Abstract
Canine infectious respiratory disease (CIRD) occurs frequently in densely housed dog populations. One of the common pathogens involved is canine respiratory coronavirus (CRCoV), however little is known regarding its pathogenesis and the role it plays in the development of CIRD. The pathogenesis of five geographically unrelated canine respiratory coronavirus (CRCoV) isolates was investigated. Following experimental infection in dogs, all five CRCoV isolates gave rise to clinical signs of respiratory disease consistent with that observed during natural infection. The presence of CRCoV was associated with marked histopathological changes in the nares and trachea, with loss and damage to tracheal cilia, accompanied by inflammation. Viral shedding was readily detected from the oropharynx up to 10 days post infection, but there was little or no evidence of rectal shedding. The successful re-isolation of CRCoV from a wide range of respiratory and mucosal associated lymphoid tissues, and lung lavage fluids demonstrates a clear tropism of CRCoV for respiratory tissues and fulfils the final requirement for Koch's postulates. By study day 14 dogs had seroconverted to CRCoV and the antibodies raised were neutralising against both homologous and heterologous strains of CRCoV in vitro, thus demonstrating antigenic homogeneity among CRCoV strains from the two continents. Defining the role that CRCoV and other agents play in CIRD is a considerable, but important, challenge if the disease is to be managed, treated and prevented more successfully. Here we have successfully developed a model for studying the pathogenicity and the role of CRCoV in CIRD.
Keywords: Canine infectious respiratory disease (CIRD), Canine respiratory coronavirus (CRCoV)
1. Introduction
Canine infectious respiratory disease (CIRD) is a disease complex which occurs frequently in densely housed dog populations such as in rehoming, training and boarding kennels, and is usually characterised by a dry hacking cough, though it may progress to a potentially fatal bronchopneumonia (Appel and Binn, 1987a). The nature of CIRD presents a number of challenges in terms of disease management and animal welfare.
Management of CIRD is problematic due to the multifactorial nature of the disease in which a number of organisms including Bordetella bronchiseptica (Bemis, 1992, Bemis et al., 1977, Keil and Fenwick, 1998), canine parainfluenza virus (Appel and Percy, 1970, Erles et al., 2004); canine adenovirus-2 (Ditchfield et al., 1962); canine herpesvirus (Erles and Brownlie, 2005, Karpas et al., 1968) and Mycoplasma spp. (Bemis, 1992, Chalker et al., 2004) are known to be involved. Despite widespread vaccination against many of these pathogens, CIRD remains a persistent problem for veterinary practitioners (Erles et al., 2004).
Since its initial discovery in 2003 (Erles et al., 2003); canine respiratory coronavirus (CRCoV) is now considered to be a significant CIRD pathogen, most frequently detected in dogs with mild respiratory clinical signs during the early stages of CIRD onset (Erles et al., 2003). Although CRCoV has been found worldwide (Decaro et al., 2007, Erles and Brownlie, 2008, Kaneshima et al., 2006, Priestnall et al., 2006, Priestnall et al., 2007, Yachi and Mochizuki, 2006, Knesl et al., 2009); little is known regarding its pathogenesis, tissue tropism or virulence differences among global isolates in the canine host.
It is postulated that CRCoV plays an important role during the early stages of CIRD by predisposing the dog to more severe clinical disease from secondary infections. Through the use of an in vitro tracheal explant culture system, a moderate reduction in ciliary function and a down-regulation of pro-inflammatory cytokine mRNA levels (TNF-α, IL-6 and the chemokine IL-8) was observed in response to CRCoV exposure (Priestnall et al., 2009). Such alterations in the mucociliary and innate immune systems could be linked to increased susceptibility to secondary infection and is consistent with the proposed role for CRCoV in CIRD. However, the limitation of this in vitro model precludes the understanding of the clinical relevance and pathogenesis of a CRCoV infection in the dog. Furthermore, given the global presence of this virus, insight into CRCoV pathogenesis among isolates originating from geographically distinct locations would be valuable to determine the need for a global vaccine.
Recently our group has collected findings from a preliminary in vivo challenge study of CRCoV. In that study we demonstrated that young dogs were susceptible to experimental infection with both CRCoV isolates, which gave rise to clinical signs of respiratory disease consistent with naturally occurring infection. CRCoV was detected in the oropharynx of infected dogs and spread rapidly to sentinel dogs which also displayed clinical signs of disease (Mitchell et al., unpublished data).
Here we extend this study to gain a better understanding of CRCoV pathogenesis in vivo. Analyses specifically focused on the histopathological changes in the canine upper and lower respiratory tissues, virulence differences among CRCoV isolates derived from CIRD cases representing wide geographical locations; UK and USA [MO, NE, UT and MI], and the demonstration of Koch's postulates. The information obtained from this study vastly enhances our understanding of CRCoV pathogenicity and its involvement in the CIRD complex.
2. Materials and methods
2.1. Viruses
Five CRCoV isolates originating from geographically distinct regions of the UK and USA were used in this study (Table 1 ). CRCoV isolates UK 4182, NP631, NP787, and NP742 were propagated in culture on human rectal tumour cells (HRT-18G; American Type Culture Collection Cell Line, Manassas, VA, USA), and used at 106 TCID50/mL for intranasal challenge. CRCoV LU298 was not was not propagated or expanded in vitro, instead 0.2 μm filtered viral fluids obtained from the tissues of a CRCoV infected dog in the USA were used to challenge animals in TO1. Prior to challenge each of the CRCoV virus and control HRT-18G cell culture fluids were satisfactorily tested for sterility and canine extraneous agents (including canine distemper virus, measles, canine adenovirus-2, canine parainfluenza virus, canine rotavirus, rabies, canine parvovirus and canine enteric coronavirus).
Table 1.
Study design. Treatment groups and euthanasia schedule. Dogs were challenged intranasally on study day 0 and 1. Two dogs were euthanized from each treatment group, on each study day 3, 6 and 14.
| Treatment group | Number of dogs | Inoculum (CRCoV isolate) | Origin of isolate | Passage of isolate | Challenge titre per dog [TCID50/mL] | Day of euthanasia |
|---|---|---|---|---|---|---|
| T1 | 6 | LU298 | MO, USA | P0 | 102 | 3 |
| 6 | ||||||
| 14 | ||||||
| T2 | 6 | UK 4182 | UK | P7 | 106 | 3 |
| 6 | ||||||
| 14 | ||||||
| T3 | 6 | NP631 | NE, USA | p2 | 106 | 3 |
| 6 | ||||||
| 14 | ||||||
| T4 | 6 | NP787 | UT, USA | p2 | 106 | 3 |
| 6 | ||||||
| 14 | ||||||
| T5 | 6 | NP742 | MI, USA | p2 | 106 | 3 |
| 6 | ||||||
| 14 | ||||||
| T6 | 6 | Control | HRTG cell culture fluid | – | – | 3 |
| 6 | ||||||
| 14 | ||||||
2.2. Study design
Thirty-six, 12–16-week old specific pathogen free (SPF) purpose bred beagle dogs were used in this study. All dogs were demonstrated as CRCoV negative and seronegative for CRCoV and B. bronchiseptica prior to the study. Dogs were housed in temperature controlled isolation rooms with dedicated shower in and out procedures, disinfection and sterilisation of all items prior to entry and a pasteurised diet were used to maintain bio-security. Colony dogs are screened quarterly to determine the SPF status. Throughout the study dogs from the same treatment group were housed in pairs to minimise stress.
