Highlights
-
•
A panel of PEDV structural and nonstructural proteins were expressed.
-
•
These PEDV antigens were tested for reactivity with sera from PEDV-infected pigs.
-
•
851 porcine sera were analyzed by ELISA with antigens showing immune-reactivity.
-
•
Pattern of neutralizing antibody was more similar to that of IgA in weaning piglets.
-
•
ORF3C and E in addition to S1 can be used as diagnostic markers for serologic detection.
Keywords: Porcine epidemic diarrhea virus, Antibodies, Diagnostic antigens, Serological assay
Abstract
Given the highly contagious and acute nature of porcine epidemic diarrhea (PED), especially in piglets, there is an urgent need for the development of rapid and sensitive diagnostic assays. The diagnostic potentials of specific porcine epidemic diarrhea virus (PEDV) accessory and nonstructural proteins, if any, have not yet been investigated. In order to determine and compare which of the viral proteins may be useful as diagnostic antigens, whole virus (WV) particles and a panel of structural and nonstructural PEDV proteins [spike subunit 1 (S1), the C-terminal part of ORF3 (ORF3C), envelope (E), nonstructural protein 1 (Nsp1), Nsp2, Ac (acidic domain of Nsp3), and ADRP (ADP-ribose-1-monophosphatase domain of Nsp3), expressed individually in bacterial and/or mammalian cells] were tested for reactivity with sera from PEDV-infected pigs by ELISA and/or western blot analysis. According to western blots, serum antibody interactions with the S1 protein were relatively more sensitive and specific than ORF3C, E and Ac. Furthermore, a total of 851 serum samples from diarrheal pigs of different ages were analyzed by ELISA, with most showing immune-reactivity towards the WV, S1, ORF3C, and E proteins. The earliest IgG antibody response was observed in the one-week-old piglets, with similar antibody ontogeny and patterns of seroconversion for S1, ORF3C, E, and WV antigens. In addition, the pattern of neutralizing antibody was more similar to that of IgA in weaning piglets after PEDV infection. Collectively, these data provide more reliable information on the host immune response to different viral proteins, which will be useful for development of novel serological assays and for design of vaccines that better stimulate protective immunity.
1. Introduction
Porcine epidemic diarrhea (PED) is characterized by severe diarrhea, vomiting, and dehydration followed by high mortality in suckling piglets (Huang et al., 2013). The causative agent, porcine epidemic diarrhea virus (PEDV), was initially identified in Europe in 1978, and its genome (˜28 kb in size) consists of seven open reading frames (ORFs) (Kocherhans et al., 2001). The 5’ two-thirds of PEDV genome encodes ORF1 (consisting of overlapping ORF1a and ORF1b), and the 3’ one-third harbors ORFs encoding four structural proteins, the spike (S), envelope (E), membrane (M) and nucleocapsid (N), and an accessory ORF3 between S and E (Huang et al., 2013; Kocherhans et al., 2001; Tian et al., 2014). RNA synthesis in PEDV is carried out by a replicase-transcriptase composed of 16 nonstructural proteins (Nsp1-16) encoded by ORF1a and ORF1b. Among them, Nsp3 comprises multiple structural domains, including a highly acidic domain at the amino terminus (Ac), and a highly conserved ADP-ribose-1-phosphatase (ADRP) macrodomain. The Ac domain of Nsp3 is essential for virion assembly and plays a critical role in interaction with the viral nucleocapsid during early infection, whereas the ADRP provides activities necessary for synthesis of genomic and subgenomic RNAs (Hurst-Hess et al., 2015). As pigs of all ages are susceptible to PEDV (Alvarez et al., 2015; Annamalai et al., 2015), there is an urgent need for the development of highly sensitive and specific diagnostic assays for use in the field (Diel et al., 2016).
Since the identification of PEDV, several diagnostic tests based on PCR detection of viral RNA have been described in the literature (Diel et al., 2016). Another common diagnostic method is serological testing for the presence of specific antibodies against viral proteins, which is also fast and convenient for epidemiologic investigations. Many tools have been developed for the detection of anti-PEDV antibodies based upon the major structural proteins (such as S, M or N proteins) in serum, colostrum, milk, feces and oral fluid, including indirect immunofluorescence assays (IFA), virus neutralization assays (SN), enzyme-linked immunosorbent assays (ELISA), and fluorescent microsphere immunoassays (FMIA) (Diel et al., 2016; Gerber et al., 2014; Gerber and Opriessnig, 2015; Gimenez-Lirola et al., 2017; Okda et al., 2015). But comparative studies of the above assays using different PEDV structural and nonstructural proteins as antigens are rarely conducted. Meanwhile, the diagnostic potentials of specific PEDV accessory and nonstructural proteins, if any, have not yet been investigated in details.
In this study, a panel of recombinant PEDV ORFs encoding structural and nonstructural proteins were expressed in mammalian and/or bacterial cells and screened for reactivity with porcine sera from seven provinces of China by ELISA and/or western blot analysis, in order to determine which antigen is most suitable as a diagnostic marker for PEDV infection. Several rabbit polyclonal antibodies against these recombinant proteins were also generated and validated for use as diagnostic tools upon PEDV infection in vitro.
2. Materials and methods
2.1. Cells and viruses
Vero cells (ATCC® CCL-81™) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 8% fetal bovine serum (FBS). The PEDV virulent strain ZJU/G2/2013 (GenBank accession no. KU558701) was used in the study (Ji et al., 2018; Qin et al., 2017).
2.2. Sample collection
A total of 851 serum samples were collected in 2015–2016 from diarrheic pigs at 24 commercial farms in the Shandong, Henan, Jiangxi, Hunan, Jiangsu, Heilongjiang and Zhejiang provinces of Eastern and Northern China. There were 295 samples from sows (76 primiparous sows, and 219 multiparous), 131 from 1-week-old piglets, 85 from 3 week-olds, 11 from 5 week-olds, 70 from 7 week-olds, 116 from 11 week-olds, 97 from 17 week-olds, and 46 from 20 week-olds.
Another 88 serum and fecal samples were obtained from weaning piglets experimentally challenged with PEDV-ZJU/G2/2013 in three PEDV challenged experiments. The procedure of the PEDV challenge has been described previously (Qin et al., 2017). The Briefly, the 3-day-old conventional piglets were inoculated with about 5 ml 106 infectious titer (TCID50) PEDV in 1 × PBS (pH 7.4). Blood and fecal samples were collected prior to inoculation and at 1, 3, 5, 7, 10, 14, 17 days post-inoculation (dpi). Samples from a total of 11 survival piglets but not from dead pigs at each time point were collected. After centrifugation at 1500 ×g for 10 min, serum was harvested, aliquoted and stored at −80 °C until use. Fecal swab samples were individually mixed with 1 ml of 1 × PBS (pH 7.4) immediately after collection, placed in a 2 ml cryogenic tube (BD Falcon™), and stored at −80 °C until use. The animal experiments were approved by the Experimental Animal Ethics Committee of Zhejiang University (approval no. ZJU20170026).
