Abstract
Canine respiratory coronavirus (CRCoV) has recently been detected in dogs; it is a group 2 coronavirus showing similarity to bovine coronavirus (BCoV) but is distinct from canine enteric coronavirus (CECoV). CRCoV may play an important role in canine infectious respiratory disease (CIRD) either by predisposing to further and potentially more serious viral and bacterial infections or possibly as a primary pathogen. The prevalence of serum antibodies to CRCoV, in a population of dogs in the south east of England, has been shown previously to be 30.1% on the first day of entry to a rehoming kennel [Erles, K., Toomey, C., Brooks, H.W., Brownlie, J., 2003. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 310, 216–223]. The purpose of this study was to establish the prevalence of CRCoV in the general canine population within as well as outside the UK. An ELISA, used to test for the presence of antibodies to CRCoV in canine serum samples, identified seropositive dogs in UK, USA, Canada, Republic of Ireland and Greece. The development of an ELISA based on CRCoV antigen and immunofluorescence assay are described here. 54.7% (547/1000) of North American and 36.0% (297/824) of United Kingdom dogs were seropositive for CRCoV. The age and geographical distribution of seropositive dogs was also assessed. The cross-reactivity demonstrated between CRCoV antibodies from different countries and a UK viral isolate suggests immunological similarity. The overall prevalence of this virus in both North America and the UK suggests that CRCoV has international significance and that further epidemiological studies are required.
Keywords: ELISA, Coronavirus, Canine, Respiratory, CIRD, CRCoV, Tracheobronchitis, Kennel cough
1. Introduction
Coronaviruses are known to cause respiratory disease in a number of species, including humans. In 2003, Erles et al. identified canine respiratory coronavirus in the respiratory tract of dogs in a large rehoming kennel where CIRD was endemic and could not be controlled by the use of vaccines against ‘kennel cough’. This finding suggested a role for the virus and possibly other unidentified agents in the pathogenesis of the disease.
CIRD is a common contagious disease of high morbidity, low mortality and is characterised by the acute onset of a dry, hacking and paroxysmal cough. The disease is often mild and recovery rapid (1–3 weeks), however in some cases interstitial pneumonitis or severe bronchopneumonia develop which can be fatal (Appel and Binn, 1987). A number of pathogens are associated with CIRD, including canine adenovirus 2 (CAV-2), canine parainfluenza virus (CPIV), Bordetella bronchiseptica, canine herpesvirus (CHV), mycoplasmas and others and disease may result from one or a combination of these agents (Bemis et al., 1977, Chalker et al., 2003, Chalker et al., 2004, Karpas et al., 1968).
CIRD is usually only a problem when groups of dogs are kept together under crowded conditions, such as rescue centres, laboratory animal units and training kennels. Despite widespread vaccination, CIRD remains a persistent global problem. In addition to the obvious welfare implications and costs of treatment, the disease also delays and disrupts rehoming and training schedules of kennels and charities.
CRCoV has been shown to be genetically very similar to BCoV and human coronavirus OC43; 98.8% and 98.4%, respectively, based on the polymerase gene. However the virus is only distantly related to canine enteric coronavirus (CECoV); 68.5% similarity based on the polymerase gene. Sequencing of the spike gene also revealed a high similarity (97.3%) to BCoV and enabled an ELISA for the detection of CRCoV antibodies to be developed based on BCoV antigen (Erles et al., 2003).
The prevalence of antibodies to CRCoV in serum samples from UK dogs, at time of entry into a rehoming kennel in London, was established as 30.1% (n = 123). The prevalence of antibodies to CRCoV was 99% after 3 weeks in the rehoming kennel, indicating that the virus was highly contagious (Erles et al., 2003). A further study found antibodies to CRCoV in 22.2% (n = 54) and 54.2% (n = 59) of serum samples obtained from dogs on the day of entry to working dog kennels in London and Warwickshire respectively (Erles and Brownlie, 2005). These data give an indication as to the prevalence of the virus in the wider canine population, although limited to the geographical catchment area of the particular kennel.