Dogs were randomly divided into six treatment groups (T1–T6) (Table 1), each consisting of six dogs. Dogs in groups T1–T5 inclusive were challenged with the CRCoV isolates as described in Table 1. Dogs in T6 were mock challenged with uninfected HRT-18G cell culture supernatant to serve as negative controls. Intranasal inoculations took place over two consecutive days (study day zero and one). On each challenge day dogs were sedated prior to intranasal administration with 1.0 mL of virus or control material (0.5 mL/nostril). Dogs were monitored throughout the trial for clinical signs of disease. On study days 3, 6, and 14, as detailed in Table 1, dogs were humanely euthanatized within each treatment group as determined by randomisation completed prior to the start of the trial, and necropsies were performed immediately. Gross pathological changes were documented throughout each necropsy and the lungs of each animal were photographed.
All experiments involving animals were carried out at a contract research organisation, in compliance with national legislation, and subject to local ethical review.
2.3. Clinical observations
General health observations were performed on each dog, twice daily, and scored for general appearance, breathing, sneezing, coughing, and ocular and nasal discharge as detailed in Table 2 . Body temperatures were recorded twice daily via implanted microchips, and dogs were weighed on study days −7, −1 and on the day of euthanasia. Appetite was recorded according to the quantity of food eaten per room.
Table 2.
Scoring system used for general health observations.
| Score | General appearance |
|---|---|
| 0 | Normal |
| 2 | Depressed |
| 3 | Lethargy |
| 20 | Death |
| Score | Breathing | Sneezing or coughing | Ocular or nasal discharge |
|---|---|---|---|
| 0 | Normal | None | None |
| 1 | Slightly audible | Occasional | Serous |
| 2 | Very audible | Moderate | Mucoid |
| 3 | – | Paroxysmal, persistent | Purulent |
2.4. Sample collection
2.4.1. Swabs
Oropharyngeal viral swabs for RT-PCR analysis (Sterilin, UK) and virus isolation (Dacron swabs, Puritan, in 3 mL virus transport medium (PAH)) were collected on study days −1 (pre challenge) 2, 3, 4, 5, 6, 8, 10, 12, and 14 from all dogs on the study. Rectal swabs were collected from each dog at necropsy. After sampling, viral swabs were frozen and stored at −70 °C until processed. Swab tips for RT-PCR analyses were immersed in 1 mL of RPMI medium (Sigma, Dorset, UK) and mixed prior to analysis.
2.4.2. Tissues
Samples from the following tissues were harvested at necropsy and stored at either −70 °C or fixed in 10% buffered formalin: nasal cavity (included ciliated area), nasal tonsil, palatine tonsil, trachea, apical lung lobe, diaphragmatic lung lobe, and bronchial lymph node. Each tissue sample was taken using a new set of sterile instruments. Lung lavage fluid samples were collected in DMEM cell culture medium and stored at −70 °C. Formalin-fixed tissues were processed and stained for histology and were examined by a veterinary pathologist as described in Section 2.9.
2.4.3. Blood and serum samples
On study days −1, 3, 6, and 14, serum and EDTA whole blood samples were collected for serological and haematological analysis as described below.
2.5. Detection of CRCoV in swabs and tissues
Swabs and tissue samples were tested for the presence of CRCoV by RT-PCR and virus isolation.
2.5.1. Cell maintenance
HRT-18G monolayers were maintained in DMEM (Dulbecco's modified eagle's medium) (Sigma–Aldrich Fine Chemicals Biosciences, St. Louis, MO, USA) supplemented with 5% FBS, 200 mM l-glutamine and 22.5 μg/mL gentamicin (final concentrations).
2.5.2. Virus isolation (VI) from swab, lung lavage and tissue samples
Prior to inoculation on to HRT-18 G cells, swab and lung lavage samples were clarified by centrifugation, and filtered (0.2 μm filter). Similarly, approximately 1 g of each tissue sample was homogenised in 3.0 mL of virus transport medium (VTM), clarified by centrifugation and filtered (0.2 μm filter). Filtered samples were inoculated onto confluent T25 or T150 monolayers of HRT-18 G cells. Inoculated cultures were maintained in DMEM supplemented with final concentrations of 200 mM l-glutamine, 22.5 μg/mL gentamicin, and 1% FBS. Inoculation media was also supplemented with trypsin. Trypsin concentrations were determined for each batch (circa 1 μg/mL) based of cell toxicity. Cell culture fluids were sampled weekly for 2 weeks to test for the presence of CRCoV by immunofluorescence assay (IFA) (Section 2.5.3).
2.5.3. Detection of CRCoV in cell culture fluids by IFA
HRT-18G cells were cultured to confluency in 96 well plates. Prior to inoculation the growth medium was discarded and replaced with 100 μL/well of DMEM containing 1% FBS. Each cell culture fluid sample derived from Section 2.5.2 was inoculated in quadruplicate at 120 μL and 30 μL/well, and incubated for 5–7 days. The monolayers were fixed with 80% acetone, washed twice in water, and stained for one hour with 50 μL/well of CRCoV monoclonal 72.1.2 fluorescent antibody conjugate (Pfizer Inc.) diluted 1:200 in FA diluent [PBS with 1% bovine serum albumin (Steris Corporation, Mentor, OH, USA) [w/v] and 0.09% sodium azide (Mallinkrodt Chemicals, Hazelwood, MO, USA) [w/v]]. Immunofluorescence was observed using an inverted microscope (Olympus IX71/IX51; Olympus, Southend-on-Sea, Essex, UK). Observation of any CRCoV FA positive cells confirmed the presence of CRCoV in the sample.
2.5.4. RNA extraction and cDNA synthesis
RNA was extracted using the RNeasy Mini Kit (Qiagen, Crawley, UK) as recommended by the manufacturer from 200 μL of the swab fluid or a 0.5 cm2 piece of tissue. RNA was transcribed into cDNA using Random Hexameres (GE Healthcare, Little Chalfont, UK) and Improm II reverse transcriptase (Promega, Southampton, UK) according to the manufacturer's protocol.
2.5.5. GAPDH PCR and CRCoV spike gene PCR
All samples were tested for the presence of the house keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by PCR as described previously (Grone et al., 1996). Results were expressed as a positive or negative outcome to ensure successful nucleic acid extraction. Samples were analysed for CRCoV using the nested spike gene PCR as described previously (Erles et al., 2003).
2.6. Histopathology
Formalin-fixed tissues were processed for histology and sections were stained using haematoxylin and eosin. The histological sections were examined by a veterinary pathologist who was blinded to the study groups. Each tissue was ascribed a histological and cilia score as described in Table 3 . A single overall histological “weighted” score was assigned to each dog, in which scores assigned to the lower respiratory tract (trachea, lungs, bronchial lymph nodes) were considered more noteworthy than those in the upper respiratory tract (nares, tonsils). This weighting of the overall score was used to take into account that histological changes (especially inflammatory reactions or lymphoid hyperplasia) in the upper respiratory tract are not uncommon, especially in young animals, and unless considerable, are considered part of the normal defences of the upper respiratory tract. Conversely, such changes in the lower respiratory tract are more likely to be clinically significant. Thus moderate to marked lymphoid hyperplasia or neutrophilic inflammation of the palatine tonsil (score 4) would have less effect on the overall histology score for that individual dog than would a similar grading of lymphoplasmacytic or neutrophilic aggregation in the tracheal mucosa or lungs.
Table 3.