2.3. Preparation of the purified PEDV virions
The PEDV-ZJU/G2/2013 strain was propagated in Vero cells in DMEM supplemented with 5 μg/ml trypsin according to the standard method (Ji et al., 2018). Briefly, a confluent cell monolayer was washed with minimum essential media (MEM) twice before infecting with the virus [MOI (multiplicity of infection) = 1] for 2 h at 37 °C, after which additional culture medium was added without removing the inoculum. Observed cytopathic effects (CPE) reached approximately 90% in 2–3 days, and the virus culture supernatant was collected after three freeze-thaw cycles, then clarified by high speed centrifugation (4000×g for 30 min) and further purified by ultracentrifugation through a 20% (wt/vol) sucrose cushion (140,000 ×g for 3 h). The purified virus was further exposed by a negative staining technique, examined using electron microscopy, then stored at −80 °C until use. Concentration of viral proteins was measured by BCA protein assay kit (Beyotime, Shanghai, China).
2.4. Expression and purification of recombinant PEDV proteins
Full-length PEDV cDNA was used as a template for amplification and cloning of various PEDV genes, including those encoding the C terminus of PEDV ORF3 (ORF3C) and the complete sequences of E, Nsp1, Nsp2, Ac, and ADRP. The constructs used for this study are listed in Table 1 . For transient expression in mammalian cells, the pcDNA-3.1 vector was used, and the expression plasmid pcDNA-PEDV-S1-Fc has been described in the previous study (Gerber et al., 2014).
Table 1.
Recombinant expression plasmids used in this study.
| Name of plasmid | Nucleotide position* | Amino acid position in ORF | Number of amino acids |
|---|---|---|---|
| pSmart-ORF3C | 25085-25468 | 98-224 | 127 |
| pSmart-E | 25449-25679 | 1-76 | 76 |
| pET-28a-nsp1 | 293-622 | 1-110 | 110 |
| pET-28a-nsp2 | 623-2977 | 111-895 | 785 |
| pET-28a-Ac | 2978-3499 | 896-1069 | 174 |
| pET-28a-ADRP | 4163-4651 | 1291-1453 | 163 |
| pcDNA-PEDV-S1-Fc | 20634-22976 | 1-781 | 781 |
Positions correspond to the PEDV ZJU/G2/2013 strain (GenBank accession no. KU558701).
For expression of His-tag fusion proteins in bacteria, PEDV target genes were cloned in-frame with N-terminal 6×His tags in the pET-28a (Novagen; for Ac, ADRP, Nsp1 and Nsp2) or the pET-28a-derived pSmart-I vector with an N-terminal SUMO (small ubiquitin-related modifier) tag (Smart-lifesciences, Changzhou, China; for ORF3C and E). The oligonucleotide primer sequences and approaches used are available upon request. The sequences of all constructs were confirmed by DNA sequencing (Huada Gene Technology Co., Ltd).
The above recombinant plasmids were transformed into BL21 (DE3) competent cells, respectively, and grown in 1 L of Luria-Bertani (LB) medium (Invitrogen) containing 100 μg/ml kanamycin at 37 °C with shaking at 220 rpm. When an OD600 of 0.6 was reached, 1 M isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to the final concentration of 0.5 mM in the 1 L of LB medium, and bacteria were grown for an additional 14 h at 20 °C. Cells were chilled at 4 °C and harvested by centrifugation at 5000 ×g for 5 min, resuspended in 30 ml lysis buffer (20 mM Tris−HCl, pH 8.0), and disrupted by ultrasonication. Crude extracts were centrifuged at 10,000 ×g for 10 min at 4 °C, and soluble expression of the His-tagged fusion peptides was confirmed by SDS-PAGE analysis prior to purification by the Ni-NTA His•Bind® Resin system (Transgen Tech, DP101, Beijing, China) according to the manufacturer’s instructions. For the polypeptides that were expressed in inclusion bodies, they were first solubilized in a denaturing buffer (20 mM Tris−HCl, with 8 M urea, pH 8.0), and purified by Ni-chelating chromatography (GE Healthcare). Elutions were pooled, dialyzed at 4 °C against 20 mM PBS (pH 7.4) with 150 mM NaCl and 4 M urea, and analyzed by SDS-PAGE and western blot.
2.5. Generation of rabbit polyclonal antibodies against recombinant PEDV proteins
Five purified, recombinant PEDV peptides (ORF3C, Ac, ADRP, Nsp1, and Nsp2) were selected and separately used to immunize two New Zealand White rabbits, using a custom antibody production service at Hangzhou Belta-Biotechnology Co., Ltd. (Hangzhou, China). Rabbits were not immunized with recombinant E protein.
2.6. SDS-PAGE and western blot analysis
The purified recombinant PEDV S1, ORF3C, E, ADRP, Nsp1, Nsp2, and Ac peptides were resolved on a 5–10% Bis-Tris polyacrylamide gel (Invitrogen) by electrophoresis and subsequently transferred onto a polyvinylidene difluoride (PVDF) membrane. Proteins were then detected using an anti-6×His MAb (1:1000 dilution; Protein-tech, Wuhan, China) or rabbit polyclonal antisera at 4 °C, followed by incubation with HRP-conjugated goat anti-mouse or anti-rabbit IgG (1:10,000 dilution; Thermo Fisher Scientific, USA), as appropriate, at room temperature. After four washing steps using Tris-buffered saline with 0.05% Tween 20 (TBST), membranes were analyzed using an enhanced chemiluminescence kit (Beyotime ECL Plus Kit, China).
For serum western blot analysis, purified S1, ORF3C, E, and Ac peptides were incubated after SDS-PAGE and membrane transfer with individual porcine sera diluted 1:1000, or with anti-His MAb or polyclonal rabbit serum as positive controls. HRP-conjugated rabbit anti-swine IgG and goat anti-mouse IgG (1:10,000 dilution; Abcam, United States) were used, as appropriate, as secondary antibodies.
2.7. Indirect ELISA
Antigen concentration and dilutions of sera and HRP-conjugated antibodies were optimized by checkerboard titrations. The optimal amount of PEDV WV, S1, ORF3C or E antigen used for coating was 7.8, 0.44, 1.56 or 0.78 ng/well/100 μL.
Microtiter plates were blocked with 300 μl/well blocking buffer (Thermo Fisher Scientific, USA) for 1.5 h at 37 °C. After coating, 100 μl of serum samples (1:100 dilution) were transferred in triplicate and incubated at 37 °C for 2 h. Afterwards, 100 μl diluted HRP-conjugated goat anti-swine IgG or IgA (1:10,000 dilution; Thermo Fisher Scientific, USA) was added to each well and incubated at 37 °C for 1 h. Wells were washed between incubation steps three times with 300 μl PBS (10 mM, pH 7.4) with 0.05% Tween-20 (PBS-T washing buffer). Finally, 100 μl TMB Color liquid (Solarbio, Beijing, China) was added to each well and incubated for 10 min at room temperature, after which the reaction was stopped by addition of 50 μl/well of 2 M sulfuric acid, and the plates were read at 450 nm using a spectrophotometer.
Initial PEDV-negative sera were obtained from the United States (a gift from Dr. Tanja Opriessnig) that was subsequently used for screening negative porcine sera in China as reported previously (Gerber et al., 2014). The ELISA positive cutoff values were calculated as the mean OD of negative controls (n = 4) plus three standard deviations. The positive and negative sera from experimentally-infected piglets were also confirmed by western blot on purified PEDV WV and S1 protein antigens as previously described (Huang et al., 2011). Positive and negative controls were run in duplicate on each ELISA plate.
