Abstract
A pantropic canine coronavirus (CCoV) strain (CB/05) has been recently associated to a fatal outbreak of systemic disease in young dogs. We report the clinical, virological and serological findings in dogs experimentally infected with strain CB/05. The dogs, three 2.5-month-old and two 6-month-old pups, were successfully infected, shedding viral RNA with their faeces for the entire observation period (21 days) and displaying systemic clinical signs resembling those observed during the course of natural infection. Leucopenia (acute lymphopenia) occurred in all infected dogs, with values dropping below 60% of the initial counts. Considering the severity of the CB/05-induced disease, two of the youngest pups were euthanized for ethical reasons at days 8–9 postinfection, whereas the other pups underwent a slow but progressive improvement of their clinical status with complete recovery. At postmortem examination, remarkable lesions were observed in the internal organs of the euthanized pups, that tested positive for CCoV by real-time RT-PCR and virus isolation on cell cultures. All pups seroconverted for CCoV, as shown by the high optical density values and antibody titres detected by ELISA and virusneutralisation tests, respectively. The present study confirms that strain CB/05 is highly pathogenic for dogs, being able to induce a severe disease (and in some cases the death) even in experimental conditions.
Keywords: Dog, Pantropic coronavirus, Experimental infection, Systemic disease
1. Introduction
Coronaviruses (CoVs) are enveloped, single-stranded RNA viruses which included in three different antigenic groups. CoVs infecting dogs comprise canine enteric coronavirus (CCoV) (Enjuanes et al., 2000) and the newly recognised canine respiratory coronavirus (CRCoV) (Erles et al., 2003, Decaro et al., 2007a), belonging to group 1 and group 2 CoVs, respectively. Two CCoV genotypes have been identified so far, namely CCoV type I and CCoV type II, which are responsible for the occurrence enteritis in dogs and are frequently associated in mixed infections (Pratelli et al., 2004, Decaro et al., 2005c). Although its tropism is restricted to the gastroenteric tract, CCoV has been recently associated to systemic disease followed by fatal outcome in pups (Buonavoglia et al., 2006). Severe clinical signs were observed in the affected pups, whereas necropsy examination revealed remarkable gross lesions in lungs, liver, spleen and kidneys. Virological and bacteriological investigations failed to detect common canine pathogens. Unexpectedly, CCoV type II RNA was detected at very high titres in the internal organs of the dead pups and the virus (strain CB/05) was isolated on canine cell cultures. The association of strain CB/05 to a severe, sometimes fatal disease of dogs, together with the isolation of the virus from organs with severe lesions, strongly suggests that CCoV has changed its tropism, acquiring the ability to spread from the enteric tract to the internal organs (Decaro et al., 2007b).
In this study, we report the results of the experimental infection with isolate CB/05 in pups with different age, showing that in contrast with classical CCoVs, this virus is able to cause systemic disease followed by fatal outcome in younger pups.
2. Materials and methods
2.1. Cells and virus
Canine fibroma A-72 cells were grown in Dulbecco's minimum essential medium supplemented with 10% foetal calf serum. Strain CB/05 was isolated from the lungs of a dead pup (117/05-C) and adapted to growth on A-72 cells. At the 3rd passage, the virus was titrated on cell cultures and inocula containing 106.25 TCID50/ml of viral suspension were stored at −70 °C. Contaminations by other canine pathogens, such as canine parvovirus type 2 (CPV-2), canine distemper virus (CDV) and canine adenoviruses (CAdVs), were ruled out by specific molecular assays (Hu et al., 2001, Decaro et al., 2005b, Elia et al., 2006).
