Abstract
An enzyme-linked immunosorbent assays (ELISA) based on recombinant nucleocapsid (N) protein generated in Escherichia coli was evaluated for its sensitivity and specificity for diagnosis of porcine epidemic diarrhea (PEDV) infection. The N gene encoding the N protein was cloned and expressed as a fusion protein with His tag protein in E. coli. The recombinant N protein was migrated at 48 kDa and reacted with six histidine tag specific monoclonal antibody by immunoblotting. Recombinant N protein ELISA (rnELISA) demonstrated 98.7% specificities among (80) PEDV-free individuals, and 98% sensitivity ranging among (103) clinical samples with PEDV. On testing 884 field samples, an overall agreement of 88.3% was generated between the SN and rnELISA. Taken together, these results indicated that nucleocapsid protein may be a useful antigen for the sera-diagnosis of PEDV and it was also suggested that the ELISA is a highly sensitive and specific test for detecting antibodies to PEDV.
Keywords: Porcine epidemic diarrhea, N gene, Recombinant N ELISA
1. Introduction
Porcine epidemic diarrhea virus (PEDV) is an important pathogen causing an enteric disease that is especially severe in piglets, among which mortality can reach up to 90% (Pensaert, 1999). The most thoroughly studied of these agents associated with PED is coronavirus (CV) 777, first identified by Pensaert and DeBouck (1978) in Belgium and the United Kingdom and characterized by acute diarrhea disease affecting pigs of all age groups. PED is one of the most important causes of economic loss in Asia countries, especially in Korea and China, mainly due to its high prevalence, compared to the rare incidence of transmissible gastroenteritis and the asymptomatic characteristics of the Rotavirus infections (Carvajal et al., 1995).
The clinical appearance of PED is similar to those of TGE except that in certain outbreaks piglets under 4–5 weeks of age do not become sick (PED Type I). Morbidity is variable in sow but high (approaching 98.7%) in suckling pigs, which are most severely affected and exhibit vomiting, watery diarrhea, and dehydration. Mortality in piglets may be 50% or higher. Most fattening pigs and weaners recover from the disease, but some may remain unthrifty. In adults, anorexia, lethargy, and diarrhea are seen, but mortality is very low. Comparing high morbidity, the mortality is usually low in adult pigs (3%), which can recover within in a week. However, when suckling pigs are involved, mortality is normally about 50% and can reach up to 90% in very severe outbreaks (Pensaert, 1999).
After an acute outbreak, the virus can disappear or persist causing an enzootic infection. Now there are no effective commercial vaccines or specific treatment available and the only measures to control the disease are those directed to preventing the entrance of the virus on the farm (Pensaert, 1999). Induction of maternal immunity by artificial spread of the virus to pregnant sow is a common procedure used to reduce mortality and economical impact of the outbreak on the farm (Pensaert, 1999). Hofmann and Wyler (1988) described PEDV isolation for the first time in cell culture using Vero cells and trypsin-supplemented medium. Since then, several diagnostic methods have been employed for the detection of both PEDV antigen and its antibodies (Callbaut et al., 1982, Carvajal et al., 1995, Debouck et al., 1981, Hofmann and Wyler, 1990, Knuchel et al., 1992, Kweon et al., 1997, Van Nieuwstadt and Zetstra, 1991). The lack of in vitro-propagated PEDV made the diagnosis of infections extremely complicated and for this reason the available tests were of limited applicability. Virus-antigen-based serologic tests were used in screening for TGEV and PEDV (Knuchel et al., 1992, de Arriba et al., 2002) but the recombinant proteins for sero-diagnosis for PEDV have not been investigated. Examination with sensitive techniques such as immunoblotting and immunoprecipitation showed that PEDV has antigenic determinants in common with feline infectious peritonitis virus and these determinants are located on the nucleocapsid protein. Therefore, it was suggested that the N protein could be a suitable candidate for the detection of PED virus specific antibodies and diagnosis of PEDV.