Antibody response in all tested samples was represented as a corrected sample-to-positive (S/P) ratio, calculated as follows: S/P ratio = (sample OD – mean OD, negative controls) / (mean OD, positive controls – mean OD, negative controls).
2.8. Immunofluorescence assay (IFA)
Vero cells were seeded in confluent monolayers in 96-well plates (Co Star™, Corning) for 24 h, then infected with 100 μl/well of PEDV (105 TCID50/ml) for 2 h at 37 °C, followed by removal of the inoculum. MEM supplemented with 0.5% (w/v) trypsin (MMT) was added to each well and incubated at 37 °C for 48 h, then cells were fixed with 4% paraformaldehyde.
For specific detection of PEDV proteins, different rabbit anti-PEDV polyclonal antibodies were used as appropriate, with a mouse anti-PEDV S1 MAb (Cat no: 9191, JBT, Korea) used as a positive control. Secondary Alexa Fluor 488-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Invitrogen) were used at a 1:1000 dilution, incubated for 1 h at room temperature. Plates were washed three times between antibody incubations with 300 μl/well of PBS-T. Nuclei were stained with 4’, 6-diamidino-2-phenylindole (DAPI; KPL, Inc.) at a 1:1000 dilution, and visualized under a fluorescence microscope.
2.9. Serum neutralization (SN) test
Sera from challenged piglets were tested for neutralizing antibodies (NA), according to the protocol with slight modification (Kusannagi et al., 1992). Briefly, serum samples were inactivated at 56 °C for 30 min and then 2-fold serial dilutions (1:4˜1:512) were prepared in 96-well plates. After mixing 50 μl of each dilution with 50 μl PEDV (105 TCID50/ml), samples were incubated for 1 h at 37 °C and used to infect monolayers of Vero cells in 96-well plates. After adsorption for 2 h at 37 °C, the inoculum was discarded, plates were washed three times with MEM, and maintenance medium (containing 5 μg/ml trypsin) was added to each well. After incubation at 37 °C for 48 h, cells were observed on an inverted microscope for CPE such as cell fusion and nuclear atrophy. SN titers were calculated using the Reed and Muench method and expressed as the reciprocal of the highest serum dilution resulting in 50% inhibition of PEDV infection, relative to controls.
2.10. Data analysis
All data were processed using SPSS (version 20.0) software and the GraphPad Prism program as described previously (Gimenez-Lirola et al., 2017; Huang et al., 2012).
The cutoff value and diagnostic performance of each PEDV antigen was determined by receiver operating characteristic (ROC) analysis (SAS Version 9.4, SAS Institute, Inc., Cary, NC, USA) based upon the ELISA results.
3. Results
3.1. The purified PEDV whole virus shows pleomorphism
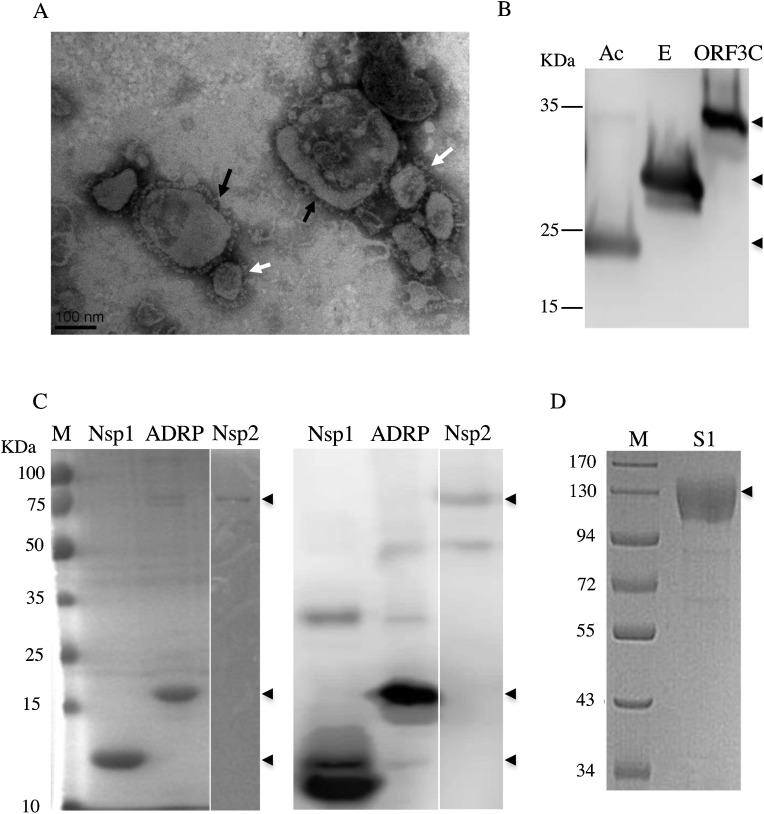
PEDV-ZJU/G2/2013 strain was propagated in Vero cells and purified by ultracentrifugation on a sucrose-gradient. Electron microscopy revealed that the purified virus was comprised of a great number of vesicles with morphological heterogeneity and envelope fragments carrying spikes (Fig. 1 A). Previously, the virions of several coronaviruses such as transmissible gastroenteritis virus (TGEV), turkey and bovine enteric coronaviruses have been observed with a diameter ranging between 60–220 nm (Dea and Garzon, 1991). To our knowledge, the pleomorphic property of PEDV virions containing not only the small or defective particles, but also the giant spherical particles ranging up to 350 nm in diameter, is reported here for the first time. Although a few of the PEDV particles had lost partial spikes, they were relatively intact. As spike glycoproteins are known to be the most immunogenic proteins of coronaviruses (Chen et al., 2016), purification by ultracentrifugation through sucrose cushions in this study proved to be reliable. Also, the level of PEDV protein was relatively high as detected by BCA protein kits, thus confirming that the quality, integrity and quantity of the purified virions were sufficient for use as the antigen in subsequent ELISA assays.
Fig. 1.
Characterization of the PEDV antigens. (A) Electron micrograph of the purified PEDV whole virus. Negatively-stained PEDV particles showing pleomorphism are labeled with black arrows whereas the “regular” virions (rounded or elongated shaped) are marked by white arrows. The bar represents 100 nm. (B) Western blot analysis of recombinant PEDV Ac, E and ORF3C peptides. Expression of PEDV Ac, E and ORF3C peptides in bacteria was confirmed with an anti-His monoclonal antibody (MAb). Approximately 150 ng of each protein were separated by SDS-PAGE and subjected to western blot. (C) SDS-PAGE and western blot analysis of recombinant PEDV Nsp1, ADRP and Nsp2 peptides. Expression of PEDV Nsp1, ADRP and Nsp2 peptides was identified using SDS-PAGE (left panel) and confirmed with an anti-His MAb (right panel). Approximately 100 ng of each protein were separated by SDS-PAGE and subjected to western blot. An HRP-conjugated goat anti-mouse IgG was used as the secondary antibody. (D) SDS-PAGE of the recombinant PEDV S1 peptide. The arrowheads indicate bands of the target proteins.
