Abstract
Group C rotaviruses (GCRVs) cause acute diarrhea in humans and animals worldwide and the evidence for a possible zoonotic role of GCRVs has been recently provided. However, there is little evidence of porcine GCRV infections or of their genetic diversity in South Korea. We examined 137 diarrheic fecal specimens from 55 farms collected from six provinces. RT-PCR utilizing primer pairs specific for the GCRV VP6 gene detected GCRV-positive reactions in 36 (26.2%) diarrheic fecal samples. Of these, 17 samples (12.4%) tested positive for porcine GCRVs alone and 19 samples (13.8%) were also positive for other pathogens. Other enteric pathogens except for GCRV were detected in 64 feces samples (46.7%) and no enteric pathogens were evident in 37 feces samples (27.0%). Phylogenetic and sequence homology analyses of GCRV partial VP6 gene between 23 Korean and other known porcine GCRVs demonstrated that Korean strains belonged to the porcine lineage. Furthermore, one Korean porcine strain shared the highest nucleotide (89.7–89.0%) and deduced amino acid sequence (92.9–93.9%) identities with bovine GCRV strains and was placed in the bovine GCRV lineage indicative of bovine origin. In conclusion, porcine GCRV infections are widespread in piglets with diarrhea in South Korea. The infecting porcine GCRVs mostly belong to the porcine lineage with the exception of one bovine-like GCRV, which possibly originated from bovine GCRV due to interspecies transmission.
Keywords: Group C rotavirus, Pigs, Diarrhea, Interspecies transmission
1. Introduction
Viruses in the genus Rotavirus (family Reoviridae) are the major etiologic agents of severe, acute dehydrating diarrhea in humans and in a wide variety of domestic animals (Estes and Kapikian, 2007). The viral particles are triple-layered and contain a genome consisting of 11 double-stranded RNA segments. Therefore, rotaviruses can undergo genetic reassortment during mixed infections, leading to progeny viruses with novel or atypical phenotypes (Estes and Kapikian, 2007). A viral protein VP6 is located in the middle layer and exposes group-specific antigens. Based on antigen characteristics of VP6, rotaviruses are classified into seven groups (A–G) with only groups A–C causing disease in both humans and animals (Estes and Kapikian, 2007). The recently reported human rotavirus strains ADRV-N and B219 have not yet been taxonomically specified (Alam et al., 2007, Nagashima et al., 2008, Yang et al., 2004).
Group C rotavirus (GCRV) was first detected in pigs in 1980 (Saif et al., 1980) and has been subsequently identified in humans, ferrets, and cattle (Rodger et al., 1982, Torres-Median, 1987, Tsunemitsu et al., 1991). Since then, the global distribution of human GCRVs has been established; they are suspected of being an emerging pathogen (Bányai et al., 2006, Caul et al., 1990, Esona et al., 2008, Iizuka et al., 2006, Kuzuya et al., 2005, Kuzuya et al., 2007, Medici et al., 2009, Steyer et al., 2006). Fecal shedding of porcine GCRVs has been reported in nursing, weaning, and post-weaning pigs with diarrhea either alone or in mixed infection with other enteric pathogens (Kim et al., 1999, Martella et al., 2007a, Morin et al., 1990, Saif and Jiang, 1994, Saif et al., 1980, Sigolo de San Juan et al., 1986). Moreover, porcine GCRVs are wide spread in swine herds; in limited surveys, antibody prevalence against GCRVs in pigs is 28–70% by 8 weeks of age, increasing with age to reach 79–100% in adult pigs (Saif and Jiang, 1994, Terrett et al., 1987, Tsunemitsu et al., 1992). However, the epidemiological significance of these observations is limited, given the limited number of studies and restricted geographical scope of such investigations (Collins et al., 2008, Janke et al., 1990, Kim et al., 1999, Martella et al., 2007a, Morin et al., 1990, Saif and Jiang, 1994, Saif et al., 1980, Sigolo de San Juan et al., 1986, Will et al., 1994). A possible explanation may be that sensitive tests for detection are not available. Diagnosis is difficult because most ELISA assays do not recognize the group C-specific antigen VP6, whereas polyacrylamide gel electrophoresis (PAGE) analysis of the double strand RNA requires the presence of at least 108 to 1010 viral particles/ml for a positive result, thus misleading the diagnosis (Kuzuya et al., 1996, Xu et al., 1990). Reverse transcription-polymerase chain reaction (RT-PCR) using group C-specific primers is a sensitive and convenient option, however, it has not been widely used (Bányai et al., 2006, Caul et al., 1990, Esona et al., 2008, Gouvea et al., 1991, Iizuka et al., 2006, Jiang et al., 1995, Kuzuya et al., 2005, Kuzuya et al., 2007, Medici et al., 2009, Qian et al., 1991, Steyer et al., 2006).
Like group A rotaviruses (GARVs), sequence comparison suggests that genetic diversity exists among GCRVs (Fielding et al., 1994, Grice et al., 1994, Jiang et al., 1999a, Jiang et al., 1999b, Kuzuya et al., 1996, Rahman et al., 2005, Tsunemitsu et al., 1992, Tsunemitsu et al., 1996). However, the molecular analysis of the porcine GCRVs has only been carried out in the United States (Jiang et al., 1999a, Jiang et al., 1999b, Jiang et al., 2000, Tsunemitsu et al., 1996), Ireland (Collins et al., 2008), and Italy (Martella et al., 2007a, Martella et al., 2007b). Therefore, it is unclear if the porcine GCRVs circulating in other countries have distinct genetic characteristics. In addition, a possible zoonotic role of animal GCRVs has been postulated based on increase sero-prevalence rates to GCRVs in human populations living in rural settings (Iturriza-Gomara et al., 2004). Direct evidence for the zoonotic potential of porcine GCRVs has been gained by analyses of archival fecal samples of Brazilian children (Gabbay et al., 2008). Furthermore, GCRV surveillance has detected interspecies transmission by GCRVs between animal species; bovine strain WD534tc is actually a porcine strain (Chang et al., 1999). Therefore, the detection of animal-like GCRVs in humans has highlighted the potential zoonotic impact of animal GCRVs for humans, stressing the need for a more in-depth study of the epidemiology of animal GCRVs, particularly in developing countries where humans and animals, or animals and animals often live in close physical contact, making mixed infections more common.