2.2. Experimental study
The experimental study was performed according to the animal health and well-being regulations and was authorised by the Ministry of Health of Italy (authorization no. 53/2005-C). Six mixed-bred female dogs including four 2.5-month-old (n = 1–4) and two 6-month-old (n = 5, 6) pups were housed at the “Infectious Disease Unit” of the Animal Hospital, Faculty of Veterinary Medicine of Bari. The dogs had tested negative for CCoV RNA by a real-time RT-PCR assay (Decaro et al., 2004) carried out on the faeces and for CCoV antibodies by an ELISA test (Pratelli et al., 2002) carried out on serum samples. All dogs were housed individually in separate boxes, fed twice daily with a commercial dry dog food and provided water ad libitum. After an acclimatization period of 5 days, 5 animals (n = 1, 2, 3, 5, 6) were administered oronasally 3 ml of a viral suspension of strain CB/05, with a titre of 106.25 TCID50 and 7.85 × 107 RNA copies per ml, whereas one dog (n = 4), 2.5-month-old, was maintained uninfected by oronasal administration of 3 ml of sterile saline solution.
2.3. Clinical score, virus isolation and real-time RT-PCR
The clinical condition of each dog was monitored daily for 21 days. A scoring system was devised taking into account rectal temperatures, total white blood cell (WBC) counts, appearance of clinical signs (vomiting, diarrhoea, depression, loss of appetite, dehydration), following the scheme adopted in a previous study (Decaro et al., 2005a) and derived by Nakamura et al. (2001), with some modifications (Table 1 ). Due to ethical reasons, dogs whose total clinical score reached a value ≥15 were euthanized by intravenous administration of 10 mg/kg of body weight of Zoletil 100 (Virbac S.r.l., Italy) followed by 0.5 ml/kg body weight of Tanax (Intervet Italia, Italy).
Table 1.
Scoring system for clinical signs of CB/05 infectiona
| Clinical signs | Daily score |
|---|---|
| Rectal temperature (°C) | |
| ≤37.6 | 3 |
| 37.7–38.7 | 0 |
| 38.8–39.7 | 1 |
| 39.8–40.0 | 2 |
| ≥40.1 | 3 |
| WBC countb | |
| 75–60 | 1 |
| 59–45 | 2 |
| 44–30 | 3 |
| ≤30 | 4 |
| Diarrhoea | |
| Mucoid | 1 |
| Fluid | 2 |
| Dysenteric | 3 |
| Haemorrhagic | 4 |
| Loss of appetite | |
| Dysorexia | 1 |
| Anorexia | 3 |
| Vomiting (episodes/day) | |
| 1 | 1 |
| >1 | 3 |
| Depression | |
| Mild | 1 |
| Moderate | 2 |
| Severe | 3 |
| Dehydration | |
| Mild | 1 |
| Moderate | 2 |
| Severe | 3 |
Adapted from Decaro et al. (2005a).
Values are expressed as percentages of the WBC counts determined prior to inoculation.
EDTA-treated blood samples were collected daily for total and differential WBC counting and for testing for CCoV RNAemia by real-time RT-PCR (Decaro et al., 2004). The presence of the viral RNA in the blood was also evaluated at hours 3, 6, 9, 12 and 18 after inoculation. Plasma samples were prepared daily to evaluate free viral RNAemia and weekly to determine CCoV antibody titres by virus neutralisation (VN) and ELISA tests (Pratelli et al., 2002).
To evaluate the viral shedding in the faeces, the rectal swabs collected daily from the control dog and from the dogs inoculated with strain CB/05 were subjected to RNA extraction using QIAamp® Viral RNA Mini Kit (Qiagen S.p.A.). In addition, tissue samples from parenchymatous organs were withdrawn from the two dead pups (Table 2 ). RNA was extracted from the WBC pellets using QIAamp® RNA Blood Mini Kit (Qiagen S.p.A.), from the plasma samples using QIAamp® Viral RNA Mini Kit and from the tissue samples using QIAamp® RNeasy Mini Kit. Attempts to isolate the virus in A-72 cells were carried out on rectal swabs of all infected pups and on organs of the sacrificed animals as described previously (Decaro et al., 2007b).
Table 2.