We have developed ELISA method using recombinant 48 kDa nucleocapsid proteins, which has antigenic determinant of PEDV (Zhou et al., 1998). This test was designed as a tool to detect antibodies for the native protein in sera from PEDV infected pigs.
2. Materials and methods
2.1. Production and purification of recombinant PEDV nucleocapsid protein
Nucleocapsid protein (N) gene was amplified from the PED Korean isolate by reverse transcriptase polymerase chain reaction (RT-PCR) using a forward primer (5′-CGC GGA TCC GCT TCT GTC AGC TTT-3′) and a reverse primer (5′-CGC GAG CTC TTA ATT TCC TGT ATC GAA GAT-3′) (positions 36–1361; GenBank accession number Z14976) containing restriction enzyme sites (BamHI and SacI) underlined. The PCR product was subcloned into the prokaryotic expression vector pQE-30 (Qiagen, Korea) on corresponding restriction enzyme sites. The recombinant plasmid, namely pQE30-PN, was transformed into Escherichia coli XL1-Blue. The PEDV nucleocapsid proteins were produced by addition of 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG, Sigma, USA). Five hours after IPTG induction, the cells were harvested and lysed by sonication. The fusion protein was purified by affinity chromatography in Ni-nitrilotriacetic acid (Ni-NTA) agarose (the poly-histidine tag at the amino terminus of the recombinant N fusion protein binds to Ni-NTA resin, Qiagen, USA), under the native condition following the manufacturer's instruction. The expressed protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie brilliant blue R250 (Amresco, USA) and confirmed by immunoblot analysis using mice-raised monoclonal antibodies against the histidine tag (Qiagen, USA) and mice-raised anti-PEDV N polyclonal antibody kindly provided by Dr. Li Yi-Jing (China, Northeast Agricultural University), whose fusion antigen proteins were expressed in E. coli. The purified protein was analyzed by SDS-PAGE and Western blotting with anti-His-tag monoclonal antibody.
2.2. SDS-PAGE and immunoblot analysis
Expressed and purified N-protein was separated by SDS-PAGE using 12% separating gel and 5% stacking gel. Gels were stained with Coomassie brilliant blue R250 (Amresco, USA) or immunoblotting was performed according to the method of Burnette (Callbaut et al., 1982). Briefly, after transferring the proteins to PVDF immunoblotting membrane (Roche Applied Science, Germany), the membrane blocking was performed with 5% Skim milk (Difco, USA) containing 5 ml of 1 M Tris–HCl, pH 8.0 solution for 1 h. The membrane was blotted with mice-raised-anti-His antibody in blocking solution at a 1:1000 dilution. Following overnight incubation, the membrane was extensively washed with washing buffer (20 mM Tris–HCl, 150 mM NaCl, 0.05% Tween 20) and incubated for over 3 h with secondary antibodies, biotinylated anti-mouse IgG (Sigma, USA). After washing, the reaction was visualized by avidin–biotin complex (Vectastain ABC Kit, Vector Lab, USA) in PBS and DAB solution (Vector Lab, USA). The typical band was observed by the naked eyes.
2.3. Sera specimens
A total of 103 swine sera from different farms confirmed PEDV by RT-PCR with feces samples and intestinal washes were examined in this study and 80 sera samples were collected from three PEDV-free pig farms as negative control (Kindly provided by JE IL FEED COMPANY, Korea).