3.2. Characterization of six recombinant PEDV proteins shows consistency with their predicted sizes
Initially, we failed to detect the expression of the complete ORF3 using the pET-28a, the pSmart or the other bacterial expression vectors under different conditions by western blot analysis (data not shown). Therefore, the 127-aa of the C terminal part of ORF3 (ORF3C) showing the predicted hydrophilicity profile was chosen as the target antigen for ORF3.
The Ac, E and ORF3C recombinant peptides were expressed in the inclusion bodies, whereas the Nsp1, ADRP and Nsp2 proteins displayed soluble expression in the cultured supernatants. Expression yields of Ac, E, and ORF3C were very low, hardly visible, when examined by Coomassie blue staining after SDS-PAGE (data not shown); but confirmation of these three peptides with predicted sizes (Ac: ˜23 KDa; E fused with a SUMO tag: ˜28 KDa; ORF3C fused with a SUMO tag: ˜34 KDa) could be done using an anti-His-tag antibody by western blot (Fig. 1B). On the other hand, SDS-PAGE and western blot analyses of purified Nsp1, ADRP and Nsp2 soluble proteins showed bands that were consistent with the predicted sizes of 13, 18 and 87 KDa, respectively (Fig. 1C). The purified S1 protein expressed in mammalian cells was also identified as a single band by SDS-PAGE (Fig. 1D) and by western blot using an anti-S1 monoclonal antibody as described previously (Gerber et al., 2014). Due to glycosylation of the S1 protein, the size of the band in the gel was larger than its predicted size (˜86 kDa).
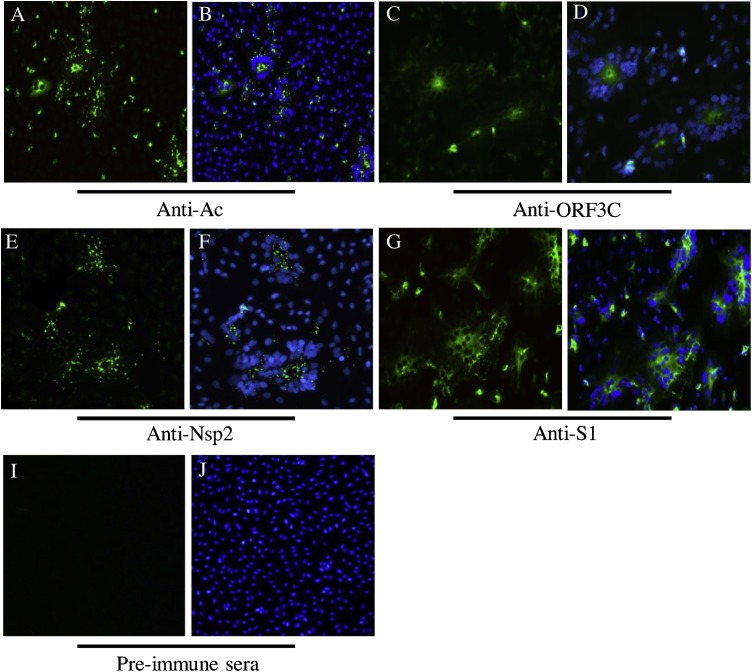
3.3. Antibodies generated against recombinant PEDV Ac, ORF3C, and Nsp2 proteins resulted in specific fluorescence in vitro
Purified recombinant PEDV peptides (ORF3C, Ac, ADRP, Nsp1, and Nsp2) were used to immunize rabbits, generating polyclonal sera that were used to detect viral proteins on PEDV-infected Vero cells by IFA. The E protein was not used to immunize rabbits in this study. Staining of anti-Ac polyclonal serum resulted in specific fluorescence at 48 h post-infection (Fig. 2 A, B) similar to the signal observed using the anti-S1 MAb as the positive control (Fig. 2G, H). Specific fluorescence was also detected with the anti-ORF3C (Fig. 2C, D) and the anti-Nsp2 polyclonal antibodies (Fig. 2E, F). In contrast, no specific fluorescence was observed when using the anti-ADRP and anti-Nsp1 polyclonal antibodies. IFA with pre-immune rabbit sera displayed no fluorescent signal in Vero cells infected with PEDV (Fig. 2I, J). The viral antigens were all detected in the cytoplasm of the infected cells. The anti-S1 Mab reacted more strongly than the three positive rabbit antisera based on a comparison of the positive cell numbers and fluorescence intensities.
Fig. 2.
Immunofluorescence assay (IFA) of PEDV-infected Vero cells. Vero cells were infected with PEDV and stained with rabbit anti-PEDV-Ac (A, B), anti-PEDV-ORF3C (C, D), or anti-PEDV-Nsp2 (E, F) antibodies at 48 h post-infection. A mouse anti-S1 monoclonal antibody (G, H) and pre-immunization rabbit serum (I, J) were used as positive and negative controls, respectively. AlexaFluor 488-conjugated goat anti-rabbit IgG and goat anti-mouse IgG (green) were used as secondary antibodies, as appropriate. Antibody staining merged with DAPI nuclear staining (blue) is shown; magnification = 20× (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Infection of Vero cells or Vero cells expressing the entry receptor porcine APN with the other swine enteric coronaviruses (Wang et al., 2018), such as swine enteric alphacoronavirus (Pan et al., 2017), porcine deltacoronavirus (PDCoV) and TGEV, had no detectable fluorescence after IFA with the anti-PEDV polyclonal antibodies described above (data not shown). Therefore, anti-PEDV-Ac, anti-PEDV-ORF3C and anti-PEDV-Nsp2 are PEDV-specific and do not cross-react with these known porcine coronaviruses.