To date, no GCRV infection either in animals or in humans has been reported from South Korea. Molecular characterization of porcine GCRVs in South Korea is needed for vaccine development efforts and evaluation, as well as for clarification of the ecology and evolution of GCRVs. Sequence data of the genes from many different countries would provide the fundamental data necessary for the development of more sensitive and specific diagnostic tools that could be used to determine the worldwide distribution of the virus. This paper reports the prevalence of porcine GCRVs in diarrheic piglets using RT-PCR, along with the genetic diversity of the porcine GCRV strains based on a partial porcine GCRV VP6 gene.
2. Materials and methods
2.1. Specimens
A total of 137 fecal specimens from 7- to 45-day-old diarrheic pigs housed on 55 farms were collected from six provinces in South Korea in the spring (41 samples from 19 farms), summer (25 samples from 9 farms), autumn (22 samples from 11 farms) and winter of 2006 (49 samples from 16 farms). Upon arrival of the fecal samples at the laboratory, they were examined for common bacterial enteric pathogens including Escherichia coli and Salmonella spp. using specific agar media, and the suspect colonies were identified based on biochemical tests. Testing for parasite eggs (Coccidium spp. and Cryptosporidium spp.) was done using standard flotation techniques. For virologic assays, fecal suspensions of each sample were prepared by diluting the feces at 1:10 in 0.01 M phosphate-buffered saline, pH 7.2. The suspensions were vortexed for 30 s, centrifuged (1200 × g for 20 min), and the supernatants along with the remaining bulk samples were collected and stored at −80oC for further testing.
2.2. RNA extraction
RNA was extracted from a 200 μl starting volume of centrifuged 10% fecal suspensions using Trizol-LS (Gibco-BRL, Grand Island, NY). The total RNA recovered was suspended in 50 μl of RNase free water and stored at −80 °C until used.
2.3. RT-PCR and nested PCR
To verify the sensitivity of RT-PCR assays for the detection of porcine GCRVs as well as other GCRVs, different primer sets from GCRV VP6 gene were designed or used (Table 1 ). For evaluating the concurrent infection of porcine GCRVs with porcine GARVs, porcine group B rotaviruses (GBRVs), porcine sapovirus (PSaV), porcine norovirus (PNoV), transmissible gastroenteritis coronavirus (TGEV), and porcine epidemic diarrhea coronavirus (PEDV) RT-PCR assays with different primer sets were performed using a standard one-step RT-PCR as previously described (Jeong et al., 2007). To increase the sensitivity and specificity of RT-PCR, a nested PCR assay with the primer pair specific to VP6 gene of PRV A (Table 1) was performed as previously described (Jeong et al., 2007). The amplification products were analyzed by 1.5 or 2% agarose gel electrophoresis and visualized by ultraviolet illumination after ethidium bromide staining.
Table 1.
RT-PCR and nested PCR primers used for the detection of the groups A, B and C rotaviruses (GARV, GBRV and GCRV), porcine sapovirus (PSaV), porcine norovirus (PNoV), transmissible gastroenteritis coronavirus (TGEV) and porcine epidemic diarrhea coronavirus (PEDV) in the fecal samples from pigs with diarrhea.
| Target viruses | Target genesa | Primer names | Primer sequences, 5′–3′b | Region (nt) | Size (bp) | Source or reference |
|---|---|---|---|---|---|---|
| GCRV | VP6 | C1 | F: CTC GAT GCT ACT ACA GAA TCA G | 997–1018 | 356 | Gabbay et al. (2008) |
| C4 | R: AGC CAC ATA GTT CAC ATT TCA TCC | 1329–1352 | Gabbay et al. (2008) | |||
| RVCF1 | F: GCA TTT AAA ATC TCA TTC ACA | 1–21 | 1352 | This study | ||
| T778a | R: AGC CAC ATA GTT CAC ATT TC | 1–1352 | Adah et al. (2002) | |||
| T729 | F: TTA ATG AAA ATA GAA GCT GG | 685–714 | 668 | Adah et al. (2002) | ||
| T778a | R: AGC CAC ATA GTT CAC ATT TC | 1333–1352 | Adah et al. (2002) | |||
| BMJ145 | F: AGT CCG TTC TAT GTG ATT C | 1014–1032 | 339 | Sanchez-Fauquier et al. (2003) | ||
| BMJ44 | R: AGC CAC ATA GTT CAC ATT TC | 1333–1352 | Sanchez-Fauquier et al. (2003) | |||
| T383 | F: AAT CTC ATT CAC AAT GGA TG | 10–29 | 311 | Adah et al. (2002) | ||
| RVCnR2 | R: TTT CAT CAT CAC ATA CAG CT | 301–320 | This study | |||
| GARV | VP6 | F: AAAGATGCTAGGGACAAAATTG | 58–78 | 308 | Elschner et al. (2002) | |
| R: TTCAGATTGTGGAGCTATTCCA | 344–365 | Elschner et al. (2002) | ||||
| nF: GACAAAATTGTCGAAGGCACATTATA | 69–94 | 121 | Elschner et al. (2002) | |||
| nR: TCGGTAGATTACCAATTCCTCCAG | 166–189 | Elschner et al. (2002) | ||||
| GBRV | NSP2 | F: CTATTCAGTGTGTCGTGAGAGG | 18–40 | 434 | Gouvea et al. (1991) | |
| R: GCAGACAAGCTAGCCCGCTTCG | 429–451 | Gouvea et al. (1991) | ||||
| PSaV and PNoV | RdRp | F: GATTACTCCAAGTGGGACTCCAC | 4568–4590 | 319 | Jiang et al., 1999a, Jiang et al., 1999b | |
| R: TGA CAATGTAAT ATCACCATA | 4865–4886 | Jiang et al., 1999a, Jiang et al., 1999b | ||||
| TGEVc | ORF1b | F: GGGTAAGTTGCTCATTAGAAATAATGG | 7968–7994 | 1006 | Kim et al. (2000) | |
| Spike | R: CTTCTTCAAAGCTAGGGACTG | 920–940 | Kim et al. (2000) | |||
| PEDV | N | F: AGGAACGTGACCTCAAAGACATCCC | 812–836 | 540 | Kubota et al. (1999) | |
| R: CCAGGATAAGCCGGTCTAACATTG | 1328–1351 | Kubota et al. (1999) | ||||
VP6: viral protein 6; NSP2: non-structural protein 2; RdRp: RNA dependent RNA polymerase; ORF1b: open reading frame 1b; N: nucleocapsid.