Scoring system used for examination of histological abnormalities and for cilia in the trachea.
| Tissue scores | Criteria for histological abnormalities | Scoring criteria for cilia |
|---|---|---|
| 0 | No significant histological abnormality is recognised (NHAIR) | Dense, long cilia |
| 1 | Minimal – mild changes | Mild shortening or irregularity of cilia |
| 2 | Modest – moderate changes | Moderate shortening/irregularity of cilia |
| 3 | Marked (multi)focal changes | Marked irregularity with focal cilia loss |
| 4 | Marked, extensive/diffuse changes | Marked irregularity with diffuse cilia loss |
2.7. Immunohistochemistry
Paraffin-embedded formalin-fixed tissues (4 mm) were mounted on SuperFrost Plus microscope slides (Menzel-Gläser, Braunschweig, Germany). Slides were heated at 60 °C for 1 h, deparaffinised and dehydrated. Endogenous peroxidase was blocked with 3% H2O2 for 10 min then slides were washed in dH2O, then incubated in pre-warmed (37 °C) protease XIV 0.05% (Sigma) in TBS for 15 min. Slides were rinsed in dH2O then incubated with blocking serum (2% normal goat serum [Vector Laboratories, Peterborough] in TBS). Staining for CRCoV was performed as described previously (Priestnall et al., 2009). Positive cells were identified microscopically by the presence of staining.
2.8. Analysis of blood samples
Haematological analysis of EDTA blood samples was performed using a Cell-Dyn 3500 automated haematology machine. A 100 cell differential count of the white blood cells was performed on blood smears stained with a Hematek® automated stainer using Modified Wright's stain, by a board certified veterinary clinical pathologist blinded to the treatment groupings. The results for day −1 were analysed for normality and reference intervals were estimated (n = 36). Total neutrophil, band neutrophil, lymphocyte and monocyte concentrations were calculated using the automated total WBC concentration, and the observed percentages of each leucocyte type determined by the differential count. The results within each treatment group for days 3, 6 and 14 were compared with each other and with those at day −1 to evaluate trends or significant differences. The paired t-test or Wilcoxon signed ranks test with repeat measures were used if distribution was normal or non-normal, respectively. Statistical significance was set at p = 0.05.
2.9. Detection of CRCoV antibodies in canine serum
HRT-18G cells were cultured to confluency in 96 well plates. Monolayers were inoculated with a USA CRCoV isolate, incubated for 5 days, and fixed with 80% acetone. Canine serum samples were diluted 1:40 in PBS supplemented with 1% bovine serum albumin (w/v) (Steris Corporation, Mentor, OH, USA) and 0.09% sodium azide (Mallinkrodt Chemicals, Hazelwood, MO, USA) followed by two-fold serial dilutions to 1:1280. Diluted serum was dispensed at 100 μL/well onto the fixed cells, incubated for 1 h, and then washed twice with water. Bound antibody was detected with 50 μL/well of fluorescein isothiocyanate-labelled secondary antibody (rabbit anti-dog IgG) (Sigma–Aldrich, Jerusalem, Israel) diluted 1:250 in PBS supplemented with 1% bovine serum albumin (w/v) and 0.09% sodium azide (w/v), incubated and washed as before. Endpoint CRCoV titres were observed using fluorescence microscopy and defined as the inverse of the last dilution of serum exhibiting definite CRCoV fluorescence. In instances where no virus-specific fluorescence at the 1:40 dilution was observed, dogs were considered seronegative or non-exposed to CRCoV.
2.10. Detection of CRCoV neutralising antibodies in canine serum
Dog sera collected on study day 14 were tested for serum neutralising antibody titres against 1 UK, and 10 USA CRCoV isolates. HRT-18G cells were grown to confluency in a 96 well plate. Cells were rinsed once in serum-free DMEM and then pre-treated for 1 hour with 100 μL/well of serum-free DMEM containing c1 μg/mL trypsin. Heat inactivated serum samples were diluted 2-fold, 1:10 through 1:1280, in DMEM containing 1% FBS. A further 1:2 dilution of the serum was made in 50–300 TCID50 of virus per 100 μL, to obtain final serum dilutions of 1:20–1:2560. Virus:serum mixtures were incubated for 1 h at room temperature. Cells were inoculated in quadruplicate with 200 μL/well of the virus: serum mixture, incubated for 5–7 days, and fixed with 80% acetone. Virus growth was detected by IFA as described previously (Section 2.5.3). The fifty percent neutralisation endpoint for each serum sample was calculated by the statistical methods of Spearman–Karber.
2.11. Bacteriology
Oropharyngeal Amies swabs (Sterilin, UK) and lung lavage fluid samples were collected from all dogs at necropsy in order to screen for the presence of bacterial pathogens. Briefly each swab was plated onto 1× chocolate agar, 1× MacConkey agar, and 2× blood agar (1× aerobic and 1× anaerobic) and submitted to the Clinical Services Division at the Royal Veterinary College for analysis. Samples were also plated onto Mycoplasma Experience Agar (Mycoplasma Experience Ltd. Surrey, UK) and incubated at 37 °C with 5% CO2 for 3 days.
3. Results
3.1.1. Clinical observations
Dogs from all six treatment groups were healthy prior to challenge. Dogs in T6 (negative control) remained healthy for the duration of the study, the only exception being one dog which had some serous ocular discharge prior to challenge, and throughout the study. All 36 dogs completed the study on the pre-assigned day. Following challenge, a number of dogs from T1 to T5 inclusive displayed mild clinical signs of respiratory disease which included nasal discharge, sneezing and coughing. Table 4 shows the total scores for each observation by treatment group. There were no significant changes in body temperature all dogs had normal or fair appetites and gained weight over the course of the study (data not shown). No consistent differences between dogs treated with different strains of the virus were observed, although respiratory signs were recorded most frequently and most severely in one dog from T3 and one from T5 (Table 4). It is worth noting that in both instances these dogs were euthanized on study day 14 and displayed the clinical signs of respiratory disease recorded throughout the 14-day study period, although the scores were highest in these two dogs on study days 10–13.
Table 4.
General health scores.
| Treatment group (isolate) | General appearance | Breathing | Sneezing or coughing | Nasal discharge | Ocular discharge | Total clinical score |
|---|---|---|---|---|---|---|
| T1 (LU298) | 0 | 0 | 4 | 4 | 0 | 8 |
| T2 (UK4182) | 0 | 0 | 3 | 12 | 0 | 15 |
| T3 (NP631) | 0 | 17 | 10 | 5 | 1 | 33a |
| T4 (NP787) | 0 | 0 | 2 | 11 | 0 | 13 |
| T5 (NP742) | 0 | 0 | 17 | 10 | 0 | 27a |
| T6 (control) | 0 | 0 | 0 | 1 | 5 | 6b |
Scores heavily weighted by one dog within the group, in both instances the dogs were euthanized on study day 14 and displayed the clinical signs of respiratory disease recorded for throughout the14 day study period. Scores were highest in these dogs on study days 10–13.
Scores awarded to one dog in this treatment group which had some serous ocular discharge prior to challenge and throughout the study.
3.2. CRCoV shedding
3.2.1. Oropharyngeal viral swabs
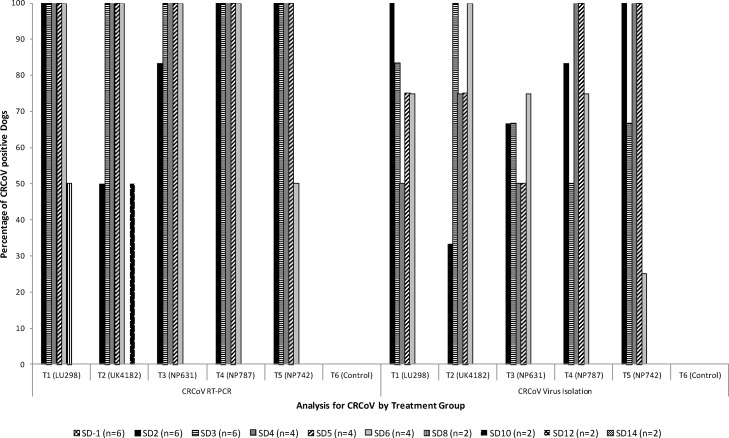
Oropharyngeal swabs were analysed for CRCoV by both RT-PCR and virus isolation (Fig. 1 ). All dogs from all six treatment groups were negative for CRCoV on study day −1 (pre-challenge) using both techniques. All dogs in T6 (negative control) remained negative for the duration of the study. Shedding of CRCoV in T1–T5 was consistently detected for up to 6 days post challenge by both RT-PCR and VI.