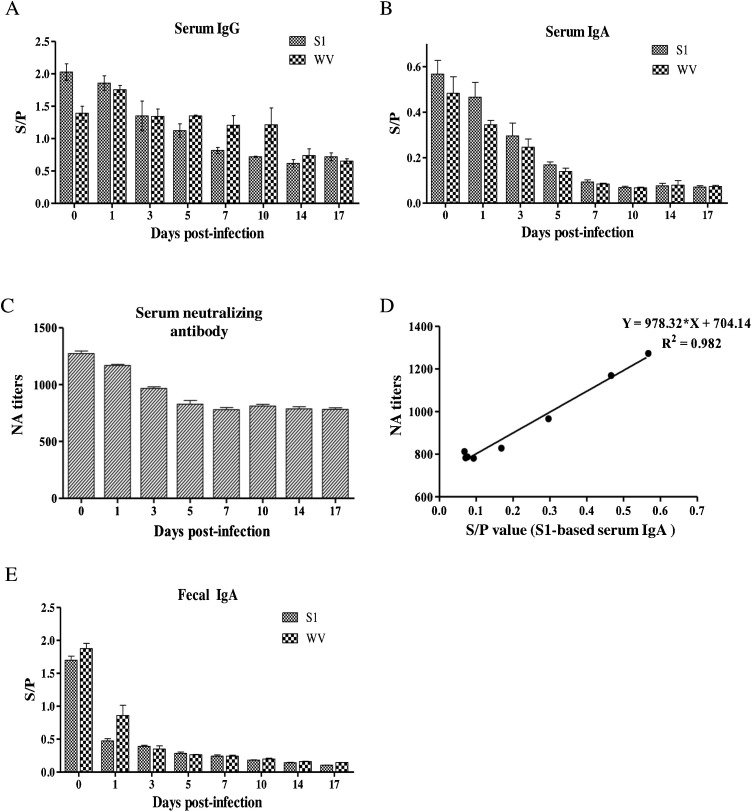
3.4. IgG and IgA responses in PEDV-infected weaning piglets varied over time
Previously, we have developed and validated indirect ELISA based on the S1 protein to monitor serum anti-PEDV IgG and serum and fecal anti-PEDV IgA antibodies in postweaning pigs (Gerber et al., 2014; Gerber and Opriessnig, 2015). In this study, in order to determine the pattern of antibody response of weaning piglets in a 17-day weaning period after PEDV infection, serum or fecal samples from experimentally-infected 3-day-old piglets were examined by ELISA based on the PEDV WV or the S1 protein, and by serum neutralization test (Fig. 3 ). The results indicated that IgG and IgA responses against both antigens were detected in serum at different time points after PEDV infection (Fig. 3A and B). Despite challenge with PEDV in these piglets, levels of serum IgG and IgA decreased from 1 dpi, reaching a minimum after 7 dpi as detected by both WV and S1 antigens (Fig. 3A, B), and the pattern or trend of neutralizing antibody (NA) was more similar to that of IgA (Fig. 3C). A good linear relationship between the S1-based IgA ELISA titers and NA titers was observed (Spearman’s rank correlation coefficient of 0.98; p < 0.001), demonstrating the correlation between them (Fig. 3D). There were some differences in the sensitivity of the antigens to detect antibodies, as levels of serum IgA were slightly higher when the S1 protein was used as the detection antigen. Levels of fecal IgA were also highest before challenge (0 dpi), and continuously declined after challenge (Fig. 3E). The specificity and sensitivity of detection of S1 and WV antigens were similar for serum IgA. The high IgG and IgA antibodies and NA detected at the early stages of the weaning piglets are presumably maternal antibodies received from sows that were not PEDV negative. In addition, piglets during weaning have not developed their own immunity to the virus. These results also demonstrated that the S1-based ELISA is an alternative (to WV-based) and ideal serological assay for detection of anti-PEDV antibodies.
Fig. 3.
ELISA detection of IgG, IgA, and neutralizing antibodies in the serum (A–D) and IgA in fecal samples (E) of weaning piglets experimentally infected with PEDV. ELISA assay based on the PEDV whole virus (WV) or S1 protein was used to measure IgG (A), IgA (B) in serum samples, and IgA in fecal samples (E). Plots represent mean ± SD of 11 samples at each time point. (C) The NA titers were expressed as the reciprocal of the highest serum dilution resulting in the 50% inhibition of PEDV infection relative to controls. (D) Scatter plots showing a correlation between the serum S1-based IgA antibodies and the serum NA (the mean values used for comparison).
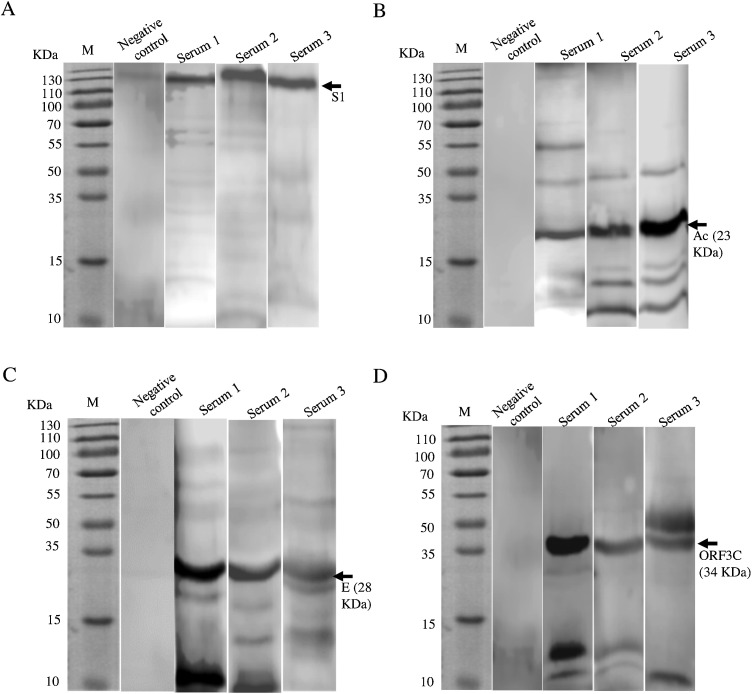
3.5. Antibodies against specific PEDV peptides were detectable in sera from naturally infected pigs
Recombinant PEDV S1, ORF3C, E, Ac and Nsp2 peptides were used as antigens in western blots to detect antibodies in porcine sera from commercial farms since they produced antibodies in rabbits reacted to PEDV antigens in infected cells except for the E protein that was not tested (Fig. 2). The criterion for determining the seropositivity to a particular antigen of a sample was whether the expected protein band was presented on the membrane. According to this, serum western blot analysis of the anti-Nsp2 antibody showed high-background results and thus further investigation of Nsp2 was not performed.
A main specific band appeared for S1, Ac, E or ORF3C protein antigen tested (arrows indicated in representative samples in Fig. 4 ), though additional “fuzzy” bands were present at low or high molecular weights that were likely from nonspecific reactivity of serum components. Compared with the other proteins, the S1 band appeared to be more specific, with a cleaner background (Fig. 4A). PEDV-positive sera were screened and verified viathis method, which were used to compare with the results of the ELISA (Fig. 5 ; see below), whereas negative sera from S1-based ELISA were also analyzed as negative controls, and none of them showed reactivity (Fig. 4, the second lanes in all panels). However, in the case of Ac, the specific 23-KDa bands also appeared in some sera that were negative by ELISA (data not shown).
Fig. 4.
Detection of antibodies against PEDV peptides in porcine sera by western blot. Representative serum samples from diarrheic pigs were used in western blots to detect PEDV S1 (A), Ac (B), ORF3C (C), and E proteins (D); 5 μg of recombinant peptides were loaded in each lane. HRP-conjugated goat anti-porcine IgG was used as the secondary antibody, and PEDV-negative serum was used as negative control.