F: forward primer for RT-PCR; R: reverse primer for RT-PCR; nF: forward primer for nested PCR; nR: reverse primer for nested PCR.
Forward primer was designed from the portion of TGEV ORF1b; reverse primer was designed from the portion of TGEV spike gene.
2.4. cDNA sequencing
To verify the specificity of PCR reaction and to obtain genomic data, 24 RT-PCR products for a portion of the porcine GCRV VP6 gene (356 bp) amplified by the C1 and C4 primers (Table 1) were selected based on the intensity of the bands by agarose gel electrophoresis and ethidium bromide visualization. The RT-PCR products were purified using a QIAEX II gel extraction kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The amplicons were sequenced directly using primers C1 and C4. DNA sequencing was carried out using an ABI system 3700 automated DNA sequencer (Applied Biosystems, Foster City, CA).
2.5. Molecular analysis
Using the DNA Basic module (DNAsis MAX, Alameda, CA), the nucleotide and deduced amino acid sequences of the partial VP6 gene (310 bp, devoid of primer pair sequences) were compared with those selected from other known GCRVs (Table 2 ). Phylogenetic analysis based on the nucleotide alignments was constructed using the neighbor-joining method and the UPGMA method of Molecular Evolutionary Genetics analysis (MEGA version 4.0) with a pairwise distance (Tamura et al., 2007). A sequence similarity search was performed for the GCRV VP6 gene using the LALIGN Query program of the GENESTREAM network server at Institut de Génétique Humaine, Montpellier, France (http://www.eng.uiowa.edu/∼tscheetz/sequence-analysis/examples/LALIGN/lalign-guess.html).
Table 2.
GenBank accession numbers of the VP6 genes of the Korean porcine group C rotavirus strains and the reference group C rotavirus strains used in phylogenetic and sequence analyses.
| Strains | Origin | Accession numbers | Strains | Origin | Accession numbers |
|---|---|---|---|---|---|
| 06-12-2 | Porcine | 1156050 | 06-268-2 | Porcine | 1156450 |
| 06-12-3 | Porcine | 1156051 | Cowden | Porcine | M94157 |
| 06-13-2 | Porcine | 1156052 | WD534tc | Bovine | AF162434 |
| 06-14 | Porcine | 1156053 | Shintoku | Bovine | M88768 |
| 06-20-1 | Porcine | 1156056 | Yamagata | Bovine | AB108680 |
| 06-20-2 | Porcine | 1156057 | V460 | Human | AY786570 |
| 06-21-1 | Porcine | 1156058 | V508 | Human | AY795898 |
| 06-21-2 | Porcine | 1156060 | V966 | Human | AY786571 |
| 06-21-3 | Porcine | 1156061 | BCN6 | Human | AM118018 |
| 06-31-4 | Porcine | 1156064 | BCN9 | Human | AM118019 |
| 06-40-2 | Porcine | 1156065 | BCN21 | Human | AM118020 |
| 06-44-2 | Porcine | 1156066 | DhakaC2 | Human | AY754827 |
| 06-44-3 | Porcine | 1156067 | DhakaC13 | Human | AY754826 |
| 06-46-1 | Porcine | 1156069 | Preston | Human | M94156 |
| 06-48-2 | Porcine | 1156070 | Bristol | Human | X59843 |
| 06-48-3 | Porcine | 1156072 | Moduganari | Human | AF325806 |
| 06-50-2 | Porcine | 1156073 | Belem | Human | M94155 |
| 06-92-2 | Porcine | 1156074 | Jajeri | Human | AF325805 |
| 06-94 | Porcine | 1156075 | 208 | Human | AB008672 |
| 06-98-1 | Porcine | 1156078 | Wu82 | Human | EF528570 |
| 06-103-2 | Porcine | 1156081 | CMH004-03 | Human | EF641110 |
| 06-114-3 | Porcine | 1156445 | SI-82-05 | Human | DQ439863 |
| 06-236-2 | Porcine | 1156446 |
3. Results
3.1. Incidence of porcine GCRVs in piglets with diarrhea in South Korea
To determine the prevalence of porcine GCRVs in diarrheic Korean piglets, a total of 137 fecal specimens from diarrheic piglets housed on 55 farms were screened by RT-PCR using five sets of primer pairs (Table 1). The fecal samples were determined to be positive if at least one sample tested positive for each primer pair. Thirty-six (26.3%) out of 137 diarrheic samples tested positive for porcine GCRVs (Table 3 ); 10 fecal samples tested positive with more than two primer pairs and the remaining 26 samples tested positive only with the C1 and C4 primer pair. Among the primer sets, the C1 and C4 primer pair (targeting a 356 bp of the GCRV VP6 gene) was the most sensitive (Table 3).
Table 3.
RT-PCR assay results for the individual swine fecal samples.
| Porcine fecal samples positive using RT-PCR assaysa (no. of positive samples/total no. of total samples; %) |
No. of total positive samplesb | ||||
|---|---|---|---|---|---|
| RVCF1/T778a | T729/T778a | C1/C4 | BMJ145/BMJ44 | T383/RVCnR2 | |
| 3/137 (2.2%) | 0/137 (0%) | 28/137 (20.4%) | 9/137 (6.6%) | 10/137 (7.3%) | 36/137 (26.3%) |
Virus and target protein for each primer pair are listed in Table 1.
Fecal samples were considered positive if at least one positive fecal sample was detected in the same sample by one of the primer pairs.
3.2. Other enteric pathogens
Of the 36 porcine GCRV-positive diarrheic fecal specimens, 17 fecal samples (12.4%) tested positive for the porcine GCRVs alone, while 19 fecal samples (13.9%) also tested positive for other enteric pathogens including porcine GARV, PSaV, TGEV, PEDV, E. coli, and Salmonella spp. (Table 4 ). Of the concurrent infections of the porcine GCRVs with the other enteric pathogens, GARVs were the most common, being found in 15 fecal samples (10.9%). In addition, 64 fecal specimens (46.7%) that tested negative for porcine GCRVs also tested positive for other enteric pathogens (Table 4). No enteric pathogens were detected in 37 fecal samples (27.0%).