Fig. 1.
CRCoV shedding: RT-PCR and virus isolation analysis of oropharyngeal swabs collected from dogs throughout the study period. For each study day the n = number of dogs samples is indicated as dogs are euthanized for necropsy on study days (SD) 3, 6 and 14.
3.2.2. Rectal swabs
All rectal swabs, collected at necropsy, were positive for the internal PCR control GAPDH. All rectal swabs collected from dogs in T6 (negative control) were negative for CRCoV by both RT-PCR and VI for the duration of the study, as was the case for dogs in groups T3, T4 and T5. In T1, two dogs euthanized on study day 3, and one dog euthanized on study day 6 were positive for CRCoV, as was one T2 dog euthanized on study day 3. The remaining dogs in these groups were negative. All rectal swabs were negative for CRCoV by VI (data not shown).
3.3. CRCoV tissue tropism
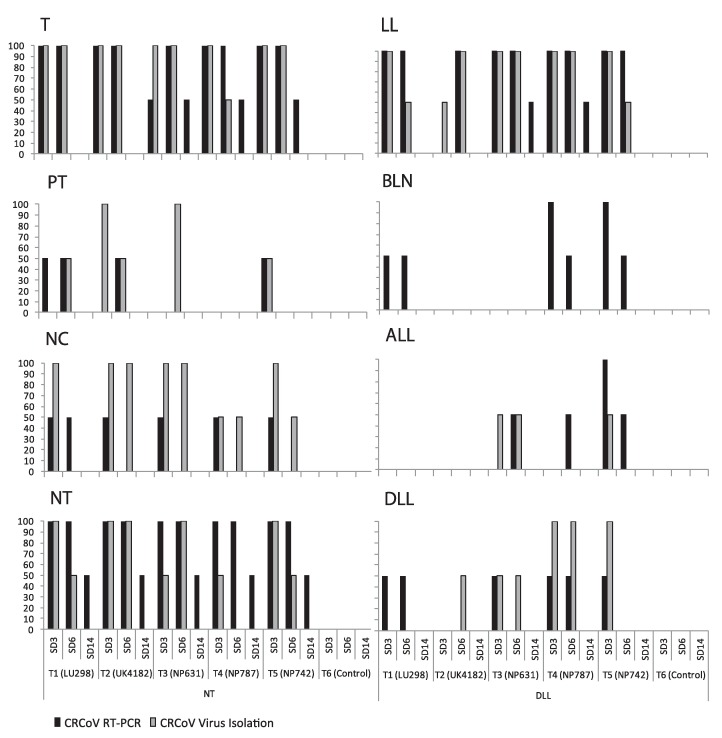
Tissues collected at necropsy were tested for the presence of CRCoV by RT-PCR and VI (Fig. 2 ). CRCoV was detected in at least five of the eight tissues [diaphragmatic lung lobe, apical lung lobe, trachea, nasal cavity, bronchial lymph node, nasal and palatine tonsil and lung lavage fluid] from groups T1–T4 by RT-PCR. In T5 CRCoV was detected in all the tissues tested. VI was successful in seven of eight tissue types from groups T1–T5 including the diaphragmatic lung lobe, apical lung lobe, trachea, nasal cavity, nasal and palatine tonsil as well as the lung lavage fluid. Only bronchial lymph nodes were negative for CRCoV by VI. Overall, CRCoV was detected most frequently from the trachea, followed by the lung lavage fluid, nasal cavity and nasal tonsil, in all five treatment groups.
Fig. 2.
CRCoV tissue tropism. Graphs show the percentage of dogs by study group and study day that are positive for CRCoV by RT-PCR and VI in different respiratory tissues. T: trachea; PT: palatine tonsil; NC: nasal cavity; NT: nasal tonsil; BLN: bronchial lymph node; LL: lung lavage; DLL: diaphragmatic lung lavage; ALL: apical lung lobe.
3.4. Pathology
3.4.1. Histopathology
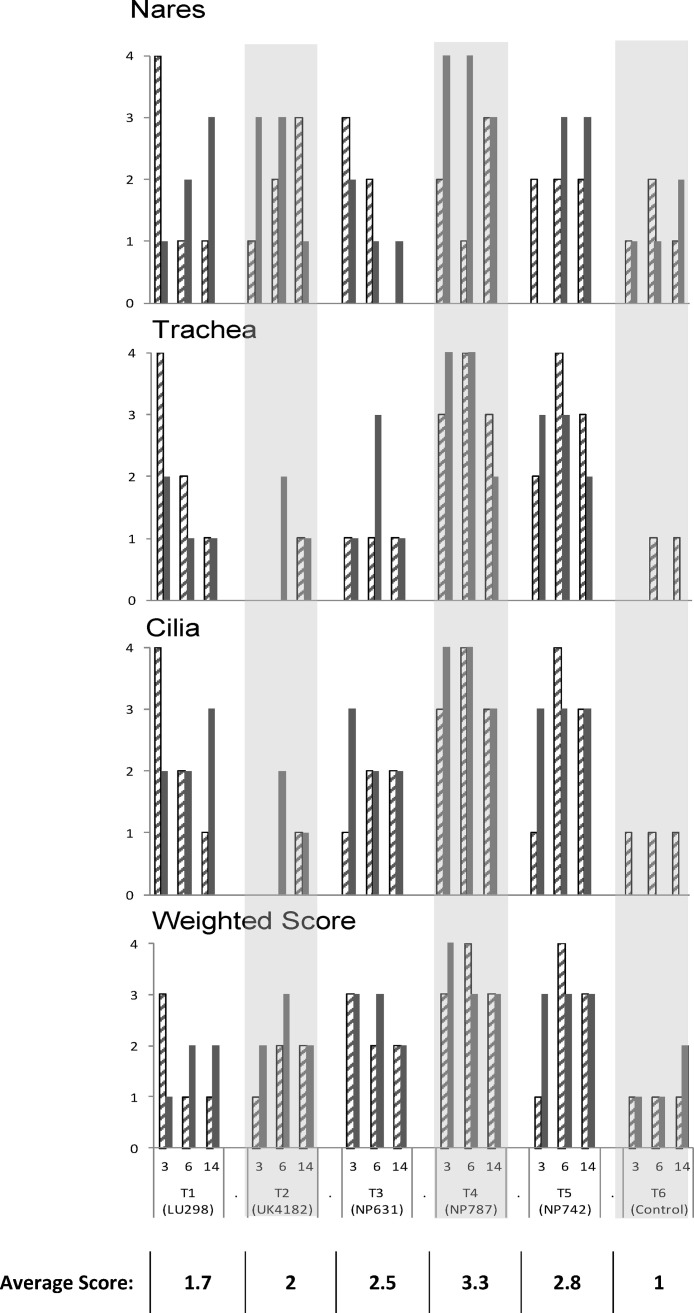
Histological examination was carried out on the palatine tonsil, external nares, nasal tonsil, trachea, apical and diaphragmatic lung lobes, and bronchial lymph node. The most significant findings were seen in the trachea and nares where inflammation, with notable changes in the length and distribution of the cilia were observed as described below and shown in Fig. 3, Fig. 4 .
Fig. 3.
The overall weighted and individual histological scores for the trachea, nares and tracheal cilia for each dog grouped according to treatment and day of necropsy (two dogs at each necropsy time point). The overall “weighted” score puts a larger emphasis on histological changes in the lower respiratory which are likely to be more clinically significant than histological changes in the upper respiratory tract are not uncommon and unless marked, are considered part of the normal defences of the upper respiratory tract. The hatched and solid bars represent each dog per necropsy time point. The table shows the average overall weighted score for each treatment group.