CCoV RNA titres in the organs of dogs succumbed to natural or experimental infection with strain CB/05
| 117/05-Aa | 117/05-Ba | 117/05-Ca | Dog 1b | Dog 3b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue/sample | RRT-PCR | VI | RRT-PCR | VI | RRT-PCR | VI | RRT-PCR | VI | RRT-PCR | VI |
| Faeces | 6.12 × 105 | + | 9.24 × 104 | + | 3.17 × 105 | + | 5.14 × 104 | + | 1.09 × 105 | + |
| Lung | 1.08 × 106 | + | 2.74 × 104 | + | 2.32 × 106 | + | 6.79 × 103 | − | 2.99 × 104 | + |
| Spleen | 4.46 × 106 | + | 1.20 × 105 | + | 6.87 × 106 | + | 5.48 × 104 | + | 3.07 × 104 | + |
| Liver | 9.02 × 104 | + | 5.98 × 104 | + | 3.12 × 105 | + | 1.93 × 104 | + | 4.70 × 103 | − |
| Kidney | 7.54 × 105 | + | 1.40 × 104 | + | 2.53 × 105 | + | 5.11 × 103 | − | 2.94 × 103 | − |
| Mesenteric lymph node | 8.09 × 104 | + | 2.01 × 104 | + | 5.17 × 104 | + | 2.19 × 104 | − | 3.61 × 104 | + |
| Brain | 5.23 × 103 | − | 9.52 × 102 | − | 1.25 × 104 | − | 3.32 × 102 | − | 6.00 × 102 | − |
RRT-PCR, real-time RT-PCR. Results are expressed as CCoV type II RNA copy number/μl of template. VI, virus isolation. Results are expressed as positive (+) or negative (−).
Dogs succumbed to natural infection (described by Decaro et al., 2007b).
Dogs euthanized after experimental infection (this study).
Real-time RT-PCR targeting the M gene of CCoV type II (GenBank accession number D13096) was carried out on the RNA extracts as described elsewhere (Decaro et al., 2005c). The genotype-specific RT-PCR assays were undertaken in an i-Cycler iQ™ Real-Time Detection System (Bio-Rad Laboratories Srl, Milan, Italy) and the data were analyzed with the appropriate sequence detector software (version 3.0). After reverse transcription, triplicates of the CCoV type II standard dilutions and RNA templates were simultaneously subjected to real-time analysis. The 50 μl reaction mixture contained 25 μl of IQ™ Supermix (Bio-Rad Laboratories Srl), 600 nM of each primers CCoVII-F (TAGTGCATTAGGAAGAAGCT) and CCoVII-R (AGCAATTTTGAACCCTTC), 200 nM of probe CCoVII-Pb (FAM-CCTCTTGAAGGTGTGCC-TAMRA) and 20 μl of c-DNA. The thermal profile consisted of activation of iTaq DNA polymerase at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 15 s, annealing at 48 °C (type II-specific assay) for 30 s and extension at 60 °C for 1 min.
2.4. Evaluation of antibody response to CCoV
Plasma samples from inoculated dogs were tested in parallel by virus neutralisation (VN) and ELISA tests (Pratelli et al., 2002).
For VN test, duplicates of serial twofold dilutions of heat-inactivated plasmas (starting from dilution 1:2) were mixed with 100 TCID50 of the isolated strain CB/05 in 96-well microtitre plates. After preincubation at room temperature for 90 min, 20,000 A-72 cells were added to each well. The plates were read after 4 days of incubation at 37 °C. VN titres were calculated with the Karber method and expressed as the highest plasma dilution that was able to neutralise the virus.
For ELISA, microtitre plates were coated with CCoV antigen (enteric strain S-378) and, after treatment with blocking solution (0.2% gelatin in carbonate buffer [15 mM Na2CO3, 35 mM NaHCO3, pH 9.6]) and repeated washing, the 1:50 dilutions of the plasma samples were added to each well. Then the plates were incubated for 90 min at 37 °C, washed four times and incubated for 60 min at 37 °C with anti-dog IgG-goat peroxidase conjugates (Sigma–Aldrich Srl, Milan, Italy). After another washing cycle, 10 mg of freshly prepared substrate, 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]diammonium salt (ABTS, Sigma–Aldrich Srl) in 50 ml of 0.05 M phosphate citrate buffer (pH 5.0) was placed in each well and the optical density at 405 nm (OD405) was determined. Positive and negative controls were used as described previously (Pratelli et al., 2002).