The other coronavirus of dogs, canine enteric coronavirus (CECoV) is known to be widespread in the dog population. Antibodies have been detected in 54% (n = 174) of a population of healthy and diarrhoeic pet dogs in UK (Tennant et al., 1991), while CECoV seroprevalence ranged from 76% (n = 63) in a rescue kennel to 100% (n = 12) in a commercial breeding colony (Tennant et al., 1993). These studies demonstrate the increased prevalence of coronavirus infection where population densities are high and there is an influx of susceptible animals and pathogens as a result of high population turnover. In the United States the seroprevalence of CECoV was 26% (n = 27) for pet dogs and up to 87% (n = 15) for kennelled dogs (Helfer-Baker et al., 1980).
Thus far the only evidence for the presence of CRCoV outside the United Kingdom has been from Canada where virus was detected in archived tissues from 2/126 cases of canine respiratory disease (Ellis et al., 2005). This study aimed to establish serological evidence for the presence of CRCoV outside the UK and to assess the seroprevalence of the virus in the wider UK canine population. The current ELISA, based on BCoV antigen, for the detection of “BCoV-like antibodies” will be assessed against an ELISA developed specifically for CRCoV and immunofluorescence assay.
2. Materials and methods
2.1. BCoV antigen ELISA
2.1.1. Samples
One thousand heat-inactivated canine sera were obtained from the College of Veterinary Medicine, Cornell University, NY. These sera had been submitted by veterinary surgeons from across North America to Cornell for the establishment of antibody titres towards predominantly canine distemper virus (CDV) and canine parvovirus (CPV), as an assessment of whether the animals needed a booster vaccination. The sera were received and stored frozen until use.
2.1.2. BCoV ELISA procedure
The BCoV ELISA was essentially as previously described (Erles et al., 2003), using freeze-dried BCoV antigen and control (Churchill Applied Biotechnology Ltd, Huntingdon). Sera were thoroughly mixed and diluted 1:100 in 5% skimmed milk powder in PBS. Blocking was performed by incubation of the plates at 37 °C for 1 h with 5% skimmed milk powder in PBS (100 μl per well). All incubation stages were performed in a humid atmosphere to prevent excess drying of the wells. Post-blocking, diluted serum was added to each of the BCoV antigen coated and control wells (50 μl per well).
Plates were incubated at 37 °C for 1 h, and then washed with PBS-Tween 20 (0.05%). Anti-dog IgG-peroxidase conjugate (Sigma-Aldrich, Poole) was diluted 1:5000 in PBS-Tween 20 (0.05%) and 50 μl added to each well, followed by incubation for 1 h at 37 °C. Plates were washed with PBS-Tween 20 (0.05%) and shaken dry. OPD peroxidase substrate (Sigma–Aldrich) was prepared according to the manufacturer's instructions and 100 μl added to each well. The plates were incubated in the dark at room temperature for 10 min. To stop the reaction, 50 μl of 2 M H2SO4 was added to each well. The optical densities of the stopped ELISAs were read on an Optimax automatic plate reader (Molecular Devices, Wokingham).
A panel of 19 canine control sera, previously established as negative for CRCoV antibodies (Erles et al., 2003), were used with each antigen batch to determine the OD cut off for positive sera based on OD > mean of negative sera + 3 × standard deviation. The 19 sera were confirmed as negative by immunofluorescence assay in this study.
2.2. Immunofluorescence
2.2.1. Preparation of fixed cells
Previous work in this laboratory has established that CRCoV grows readily in human rectal tumour (HRT-18) cells (Benfield and Saif, 1990) without requiring adaptation (Erles, submitted for publication). Cultured HRT-18 cells (ECACC, Salisbury) were infected with CRCoV UK isolate 4182 (MOI = 0.1), obtained from a clinical case of CIRD (Erles, submitted for publication). Cells were counted and seeded onto 10-well slides at 105 cells/slide (30 μl/well). The slides were incubated at 37 °C in a humid 5% CO2 atmosphere overnight for the cells to adhere. Excess media was removed in PBS and the cells were fixed in methanol/acetone (2:1) for 10 min at −20 °C. The slides were briefly air-dried and stored at −20 °C.
2.2.2. IFA procedure
Canine sera were diluted 1:30 in PBS to a final volume of 30 μl. Fixed infected cells were incubated with diluted sera in a humidified atmosphere at 37 °C for 1 h. Slides were washed 3 × 5 min in PBS. Thirty microlitres anti-dog IgG FITC-conjugate (Sigma-Aldrich), diluted 1:100 in PBS, was applied to each well. Incubation was performed for 1 h at 37 °C in the dark. Slides were washed 3 × 5 min in PBS and coverslips mounted with Vectashield mounting medium (Vector Laboratories, Peterborough) to preserve fluorescence. Slides were viewed under an ultraviolet microscope and the sample determined by two independent observers to be either positive or negative.