Table 4.
Summary of enteric pathogens present in the fecal samples obtained from pigs with diarrhea.
| Enteric pathogens presenta | No. of samples (%)b |
|---|---|
| GCRV alone | 17 (12.4) |
| GCRV plus GARV | 6 (4.38) |
| GCRV plus E. coli | 1 (0.73) |
| GCRV plus Salmonella | 2 (1.46) |
| GCRV, GARV plus PSaV | 4 (2.92) |
| GCRV, GARV plus Salmonella | 2 (1.46) |
| GCRV, GARV plus E. coli | 2 (1.46) |
| GCRV plus PSaV | 1 (0.73) |
| GCRV, GARV, PSaV plus Salmonella | 1 (0.73) |
| Other enteric pathogens detectedc | 64 (46.7) |
| No enteric pathogens detected | 37 (27.0) |
| Total | 137 (100) |
GCRV: group C rotavirus; GARV: group A rotavirus; PSaV: porcine sapovirus; TGEV: transmissible gastroenteritis coronavirus; PEDV: porcine epidemic diarrhea virus.
Number of positive fecal samples.
These fecal samples were negative for GCRV infection, but positive for other enteric pathogens including the GARV, GBRV, PSaV, PEDV, Salmonella and E. coli, either alone or in combination.
3.3. Seasonal distribution of porcine GCRVs in piglets with diarrhea in South Korea
Seasonally, porcine GCRV infections were more prevalent in fecal samples of pigs in spring and winter than in the other seasons: 16 (44.0%) out of 41 fecal samples were positive in spring, 6 (17.0%) out of 25 fecal samples were positive in summer, 1 (3.0%) out of 22 fecal samples was positive in autumn, and 13 (36.0%) out of 49 fecal samples were positive in winter.
3.4. Molecular analysis of the VP6 genes of identified strains
The genetic diversity of the porcine GCRVs was investigated by sequencing 356 bp of the nt 997–1352 VP6 gene from the 24 GCRVs amplified strongly by RT-PCR. The phylogenetic analysis between our and other known GCRVs was performed with a 310 bp fragment (excluding the primer sequences). Alignments indicated that the Korean GCRVs belonged to the porcine and bovine lineages, respectively; 23 Korean GCRVs grouped with the porcine strain Cowden and bovine strain WD534tc; the latter is believed to be of porcine origin (Fig. 1 ). In this porcine lineage, 23 Korean GCRV strains were placed on the separate branch from the other known GCRV strains, Cowden and WD534tc. The remaining strain, 06-48-2, clustered with the bovine GCRV Shintoku and Yamagata strains (Fig. 1). None of Korean porcine GCRV sequences was closely related to human GCRV strains (Fig. 1).
Fig. 1.
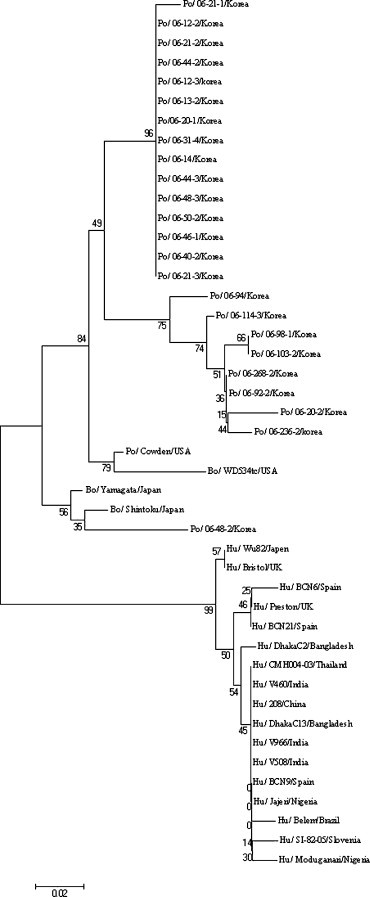
Phylogenetic tree of a VP6 peptide (aa 334–395) of the Korean porcine group C rotavirus strains showing its genetic relationship with the other group C rotavirus strains. The names of group C rotavirus strains are listed in Table 2.
Comparisons of nucleotide and deduced amino acid sequences of the partial VP6 gene between all Korean porcine GCRVs and other known porcine, bovine, and human GCRVs are shown in Table 5 . Among the GCRVs examined, 23 Korean GCRVs that clustered in the porcine lineage had the highest nucleotide (86.5–100%) and deduced amino acid (89.9–100%) identities with each other. These viruses also shared relatively higher nucleotide and deduced amino acid identities with the porcine Cowden and bovine WD534ct strains (85.8–96.0% nucleotide and 89.8–96.0% deduced amino acid sequence identities). The remaining Korean GCRV strain, 06-48-2, had the highest nucleotide (89.0–89.7%) and deduced amino acid (92.9–93.9%) identities with the bovine GCRV strains, Shintoku and Yamagata.
Table 5.
Nucleotide and deduced amino acid sequence comparison of the VP6 of the Korean group C rotavirus strains with that of the other strains.
| Strain | Origin | % identity with strainsa |
|||
|---|---|---|---|---|---|
| 23 Korean strainsb |
One Korean strainc |
||||
| nt | aa | nt | aa | ||
| Cowden | Porcine | 87.4–89.4 | 91.9–96.0 | 84.8 | 90.9 |
| WD534tc | Bovine | 85.8–87.7 | 89.8–94.9 | 83.5 | 88.8 |
| Shintoku | Bovine | 85.8–87.4 | 89.9–92.9 | 89.7 | 93.9 |
| Yamagata | Bovine | 84.8–87.1 | 90.9–93.9 | 89.0 | 92.9 |
| V996 | Human | 82.3–84.8 | 81.8–83.8 | 83.9 | 85.9 |
| DhakaC2 | Human | 82.6–84.8 | 81.8–83.8 | 83.5 | 84.8 |
| BCN6 | Human | 82.3–84.5 | 80.8–82.8 | 83.9 | 88.8 |
| Bristol | Human | 82.9–85.2 | 82.8–84.8 | 83.9 | 84.8 |
| Belem | Human | 81.9–83.9 | 80.8–82.8 | 84.5 | 84.8 |
| Jajeri | Human | 83.2–85.2 | 81.8–83.8 | 84.2 | 85.9 |
| Wu82 | Human | 81.9–84.5 | 82.8–84.8 | 83.5 | 84.8 |
| SI-82-05 | Human | 81.6–84.2 | 81.6–82.8 | 83.9 | 84.8 |
The classification of Korean GCRV strains into 23 and 1 is based on the phylogenetic data in which they clustered on the separate branches (Fig. 1).