3. Results
3.1. Clinical signs and WBC counts
Neither clinical signs or viral shedding were observed in the control dog, whose leukocyte counts did not draw away from the baseline values. All the inoculated animals displayed severe clinical signs similar to those observed in dogs infected naturally, although the outcome of the disease was different on the basis of the age (Fig. 1 ). In fact, two out of the three youngest pups (dogs 1 and 3) had to be euthanized for ethical reasons, at days 8 and 9 postinfection (p.i.), respectively, while both the 6-month-old pups recovered from the disease, albeit very slowly. Irrespective of the final outcome, i.e, euthanasia or recovery, clinical signs were remarkably similar in all inoculated animals.
Fig. 1.
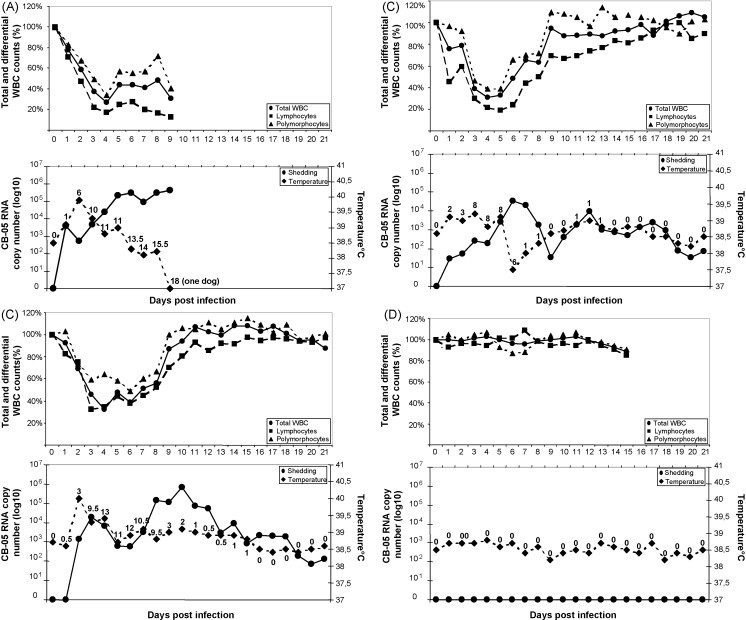
Disease progression in dogs infected experimentally with strain CB/05. (A) 2.5-Month-old dogs that were euthanized (dogs 1 and 3, means); (B) 2.5-month-old dog that survived (dog 2); (C) 6-month old dogs (dogs 5 and 6, means); (D) control dog (dog 2). Dogs inoculated oronasally with CB-05 strain were monitored for up to 21 days for total WBC, lymphocyte and polymorphocyte counts (top graphs). In addition, fever, viral RNA shed in faeces and clinical score where determined (bottom graphs). Total WBC, lymphocyte and polymorphocyte counts are presented as percentages of the cell counts determined at day 0. Viral RNA titres as determined by real-time RT-PCR are expressed as log copy numbers (log10) per μl of template. Clinical scores were calculated as shown in Table 1 and are reported for each day in correspondence of the temperature curves.
The 2.5-month-old dogs that underwent a fatal outcome (Fig. 1A) showed fever at days 1–2 p.i., with a peak of 39.9 °C at day 2 p.i. (dog 1), and from days 1 to 6 p.i., with a peak of 40.0 °C at day 3 p.i. (dog 3). Depression (days 3–8 p.i.), anorexia/dysorexia (days 3–9 p.i.), haemorrhagic diarrhoea (days 2–7 p.i.) and vomiting (days 4–5 p.i.) also occurred. Leukopenia appeared at day 3 (dog 1) or 2 p.i. (dog 3), with total WBC counts remaining below 60% of the baseline values until euthanasia. Acute lymphopenia was observed in both dogs, with lymphocyte numbers dropping below 60% of the initial counts from day 3 p.i. until death (mean, 16.3%; 0.9 × 103 lymphocytes/μl at day 8 p.i.). Postmortem examination revealed severe changes in the intestines and major organs, which were very similar to those observed in dogs infected naturally (data not shown).