2.3. CRCoV antigen ELISA
2.3.1. CRCoV antigen preparation
Two confluent 175 cm2 flasks (Nunc, Rochester, NY) of HRT-18 cells were prepared. Cells were washed with serum-free RPMI-1640 medium (Sigma–Aldrich) containing trypsin (Sigma–Aldrich) at 1 μg/ml. CRCoV isolate 4182 (MOI = 0.1) diluted in serum-free RPMI plus trypsin (1 μg/ml) was added to one flask, the other flask was mock infected with RPMI plus trypsin only, the flasks were incubated at 37 °C (5% CO2) for 1 h. The inoculum was removed and 25 ml fresh serum-free RPMI plus trypsin (1 μg/ml) was added, followed by incubation at 37 °C (5% CO2) for 6 days.
Following incubation, the medium was removed from each flask and replaced with 10 ml PBS. The cell monolayers from each flask were scraped off, transferred to a centrifuge tube and spun at 500 × g for 10 min at 4 °C. The supernatant was discarded, the pellet resuspended in 10 ml PBS and the centrifugation step repeated. The supernatant was discarded and the cell pellet resuspended in 0.5 ml 1.0% Igepal CA-630 (Sigma-Aldrich) followed by mixing at room temperature for 1 h. The cell lysate was then centrifuged at 10,000 × g for 1 min at room temperature. The supernatant was retained and the cell debris discarded.
2.3.2. Determining the protein concentration
The protein concentration of the prepared ELISA antigen and control was established using a colorimetric protein assay (Micro BCA Protein Assay Kit, Pierce Biotechnology Inc., Rockford, IL) according to the manufacturer's instructions. The optical density of the samples was determined using an Optimax automatic plate reader (Molecular Devices) and this was used to estimate the protein concentration (μg/ml) based on a standard curve of bicinchoninic acid (BCA). The antigen and control were aliquoted into 50 μl and stored at −20 °C until required.
2.3.3. Samples
Eight hundred and ninety-six canine serum samples were obtained from the diagnostic haematology and biochemistry section of Axiom Veterinary Laboratories, Teignmouth, Devon. The samples were received by the laboratory from veterinary practices across the United Kingdom and Ireland, for various biochemical or haematological screens. On receipt the samples were aliquoted and frozen at −20 °C.
2.3.4. CRCoV ELISA procedure
CRCoV antigen and control were used at a protein concentration of 20 μg/ml, however the remainder of the procedure was identical to the BCoV antigen ELISA.
2.3.5. Analysis of data
The influence of age on the presence or absence of antibodies to CRCoV was investigated using the Fisher's exact test.
3. Results
3.1. CRCoV antibody prevalence in North America
3.1.1. Overall CRCoV prevalence
One thousand canine sera were tested by BCoV antigen ELISA; of these 547 dogs (54.7%) were found to possess antibodies towards a BCoV-like virus. Nine hundred and fifty-six dogs were from the United States and 44 were from Canada; of these 54.5% (521/956) and 59.1% (26/44) were positive, respectively.
3.1.2. CRCoV prevalence by state
Samples of sera were obtained from 33 states (Table 1 ). For each state the percentage of positive dogs was determined (Fig. 1 ). Individual state seropositivity rates ranged from 0% to 100%. Sample numbers from each state varied considerably; 1 sample (Arizona, Delaware, Louisiana and Oregon) to 140 samples (New York), thus for some states the data can only be used to indicate those states with or without serological evidence of CRCoV, rather than true seroprevalence.
Table 1.