The nucleotide and deduced amino acid sequence identities of VP6 among the Korean 23 strains were 86.5–100 and 89.9–100%, respectively.
The nucleotide and deduced amino acid sequence identities of VP6 between the Korean 23 and 1 strains were 82.9–84.8 and 85.9–88.9%, respectively.
4. Discussion
Since porcine GCRV infections have only been reported in a few countries, their epidemiological details are unclear. Although presently five sets of primer pairs were used to detect the porcine GCRV strains, it is likely that some strains escaped detection. Among those primer pairs, the C1 and C4 primer pair (Gabbay et al., 2008), showed high sensitivity in detecting porcine GCRVs. When fecal samples were determined to be positive based on at least one positive fecal sample detected by each primer pair, porcine GCRVs were detected in 26.3% fecal samples obtained from six provinces. Overall, the high prevalence and widespread geographical distribution of porcine GCRVs suggest that these viruses are widespread in piglets with diarrhea in South Korea, similar to that documented in Italy (Martella et al., 2007a). In addition, it is reported that porcine GCRVs were not infrequently detected in asymptomatic piglets (Collins et al., 2008). Because the present data were analyzed with the fecal samples collected from piglets with diarrhea, further studies will be needed to elucidate their precise ecology in fecal samples from asymptomatic piglets.
Porcine GCRVs have been detected in diarrheic fecal samples in nursing, weaning, and post-weaning pigs either alone or in combination of other enteric pathogens (Kim et al., 1999, Martella et al., 2007a, Morin et al., 1990, Saif and Jiang, 1994, Saif et al., 1980, Sigolo de San Juan et al., 1986). In this study, 12.4% diarrheic fecal samples tested positive for porcine GCRVs alone, while 13.9% were positive for not only porcine GCRVs but also other enteric pathogens including GARV, PSaV, PEDV, TGEV, E. coli, and Salmonella spp. This suggests that a number of enteric pathogens, either singly or in combination, can augment the clinical course of porcine GCRV infections (Martella et al., 2007a). This hypothesis is supported by the observation that experimental coinfection of calves with group A rotaviruses enhances fecal shedding of a bovine group C rotavirus and the extent of histopathological lesions in the small intestine (Chang et al., 1999). In addition, we detected porcine GCRVs more commonly in the spring (44%) and winter (36%) than autumn (3%) and summer (17%). To our knowledge, there is no report showing a clear seasonal distribution of porcine GCRV infections in pigs. Further epidemiological studies throughout the world will be needed to properly understand the seasonal pattern of porcine GCRV infections and to establish porcine GCRV surveillance programs to prevent porcine GCRV infections.
The geographical genetic divergence of porcine GCRV VP6 gene is unclear because the available GenBank sequence data involve only a few countries. In this study, genetically variable porcine GCRVs were detected in South Korea and their genetic relationship with other porcine, bovine, and human GCRVs was determined. Analyses of the partial VP6 gene of the 24 porcine GCRVs showed that they share low nucleotide and deduced amino acid sequence identities with human GCRVs, consistent with a previous report (Martella et al., 2007a). This result indicates that the Korean porcine GCRVs belong to different genetic clusters with human GCRVs. In addition, phylogenetic analysis of GCRV partial VP6 gene between the 23 Korean and other known porcine GCRVs within the porcine linage revealed two subclusters consisting of only Korean porcine GCRVs and composed of American porcine Cowden strain and bovine WD534ct strain. However, genetic distances were variable among the Korean GCRVs strains (86.5–100% nucleotide and 89.9–100% deduced amino acid identities) and between the Korean and other known strains (85.8–96.0% nucleotide and 89.8–96.0% deduced amino acid sequence identities). From these results, it is unclear whether there are different sublineages within the porcine GCRV lineage. Therefore, more in-depth epidemiological analysis of porcine GCRVs throughout the world will be needed to understand their diversity and evolution as well as to develop classification schemes.
There is now increasing evidence that the transmission of group A rotaviruses can occur from animal-to-human as well as from animal-to-animal by direct transmission of the virus or by the contribution of one or several genes to reassortants (Ghosh et al., 2007, Griffin et al., 2002, Martella et al., 2006, Matthijnssens et al., 2006, Palombo et al., 2000, Pongsuwanna et al., 1996). Compared to group A rotaviruses, there is a paucity of information regarding the sequence and phylogenetic data on all 11 genomic segments of GCRVs. Therefore, it is largely unknown whether these genomic segments are totally homologous to those of the original species or reassortants from other species. However, GCRVs are thought to be able to cause interspecies transmission. Good examples are increasing sero-prevalence rates to GCRVs in human populations living in rural settings (Iturriza-Gomara et al., 2004), porcine-like GCRVs in Brazilian children (Gabbay et al., 2008), and porcine-like GCRV strain (WD534tc) in cattle (Chang et al., 1999). In this investigation, one strain (Po/06-48-2) of the 24 Korean porcine GCRVs displayed the highest nucleotide (89.7–89.0%) and deduced amino acid sequence identities (92.9–93.9%) with the bovine GCRV Shikoku and Yamagata strains. Phylogenetically, this strain was placed in the lineage of bovine GCRV strains. These results suggest that the Korean Po/06-48-2 strain might be of bovine origin. It is perhaps not surprising that emergence of bovine-like porcine GCRV occurred in South Korea because, in 2007, there were approximately 10,000 pig farms harboring nearly 10 million pigs, which tend to be located in the same geographic boundaries with approximately 19,200 cattle farms harboring over 2.5 million animals. Therefore, direct transmission between swine and cattle, or generation of bovine-porcine reassortant GCRVs can be envisioned via direct contact between the animals and the contamination of the environment, food and water, and fomites as well as farmers (Cook et al., 2004). Although there is no experimental evidence for the generation of human-like porcine or porcine-like human GCRVs, the close contact between humans and pigs in nature may induce the generation of human-like porcine GCRVs or vice versa. Therefore, a more in-depth study of the epidemiology of animal-like human GARVs or vice versa should be performed to provide an understanding of interspecies transmission and face new challenges for rotavirus vaccine development in South Korea.