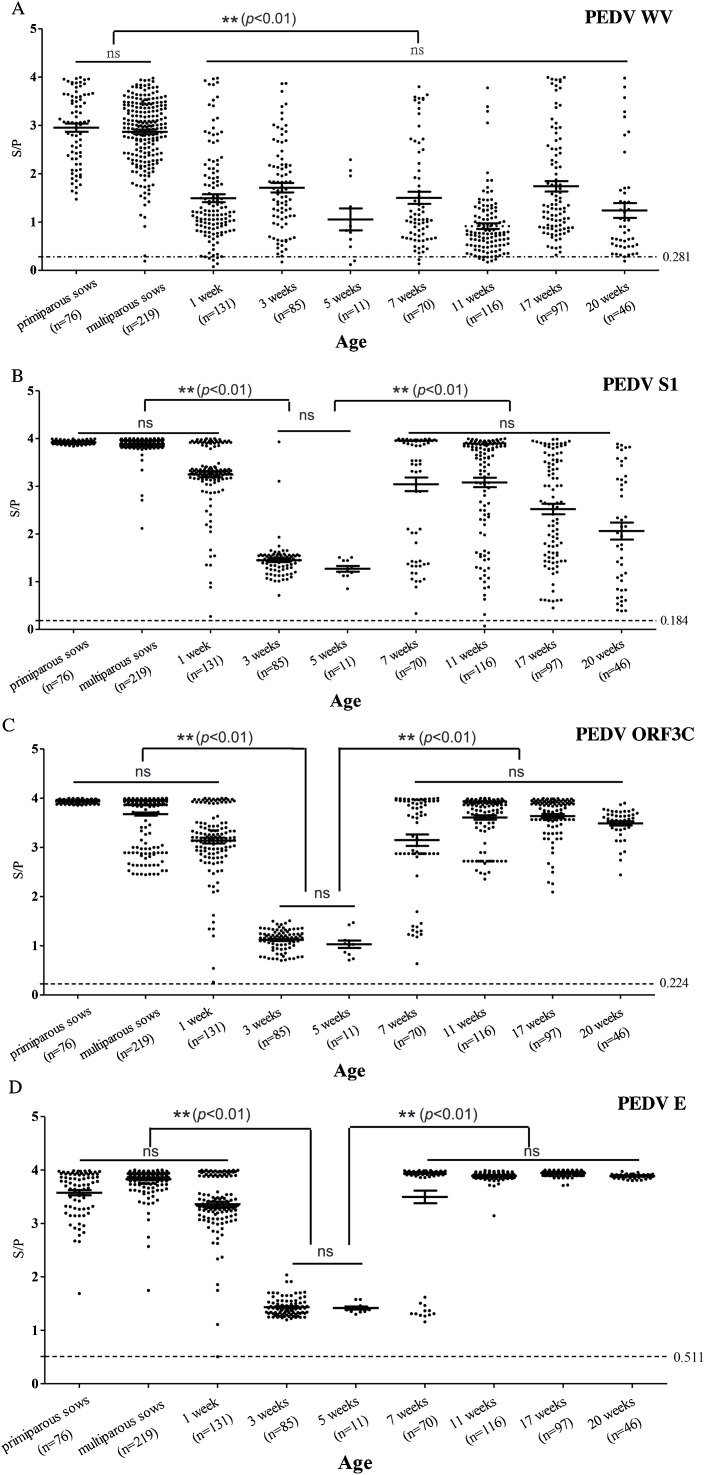
Fig. 5.
Distribution of cumulative IgG ELISA sample-to-positive (S/P) ratios in serum samples collected from diarrheic pigs at commercial pig farms. Various indirect ELISA assays based on PEDV whole virus (WV) (A), S1 (B), ORF3C (C), and E proteins (D) were used to test serum samples from commercial sows and pigs with diarrhea at different ages with unknown PEDV infectious status. Sera from naïve piglets were used as negative controls; samples above the determined S/P cutoff (dashed line) were considered positive. One-way analysis of variance (ANOVA) was used for multiple comparisons between different ages among the individual antigens with an alpha value of 0.01. The “ns” denoted no statistical differences.
3.6. Sera from diarrheic pigs of different ages had variable reactivity with PEDV antigens
Furthermore, the specific IgG antibody responses in sera from a total of 851 diarrheic pigs from farms in China were analyzed using ELISA assays based on PEDV WV, S1, ORF3C or E proteins (Fig. 5A–D). The results indicated good correlations among these antigens. Overall, the levels of antibodies against PEDV WV and the three proteins antigens were generally higher in primiparous and multiparous sows than those in 3–5 weeks-old pigs (p < 0.01). The trend of serum IgG response to each PEDV antigen from the 1-week-old to the 3-week-old pigs was similar to that of the experimentally-infected weaning piglets (Fig. 3A). Antibodies were highest in the 1 week-old piglets (p < 0.01) except for WV antigen, and then declined to a minimum by 5 weeks-of-age significantly (p < 0.01) before increasing again to a relatively stable level from 7 to 20 weeks-of-age (Fig. 5B,C; p < 0.01). This pattern was in agreement with a recent report about the prevalence of PEDV antibodies in swine farms (Bertasio et al., 2016), which might reflect that newborn piglets receive maternal IgG antibodies from sows, though the level of the antibodies decline quickly, and the weaned piglets must begin to develop their own immunity to PEDV.
However, there were some differences in reactivity to the antigens used in the ELISAs. IgG detected by WV-based ELISA had a similar pattern to that detected by the other antigens, as the antibodies were in a relatively dispersed distribution, although its cutoff value (0.281) was closest to the ORF3C ELISA (0.224). Antibody levels against the PEDV E protein were higher with a relatively concentrated distribution, especially in pigs older than 7 weeks. However, the E protein-based ELISA had the highest cutoff value (0.511), and the distribution of antibodies from 1 to 20 weeks-of-age was obviously different from the S1-based ELISA, which had the lowest cutoff (0.184). Collectively, the data indicated that the PEDV S1 protein was more specific and accurate as a detection antigen in ELISA tests.
4. Discussion
With the introduction of PEDV into the North American herd in 2013–2014 (Huang et al., 2013; Tian et al., 2014), the need for a suitable diagnostic marker for the accurate, rapid, and early diagnose of PEDV infection has become much more urgent. PEDV herd-infection status is very important for biosecurity and the control of PED. Compared to RT-PCR and other nuclei acid detection assays, serological tests are advantageous to study immune responses related to vaccination, wild-type virus infection, and to determine whether sow immunity is adequate in individual litters after PEDV exposure (Diel et al., 2016). PEDV infection is not always obvious in finishing pigs, which increases the risk of widespread disease in pigs of all ages (Bertasio et al., 2016). Thus, sensitive serological tests would allow detection of recent infections, to avoid the introduction of these animals into naïve herds. However, there are many different structural and non-structural proteins to choose from when selecting an antigen to use in novel diagnostic assays. Previously, we have developed and validated indirect ELISA based on the S1 protein to detect anti-PEDV IgG and IgA antibodies in postweaning pigs (Gerber et al., 2014). The present study was set up to investigate diagnostic potentials of specific PEDV accessory and nonstructural proteins, which have not yet been reported systematically.
The sera and feces from the experimental infected piglets at weaning were first used for detection of antibodies using the PEDV WV and S1 protein-based indirect ELISAs. The pattern of change in anti-PEDV IgG/IgA from serum samples and in anti-PEDV IgA fecal samples (immediate decline post-infection) indicates that the piglets had obtained maternal antibodies at birth, and did not produced antibodies even after PEDV challenge until their immune system matured. Additionally, levels of NA were more closely correlated with IgA than IgG (Fig. 3B-D), as seen in other studies (Paudel et al., 2014). Abundant anti-PEDV NA have been demonstrated in colostrum on the day of birth, decreasing rapidly in milk by day 3, and gradually declining further from days 3–19 post-farrowing, which may contribute to variable protective capacity (Song et al., 2016). The current study showed a similar decline, further confirming the reliability of the S1-based ELISA assays applicable to weaning pigs in addition to postweaning pigs (Gerber et al., 2014; Gerber and Opriessnig, 2015), and also highlighting the importance of accurate diagnosis in a short window for proper immunization of sows and piglets.