Summary of CRCoV antibody status by state of origin (n = 1000)
| State | Number of dogs tested, n | Number CRCoV positive | CRCoV positive (%) |
|---|---|---|---|
| Arkansas | 2 | 1 | 50.0 |
| Arizona | 1 | 0 | 0.0 |
| California | 8 | 3 | 37.5 |
| Colorado | 5 | 4 | 80.0 |
| Connecticut | 22 | 7 | 31.8 |
| Delaware | 1 | 0 | 0.0 |
| Florida | 44 | 18 | 40.9 |
| Georgia | 11 | 7 | 63.6 |
| Iowa | 5 | 2 | 40.0 |
| Illinois | 21 | 14 | 66.7 |
| Indiana | 10 | 6 | 60.0 |
| Kentucky | 16 | 14 | 87.5 |
| Louisiana | 1 | 1 | 100 |
| Massachusetts | 58 | 28 | 48.3 |
| Maryland | 30 | 16 | 53.3 |
| Maine | 16 | 5 | 31.3 |
| Michigan | 10 | 4 | 40.0 |
| Minnesota | 44 | 17 | 38.6 |
| Missouri | 9 | 7 | 77.8 |
| Montana | 2 | 2 | 100 |
| North Carolina | 83 | 54 | 65.1 |
| New Hampshire | 26 | 19 | 73.1 |
| New Jersey | 15 | 9 | 60.0 |
| New York | 140 | 75 | 53.6 |
| Ohio | 94 | 58 | 61.7 |
| Oregon | 1 | 0 | 0.0 |
| Pennsylvania | 61 | 30 | 49.2 |
| Texas | 2 | 0 | 0.0 |
| Utah | 2 | 2 | 100 |
| Virginia | 76 | 33 | 43.4 |
| Vermont | 7 | 7 | 100 |
| Washington | 3 | 3 | 100 |
| Wisconsin | 130 | 75 | 57.7 |
| 956 | 521 | 54.5 | |
| Canada | 44 | 26 | 59.1 |
Fig. 1.
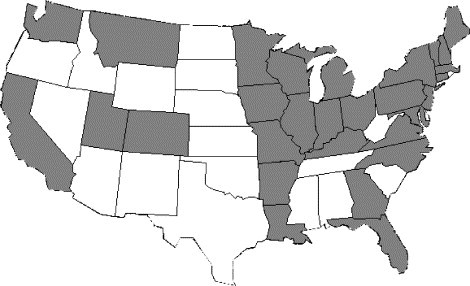
Diagrammatic representation of states with CRCoV seropositive dogs. Grey states have one or more CRCoV seropositive dogs and white states have either no seropositive dogs or no data.
3.1.3. CRCoV prevalence by age
The age at the time of blood sample collection was known for 943 dogs. The relationship between age of dog and the presence of CRCoV antibodies was determined (Fig. 2 ). 23.6% (13/55) of dogs <6 months of age were found to be seropositive for CRCoV. Seropositivity rates were highest, 68.4% (52/76), amongst the 7–8 years age group. 41.8% (23/55) of dogs >12 years were seropositive. Age appeared to influence the prevalence of antibodies to CRCoV, with dogs less than 1 year of age more likely to be seronegative for CRCoV compared to older dogs (P < 0.01).
Fig. 2.
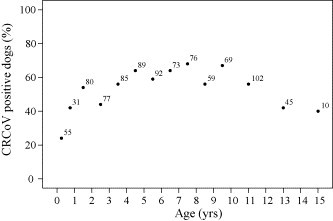
Relationship of CRCoV antibody status to age in canine samples from North America (n = 943). Numbers indicate the quantity of samples associated with each data point. Each data point represents the average age within an age range, i.e., 0–6 months: 0.25, 6 months–1 year: 0.75, 1–2 years: 1.5 and so on until 10–12 years: 11, 12–14 years: 13 and 14–16 years: 15.
3.2. CRCoV antibody prevalence in the United Kingdom and Ireland
3.2.1. Overall prevalence
Eight hundred and ninety-six canine sera were tested by CRCoV antigen ELISA; of these 319 dogs (35.6%) were found to possess antibodies towards the virus. The overall prevalences for the United Kingdom and Republic of Ireland were 36.0% and 30.3%, respectively.
3.2.2. CRCoV prevalence by region and county
Prevalence was determined by region and county of the United Kingdom and the Republic of Ireland (ROI) (Table 2 ). Small numbers of samples from other countries (Greece and Malta) were also assayed (Table 2). The presence of CRCoV antibodies was assessed by county (Fig. 3 ), however within the United Kingdom and ROI a number of counties had a low number of samples, thus only counties with evidence of CRCoV seropositivity are displayed. CRCoV prevalence was generally below average in southwest England, the Midlands and in Wales. Central southern England, parts of the West Country, East Anglia and northern England had a higher than average prevalence. The prevalence in Scotland was 61.1%. Antibodies to CRCoV were also detected in two samples from Greece.
Table 2.