2.4. Recombinant N protein ELISA (rnELISA)
Swine sera were tested for IgG against PEDV by conventional enzyme-linked immunosorbent assay (ELISA) methods. Briefly, the microtitration Plates (NUNC Brand Products, Denmark) were coated with 100 ng/100 μl of purified PEDV N protein, in bicarbonate buffer (pH 9.6) for overnight. Plates were washed five times with PBST (PBS containing 0.05% Tween 20, Bio-Rad) and then incubated with blocking solution 10% Skim milk for 2 h at room temperature. Wells were washed and into each well, 100 μl of diluted sera, either positive or negative, was added and incubated for 2 h at 37 °C. After washing five times with PBST, the plates were incubated with 100 μl of 1:3000 diluted secondary antibodies, anti-pig-IgG antibodies conjugated to horseradish peroxidase (Sigma, USA) for 1 h at 37 °C. After washing, 100 μl/well of 0.2% H2O2 substrate (Sigma, USA) solution containing 4 μg/ml of o-pheylendiamine (OPD, Sigma, USA) as chromogen was added into the wells. The plates were incubated for 15 min in the dark at room temperature. The color development was stopped by adding 50 μl of 2N H2SO4. The absorbance at 450 nm was measured and recorded for the statistics.
2.5. Determination of optimal N protein concentration and serum dilution
Initial assays were performed to determine the antigen concentration that best discriminated between ELISA reactions of serum sample from confirmed PED cases (SN titres ≥ 1:16) and PEDV-free individuals. The checkerboard titration was carried out by the method of Hass et al. (1999). In subsequent assays, plates were coated with the predetermined antigen concentrations and serum dilution.
2.6. Negative cut-off value
Pig sera were collected randomly from three confirmed PEDV-free farms as negative control (SN titres < 1:2). A cut-off value for each recombinant-antigen ELISA was defined as absorbance values among serum samples (n = 80) from field according to the mean value plus two standard deviations. Sensitivity was determined as the percentage of laboratory-confirmed (RT-PCR) cases of PED (n = 103) whose serum samples had mean absorbance of samples greater than the cut-off value. Specificity was calculated as the percentage of PEDV-free individuals whose samples had mean absorbance below the cut-off value. To evaluate the agreement between rnELISA and other serum method, the serum neutralization (SN) tests were done with 884 field sow samples from 32 commercial swine farms of undefined PED status (kindly provided by Haerbin Veterinary Institute). All samples were tested on the PEDV SN (Oh et al., 2005) according to the standard protocol routinely performed at our laboratory.
3. Results
3.1. Production and purification of recombinant PEDV N protein
The PEDV N nucleoprotein isolated and amplified by RT-PCR (data not shown). The 1326 bp PED N gene was sub-cloned into the pQE-30 expression vector. The cloning was confirmed by enzyme restriction and sequencing analysis (data not shown).
The cloned N genes of PED were expressed as a soluble protein with an N-terminal His6 tag downstream of a T5 promoter, yielding a fusion protein of 442 amino acids, which gave a band corresponding to the expected molecular mass of about 48 kDa (Fig. 1 ). Expressed PEDV N antigens were purified by affinity chromatography using the following manufacturer's instruction (QIAGEN). The recombinant N protein bound to Ni-NTA agarose was eluted with 250 mM imidazole. The purified N protein was detected by SDS-PAGE staining with Coomassie brilliant blue R250 and Western blot using anti-His-tag monoclonal antibody (Fig. 2 ).
Fig. 1.
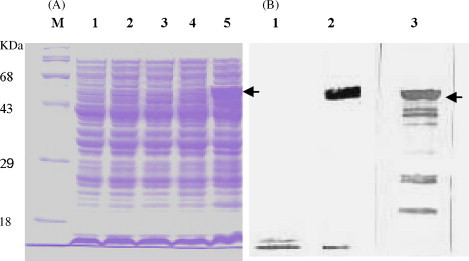
Expression of PED N protein stained with Coomassie brilliant blue R250 (A) and immunoblotting with anti-His-tag monoclonal antibody and anti-PEDN polyclonal antibody (B). The recombinant N proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The membrane was incubated with a 1:1000 dilution of anti-His-tag or followed by horseradish peroxidase-conjugated anti-mouse IgG (1:3000 dilution). (A) Lane 1, transformed cells XL1-Blue before induction; lane 2, Vector cells pQE30 before induction; lane 3, cells containing PEDN protein before IPTG induction; lane 4, pQE30 cells 5 h after induction; lane 5, cells containing PEDN protein (48.62 kDa) 5 h after induction. (B) Lane 1, pQE30 cells 5 h after induction; lane 2, cells containing PEDN protein 5 h after induction (anti-His-tag monoclonal antibody); lane 3, cells containing PEDN protein 5 h after induction (anti-PEDN polyclonal antibody).