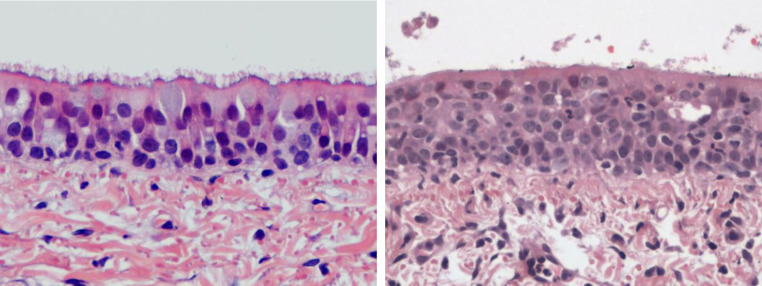
Fig. 4.
Representation of the typical histopathological presentation observed in the trachea of control in CRCoV infected dogs. Left: tracheal epithelium from dog from T6 (Control). There is a full covering of long slender cilia. Epithelium is orderly and contains full goblet (mucus secreting cells are indicated by the arrow). ×400, haematoxylin and eosin. Right: tracheal epithelium from dog from T4 (NP787), necropsy day 6. There is surface debris of cells and proteinaceous material. Cilia are almost completely denuded. Epithelial cells are disorderly and devoid of goblet cells. There are inflammatory cells within the epithelium and in the subjacent lamina propria. ×400, haematoxylin and eosin.
In T6 the overall weighted histology score was consistently minimal (average 1.1), with minimal changes in all the tissues examined.
In T1 the weighted score indicated minimal to modest abnormalities (average 1.7), including inflammatory aggregates in the nares and inflammation in the trachea which was associated with loss of cilia from the mucosal surface. One dog in particular on day 3 showed marked changes (scoring 3 or 4) in all of the tissues examined, with the exception of the diaphragmatic lung lobe and nasal tonsil where only minimal to modest changes were observed.
In T2 and T3 the overall weighted score showed modest to marked histological abnormalities with average scores of 2 and 2.5, respectively. In the nares modest to marked inflammation was seen, which in T3 trailed off in the later stages of the trial period, whilst inflammation in the trachea of dogs from both groups was mild but with moderate shortening and irregularity of the cilia.
In T4, with the exception of two dogs (one each on days 3 and 6), marked histological changes in the nares were observed throughout the trial period. In addition marked changes were also observed in the trachea and cilia. One dog on day 6 also had marked changes in the bronchial lymph node and diaphragmatic lung lobe (data not shown). The overall weighted scores indicated consistently marked abnormal histological changes in all dogs in this group with an average score of 3.3.
In T5 modest to marked inflammation was observed in the trachea of dogs in T5 which was reflected in the condition of the cilia, whilst only modest changes were observed in the nares. On study day 14 marked changes were also seen in the bronchial lymph nodes. The overall weighted score showed consistently marked abnormal histological changes (average 2.8), with the exception of one of the dogs at the first time point which had a score of 1.
In T1–T5 inclusive histological abnormalities in the lungs, although not marked, were variable and tended to be lymphoid aggregates adjacent to airways or blood vessels, an observation which was not seen in the broth control which yielded consistently mild scores. In all groups, including the broth control, the palatine tonsils had medium to high histological scores (data not shown).
3.4.2. Immunohistochemistry
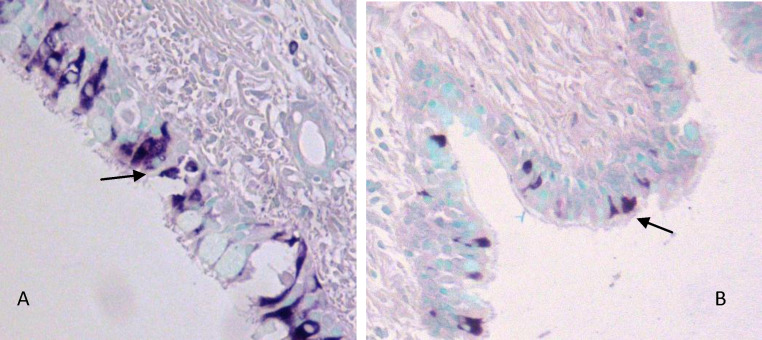
Coronavirus antigen-positive cells were detected within the epithelium of the trachea and bronchioles of the infected dogs (Fig. 5 ). Positive staining was present in the cytoplasm of ciliated columnar epithelial and goblet cells. Positive cells were widespread but often found in focal clusters, and often associated with small aggregates on the luminal surface of the trachea. Positive cells were surrounded by areas of vacuolation within the epithelium, possibly representing a cytopathic effect of the virus.
Fig. 5.
Representation of the type of immunohistochemical staining of CRCoV seen in (A) the trachea and (B) the bronchioles of CRCoV infected dogs. Coronavirus antigen positive epithelial cells are indicated by arrows. Counter staining with methyl green shows cell nuclei. Images were taken at 200× magnification.
3.5. Serology
3.5.1. Seroconversion and cross-neutralisation
Seroconversion to CRCoV was measured by IFA. All dogs were seronegative (IFA < 40) for CRCoV on study days −1, 3 and 6, and all dogs in the T6 control group remained seronegative throughout the study whilst all dogs in the CRCoV challenge groups seroconverted by study day 14.
CRCoV serum cross-neutralisation antibodies from serum collected on study day 14 were tested against 1 UK and 10 USA CRCoV isolates (Table 5 ). All serum samples tested from dogs in CRCoV treatment groups were shown to be serum neutralisation positive against all the CRCoV isolates tested. Serum collected from T6 control dogs showed no neutralising activity against any of the CRCoV strains.
Table 5.
Study day 14 cross neutralisation titres. Two dogs remained on the study at day 14 in each treatment group. Samples with SN values of 14 were considered negative at the 1:20 starting dilution. Serum samples collected at earlier time points were negative for CRCoV antibody.
| Cross serum neutralisation strain |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group (isolate) | LU131 | LU172 | LU189 | LU295 | LU317 | NP599 | NP604 | NP617 | NP631 | NP634 | UK4182 |
| T1 (LU295) | 320 | 80 | 95 | 113 | 113 | 190 | 135 | 135 | 95 | 113 | 80 |
| 381 | 135 | 80 | 160 | 160 | 226 | 160 | 135 | 135 | 135 | 113 | |
| T2 (UK4182) | 369 | 80 | 48 | 57 | 113 | 113 | 95 | 113 | 40 | 135 | 57 |
| 67 | 34 | 28 | 40 | 40 | 28 | 28 | 17 | 40 | 57 | 57 | |
| T3 (NP631) | 40 | 28 | 48 | 28 | 28 | 28 | 34 | 17 | 34 | 48 | 28 |
| 95 | 95 | 80 | 95 | 67 | 48 | 95 | 48 | 67 | 113 | 48 | |
| T4 (NP787) | 226 | 80 | 80 | 80 | 113 | 80 | 80 | 67 | 80 | 95 | 67 |
| 80 | 40 | 48 | 34 | 48 | 48 | 48 | 34 | 40 | 40 | 40 | |
| T5 (NP742) | 160 | 48 | 48 | 57 | 113 | 80 | 67 | 80 | 67 | 57 | 48 |
| 761 | 269 | 160 | 160 | 320 | 190 | 269 | 226 | 190 | 381 | 135 | |
| T6 (control) | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | |
3.5.2. Leukogram analysis
The mean concentrations of lymphocytes, neutrophils and monocytes, for each group on study days −1, 3, 6 and 14 were determined (data not shown). In T6, and T3 no statistical differences in the lymphocyte, neutrophil or monocyte concentrations were detected. In T1, T2, T4 and T5 increases in total white blood cell concentrations were observed as a result of lymphocytosis which was significant in T1 (p = 0.002) on day 6 and T5 (p = 0.027) on day 3. In T2 a marked lymphocytosis was seen on day 14. In T4 there was moderate lymphocytosis accompanied by significant changes in monocyte concentrations which peaked on day 6 [>day 3 (p = 0.003), >day 14 (p = 0.006)]. In contrast there was a significant decrease in neutrophil concentration on day 3 in T1, T4 and T5 (p = 0.004, p = 0.001 and p = 0.005, respectively) compared to day −1, which in T1 resulted in 3 of the dogs becoming neutropenic.