Summary of CRCoV antibody status by region and county of origin (n = 896)
| Region | County | Number of dogs tested, n | Number of CRCoV positive | CRCoV positive (%) (with average for region) |
|---|---|---|---|---|
| England | Bedfordshire | 2 | 0 | 0 |
| Berkshire | 17 | 5 | 29.4 | |
| Bristol | 47 | 20 | 42.6 | |
| Cambridgeshire | 5 | 1 | 20.0 | |
| Cheshire | 6 | 4 | 66.7 | |
| Cornwall | 38 | 9 | 23.7 | |
| Cumbria | 7 | 0 | 0 | |
| Derbyshire | 5 | 0 | 0 | |
| Devon | 132 | 40 | 30.3 | |
| Dorset | 24 | 9 | 37.5 | |
| East Sussex | 1 | 0 | 0 | |
| Essex | 5 | 3 | 60.0 | |
| Gloucestershire | 51 | 20 | 39.2 | |
| Hampshire | 32 | 12 | 37.5 | |
| Hereford and Worcester | 12 | 5 | 41.7 | |
| Hertfordshire | 9 | 3 | 33.3 | |
| Isle of Mann | 4 | 1 | 25.0 | |
| Isle of Wight | 1 | 0 | 0 | |
| Kent | 28 | 9 | 32.1 | |
| Lancashire | 5 | 2 | 40.0 | |
| Leicestershire | 5 | 1 | 20.0 | |
| Lincolnshire | 2 | 1 | 50.0 | |
| London | 65 | 33 | 50.8 | |
| Merseyside | 4 | 1 | 25.0 | |
| Norfolk | 24 | 9 | 37.5 | |
| North Yorkshire | 1 | 1 | 100 | |
| Northamptonshire | 4 | 1 | 25.0 | |
| Nottinghamshire | 21 | 3 | 14.3 | |
| Oxfordshire | 28 | 9 | 32.1 | |
| Shropshire | 3 | 0 | 0 | |
| Somerset | 28 | 8 | 28.6 | |
| Suffolk | 6 | 3 | 50.0 | |
| Surrey | 43 | 21 | 48.8 | |
| Tyne and Wear | 2 | 2 | 100 | |
| Warwickshire | 5 | 1 | 20.0 | |
| West Midlands | 1 | 0 | 0 | |
| West Sussex | 4 | 1 | 25.0 | |
| West Yorkshire | 17 | 12 | 70.6 | |
| Wiltshire | 43 | 17 | 39.5 | |
| 737 | 267 | 36.2 | ||
| Wales | Dyfed | 4 | 1 | 25.0 |
| Glamorgan—mid/west | 16 | 5 | 31.3 | |
| Glamorgan—south | 6 | 1 | 16.7 | |
| Gwent | 15 | 3 | 20.0 | |
| Gwynedd | 9 | 2 | 22.2 | |
| 50 | 12 | 24.0 | ||
| Scotland | Ayrshire | 14 | 7 | 50.0 |
| Lanarkshire | 4 | 4 | 100 | |
| 18 | 11 | 61.1 | ||
| Channel Is. | Guernsey | 8 | 2 | 25.0 |
| Northern Ireland | Co. Antrim | 7 | 1 | 14.3 |
| Co. Down | 2 | 2 | 100 | |
| Co. Fermanagh | 2 | 2 | 100 | |
| 11 | 5 | 45.5 | ||
| Republic of Ireland | Co. Clare | 1 | 1 | 100 |
| Co. Cork | 11 | 5 | 45.5 | |
| Co. Donegal | 1 | 1 | 100 | |
| Co. Dublin | 38 | 9 | 23.7 | |
| Co. Galway | 3 | 1 | 33.3 | |
| Co. Kerry | 1 | 1 | 100 | |
| Co. Offaly | 1 | 0 | 0 | |
| Co. Tipperary | 3 | 0 | 0 | |
| Co. Westmeath | 1 | 0 | 0 | |
| Co. Wicklow | 6 | 2 | 33.3 | |
| 66 | 20 | 30.3 | ||
| Malta | 1 | 0 | 0 | |
| Greece | 5 | 2 | 40.0 | |
Fig. 3.