The 2.5-month-old dog that survived (Fig. 1B) displayed less severe symptoms, consisting of fever (up to 39.2 °C) from days 1 to 5 p.i. and at days 11–12 p.i., and mucoid diarrhoea (days 2–6 p.i.). Total WBC counts dropped below the 60% of the initial counts from days 3 to 6 p.i., whereas severe lymphopenia (below 60% of baseline values) was registered from days 1 to 8 p.i., with a peak at day 5 (19%; 1.6 × 103 lymphocytes/μl). Subsequently, the total number of peripheral blood lymphocytes started to rise again and the clinical signs subsided. A mild loss of appetite occurred from days 1 to 6 p.i.
In the two 6-month-old pups (Fig. 1C), fever showed a biphasic course, with a first peak at day 2 p.i. of 39.8 and 40.1 °C in dogs 5 and 6, respectively. A transient remission was observed from days 5 to 8 p.i. (dog 5) and at day 5 p.i. (dog 6), and a second episode of pyrexia occurred from days 9 to 14 p.i. with a peak of 39.5 °C at day 10 p.i. (dog 5), and from days 6 to 8 p.i. with a peak of 39.4 °C at day 7 p.i. (dog 6). Depression was observed between days 3 and 8 p.i., whereas vomiting appeared only sporadically in the same period (days 3, 4, 8 p.i. in dog 5; days 1, 3, 8 p.i. in dog 6), with 1–4 episodes per day. Both dogs displayed anorexia (days 3–7 p.i.), mucoid or fluid diarrhoea (days 3–10), and leucopenia, with WBC values dropping below 60% of the baseline from days 3 to 6 (dog 5) or 3 to 8 p.i. (dog 6). Lymphocytes dropped below 60% of the initial cell counts from days 3 to 6 p.i. in dog 5 and from days 3 to 8 p.i. in dog 6 (mean, 32.2%; 1.6 × 103 lymphocytes/μl at day 3 p.i.). Starting from day 10 (dog 5) or 9 p.i. (dog 6), the clinical conditions of the dogs improved progressively, with a complete recovery at days 15–16 p.i.
The control (uninfected) pup (Fig. 1D) remained in a good clinical status during the entire observation period and no variations in total WBC and lymphocyte counts were observed.
3.2. Detection of CCoV RNA and CCoV antibodies
The uninfected dog tested constantly negative for CCoV RNA. All the CCoV-infected dogs tested negative for other common pathogens of dogs, including CCoV type I (Decaro et al., 2005c), CDV (Elia et al., 2006), CAdVs (Hu et al., 2001) and CPV-2 (Decaro et al., 2005b).
The faecal shedding of the infected dogs followed the similar pattern, although higher viral RNA titres were obtained from the two sacrificed animals in comparison to survivors (Fig. 1). The pups that succumbed shed virus starting between days 1 and 3 p.i. and lasting until the day of death, with a peak at day 6 (titre of 4.97 × 105 RNA copy numbers/μl of template) or 9 (titre of 8.72 × 105 RNA copy numbers/μl of template). After their death, CCoV type II RNA was detected in the organs at titres slightly lower than those observed in the dogs of the natural outbreak (Table 2). The 2.5-month-old pup that recovered shed CCoV RNA starting from day 1 p.i. (titre of 2.97 × 101 RNA copy numbers/μl of template) and lasting for the entire observation period (21 days), with a peak at day 6 p.i. (3.24 × 104 RNA copy numbers/μl of template). Shedding of CCoV in the faeces of the two 6-month-old dogs was observed from day 2 p.i. (mean titre, 1.40 × 103 RNA copy numbers/μl of template) to day 21 p.i. (last day of observation) reaching the maximal mean value of 6.79 × 105 RNA copy numbers/μl of template at day 10 p.i.
Surprisingly, CCoV RNA was never detected in the blood of the 6-month-old pups, as well as in the euthanized animals, in whose organs remarkable viral RNA titres were found. Traces of CCoV RNA were detected only in the blood of the survived 2.5-month-old pup between days 7 and 10 p.i., with plasma viral titres ranging from 5.54 × 100 to 9.30 × 101 RNA copies/μl of template.
The virus was successfully isolated on cell cultures from the rectal swabs of all inoculated dogs (data not shown) and from some organs of the euthanized pups (Table 2).