In summary, this study demonstrates that porcine GCRV infections are widespread in piglets with diarrhea in South Korea. In addition, the infecting strains mostly belong to the porcine lineage but have one bovine-like GCRV, which possibly originated from bovine GCRV due to interspecies transmission.
Acknowledgements
This study was supported by Technology Development Program of Agriculture and Forestry, Ministry for Agriculture, Forestry and Fisheries, and the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE), Republic of Korea. The authors acknowledge a graduate fellowship from the Korean Ministry of Education and Human Resources Development through the Brain Korea 21 project.
Footnotes
The GenBank accession numbers of the Korean partial VP6 gene sequences are listed in Table 2.
References
- Adah M.I., Wade A., Oseto M., Kuzuya M., Taniguchi K. Detection of human group C rotaviruses in Nigeria and sequence analysis of their genes encoding VP4, VP6, and VP7 proteins. J. Med. Virol. 2002;66:269–275. doi: 10.1002/jmv.2141. [DOI] [PubMed] [Google Scholar]
- Alam M.M., Kobayashi N., Ishino M., Ahmed M.S., Ahmed M.U., Paul S.K., Muzumdar B.K., Hussain Z., Wang Y.H., Naik T.N. Genetic analysis of an ADRV-N-like novel rotavirus strain B219 detected in a sporadic case of adult diarrhea in Bangladesh. Arch. Virol. 2007;152:199–208. doi: 10.1007/s00705-006-0831-y. [DOI] [PubMed] [Google Scholar]
- Bányai K., Jiang B., Bogdán A., Horváth B., Jakab F., Meleg E., Martella V., Magyari L., Melegh B., Szuces G. Prevalence and molecular characterization of human group C rotaviruses in Hungary. J. Clin. Virol. 2006;37:317–322. doi: 10.1016/j.jcv.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Caul E.O., Ashley C.R., Darville J.M., Bridger J.C. Group C rotavirus associated with fatal enteritis in a family outbreak. J. Med. Virol. 1990;30:201–205. doi: 10.1002/jmv.1890300311. [DOI] [PubMed] [Google Scholar]
- Chang K.O., Nielsen P.R., Ward L.A., Saif L.J. Dual infection of gnotobiotic calves with bovine strains of group A and porcine-like group C rotaviruses influences pathogenesis of the group C rotavirus. J. Virol. 1999;73:9284–9293. doi: 10.1128/jvi.73.11.9284-9293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P.J., Martella M., O'Shea H. Detection and characterization of group C rotaviruses in asymptomatic piglets in Ireland. J. Clin. Microbiol. 2008;46:2973–2979. doi: 10.1128/JCM.00809-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N., Bridger J., Kendall K., Iturriza Gomara M., El-Attar L., Gray J. The zoonotic potential of rotavirus. J. Infect. 2004;48:289–302. doi: 10.1016/j.jinf.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Elschner M., Prudlo J., Hotzel H., Otto P., Sachse K. Nested reverse transcriptase-polymerase chain reaction for the detection of group A rotaviruses. J. Vet. Med. B. 2002;49:77–81. doi: 10.1046/j.1439-0450.2002.00510.x. [DOI] [PubMed] [Google Scholar]
- Esona M.D., Humphrey C.D., Dennehy P.H., Jiang B. Prevalence of group C rotavirus among children in Rhode Island, United States. J. Clin. Virol. 2008;42:221–224. doi: 10.1016/j.jcv.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Estes M.K., Kapikian A.Z. Rotaviruses. In: Knipe D.M., Griffin D.E., Lamb R.A., Straus S.E., Howley P.M., Martin M.A., Roizman B., editors. Fields Virology. fifth ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 1917–1974. [Google Scholar]
- Fielding P.A., Lambden P.R., Caul E.O., Clarke I.N. Molecular characterization of the outer capsid spike protein (VP4) gene from human group C rotavirus. Virology. 1994;204:442–446. doi: 10.1006/viro.1994.1551. [DOI] [PubMed] [Google Scholar]
- Gabbay Y.B., Borges A.A., Oliveria D.S., Linhares A.C., Mascarenhas J.D., Barardi C.R., Simões C.M., Wang Y., Glass R.I., Jiang B. Evidence for zoonotic transmission of group C rotaviruses among children in Belém. Braz. J. Med. Virol. 2008;80:1666–1674. doi: 10.1002/jmv.21250. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Varghese V., Samajdar S., Bhattacharya S.K., Kobayashi N., Naik T.N. Evidence for independent segregation of the VP6- and NSP4-encoding genes in porcine group A rotavirus G6P[13] strains. Arch. Virol. 2007;152:423–429. doi: 10.1007/s00705-006-0848-2. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Allen J.R., Glass R.I., Fang Z., Bremont M., Cohen J., McCrae M.A., Saif L.J., Sinarachatanant P., Caul E.O. Detection of group B and C rotaviruses by polymerase chain reaction. J. Clin. Microbiol. 1991;29:519–523. doi: 10.1128/jcm.29.3.519-523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice A.S., Lambden P.R., Caul E.O., Clarke I.N. Sequence conservation of the major outer capsid glycoprotein of human group C rotaviruses. J. Med. Virol. 1994;44:166–171. doi: 10.1002/jmv.1890440209. [DOI] [PubMed] [Google Scholar]
- Griffin D.D., Nakagomi T., Hoshino Y., Nakagomi O., Kirkwood C.D., Parashar U.D., Glass R.I., Gentsch J.R. Characterization of nontypeable rotavirus strains from the United States: identification of a new rotavirus reassortant (P2A[6]G12) and rare P3[9] strains related to bovine rotaviruses. Virology. 2002;294:256–269. doi: 10.1006/viro.2001.1333. [DOI] [PubMed] [Google Scholar]