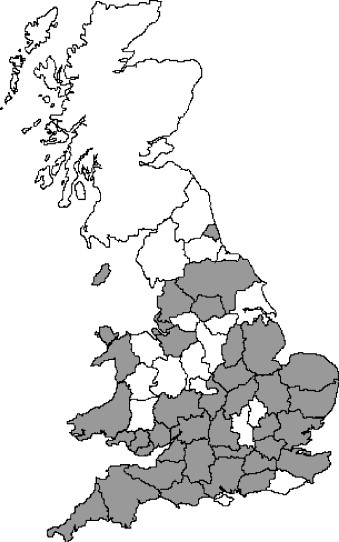
Diagrammatic representation of counties with CRCoV seropositive dogs. Scotland, Northern Ireland and the Republic of Ireland were omitted since low sample numbers prohibited meaningful representation. Grey counties have one or more CRCoV seropositive dogs and white counties have either no seropositive dogs or no data.
3.2.3. CRCoV prevalence by age
The age at the time of blood sampling was known for 887 dogs. The relationship between age of dog and the presence of CRCoV antibodies was determined (Fig. 4 ). Zero percent (0/13) of dogs below 6 months of age were found to be seropositive for CRCoV. Seropositivity rates were highest around 2–4 years (40.5–45.0%) and again around 11–12 years of age (40.9–47.4%), however these differences are not statistically significant (P = 0.444 and 0.144 respectively). Again age appeared to influence the prevalence of antibodies to CRCoV, with dogs less than 6 months of age more likely to be seronegative for CRCoV compared to older dogs (P < 0.05).
Fig. 4.
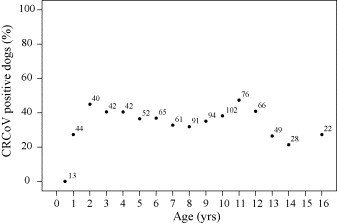
Relationship of CRCoV antibody status to age in canine samples from UK, Republic of Ireland, Greece and Malta (n = 887). Numbers indicate the quantity of samples associated with each data point.
3.2.4. Immunofluorescence assay
Immunofluorescence assay (IFA) was performed on HRT-18 cells, infected with CRCoV isolate 4182, with 90 USA serum samples, selected at random, to validate and compare the ELISA results using BCoV or CRCoV antigen (Table 3 and Fig. 5 ). IFA was taken to be the ‘gold standard’ for detection and thus ELISA sensitivity and specificity were calculated for BCoV and CRCoV antigens (Table 3).
Table 3.
Comparison of BCoV and CRCoV ELISA antigens with IFA
| IFA positive, n = 55 (%) | IFA negative, n = 35 (%) | Assay specificity (%) | Assay sensitivity (%) | |
|---|---|---|---|---|
| BCoV antigen ELISA | 53/90 (58.9) | 37/90 (41.1) | 96.7 | 94.4 |
| CRCoV antigen ELISA | 54/90 (60.0) | 36/90 (40.0) | 96.7 | 95.6 |
Fig. 5.
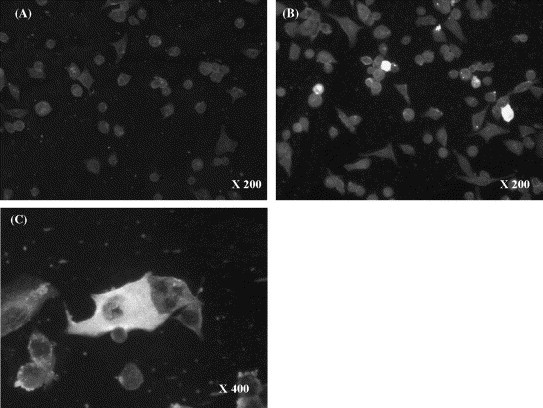
Immunofluorescence assay—human rectal tumour (HRT-18) cells infected with CRCoV (UK isolate 4182). (A) ELISA negative serum, (B) and (C) ELISA positive serum.