All the infected animals seroconverted for CCoV, whereas antibodies were not detected in the control dog (Fig. 2D). In the dogs that were euthanized the antibody titres were determined only at day 7 p.i., with VN titres of 1:8 and OD values of 0.089 (geometric means, Fig. 2A). In survivors, the maximal antibody titres were reached at days 14 and 21 p.i. by VN and ELISA test, respectively (Fig. 2B and C).
Fig. 2.
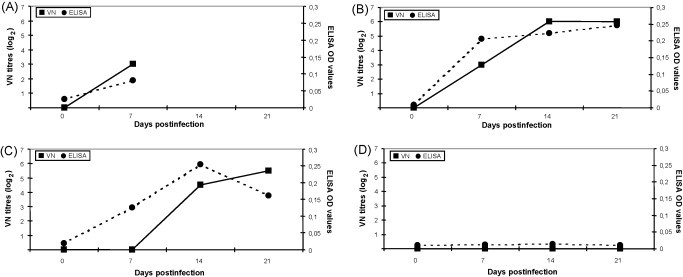
Antibody responses in dogs infected experimentally with strain CB/05. (A) 2.5-Month-old dogs that were euthanized (dogs 1 and 3, means); (B) 2.5-month-old dog that survived (dog 2); (C) 6-month-old dogs (dogs 5 and 6, means); (D) control dog (dog 4). Antibody responses are presented as geometric means of virus neutralising (VN) titres and ELISA optical density (OD) values.
4. Discussion
In a previous study, a pantropic variant of CCoV was associated to a fatal disease of dogs, characterised by leucopenia, gastroenteritis and severe changes in the internal organs (Buonavoglia et al., 2006). The disease induced by strain CB/05 in pups of the natural outbreak was reproduced in dogs infected experimentally. All inoculated dogs were successfully infected, as shown by the occurrence of faecal shedding, seroconversion and severe clinical signs. The viral excretion was similar to those observed during enteric CCoV infection (Decaro et al., 2004, Decaro et al., 2005c), but the course of disease was more severe, as clinical signs characteristic of systemic infection were observed in the infected dogs. The pantropism of the virus was confirmed by the presence of gross lesions in the internal organs of the dead dogs, as well as by the detection of viral RNA in those tissues. Interestingly, CCoV RNA was detected also in the brain of the dead dogs. In contrast, enteric CCoV has been never associated to systemic infection, although the virus has been isolated previously from some tissues (tonsils, lungs and liver) of experimentally infected pups (Tennant et al., 1991).
Two of the three inoculated 2.5-month-old pups had to be euthanized after few days of postinfection, whereas the other dogs (the remaining dog of the same age and the two dogs 6-month of age) recovered after a severe disease. Considering that dogs infected naturally were all between 45 and 56 days of age, it could be hypothesised that the age of the infected animals plays a role in determining the fate of CB/05 infection, with a very severe clinical course in the youngest pups.
Moreover, in the experimental infection, the organs of the dead dogs contained lower CCoV RNA titres with respect to dogs infected naturally, so that virus isolation was not obtained from all PCR-positive tissues. Despite the drop of the WBC counts registered in all infected dogs and the detection of the viral RNA in the internal organs of the sacrificed dogs, free or cell-associated CCoV RNAemia was not found at any time either in euthanized or survived dogs, with the exception of the recovered 2.5-month-old pup which showed very low RNA viral titres in the plasma between days 7 and 10 p.i. Thus, at this moment, the mechanisms of lymphopenia and viral spread to internal organs remained unknown. Albeit strange, our findings are compatible with those obtained from cats experimentally infected with feline infectious peritonitis virus (FIPV). Cats succumbed to FIPV display very high-viral titres in the haemolymphatic tissues (Kipar et al., 2006), in contrast with the low loads detected in the blood (de Groot-Mijnes et al., 2005). In our study, CB/05-infected dogs were found to contain lower viral loads in the lymphoid tissues in comparison to FIPV-infected cats, thus likely accounting for the undetected viral RNAemia in euthanized dogs.