Across the groups seven dogs had rare to occasional toxic neutrophils exhibiting foamy cytoplasm, mild cytoplasmic basophilia or rare Dohle bodies, and detectable levels of band neutrophils. In group T1, all dogs had detectable levels of band neutrophils on day 3, which was a significant increase when compared to day −1 (p = 0.031). On day 3 one dog in each of T2, T4, and T5 had band concentrations higher than the estimated upper reference limit; however this did not result in statistically significant changes at a group level and no other significant changes could be detected.
3.6. Bacteriology
Oropharyngeal Amies swabs collected from all dogs on day −1 and at euthanasia yielded growth of normal respiratory flora. A majority of dogs also yielded scanty growth of either Streptococcus canis or Staphylococcus intermedius. In the vast majority of dogs the lungs were sterile for bacterial growth, with the exception of the growth of Mycoplasma spp. in four dogs, one from each treatment group T2–T5 inclusive (data not shown).
4. Discussion
Previous publications have collectively demonstrated the global distribution of CRCoV and its association with respiratory disease in dogs under field conditions (Erles et al., 2003, Decaro et al., 2007, Kaneshima et al., 2006, Priestnall et al., 2006, Priestnall et al., 2007, Yachi and Mochizuki, 2006, Knesl et al., 2009). This is the first publication to report experimental infection of dogs using CRCoV, and the comparison of different CRCoV isolates from wide geographic origins. Each CRCoV isolate was derived from dogs with respiratory disease in MO, MI, UT and NE in the USA, and one isolate from London, UK.
This study builds upon a preliminary experimental challenge study (Mitchell et al., unpublished data). In that study we demonstrated rapid shed-spread of CRCoV from experimentally infected dogs to sentinel dogs within 4 days of exposure. Peak viral loads were detected in oropharyngeal swabs at 4 and 6 days post infection in experimentally infected, and sentinel dogs, respectively; with shedding lasting for 8–10 days in both groups. Both inoculated and sentinel dogs displayed clinical signs of mild respiratory disease, and seroconverted to the virus.
In the current study prolific shedding of CRCoV from the oropharynx of dogs in all treatment groups (detected by RT-PCR and VI from oral swabs) was seen by day 2. In a majority of dogs viral shedding ceased after day 6, although CRCoV was detected up to 10 days post infection by RT-PCR in one dog. Small differences in the duration of viral shedding between the two studies are most likely to be explained by differences in the age of the dogs used in each of the studies (preliminary study: 1–3 weeks old, current study: 12–16 weeks old). Despite differences, both studies clearly illustrate that CRCoV is a quick-hit respiratory pathogen, and supports field data in which the rapid spread of CRCoV throughout a large rehoming centre was observed (Erles et al., 2003).
Consistent with observations made during naturally occurring infection, dogs in this study also displayed clinical signs of mild respiratory disease following viral challenge (nasal discharge, sneezing, and coughing); whilst the control group remained healthy. There appeared to be no profound difference in the clinical observations made between groups of dogs challenged with the different strains. Two dogs however (one each in T3 and T5) showed more severe and prolonged clinical signs compared to the others. This included increased respiratory noise, and more frequent and prolonged coughing and sneezing. The reason for this was unclear. Disease in these two dogs did not appear to be associated with any secondary bacterial infections in the lung, nor with notably different histopathological changes when compared to other challenge dogs in the same group (data not shown).
Given its multifactorial aetiology; modelling CIRD under controlled conditions, to mimic disease as it appears in the field is difficult to achieve. When studied in isolation dogs infected with other viruses involved in the CIRD complex, such as CPIV and CAV-2, also cause only mild respiratory disease such as a dry cough and nasal discharge (Appel and Binn, 1987a, Appel and Binn, 1987b, Castleman, 1985, Buonavoglia and Martella, 2007). More severe clinical signs, representative of the disease complex, are likely to occur when other viruses or bacterial respiratory pathogens are also present, and when environmental stresses such as those encountered in rehoming facilities are experienced (Appel and Binn, 1987a, Appel and Binn, 1987b, Buonavoglia and Martella, 2007).
Overall there appeared to be little difference in tissue tropism, when comparing the different CRCoV isolates investigated. CRCoV was detected in at least five of the eight necropsy samples examined (lung lavage, diaphragmatic lung lobes, trachea, nasal tonsils and nasal cavity) in dogs from all five challenge groups. Crucially, isolation of CRCoV was achieved from six of the seven tissues collected (the bronchial lymph node remained negative) as well as the lung lavage fluid. Re-isolation of virus from experimentally infected dogs displaying clinical signs of disease, signifies a causal relationship between CRCoV and respiratory disease, which until now has been best demonstrated through epidemiological surveys (Erles et al., 2004, Erles et al., 2003, Decaro et al., 2007, Kaneshima et al., 2006, Priestnall et al., 2006, Priestnall et al., 2007, Knesl et al., 2009).
In all treatment groups CRCoV was detected most frequently in the trachea, nasal tonsil and lung lavage fluid, and these same tissues also exhibited the highest viral titres (data not shown). This is consistent with previous findings from a small cohort of naturally infected dogs in which the highest viral loads, detected by quantitative RT-PCR, were seen in the trachea and nasal tonsil (Mitchell et al., 2009).
Importantly, histological analyses were consistent with our molecular and virological findings. IHC revealed coronavirus positive staining in the cytoplasm of ciliated epithelial and goblet cells of the trachea and bronchioles of CRCoV infected dogs. This pattern of staining is consistent with previous work which identified coronavirus antigen-positive epithelial cells within the trachea of dogs naturally infected with CRCoV (Ellis et al., 2005, Priestnall, 2007), and in CRCoV infected tracheal sections maintained in culture (Priestnall et al., 2009, Priestnall, 2007). Furthermore, histopathological analysis showed a clear association between exposure to CRCoV and inflammation in the nares and trachea, with loss or damage to cilia in the latter. These changes were particularly marked in dogs belonging to T4 and T5, which may indicate a higher degree of pathogenicity for those CRCoV strains; although, with the exception of one dog in T5 this was not apparent from the clinical signs. Ciliary clearance is a key strategy for the removal of pathogens from the respiratory tract. Reductions in the efficiency of ciliary clearance would potentiate infection with secondary respiratory pathogens, leading to increased severity and duration of disease.
In addition to the trachea, the nasal tonsil may also represent an important site for CRCoV infection and pathogenicity; given the consistency and duration of CRCoV isolation and detection in this tissue. Preliminary IHC analysis has shown that CRCoV infection in the nasal tonsil is associated with the epithelium, and with large mononuclear cells which have the appearance of macrophages (Priestnall, 2007). A clear association of CRCoV with macrophages is yet to be confirmed, but this could present another interesting area given the importance of macrophages in the pathogenesis of disease associated with other coronaviruses, such as feline infectious peritonitis virus (FIPV), severe acute respiratory syndrome coronavirus (SARS-CoV) and human coronavirus 229E (HCoV-229E) (Desforges et al., 2007, Rottier et al., 2005, Shieh et al., 2005).