4. Discussion
This study for the first time revealed the presence of canine antibodies to CRCoV outside the United Kingdom. Serological evidence for the existence of CRCoV in the United States, Canada, the Republic of Ireland and Greece is presented here. The BCoV ELISA had previously been shown to be specific for CRCoV and there was no cross-reactivity with CECoV antibodies (Erles et al., 2003). The cross-reactivity of BCoV ELISA antigen with canine antibodies suggests immunological, as well as genetic, similarity with CRCoV. In addition antibodies from dogs originating from other countries, bound to CRCoV antigen, produced from a United Kingdom isolate (4182), suggesting a high degree of geographical immunological cross-reactivity. This implies either restricted epitopic diversity or conservation of immunologically dominant domains. Cross-reactivity between CRCoV and CECoV has not definitively been ruled out. The genetic similarity of BCoV and CRCoV based on the translated amino acid sequence of the main antigenic determinant, the spike protein, has been established as 96% compared with a 21% identity to CECoV (Erles et al., 2003). Furthermore it is possible (and sometimes quite likely given the epidemiology of the two viruses) that antibodies to both viruses could occur in the same serum sample. In order to definitively rule out the possibility of any cross-reactivity between the two coronaviruses, inoculation of experimental animals with either CRCoV or CECoV, followed by cross-neutralisation, would have to be performed.
Overall seroprevalence of CRCoV in North America was 54.7%, compared to 36.0% in the United Kingdom. This data indicates that the prevalence of CRCoV may be around a third greater in North America. Prevalence in Ireland (Republic of Ireland [ROI] and Northern Ireland) was 32.5%. Previously CRCoV antibodies were found in 30.1% of dogs on the day of entry to a rehoming kennel in the London area (Erles et al., 2003), this work suggests that this estimate may be low compared to the overall prevalence for London of 50.8%. However seropositivity had risen to 99% after 21 days in the kennel, i.e. almost all dogs negative on entry showed seroconversion to CRCoV within 3 weeks, indicating that the virus was highly contagious (Erles et al., 2003) and prevalent in the kennel environment.
CRCoV prevalence appears to correlate to some degree with population density in the UK and Ireland. In regions with low population density (and possibly low canine density) the seroprevalence of CRCoV is generally low; the southwest of England and Wales in particular have a low prevalence of antibodies. The main urban areas appear to have a greater incidence of CRCoV antibodies. Exceptions would include East Anglia, North Yorkshire and in particular Scotland however, where the bulk of samples originated from one large town or city (e.g. Glasgow) in an otherwise sparsely populated region, thus the regions appear to have an overall high CRCoV seroprevalence. Diagnostic laboratories often receive samples from a selective number of veterinary practices, thus a certain degree of geographical selection might be expected in such studies and would account for findings such as in Ayrshire, Scotland where the 61.1% seropostivity more accurately reflects that of Glasgow.
Sample numbers for much of the western USA were relatively low and thus these data can only realistically be used to indicate those states that do have evidence of CRCoV. Many more samples were available from the Midwest and eastern USA allowing for a more accurate representation of the seroprevalence within a particular state. The Midwest (including Iowa, Illinois, Indiana, Michigan, Minnesota, Missouri, Ohio and Wisconsin) appeared to have a slightly higher prevalence (56.7%) than surrounding regions (Northeast: 52.1% and Southeast: 54.8%), however the difference is not statistically significant (P = 0.254 and 0.603, respectively using Fisher's exact test). This does show however that there is widespread evidence for the virus across the eastern USA and that seroprevalence does not appear to vary dramatically with geographical location.
The percentage of dogs with antibodies to CRCoV increased with age. In North America 23.6% of dogs less than 6 months of age had antibodies to CRCoV; this suggests that dogs are challenged from a young age, possibly via the dam, and subsequently develop an immune response. However in both the UK and North America, dogs under 6 months of age were significantly less likely to have antibodies to CRCoV than older dogs. This finding is similar to the situation with CECoV where seropositivity is significantly lower at less than 6 months of age (Tennant et al., 1991). Both North American and United Kingdom/ROI dogs showed a marked increase in antibodies to CRCoV from 6 months of age onwards, this likely corresponds to the beginning of socialisation and mixing with other dogs. United Kingdom/ROI dogs appear to have two peaks in CRCoV antibody prevalence, around 3 years and again at 12 years of age, however the differences are not statistically significant (P = 0.444 and 0.144 respectively) thus may be artefactual and disappear with an increased number of samples. Samples from North American dogs show a distribution with a gradual rise in seroprevalence to a peak between 7 and 8 years. In both North America and UK/ROI seroprevalence slowly declines with increasing age after 10–12 years. This could be explained by an age-related fall in the efficiency of the immune system and antibody production.
IFA was successfully used to validate the ELISA results based on BCoV or CRCoV antigen. The sensitivity and specificity of both the CRCoV antigen based ELISA and the BCoV antigen ELISA, taking immunofluorescence assay to be the ‘gold standard’, were high, thus endorsing the seroprevalence data presented. The ability to culture CRCoV in HRT-18 cells has allowed the development of a successful, specific CRCoV antigen ELISA and this will be useful in further epidemiological surveys of CRCoV.