- Iizuka S., Tabara K., Kawamukai A., Itogawa H., Hoshina K. An outbreak of group C rotavirus infection in an elementary school in Shimane prefecture, Japan, February 2006. Jpn. J. Infect. Dis. 2006;59:350–351. [PubMed] [Google Scholar]
- Iturriza-Gomara M., Clarke I., Desselberger U., Brown D., Thomas D., Gray J. Seroepidemiology of group C rotavirus infection in England and Wales. Eur. J. Epidemiol. 2004;19:589–595. doi: 10.1023/b:ejep.0000032381.36658.cb. [DOI] [PubMed] [Google Scholar]
- Janke B.H., Nelson J.K., Benfield D., Nelson E.A. Relative prevalence of typical and atypical strains among rotaviruses from diarrheic pigs in conventional swine herds. J. Vet. Diagn. Invest. 1990;2:308–311. doi: 10.1177/104063879000200410. [DOI] [PubMed] [Google Scholar]
- Jeong C., Park S.I., Park S.H., Kim H.H., Park S.J., Jeong J.H., Choy H.E., Saif L.J., Kim S.K., Kang M.I., Hyun B.H., Cho K.O. Genetic diversity of porcine sapoviruses. J. Vet. Med. 2007;122:246–257. doi: 10.1016/j.vetmic.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Saif L.J., Gentsch J.R., Glass R.I. Completion of the four large gene sequences of porcine group C Cowden rotavirus. Virus Genes. 2000;20:193–194. doi: 10.1023/a:1008187002183. [DOI] [PubMed] [Google Scholar]
- Jiang B., Gentsch J.R., Tsunemitsu H., Saif L.J., Glass R.I. Sequence analysis of the gene encoding VP4 of a bovine group C rotavirus: molecular evidence for a new P genotype. Virus Genes. 1999;19:85–88. doi: 10.1023/a:1008196824879. [DOI] [PubMed] [Google Scholar]
- Jiang X., Espul C., Zhong W.M., Cuello H., Matson D.O. Characterization of a novel human calicivirus that may be a naturally occurring recombinant. Arch. Virol. 1999;144:2377–2387. doi: 10.1007/s007050050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Dennehy P.H., Spangenberger S., Gentsch J.R., Glass R.I. First detection of group C rotavirus in fecal specimens of children with diarrhea in the United States. J. Infect. Dis. 1995;172:45–50. doi: 10.1093/infdis/172.1.45. [DOI] [PubMed] [Google Scholar]
- Kim L., Chang K.O., Sestak K., Parwani A., Saif L.J. Development of a reverse transcription-nested polymerase chain reaction assay for differential diagnosis of transmissible gastroenteritis virus and porcine respiratory coronavirus from feces and nasal swabs of infected pigs. J. Vet. Diagn. Invest. 2000;12:385–388. doi: 10.1177/104063870001200418. [DOI] [PubMed] [Google Scholar]
- Kim Y., Chang K.O., Straw B., Saif L.J. Characterization of group C rotaviruses associated with diarrhea outbreak in feeder pigs. J. Clin. Microbiol. 1999;37:1484–1488. doi: 10.1128/jcm.37.5.1484-1488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S., Sasaki O., Amimoto K., Okada N., Kitazima T., Yasuhara H. Detection of porcine epidemic diarrhea virus using polymerase chain reaction and comparison of the nucleocapsid protein genes among strains of the virus. J. Vet. Med. Sci. 1999;61:827–830. doi: 10.1292/jvms.61.827. [DOI] [PubMed] [Google Scholar]
- Kuzuya M., Fujii R., Hamano M., Nishijima M., Ogura H. Detection and molecular characterization of human group C rotaviruses in Okayama prefecture, Japan, between 1986 and 2005. J. Med. Virol. 2007;79:1219–1228. doi: 10.1002/jmv.20910. [DOI] [PubMed] [Google Scholar]
- Kuzuya M., Hamano M., Nishijima M., Fujii R., Ogura H., Tanaka M., Oda A., Kusaka S., Naitou M. An outbreak of acute gastroenteritis caused by human group C rotavirus in a welfare institution in Okayama prefecture. Jpn. J. Infect. Dis. 2005;58:255–257. [PubMed] [Google Scholar]
- Kuzuya M., Fujii R., Hamano M., Nakamura J., Yamada M., Nii S., Mori T. Molecular analysis of outer capsid glycoprotein (VP7) genes from two isolates of human group C rotavirus with different genome electropherotypes. J. Clin. Microbiol. 1996;34:3185–3189. doi: 10.1128/jcm.34.12.3185-3189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Bányai K., Lorusso E., Bellacicco A.L., Decaro N., Camero M., Bozzo G., Moschidou P., Arista S., Pezzotti G., Lavazza A., Buonavoglia C. Prevalence of group C rotaviruses in weaning and post-weaning pigs with enteritis. Vet. Microbiol. 2007;123:26–33. doi: 10.1016/j.vetmic.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Martella V., Bányai K., Lorusso E., Decaro N., Bellacicco A., Desario C., Corrente M., Greco G., Moschidou P., Tempesta M., Arista S., Ciarlet M., Lavazza A., Buonavoglia C. Genetic heterogeneity in the VP7 of group C rotaviruses. Virology. 2007;367:358–366. doi: 10.1016/j.virol.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Martella V., Bányai K., Ciarlet M., Iturriza Gómara M., Lorusso E., de Grazia S., Arista S., Decaro N., Elia G., Cavalli A., Corrente M., Lavazza A., Baselga R., Buonavoglia C. Relationships among porcine and human P[6] rotaviruses: evidence that the different human P[6] lineages have originated from multiple interspecies transmission events. Virology. 2006;344:509–519. doi: 10.1016/j.virol.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Rahman M., Martella V., Xuelei Y., de Vos S., de Leener K., Ciarlet M., Buonavoglia C., Van Ranst M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J. Virol. 2006;80:3801–3810. doi: 10.1128/JVI.80.8.3801-3810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici M.C., Abelli L.A., Martinelli M., Martella V., Dettori G., Chezzi C. Molecular characterization of group C rotaviruses detected in children in Italy. J. Clin. Virol. 2009;44:62–65. doi: 10.1016/j.jcv.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Morin M., Magar R., Robinson Y. Porcine group C rotavirus as a cause of neonatal diarrhea in a Quebec swine herd. Can. J. Vet. Res. 1990;54:385–389. [PMC free article] [PubMed] [Google Scholar]