Compared to the PEDV WV, S1-based assays showed good reactivity and high sensitivity/specificity (Figs. 3, 4A and 5 B). It is worth noting that the recombinant S1 protein was expressed in a eukaryotic expression system and should display a natural conformation with high glycosylation, as shown in Fig. 1D, which may be one reason for its higher detection sensitivity. On the other hand, WV was mainly purified by sucrose density gradient centrifugation, differential centrifugation or polyethylene glycol (PEG) precipitation (Hoffmann and Robert, 1990). These methods would damage the integrity of the virus, especially the surface spike glycoprotein. Therefore, the eukaryotic-expressed S1 protein is likely more advantageous than the WV as the antigen for PEDV serological assays. For another two major structural proteins M and N, due to common epitopes shared by PEDV, TGEV and PDCoV, several studies have previously demonstrated that PEDV M or N presented some cross-reactivity to TGEV or PDCoV (Gimenez-Lirola et al., 2017; Lin et al., 2015; Ma et al., 2016). In contrast, recombinant PEDV-S1 had no cross-reactivity with sera from these porcine coronaviruses, showing the best diagnostic sensitivity (Gimenez-Lirola et al., 2017). Therefore, we did not pursue the development of serological assays based on M or N proteins in this study.
The accessory ORF3 protein is thought to have high potassium channel activity and may be associated with the virulence of PEDV (Wang et al., 2012). The small structural E protein has important roles in the assembly of coronavirus virions, virus egress and in the host stress response (Ruch and Machamer, 2012). Besides the structural proteins, PEDV has several non-structural proteins (Nsp1, Nsp2, Nsp3, among others) that express in the early stage of virus infection and have important functions in the viral replication cycle. The coronavirus Nsp3 is a conserved component of the viral protein processing machinery, and may be incorporated in the virion viaits intimate association with viral RNA (Neuman et al., 2008). The Nsp3 is known as the largest replicase subunit, consisting of numerous distinct structural domains separated by disordered linkers. Some of these, such as the Ac and ADRP (macrodomain), are well conserved across all genera of coronaviruses, though there have been no reports about serological assays based on PEDV Ac and ADRP domains. Considering their potential use in the study of host immune response, these proteins were specifically included in the current study.
Recombinant ORF3C, E, Ac, ADRP, Nsp1 and Nsp2 peptides were expressed and purified (Fig. 1); however, only ORF3C, Ac and Nsp2 produced functional rabbit antibodies recognizing PEDV antigens in infected cells (Fig. 2; the anti-E was not generated and tested). Subsequently, they were used individually as detection antigens in western blot and/or indirect ELISAs to detect anti-PEDV IgG antibodies in sera from diarrheic pigs (Fig. 5). The PEDV WV and the recombinant S1 expressed from mammalian cells was used for comparison. The S1, ORF3C, and E peptides each reacted strongly with the sera, reflecting expected distributions of PEDV-specific antibodies. The reactivity of Ac, ADRP, Nsp1 and Nsp2 peptides was less pronounced (data not shown), thus they were discarded from consideration as novel diagnostic antigens. The generally high level of IgG against S1, ORF3C, and E proteins in old-age pigs was consistent with recent reports that anti-PEDV IgG in infected pigs persisted for over than 17 weeks after the onset of diarrhea symptoms (Lin et al., 2016). All antibody levels declined to their lowest levels at 3 weeks-of-age, which was consistent with the result from experimentally-infected piglets at weaning (Fig. 3). Western blot was implemented to confirm the ELISAs, with additional “fuzzy” bands appeared when the ORF3C, Ac and E were used as detection antigens, indicating non-specific recognition (Fig. 4). This may be related to differences in antigens used and/or the sensitivity of the assays for detecting anti-PEDV antibodies.
The ORF3C antigen has moderate immune reactivity as evidenced by staining of anti-ORF3C in PEDV-infected cells displaying specific fluorescence in IFA (Fig. 2C and D), by serum western blot (Fig. 4D), and by indirect ELISA (Fig. 5C). In future studies, we plan to employ the PEDV mutants with the ORF3 deletion generated by reverse genetics (Ji et al., 2018; Zhao et al., 2017) in comparison with the wild type PEDV for more detailed evaluation of the protein for use as a marker in diagnostic assays. The accessory protein E showed intermediate sensitivity in western blot and extremely high sensitivity in ELISA assays compared with the other antigens (Fig. 4C and 5 D). The ELISA sensitivity was too strong, as nearly all of the sera from sows and 0–1 week-old piglets were strongly positive, thus it could not properly reflect the trend of PEDV-specific antibodies. To our knowledge, this is the first report about the use of an ORF3- or E-based ELISA on such a large scale.
Of the non-structural proteins, Ac displayed strong immune reactivity (Figs. 2A and 4 B), suggesting that it may be released into circulation or is picked up by antigen-presenting cells (Hurst-Hess et al., 2015). Therefore, the study of anti-Ac antibodies may contribute to a better understanding of the detailed function of Nsp3. Our results also complement previous mass spec identification of Nsp3 within purified virions (Neuman et al., 2008).
In summary, this study is the first to dissect the range of antibody responses against PEDV during infection, using different assays (ELISA, western blot, SN) to comprehensively analyze PEDV antibodies in porcine sera from China. The results confirmed high PEDV prevalence in China (Sun et al., 2016). The antibody profiles provided by the study offer more reliable information on the host immune response to different viral proteins, and will be useful for design of vaccines that better stimulate protective immunity. Above all, our data identified that besides S1, the recombinant ORF3C and E proteins can also be used as diagnostic markers; but S1 represents greater sensitivity for a wide range of PEDV-specific antibodies.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by The National Key Research and Development Program of China (2016YFD0500102), The Key Research and Development Program of Zhejiang province (2015C02021), and The National Natural Science Foundation of China (31872488). We thank the professional editing service NB Revisions for technical preparation of the text prior to submission.
