney tissues following coronavirus avian infectious bronchitis virus infection. BMC Genomics. 2013;14:743. doi: 10.1186/1471-2164-14-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Hu Y., Ma Z., Yu Q., Zhao J., Liu X., Zhang G. Virulent avian infectious bronchitis virus, People's Republic of China. Emerg. Infect. Dis. 2012;18:1994–2001. doi: 10.3201/eid1812.120552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo R.A., van Santen V.L., Toro H. Effects of chicken anaemia virus and infectious bursal disease virus-induced immunodeficiency on infectious bronchitis virus replication and genotypic drift. Avian Pathol. 2012;41:451–458. doi: 10.1080/03079457.2012.702889. [DOI] [PubMed] [Google Scholar]
- Gravendyck M., Tritt S., Spenkoch-Piper H., Kaleta E.F. Antigenic diversity of psittacine herpesviruses: cluster analysis of antigenic differences obtained from cross-neutralization tests. Avian Pathol. 1996;25(2):345–357. doi: 10.1080/03079459608419145. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56(4):634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Ji J., Xie J., Chen F., Shu D., Zuo K., Xue C., Qin J., Li H., Bi Y., Ma J., Xie Q. Phylogenetic distribution and predominant genotype of the avian infectious bronchitis virus in China during 2008–2009. Virol. J. 2011;8:184. doi: 10.1186/1743-422X-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler C.L. Jr., Reed K.L., Nix W.A., Gelb J.Jr. Serotype identification of avian infectious bronchitis virus by RT-PCR of the peplomer (S-1) gene. Avian Dis. 1998;42(2):275–284. [PubMed] [Google Scholar]
- Li M., Wang X.Y., Wei P., Chen Q.Y., Wei Z.J., Mo M.L. Serotype and genotype diversity of infectious bronchitis viruses isolated during 1985–2008 in Guangxi, China. Arch. Virol. 2012;157:467–474. doi: 10.1007/s00705-011-1206-6. [DOI] [PubMed] [Google Scholar]
- Liu X.L., Su J.L., Zhao J.X., Zhang G.Z. Complete genome sequence analysis of a predominant infectious bronchitis virus (IBV) strain in China. Virus Genes. 2009;38:56–65. doi: 10.1007/s11262-008-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M.L., Hong S.M., Kwon H.J., Kim I.H., Song C.S., Kim J.H. Genetic diversity of spike, 3a, 3b and e genes of infectious bronchitis viruses and emergence of new recombinants in Korea. Viruses. 2013;5(2):550–567. doi: 10.3390/v5020550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqi S., Gay K., Patalla P., Mondal S., Liu R. Establishment of persistent avian infectious bronchitis virus infection in antibody-free and antibody-positive chickens. Avian Dis. 2003;47:594–601. doi: 10.1637/6087. [DOI] [PubMed] [Google Scholar]
- Neuman B.W. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174(1):11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteros J.A., Markham P.F., Lee S.W., Hewson K.A., Hartley C.A., Legione A.R., Coppo M.J., Vaz P.K., Browning G.F. Analysis of the complete genomic sequences of two virus subpopulations of the Australian infectious bronchitis virus vaccine VicS. Avian Pathol. 2015;44(3):182–191. doi: 10.1080/03079457.2015.1022857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H.A. Simple method of estimating fifty percent endpoint. Am. J. Epidemiol. 1938;27(3):493–497. [Google Scholar]
- Sapats S.I., Wright P.J. Novel variation in the N protein of avian infectious bronchitis virus. Virology. 1996;226(2):412–417. doi: 10.1006/viro.1996.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Han Z., Ma H., Zhang Q., Yan B., Shao Y., Xu J., Kong X., Liu S. Phylogenetic analysis of infectious bronchitis coronaviruses newly isolated in China, and pathogenicity and evaluation of protection induced by Massachusetts serotype H120 vaccine against QX-like strains. Avian Pathol. 2011;40:43–54. doi: 10.1080/03079457.2010.538037. [DOI] [PubMed] [Google Scholar]
- Valastro V., Monne I., Fasolato M., Cecchettin K., Parker D., Terregino C., Cattoli G. QX-type infectious bronchitis virus in commercial flocks in the UK. Vet. Rec. 2010;167(22):865–866. doi: 10.1136/vr.c6001. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Huang Y.C. Relationship between serotypes and genotypes based on the hypervariable region of the S1 gene of infectious bronchitis virus. Arch. Virol. 2000;145:291–300. doi: 10.1007/s007050050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe I.N., de Vries R.P., Gröne A., de Haan C.A., Verheije M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011;85(17):8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington K.J., Jones R.C. New genotype of infectious bronchitis virus in chickens in Scotland. Vet. Rec. 2006;159(9):291–292. doi: 10.1136/vr.159.9.291-b. [DOI] [PubMed] [Google Scholar]
- Worthington K.J., Currie R.J., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37(3):247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Xu C., Zhao J., Hu X., Zhang G. Isolation and identification of four infectious bronchitis virus strains in China and analyses of their S1 glycoprotein gene. Vet. Microbiol. 2007;122:61–71. doi: 10.1016/j.vetmic.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Xu G., Liu X., Zhao Y., Chen Y., Zhao J., Zhang G. Characterization and analysis of an infectious bronchitis virus strain isolated from southern China in 2013. Virol. J. 2016;13:1–9. doi: 10.1186/s12985-016-0497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S.H., Chen Y., Zhao J., Xu G., Zhao Y., Zhang G. Pathogenicity of a TW-Like strain of infectious bronchitis virus and evaluation of the protection induced against it by a QX-Like strain. Front. Microbiol. 2016;7:402537. doi: 10.3389/fmicb.2016.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Jiang Y., Low S., Wang Z., Nam S.J., Liu W., Kwangac J. Characterization of three infectious bronchitis virus isolates from China associated with proventriculus in vaccinated chickens. Avian Dis. 2001;45:416–424. [PubMed] [Google Scholar]
- Zhao Y., Liu X.Y., Cheng J.L., Wu Y.P., Zhang G.Z. Molecular characterization of an infectious bronchitis virus strain isolated from northern China in 2012. Arch. Virol. 2014;159:3457–3461. doi: 10.1007/s00705-014-2213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Cheng J., Liu X., Zhao J., Hu Y., Zhang G. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet. Microbiol. 2015;180:49–58. doi: 10.1016/j.vetmic.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhang H., Zhao J., Zhong Q., Zhang G.Z. Evolution of infectious bronchitis virus in China over the past two decades. J. Gen. Virol. 2016;97(7):1566–1574. doi: 10.1099/jgv.0.000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q., Hu Y.X., Jin J.H., Zhao Y., Zhao J., Zhang G.Z. Pathogenicity of virulent infectious bronchitis virus isolate YN on hen ovary and oviduct. Vet. Microbiol. 2016;193:100–105. doi: 10.1016/j.vetmic.2016.08.017. [DOI] [PubMed] [Google Scholar]