The detection and re-isolation of CRCoV from the lungs indicates the virus can also establish an infection in the lower airways. Gross and histopathological analysis showed that both the control and challenged dogs had focal reddening of the lungs at necropsy, however no significant histological abnormalities were associated specifically with this reddening, and such reddening is assumed to be an artefact of barbiturate euthanasia (Lopez, 2001). Nonetheless, in most cases there were microscopic differences between the lungs of these dogs, which could not be appreciated grossly, and therefore the gross appearance of the lungs was not considered an accurate predictor of histopathological changes in these experimental conditions.
Pulmonary inflammation in CRCoV challenge dogs was associated with lymphoid aggregates adjacent to the airways or blood vessels. The significance of this is uncertain, but notably these aggregates were not a striking feature of the control dogs. Histological changes in the lungs and bronchial lymph nodes of dogs in T6 tended to be more consistent and mild; whilst scores for dogs in CRCoV challenge groups, although not marked, were more variable throughout the study. Histological changes in the lymphoid tissues of most dogs in the study (T1–T6 inclusive) included hyperplasia. Lymphoid hyperplasia is not uncommon in dogs at this age, particularly in the palatine tonsil. Acute inflammatory responses were evident; however in some dogs this may have been associated with the presence of fragments of foreign material (e.g. hair) in the tonsillar crypts. Where marked lymphoid hyperplasia was seen, this was reflected in the histological scores, which were more variable in the challenge dogs compared to those in the broth control animals throughout the trial period.
The close genetic relationship between CRCoV and bovine coronavirus (BCoV) (Erles et al., 2003, Erles et al., 2007), raised the question as to whether CRCoV may have an extended tropism which involves the gastrointestinal tract, as seen with some BCoV strains (Park et al., 2007). The molecular detection of CRCoV in the rectal swabs of some dogs from T1 is interesting, and consistent with findings from a number of previous reports of dogs naturally infected with CRCoV (Decaro et al., 2007, Yachi and Mochizuki, 2006, Mitchell et al., 2009). Dogs in the T1 challenge group were unique in that they were the only dogs to be challenged with virus that had not been grown in vitro. The inability to detect CRCoV from dogs in other challenge groups may therefore be a result of cell culture adaptation; alternatively, this may be a strain specific phenomenon. At present there is no conclusive evidence that CRCoV displays a true dual tropism for respiratory and enteric tissues. The failure to isolate CRCoV from enteric samples collected during the peak oropharyngeal shedding period, suggests that CRCoV may pass through the canine gut as a bystander, without infection. Nevertheless, enteric shedding could have implications for managing the spread of disease, and therefore further investigation is warranted.
In addition to the histological changes observed in the tissues, significant changes in the leukogram can also be detected in association with CRCoV infection. This is summarised by a statistically and also clinically relevant lymphocytosis between days 6 and 14; most clearly seen in groups T1, T2, and T5. This observation is not unexpected due to the viral antigenic stimulation, and the lymphocytic reactions found in various tissues. What was somewhat less expected is the high number of dogs with decreased neutrophil concentrations, including a number which developed neutropenia, mild left shift and toxicity, best seen in groups T1, T2, T4, and T5. These changes suggest an acute inflammatory reaction with a high demand for neutrophils and accelerated production in the marrow.
Transient neutropenia is not uncommon during infections with some viruses; however, there is currently limited data relating to the leukogram profile following infections with other beta coronaviruses. In one report detailing the experimental infection of cows with BCoV, significant reductions in neutrophil concentrations were observed at 2 days post infection, followed by neutrophilia at 14 days post infection (Traven et al., 2001). In SARS coronavirus infected patients the picture is mixed. Neutropenia has been reported in some cases, however, in most cases high blood neutrophil concentrations were observed, and this is often associated with a poor clinical outcome (Lee et al., 2003, Manocha et al., 2003, Wong et al., 2003). Neutrophil infiltration of tissues infected with different coronaviruses such as SARS, MHV and Rat CoV has also been described (Bhatt and Jacoby, 1977, Iacono et al., 2006, Ding et al., 2003); however, their role in viral clearance and possible immune pathology is largely unknown. Considering the presence of mostly lymphoid aggregates in CRCoV infected tissues in this study, the observed changes are intriguing, and a direct effect of CRCoV on the myeloid series in the bone marrow cannot be ruled out.
Seroconversion to CRCoV and the acquisition of neutralising antibodies to heterologous strains from distinct geographical locations within the USA and UK occurred in all challenge dogs remaining on the study at day 14. This degree of serological cross reactivity is not unexpected given the high degree of amino acid similarity seen in the spike protein of different isolates published to date. Whilst the correlation between neutralising titres and protection in vivo is yet to be determined, this finding supports published data which demonstrated that the presence of CRCoV specific antibodies in dogs significantly decreased the risk of developing respiratory disease upon entry to the kennel (Erles et al., 2003). These findings support a possible role for vaccinating against CRCoV as part of a preventative strategy for respiratory disease in kennelled dogs. Moreover, based on the high degree of cross neutralisation and high degree of amino acid identity among the CRCoV spike proteins described to date, it is anticipated that a single CRCoV isolate as a vaccine antigen will be sufficient to provide protection against CRCoV induced respiratory disease.
In summary, this is the first study to describe the development of a model for studying CRCoV pathogenesis; and to fully demonstrate Koch's postulates through the successful re-isolation of CRCoV from experimentally infected dogs with clinical signs of respiratory disease. Isolation of CRCoV was achieved from a wide variety of respiratory and mucosal associated lymphoid tissues, lung lavage fluids and swabs collected over the 2-week period, thus providing clear evidence of tropism for the canine respiratory tract, accompanied by respiratory shedding. Moreover, we have shown CRCoV infection is associated with marked histopathological changes in the nares and trachea, with loss and damage to tracheal cilia alongside inflammatory responses. Significant effects on the leukogram, in the form of clinically relevant lymphocytosis and neutrophil changes were also documented. Strong serological and cross neutralising reactivity between heterologous CRCoV isolates demonstrates antigenic homogeneity among CRCoV from the two continents. This study provides vital evidence supporting a role for CRCoV in the CIRD complex. Defining the role that CRCoV and other agents play in CIRD is a considerable, but important, challenge if the disease is to be managed, treated and prevented more successfully.
Acknowledgements
This study was funded by Pfizer Animal Health. The authors would like to thank M. Ikeh for technical assistance. RVC Manuscript ID number: PID_00399.
Contributor Information
Judy A. Mitchell, Email: jmitchell@rvc.ac.uk.
Harriet W. Brooks, Email: hbrooks@rvc.ac.uk.
Balázs Szladovits, Email: bszladovits@rvc.ac.uk.
Kerstin Erles, Email: kerles@rvc.ac.uk.
Rachel Gibbons, Email: rachel.gibbons@pfizer.com.
Shelly Shields, Email: shelly.shields@pfizer.com.
Joe Brownlie, Email: jbrownlie@rvc.ac.uk.