References
- Alvarez J., Sarradell J., Morrison R., Perez A. Impact of porcine epidemic diarrhea on performance of growing pigs. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai T., Saif L.J., Lu Z., Jung K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immun. 2015;168:193–202. doi: 10.1016/j.vetimm.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertasio C., Giacomini E., Lazzaro M., Perulli S., Papetti A., Lavazza A., Lelli D., Alborali G., Boniotti M.B. Porcine epidemic diarrhea virus shedding and antibody response in swine farms: a longitudinal study. Front. Microbiol. 2016;7:1–9. doi: 10.3389/fmicb.2016.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Thomas J.T., Giménez-Lirola L.G., Hardham J.M., Gao Q., Gerber P.F., Opriessnig T., Zheng Y., Li G., Gauger P.C., Madson D.M., Magstadt D.R., Zhang J. Evaluation of serological cross-reactivity and cross-neutralization between the United States porcine epidemic diarrhea virus prototype and S-INDEL-variant strains. BMC Vet. Res. 2016;12:70. doi: 10.1186/s12917-016-0697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S., Garzon S. Identification of coronaviruses by the use of indirect protein A-gold immunoelectron microscopy. J. Vet. Diagn. Invest. 1991;3:297–305. doi: 10.1177/104063879100300405. [DOI] [PubMed] [Google Scholar]
- Diel D.G., Lawson S., Okda F., Singrey A., Clement T., Fernandes M.H., Christopher-Hennings J., Nelson E.A. Porcine epidemic diarrhea virus: An overview of current virological and serological diagnostic methods. Virus Res. 2016;226:60–70. doi: 10.1016/j.virusres.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P.F., Gong Q., Huang Y.W., Wang C., Holtkamp D., Opriessnig T. Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. J. Vet. 2014;202:33–36. doi: 10.1016/j.tvjl.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P.F., Opriessnig T. Detection of immunoglobulin (Ig) A antibodies against porcine epidemic diarrhea virus (PEDV) in fecal and serum samples. MethodsX. 2015;2:368–373. doi: 10.1016/j.mex.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Lirola L.G., Zhang J., Carrillo-Avila J.A., Chen Q., Magtoto R., Poonsuk K., Baum D.H., Pineyro P., Zimmerman J. Reactivity of porcine epidemic diarrhea virus structural proteins to antibodies against porcine enteric coronaviruses: diagnostic implications. J. Clin. Microbiol. 2017;55:1426–1436. doi: 10.1128/JCM.02507-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Robert W. Enzyme-linked immunosorbent assay for the detection of porcine epidemic diarrhea coronavirus antibodies in swine sera. Vet. Microbiol. 1990;21:263–273. doi: 10.1016/0378-1135(90)90037-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Dickerman A.W., Piñeyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the united states. mBio. 2013;4:e00737–00713. doi: 10.1128/mBio.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Harrall K.K., Dryman B.A., Beach N.M., Kenney S.P., Opriessnig T., Vaughn E.M., Roof M.B., Meng X.J. Expression of the putative ORF1 capsid protein of Torque teno sus virus 2 (TTSuV2) and development of Western blot and ELISA serodiagnostic assays: correlation between TTSuV2 viral load and IgG antibody level in pigs. Virus Res. 2011;158:79–88. doi: 10.1016/j.virusres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Harrall K.K., Dryman B.A., Opriessnig T., Vaughn E.M., Roof M.B., Meng X.J. Serological profile of torque teno sus virus species 1 (TTSuV1) in pigs and antigenic relationships between two TTSuV1 genotypes (1a and 1b), between two species (TTSuV1 and -2), and between porcine and human anelloviruses. J. Virol. 2012;86:10628–10639. doi: 10.1128/JVI.00176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst-Hess K.R., Kuo L., Masters P.S. Dissection of amino-terminal functional domains of murine coronavirus nonstructural protein 3. J. Virol. 2015;89:6033–6047. doi: 10.1128/JVI.00197-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C.M., Wang B., Zhou J., Huang Y.W. Aminopeptidase-N-independent entry of porcine epidemic diarrhea virus into Vero or porcine small intestine epithelial cells. Virology. 2018;517:16–23. doi: 10.1016/j.virol.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusannagi K., Hiroyoshi K., Nunoya T., Ishikawa Y., Samejima T., Tajima M. Isolation and serial propagation of porcine epidemic diarrhea virus in cell cultures and partial characterization of the isolate.pDf. J. Vet. Med. Sci. 1992;54:313–318. doi: 10.1292/jvms.54.313. [DOI] [PubMed] [Google Scholar]
- Lin C.M., Gao X., Oka T., Vlasova A.N., Esseili M.A., Wang Q., Saif L.J. Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J. Virol. 2015;89:3332–3342. doi: 10.1128/JVI.03196-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.M., Saif L.J., Marthaler D., Wang Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016;226:20–39. doi: 10.1016/j.virusres.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang Y., Liang X., Oglesbee M., Krakowka S., Niehaus A., Wang G., Jia A., Song H., Li J. Two-way antigenic cross-reactivity between porcine epidemic diarrhea virus and porcine deltacoronavirus. Vet. Microbiol. 2016;186:90–96. doi: 10.1016/j.vetmic.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman B.W., Joseph J.S., Saikatendu K.S., Serrano P., Chatterjee A., Johnson M.A., Liao L., Klaus J.P., Yates J.R., 3rd, Wuthrich K., Stevens R.C., Buchmeier M.J., Kuhn P. Proteomics analysis unravels the functional repertoire of coronavirus nonstructural protein 3. J. Virol. 2008;82:5279–5294. doi: 10.1128/JVI.02631-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okda F., Liu X., Singrey A., Clement T., Nelson J., Christopher-Hennings J., Nelson E.A., Lawson S. Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of Porcine Epidemic Diarrhea Virus. BMC Vet. Res. 2015;11:180. doi: 10.1186/s12917-015-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Tian X., Qin P., Wang B., Zhao P., Yang Y.L., Wang L., Wang D., Song Y., Zhang X., Huang Y.W. Discovery of a novel swine enteric alphacoronavirus (SeACoV) in southern China. Vet. Microbiol. 2017;211:15–21. doi: 10.1016/j.vetmic.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel S., Park J.E., Jang H., Shin H.J. Comparison of serum neutralization and enzyme-linked immunosorbent assay on sera from porcine epidemic diarrhea virus vaccinated pigs. Vet. Res. Forum. 2014;34:218–223. doi: 10.1080/01652176.2014.979512. [DOI] [PubMed] [Google Scholar]
- Qin P., Li H., Wang J.W., Wang B., Xie R.H., Xu H., Zhao L.Y., Li L., Pan Y., Song Y., Huang Y.W. Genetic and pathogenic characterization of a novel reassortant mammalian orthoreovirus 3 (MRV3) from a diarrheic piglet and seroepidemiological survey of MRV3 in diarrheic pigs from east China. Vet. Microbiol. 2017;208:126–136. doi: 10.1016/j.vetmic.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch T.R., Machamer C.E. The coronavirus E protein: assembly and beyond. Viruses. 2012;4:363–382. doi: 10.3390/v4030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Stone S., Drebes D., Greiner L.L., Dvorak C.M., Murtaugh M.P. Characterization of anti-porcine epidemic diarrhea virus neutralizing activity in mammary secretions. Virus Res. 2016;226:85–92. doi: 10.1016/j.virusres.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Wang X., Wei S., Chen J., Feng L. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini-review. J. Vet. Med. Sci. 2016;78:355–363. doi: 10.1292/jvms.15-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P.F., Jin Y.L., Xing G., Qv L.L., Huang Y.W., Zhou J.Y. Evidence of recombinant strains of porcine epidemic diarrhea virus, United States, 2013. Emerg Infect Dis. 2014;20:1735–1738. doi: 10.3201/eid2010.140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Liu Y., Ji C.M., Yang Y.L., Liang Q.Z., Zhao P., Xu L.D., Lei X.M., Luo W.T., Qin P., Zhou J., Huang Y.W. Porcine deltacoronavirus engages the transmissible gastroenteritis virus functional receptor porcine aminopeptidase N for infectious cellular entry. J. Virol. 2018;92:e00318–318. doi: 10.1128/JVI.00318-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Lu W., Chen J., Xie S., Shi H., Hsu H., Yu W., Xu K., Bian C., Fischer W.B., Schwarz W., Feng L., Sun B. PEDV ORF3 encodes an ion channel protein and regulates virus production. FEBS Lett. 2012;586:384–391. doi: 10.1016/j.febslet.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Wang B., Ji C.M., Cong X., Wang M., Huang Y.W. Identification of a peptide derived from the heptad repeat 2 region of the porcine epidemic diarrhea virus (PEDV) spike glycoprotein that is capable of suppressing PEDV entry and inducing neutralizing antibodies. Antiviral Res. 2017;150:1–8. doi: 10.1016/j.antiviral.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]