Fig. 2.

Purification of PEDV N protein analyzed by SDS-PAGE with Coomassie brilliant blue R250 (A) and Western blotting with mouse anti-Histidine monoclonal antibody (B, 12% gel). The molecular mass of each one is indicated in the left margin. Lane 1, transformed cells XL1-Blue as control; lane 2, Vector cells pQE30 as control; lane 3, the washing sample before elution as control; lanes 4–6, purified protein (48.62 kDa) after eluting from Ni-NTA agarose for three times.
3.2. Determination of optimal concentration of recombinant His6-N protein and optimal dilution of test sera
To determine the specificity and sensitivity of ELISA using recombinant purified N protein, different antigen concentrations and serum dilutions were examined as shown in Fig. 3, Fig. 4 . Sample absorbance in the ELISA was positively correlated with antigen concentration from 25 to 100 ng/well in dose-dependent manner. In contrast, maximum absorbance value was determined at a concentration of 100 ng/well, while absorbance decreased with increasing antigen concentrations above 100 ng/well (Fig. 3). As the results of comparison for the sensitivity and specificity, a concentration of 100 ng/well was used for recombinant N protein ELISA. Because the absorbance values between positive and negative sample was not significantly different at 50,100-fold dilutions of primary sera, a 100-fold dilution was chosen for subsequent assays (Fig. 4).
Fig. 3.
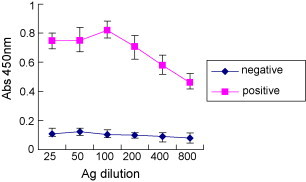
Optimal concentration of recombinant His6-N protein used for ELISA. The serum was diluted 1:100. The conjugation was diluted 1:3000. The SN titre of positive serum was ≥1:16. The SN titres of negative sera were ≤1:2.
Fig. 4.
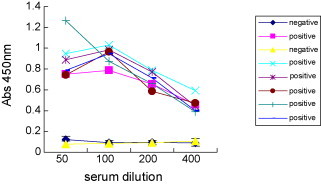
Optimal sera dilution of test sera used for PEDV–ELISA. The SN titres of positive sera were ≥1:16. The SN titres of negative sera were ≤1:2. Ag, 100 ng/well; conjugation, 1:3000.
3.3. Determination of negative cut-off values of ELISA
A negative cut-off value of ELISA was determined based on the reactivity with negative sera. The cut-off value for each recombinant-antigen ELISA was defined as absorbance values among serum samples (n = 80) from field according to the mean value plus two standard deviations. The mean value was 0.147 and SD (standard deviations) was 0.0525. Therefore, the negative cut-off value determined as 0.252. Consequently, ELISA reaction within the range of 0.252 was regarded as negative and higher of the cut-off value was regarded as positive. The recombinant N protein ELISA demonstrated 98.7% specificities among (80) PEDV-free individuals, and 98% sensitivity ranging among (103) clinical samples with PEDV (Table 1 and Fig. 5 ). Table 2 suggests that the overall agreement between SN and rnELISA is 88.3%. The maximum agreement was obtained from the serum samples of 1:≥32 of SN titre. But when the SN titre was 1:4, the agreement with rnELISA is only 30%.
Table 1.
Comparison of the sensitivity and specificity of the two methodologies
| Detection | Positive | Negative |
|---|---|---|
| RT-PCR | 103 | 80 |
| rnELISA positive | 101 | 2 |
| rnELISA negative | 1 | 79 |
| rnELISA sensitivitya | 98% | |
| rnELISA specificityb | 98.7% |
(number of ELISA positive sera)/(number of RT-PCR positive) × 100%.