- Nagashima S., Kobayashi N., Ishino M., Alam M.M., Ahmed M.U., Paul S.K., Ganesh B., Chawla-Sarkar M., Krishnan T., Naik T.N., Wang Y.H. Whole genomic characterization of a human rotavirus strain B219 belonging to a novel group of the genus rotavirus. J. Med. Virol. 2008;80:2023–2033. doi: 10.1002/jmv.21286. [DOI] [PubMed] [Google Scholar]
- Palombo E., Clark R., Bishop R.F. Characterization of a “European-like” serotype G8 human rotavirus isolated in Australia. J. Med. Virol. 2000;60:56–62. [PubMed] [Google Scholar]
- Pongsuwanna Y., Taniguchi K., Chiwakul M., Urasawa T., Wahasugi F., Jayavasu C., Urasawa S. Serological and genomic characterization of porcine rotaviruses in Thailand: detection of a G10 porcine rotavirus. J. Clin. Microbiol. 1996;31:2010–2015. doi: 10.1128/jcm.34.5.1050-1057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Jiang B., Saif J.J., Kang S.Y., Ishimaru Y., Yamashita Y., Oseto M., Green K.Y. Sequence conservation of gene 8 between human and porcine group C rotaviruses and its relationship to the VP7 gene of group A rotaviruses. Virology. 1991;182:562–569. doi: 10.1016/0042-6822(91)90597-5. [DOI] [PubMed] [Google Scholar]
- Rahman M., Banik S., Faruque A.S., Taniguchi K., Sack D.A., Van Ranst M., Azim T. Detection and characterization of human group C rotaviruses in Bangladesh. J. Clin. Microbiol. 2005;43:4460–4465. doi: 10.1128/JCM.43.9.4460-4465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S.M., Bishop R.F., Holmes I.H. Detection of a rotavirus-like agent associated with diarrhea in an infant. J. Clin. Microbiol. 1982;16:724–726. doi: 10.1128/jcm.16.4.724-726.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Jiang B. Nongroup A rotaviruses of humans and animals. Curr. Top. Microbiol. Immunol. 1994;185:339–371. doi: 10.1007/978-3-642-78256-5_11. [DOI] [PubMed] [Google Scholar]
- Saif L.J., Bohl E.H., Theil K.W., Cross R.F., House J.A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J. Clin. Microbiol. 1980;12:105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fauquier A., Roman E., Colomina J., Wilhemi I., Glass R.I., Jiang B. First detection of group C rotavirus in children with acute diarrhea in Spain. Arch. Virol. 2003;148:399–404. doi: 10.1007/s00705-002-0921-4. [DOI] [PubMed] [Google Scholar]
- Sigolo de San Juan C., Bellinzoni R.C., Mattion N., La Torre J., Scodeller E.A. Incidence of group A and atypical rotaviruses in Brazilian pig herds. Res. Vet. Sci. 1986;41:270–272. [PubMed] [Google Scholar]
- Steyer A., Poljsak-Prijatelj M., Bufon T., Sedmak M., Vidmar L., Mijovski J.Z., Marin J. First detection of group C rotavirus in patients with gastroenteritis in Slovenia. J. Med. Virol. 2006;78:1250–1255. doi: 10.1002/jmv.20687. [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Terrett L.A., Saif L.J., Theil K.W., Kohler E.M. Physicochemical characterization of porcine pararotavirus and detection of virus and viral antibodies using cell culture immunofluorescence. J. Clin. Microbiol. 1987;25:268–272. doi: 10.1128/jcm.25.2.268-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Median A. Isolation of an atypical rotavirus causing diarrhea in neonatal ferrets. Lab. Anim. Sci. 1987;37:167–171. [PubMed] [Google Scholar]
- Tsunemitsu H., Jiang B., Saif L.J. Sequence comparison of the VP7 gene encoding the outer capsid glycoprotein among animal and human group C rotaviruses. Arch. Virol. 1996;141:705–713. doi: 10.1007/BF01718328. [DOI] [PubMed] [Google Scholar]
- Tsunemitsu H., Jiang B., Yamashita Y., Oseto M., Ushijima H., Saif L.J. Evidence of serologic diversity within group C rotaviruses. J. Clin. Microbiol. 1992;30:3009–3012. doi: 10.1128/jcm.30.11.3009-3012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H., Saif L.J., Jiang B.M., Shimizu M., Hiro M., Yamaguchi H., Ishiyama T., Hirai T. Isolation, characterization, and serial propagation of a bovine group C rotavirus in a monkey kidney cell line (MA104) J. Clin. Microbiol. 1991;29:2609–2613. doi: 10.1128/jcm.29.11.2609-2613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will L.A., Paul P.S., Proescholdt T.A., Aktar S.N., Flaming K.P., Janke B.H., Sacks J., Lyoo Y.S., Hill H., Hoffman L.J., Wu L.L. Evaluation of rotavirus infection and diarrhea in Iowa commercial pigs based on an epidemiologic study of a population represented by diagnostic laboratory cases. J. Vet. Diagn. Invest. 1994;6:416–422. doi: 10.1177/104063879400600403. [DOI] [PubMed] [Google Scholar]
- Xu L., Harbour D., McCrae M.A. The application of polymerase chain reaction to the detection of rotaviruses in faeces. J. Virol. Methods. 1990;27:29–38. doi: 10.1016/0166-0934(90)90143-4. [DOI] [PubMed] [Google Scholar]
- Yang H., Makeyev E.V., Kang Z., Ji S., Bamford D.H., van Dijk A.A. Cloning and sequence analysis of dsRNA segments 5, 6 and 7 of a novel non-group A, B, C adult rotavirus that caused an outbreak of gastroenteritis in China. Virus Res. 2004;106:15–26. doi: 10.1016/j.virusres.2004.05.011. [DOI] [PubMed] [Google Scholar]