The sera used in this study were obtained from diagnostic laboratories, which may not be considered as an entirely random population sample. Animals presented to a veterinary surgeon and subsequently blood-sampled are unlikely to be completely healthy, thus some bias towards unhealthy animals (although not necessarily suffering from infectious disease) is always going to be an issue. However the North American sera were from clinically healthy dogs, whose serum had been submitted to determine the antibody status of the dogs as an assessment of whether the animals needed a booster vaccination, thus should give a reasonably accurate estimate of seroprevalence.
Interestingly, CRCoV has recently been detected, using immunohistochemistry, in two Canadian dogs associated with a suppurative bronchiolitis (Ellis et al., 2005), this may be further evidence that CRCoV is aetiologically associated with CIRD. The health status of the dogs tested was unknown therefore no association with any diseases can be made; furthermore the data gives no information regarding current infection with CRCoV, only evidence of infections in the past.
5. Conclusion
In summary, CRCoV has an international presence and there is a growing wealth of evidence to suggest the role of novel pathogens in the widespread and complex problem of CIRD; coronaviruses may just be the tip of the iceberg, however the elucidation of their involvement in the disease could open up new ways of thinking with regard to the traditional views on the causes of canine infectious respiratory disease. Further studies involving dogs with CIRD and in other countries are required to determine the role of CRCoV.
Acknowledgements
This study was supported by a grant (K. Erles) and a PhD scholarship (S. Priestnall) from The Royal Veterinary College. We thank S. Greaves for technical support.
References
- Appel M.J., Binn L.N. Virus Infections of Carnivores. Elsevier Science Publishing Co.; New York: 1987. Canine infectious tracheobronchitis short review: kennel cough. pp. 201–211. [Google Scholar]
- Bemis D.A., Greisen H.A., Appel M.J. Pathogenesis of canine bordetellosis. J. Infect. Dis. 1977;135:753–762. doi: 10.1093/infdis/135.5.753. [DOI] [PubMed] [Google Scholar]
- Benfield D.A., Saif L.J. Cell culture propagation of a coronavirus isolated from cows with winter dysentery. J. Clin. Microbiol. 1990;28:1454–1457. doi: 10.1128/jcm.28.6.1454-1457.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker V.J., Owen W.M., Paterson C., Barker E., Brooks H., Rycroft A.N., Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- Chalker V.J., Toomey C., Opperman S., Brooks H.W., Ibuoye M.A., Brownlie J., Rycroft A.N. Respiratory disease in kennelled dogs: serological responses to Bordetella bronchiseptica lipopolysaccharide do not correlate with bacterial isolation or clinical respiratory symptoms. Clin. Diagn. Lab. Immunol. 2003;10:352–356. doi: 10.1128/CDLI.10.3.352-356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J.A., McLean N., Hupaelo R., Haines D.M. Detection of coronavirus in cases of tracheobronchitis in dogs: a retrospective study from 1971 to 2003. Can. Vet. J. 2005;46:447–448. [PMC free article] [PubMed] [Google Scholar]
- Erles K., Brownlie J. Investigation into the causes of canine infectious respiratory disease: antibody responses to canine respiratory coronavirus and canine herpesvirus in two kennelled dog populations. Arch. Virol. 2005;150:1493–1504. doi: 10.1007/s00705-005-0533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer-Baker C., Evermann J.F., McKeirnan A.J., Morrison W.B. Serological studies on the incidence of canine enteritis viruses. Canine Pract. 1980;7:37–42. [Google Scholar]
- Karpas A., Garcia F.G., Calvo F., Cross R.E. Experimental production of canine tracheobronchitis (kennel cough) with canine herpesvirus isolated from naturally infected dogs. Am. J. Vet. Res. 1968;29:1251–1257. [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Jones R.C., Gaskell C.J. Prevalence of antibodies to four major canine viral diseases in dogs in a Liverpool hospital population. J. Small Anim. Pract. 1991;32:175–179. [Google Scholar]
- Tennant B.J., Gaskell R.M., Jones R.C., Gaskell C.J. Studies on the epizootiology of canine coronavirus. Vet. Rec. 1993;132:7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]