(number of ELISA negative sera)/(number of RT-PCR negative) × 100%.
Fig. 5.

Determination of negative cut-off value for PEDV–ELISA. The samples were negative sera which SN titres were less than 1:2. The coating antigen was 100 ng per well. The negative sera diluted 1:100 and the conjugation diluted 1:3000.
Table 2.
Comparison of PEDV rnELISA with serum neutralization (SN) by 884 field sow serum samples
| SN resultsa | Total tested | rnELISA+ | rnELISA− | % agreement | % overall agreement |
|---|---|---|---|---|---|
| ≥64 | 217 | 217 | 0 | 100 | 88.3 |
| 32 | 140 | 140 | 0 | 100 | |
| 16 | 135 | 132 | 3 | 97.8 | |
| 8 | 105 | 75 | 30 | 71.4 | |
| 4 | 74 | 22 | 52 | 30.0 | |
| ≤2 | 213 | 18 | 195 | 91.5 |
The cut-off value for SN was 1:4. ELISA results ≥0.252 was considered as positive.
4. Discussion
Since it was not possible until 1988 to propagate porcine epidemic diarrhea virus in cell culture, the diagnostic tests for detection of anti-PEDV antibodies were complicated to establish and be expensive to perform (Knuchel et al., 1992). But PEDV is still difficult to isolate in cell culture. Therefore, their application was clearly limited and large-scale serological surveys could not be performed. The application of ELISA for serological survey was firstly reported by Hofmann and Wyler (1990). Their approach, however, had a disadvantage that PEDV particles were purified by sucrose density-gradient centrifugation before being used as ELISA antigens. A less complicated method of antigen preparation would facilitate the application of ELISA for large-scale serological testing. For the antibody ELISA, antigen consists of semi-purified virus, pig cell cultivated (Callbaut et al., 1982, Carvajal et al., 1995, Kweon et al., 1994) or S, E and N viral protein extracted from infected Vero cells (Knuchel et al., 1992). It is difficult to use this method under practical diagnostic circumstances because of the absence of the cell line adapted PEDV.
N protein is a structural protein of coronavirus that forms a helical nucleocapsid with genomic RNA and is the predominant antigen produced in coronavirus-infected cells, thus making it a major viral target (Sturman and Holmes, 1983). Therefore, we developed specific and sensitive ELISA for the detection of PED using the N protein as a sensitive diagnostic antigen. We found that the level of expression of N protein of PEDV was higher compared to the level of expression of the spike protein in E. coli expression system (data not shown). Recombinant protein-based serologic tests have high sensitivity and specificity because of the high concentration of immunoreactive antigens.
The specificity and the sensitivity of the assays were determined by evaluation of serum samples from PEDV-free individuals (n = 80) and from clinical samples with PEDV (n = 103). Recombinant N protein IgG ELISA demonstrated 98.7% specificities and 98% sensitivity, but these specificities and sensitivity are only comparisons of methodology between ELISA and RT-PCR. If the SN is used as the “gold standard”, the rnELISA would generate 12.7% false-negative results by missing 85 of 671 SN-positive samples. On the other hands, the rnELISA would produce 8.5% false-positive results by detecting “antibody” in 18 of 213 SN-negative samples. The maximum agreement was obtained from the serum samples of 1:≥32 of SN titre, however, the least 30% was from the serum samples of 1:4 of SN titre. These findings indicated that nucleocapsid may be a useful antigen for the sero-diagnosis of PEDV and it was also suggested that the ELISA is a simple and rapid test for screening a large number of swine sera for the anti-PEDV antibodies.
Acknowledgements
This project was supported by the Nature Science Foundation of Heilongjiang province (TC2005-10) and the fund of the 10th Five-Year-Plan in Key Science and Technology Research in Heilongjiang province of China (GB05B501-2). JE IL FEED COMPANY supported this research.