References
- Appel M.J., Binn L.N. Canine infectious tracheobronchitis short review: kennel cough. In: Carnivors Vio, editor. Virus Infections of Carnivors. Elseiver Science Publishing Co.; New York: 1987. pp. 201–221. [Google Scholar]
- Appel M.J., Binn L.N. Canine infectious tracheobronchitis short review: kennel cough. In: Carnivors Vio, editor. Virus Infections of Carnivors. Elseiver Science Publishing Co.; New York: 1987. pp. 45–51. [Google Scholar]
- Appel M.J., Percy D.H. SV-5-like parainfluenza virus in dogs. J. Am. Vet. Med. Assoc. 1970;156:1778–1781. [PubMed] [Google Scholar]
- Bemis D.A. Bordetella and Mycoplasma respiratory infections in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 1992;22:1173–1186. doi: 10.1016/s0195-5616(92)50308-4. [DOI] [PubMed] [Google Scholar]
- Bemis D.A., Greisen H.A., Appel M.J. Pathogenesis of canine bordetellosis. J. Infect. Dis. 1977;135:753–762. doi: 10.1093/infdis/135.5.753. [DOI] [PubMed] [Google Scholar]
- Bhatt P.N., Jacoby R.O. Experimental infection of adult axenic rats with Parker's rat coronavirus. Arch. Virol. 1977;54:345–352. doi: 10.1007/BF01314779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Martella V. Canine respiratory viruses. Vet. Res. 2007;38:355–373. doi: 10.1051/vetres:2006058. [DOI] [PubMed] [Google Scholar]
- Castleman W.L. Bronchiolitis obliterans and pneumonia induced in young dogs by experimental adenovirus infection. Am. J. Pathol. 1985;119:495–504. [PMC free article] [PubMed] [Google Scholar]
- Chalker V.J., Owen W.M., Paterson C., Barker E., Brooks H., Rycroft A.N., Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Mari V., Lucente M.S., Cordioli P., Colaianni M.L., Martella V., Buonavoglia C. Serological and molecular evidence that canine respiratory coronavirus is circulating in Italy. Vet. Microbiol. 2007;121:225–230. doi: 10.1016/j.vetmic.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Miletti T.C., Gagnon M., Talbot P.J. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130:228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield J., Macpherson L.W., Zbitnew A. Association of canine adenovirus (Toronto A 26/61) with an outbreak of laryngotracheitis (“Kennel Cough”): a preliminary report. Can. Vet. J. 1962;3:238–247. [PMC free article] [PubMed] [Google Scholar]
- Ellis J.A., McLean N., Hupaelo R., Haines D.M. Detection of coronavirus in cases of tracheobronchitis in dogs: a retrospective study from 1971 to 2003. Can. Vet. J. 2005;46:447–448. [PMC free article] [PubMed] [Google Scholar]
- Erles K., Brownlie J. Investigation into the causes of canine infectious respiratory disease: antibody responses to canine respiratory coronavirus and canine herpesvirus in two kennelled dog populations. Arch. Virol. 2005;150:1493–1504. doi: 10.1007/s00705-005-0533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Brownlie J. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Vet. Clin. North Am. Small Anim. Pract. 2008;38:815–825. doi: 10.1016/j.cvsm.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Dubovi E.J., Brooks H.W., Brownlie J. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 2004;42:4524–4529. doi: 10.1128/JCM.42.10.4524-4529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Shiu K.B., Brownlie J. Isolation and sequence analysis of canine respiratory coronavirus. Virus Res. 2007;124:78–87. doi: 10.1016/j.virusres.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone A., Weckmann M.T., Capen C.C., Rosol T.J. Canine glyceraldehyde-3-phosphate dehydrogenase complementary DNA: polymerase chain reaction amplification, cloning, partial sequence analysis, and use as loading control in ribonuclease protection assays. Am. J. Vet. Res. 1996;57:254–257. [PubMed] [Google Scholar]
- Iacono K.T., Kazi L., Weiss S.R. Both spike and background genes contribute to murine coronavirus neurovirulence. J. Virol. 2006;80:6834–6843. doi: 10.1128/JVI.00432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshima T., Hohdatsu T., Satoh K., Takano T., Motokawa K., Koyama H. The prevalence of a group 2 coronavirus in dogs in Japan. J. Vet. Med. Sci. 2006;68:21–25. doi: 10.1292/jvms.68.21. [DOI] [PubMed] [Google Scholar]
- Karpas A., King N.W., Garcia F.G., Calvo F., Cross R.E. Canine tracheobronchitis: isolation and characterization of the agent with experimental reproduction of the disease. Proc. Soc. Exp. Biol. Med. 1968;127:45–52. doi: 10.3181/00379727-127-32618. [DOI] [PubMed] [Google Scholar]
- Keil D.J., Fenwick B. Role of Bordetella bronchiseptica in infectious tracheobronchitis in dogs. J. Am. Vet. Med. Assoc. 1998;212:200–207. [PubMed] [Google Scholar]
- Knesl O., Allan F.J., Shields S. The seroprevalence of canine respiratory coronavirus and canine influenza virus in dogs in New Zealand. N.Z. Vet. J. 2009;57:295–298. doi: 10.1080/00480169.2009.58624. [DOI] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lopez A. In: Respiratory System, Thoracic Cavity and Pleura in Thomson's Special Veterinary Pathology. McGavin M.C., Carlton W.W., Zachary J.F., editors. Mosby Inc.; St Louis, MO, USA: 2001. p. 148. [Google Scholar]
- Manocha S., Walley K.R., Russell J.A. Severe acute respiratory distress syndrome (SARS): a critical care perspective. Crit. Care Med. 2003;31:2684–2692. doi: 10.1097/01.CCM.0000091929.51288.5F. [DOI] [PubMed] [Google Scholar]
- Mitchell J.A., Brooks H., Shiu K.B., Brownlie J., Erles K. Development of a quantitative real-time PCR for the detection of canine respiratory coronavirus. J. Virol. Methods. 2009;155:136–142. doi: 10.1016/j.jviromet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Kim G.Y., Choy H.E., Hong Y.J., Saif L.J., Jeong J.H., Park S.I., Kim H.H., Kim S.K., Shin S.S., Kang M.I., Cho K.O. Dual enteric and respiratory tropisms of winter dysentery bovine coronavirus in calves. Arch. Virol. 2007;152:1885–1900. doi: 10.1007/s00705-007-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestnall, S.L., The role of a novel coronavirus in canine infectious respiratory disease. Royal Veterinary College, University of London, London, PhD Thesis, 2007.
- Priestnall S.L., Brownlie J., Dubovi E.J., Erles K. Serological prevalence of canine respiratory coronavirus. Vet. Microbiol. 2006;115:43–53. doi: 10.1016/j.vetmic.2006.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestnall S.L., Pratelli A., Brownlie J., Erles K. Serological prevalence of canine respiratory coronavirus in southern Italy and epidemiological relationship with canine enteric coronavirus. J. Vet. Diagn. Invest. 2007;19:176–180. doi: 10.1177/104063870701900206. [DOI] [PubMed] [Google Scholar]
- Priestnall S.L., Mitchell J.A., Brooks H.W., Brownlie J., Erles K. Quantification of mRNA encoding cytokines and chemokines and assessment of ciliary function in canine tracheal epithelium during infection with canine respiratory coronavirus (CRCoV) Vet. Immunol. Immunopathol. 2009;127:38–46. doi: 10.1016/j.vetimm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P.J., Nakamura K., Schellen P., Volders H., Haijema B.J. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 2005;79:14122–14130. doi: 10.1128/JVI.79.22.14122-14130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W.J., Hsiao C.H., Paddock C.D., Guarner J., Goldsmith C.S., Tatti K., Packard M., Mueller L., Wu M.Z., Rollin P., Su I.J., Zaki S.R. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum. Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traven M., Naslund K., Linde N., Linde B., Silvan A., Fossum C., Hedlund K.O., Larsson B. Experimental reproduction of winter dysentery in lactating cows using BCV – comparison with BCV infection in milk-fed calves. Vet. Microbiol. 2001;81:127–151. doi: 10.1016/S0378-1135(01)00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K., Chan P.K., Ng M.H., Yu L.M., Hui D.S., Tam J.S., Cheng G., Sung J.J. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachi A., Mochizuki M. Survey of dogs in Japan for group 2 canine coronavirus infection. J. Clin. Microbiol. 2006;44:2615–2618. doi: 10.1128/JCM.02397-05. [DOI] [PMC free article] [PubMed] [Google Scholar]