In conclusion, we have confirmed the pantropism of strain CB/05, reproducing the natural disease even in experimental conditions. Further studies will contribute to better understand the epidemiological distribution and the pathogenetic mechanisms of the virus, including the possible involvement of the different lymphocyte classes.
Acknowledgements
This work was supported by grants from Ministero dell’Istruzione, dell’Università e della Ricerca: PRIN 2005 (N.D.), project “Il coronavirus del cane: aspetti molecolari e patogenetici”; PRIN 2006 (C.B.), project “Infezione del cane da coronavirus pantropico: aspetti epidemiologici, patogenetici e molecolari”.
References
- Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot-Mijnes J.D., van Dun J.M., van der Most R.G., de Groot R.J. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J. Virol. 2005;79:1036–1044. doi: 10.1128/JVI.79.2.1036-1044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Pratelli A., Campolo M., Elia G., Martella V., Tempesta M., Buonavoglia C. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J. Virol. Methods. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Desario C., Elia G., Martella V., Lorusso E., Buonavoglia C. Maternally-derived antibodies in pups and protection from canine parvovirus infection. Biologicals. 2005;33:259–265. doi: 10.1016/j.biologicals.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Desario C., Campolo M., Di Trani L., Tarsitano E., Tempesta M., Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs. Vet. Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Decaro N., Martella V., Ricci D., Elia G., Desario C., Campolo M., Cavaliere N., Di Trani L., Tempesta M., Buonavoglia C. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J. Virol. Methods. 2005;130:72–78. doi: 10.1016/j.jviromet.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Mari V., Lucente M.S., Cordioli P., Colaianni M.L., Martella V., Buonavoglia C. Serological and molecular evidence that canine respiratory coronavirus is circulating in Italy. Vet. Microbiol. 2007;121:225–230. doi: 10.1016/j.vetmic.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Elia G., Campolo M., Desario C., Cirone F., Tempesta M., Buonavoglia C. Molecular characterisation of the virulent canine coronavirus CB/05 strain. Virus Res. 2007;125:54–60. doi: 10.1016/j.virusres.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., Di Trani L., Buonavoglia C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods. 2006;136:171–176. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Brian D., Cavanagh D., Holmes K., Lai M.M.C., Laude H., Masters P., Rottier P., Siddell S., Spaan W.J.M., Taguchi F., Talbot P. Family Coronaviridae. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R., Wickner R.B., editors. Virus Taxonomy, Classification and Nomenclature of Viruses. Academic Press; New York: 2000. pp. 835–849. [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R.L., Huang G., Qiu W., Zhong Z.H., Xia X.Z., Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet. Res. Commun. 2001;25:77–84. doi: 10.1023/a:1006417203856. [DOI] [PubMed] [Google Scholar]
- Kipar A., Baptiste K., Barth A., Reinacher M. Natural FCoV infection: cats with FIP exhibit significantly higher viral loads than healthy infected cats. J. Feline Med. Surg. 2006;8:69–72. doi: 10.1016/j.jfms.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Sakamoto M., Ikeda Y., Sato E., Kuwakami K., Miyazawa T., Tohya Y., Takahashi E., Mikami T., Mochizuki M. Pathogenic potential of canine parvovirus types 2a and 2c in domestic cats. Clin. Diagn. Lab. Immunol. 2001;8:663–668. doi: 10.1128/CDLI.8.3.663-668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Elia G., Martella V., Palmieri A., Cirone F., Tinelli A., Corrente M., Buonavoglia C. Prevalence of canine coronavirus antibodies by an enzyme-linked immunosorbent assay in dogs in the south of Italy. J. Virol. Methods. 2002;102:67–71. doi: 10.1016/S0166-0934(01)00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Decaro N., Tinelli A., Martella V., Elia G., Tempesta M., Cirone F., Buonavoglia C. Two genotypes of canine coronavirus simultaneously detected in fecal samples of dogs with diarrhea. J. Clin. Microbiol. 2004;42:1797–1799. doi: 10.1128/JCM.42.4.1797-1799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Kelly D.F., Carter S.D., Gaskell C.J. Canine coronavirus infection in the dog following oronasal inoculation. Res. Vet. Sci. 1991;51:11–18. doi: 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]