References
- Callbaut P., DeBouck P., Pensaert M. Enzyme-linked immunosorbent assay for detection of the coronavirus-like agent and its antibodies in pigs with porcine epidemic diarrhea. Vet. Microbiol. 1982;7:295–306. doi: 10.1016/0378-1135(82)90009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal A., Diego R., Lanza I., Diego R., Rubio P., Carmenes P. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J. Vet. Diagn. Invest. 1995;7:60–64. doi: 10.1177/104063879500700109. [DOI] [PubMed] [Google Scholar]
- de Arriba M.L., Carvajal A., Pozo J., Rubio P. Mucosal and systemic isotype-specific antibody responses and protection in conventional pigs exposed to virulent or attenuated porcine epidemic diarrhea virus. Vet. Immunol. Immunopathol. 2002;85:85–97. doi: 10.1016/s0165-2427(01)00417-2. [DOI] [PubMed] [Google Scholar]
- Debouck P., Callebaut P., Pensaert M. The diagnosis of coronavirus-like agent (CVLA) diarrhea in suckling pigs. Curr. Top. Vet. Med. Anim. Sci. 1981;13:59–61. [Google Scholar]
- Hass B., Hinz K.H., Glunder G. Biotin–streptavidin enzyme-linked immunosorbent assay for the detection of antibodies to Campylobacter jejuni and C. coli in chickens. J. Vet. Med. 1999;46:163–171. doi: 10.1046/j.1439-0450.1999.00217.x. [DOI] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Enzyme-linked immunosorbent assay for the detection of porcine epidemic diarrhea coronavirus antibodies in swine sera. Vet. Microbiol. 1990;21:263–273. doi: 10.1016/0378-1135(90)90037-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuchel M., Ackermann M., Muler H.K., Kihm U. An ELISA for detection of antibodies against porcine epidemic diarrhea virus (PEDV) based on the specific solubility of the viral surface glycoprotein. Vet. Micobiol. 1992;32:117–134. doi: 10.1016/0378-1135(92)90100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon C.H., Lee J.G., Han M.G., Kang Y.B. Rapid diagnosis of porcine epidemic diarrhea virus infection by polymerase chain reaction. J. Vet. Med. Sci. 1997;59:231–232. doi: 10.1292/jvms.59.231. [DOI] [PubMed] [Google Scholar]
- Kweon C.H., Kwon B.J., Kang Y.B., An S.H. Cell adaptation of KPEDV-9 and serological survey on porcine epidemic diarrhea virus (PEDV) in Korea. Kor. J. Vet. Res. 1994;33:249–254. [Google Scholar]
- Oh J.S., Song D.S., Yang J.S., Song J.Y., Moon H.J., Kim T.Y. Comparison of an enzyme-linked immunosorbent assay with serum neutralization test for serodiagnosis of porcine epidemic diarrhea virus infection. J. Vet. Sci. 2005;6:349–352. [PubMed] [Google Scholar]
- Pensaert M.B. Porcine epidemic diarrhea. In: Straw B.E., Allaire S.D., Mengeling W.L., Taylor D.J., editors. Diseases of Swine. Iowa State University Press; Iowa, EEUU: 1999. pp. 179–185. [Google Scholar]
- Pensaert M.B., DeBouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;68:45–52. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L.S., Holmes K.V. The molecular biology of coronaviruses. Adv. Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwstadt A.P., Zetstra T. Use of two enzyme-linked immunosorbent assays to monitor antibody responses in swine with experimentally induced infection with porcine epidemic diarrhea virus. Am. J. Vet. Res. 1991;52:1044–1050. [PubMed] [Google Scholar]
- Zhou Y.L., Ederveen J., Egberink H., Pensaert M., Horzinek M.C. Porcine epidemic diarrhea virus (CV777) and feline infectious peritonitis virus (FIPV) are antigenically related. Arch. Virol. 1998;102:63–71. doi: 10.1007/BF01315563. [DOI] [PMC free article] [PubMed] [Google Scholar]


