Abstract
Porcine group A rotavirus (GARV) is considered to be an important animal pathogen due to their economic impact in the swine industry and its potential to cause heterologous infections in humans. This study examined 475 fecal samples from 143 farms located in 6 provinces across South Korea. RT-PCR and nested PCR utilizing primer pairs specific for the GARV VP6 gene detected GARV-positive reactions in 182 (38.3%) diarrheic fecal samples. A total of 98 porcine GARV strains isolated from the GARV-positive feces were analyzed for G and P genotyping. Based on the sequence and phylogenetic analyses, the most predominant combination of G and P genotypes was G5P[7], found in 63 GARV strains (64.3%). The other combinations of G and P genotypes were G8P[7] (16 strains [16.3%]), G9P[7] (7 strains [7.1%]), G9P[23] (2 strains [2.0%]), and G8P[1] (1 strain [1.0%]). The counterparts of G or P genotypes were not determined in three G5, five P[7], and one P[1] strains. Interestingly, phylogenetic analysis indicated that all Korean G9 strains were more closely related to lineage VI porcine and human viruses than to other lineages (I–V) of GARVs and to Korean human G9 strains (lineage III). These results show that porcine GARV infections are common in diarrheic piglets in South Korea. The infecting strains are genetically diverse, and include homologous (G5P[7]), heterologous (G8P[1]), and reassortant (G8P[7]), as well as emerging G9 GARV strains.
Keywords: Rotavirus, Prevalence, Genetic diversity, Reassortant
1. Introduction
Group A rotavirus (GARV), a member of the Reoviridae family, is one of the major pathogens that cause severe, acute dehydrating diarrhea in young children and in a wide variety of domestic animals (Estes and Kapikian, 2007, Gentsch et al., 2005, Glass et al., 1997). The rotavirus genome consists of 11 segments of double-stranded (ds) RNA enclosed in a trilaminar capsid and encodes six structural (VP1–VP4, VP6, and VP7) and six nonstructural proteins (NSP1–NSP6) (Estes and Kapikian, 2007, Gentsch et al., 2005, Parashar et al., 2006). Due to the segmented nature of the genome, GARVs can undergo genetic reassortment if two different GARVs of the same group co-infect one cell (Estes and Kapikian, 2007, Gentsch et al., 2005, Parashar et al., 2006).
Recently, a new rotavirus classification system was proposed, in which nucleotide percentage identity cut-off values define different genotypes for all the 11 genomic RNA segments (Matthijnssens et al., 2008). The VP7 and VP4 outer capsid proteins independently elicit neutralizing antibody responses and are used to classify GARVs into G (for glycoprotein) and P (for protease-sensitive) types (Ciarlet and Estes, 2002, Estes and Kapikian, 2007, Glass et al., 1997). Currently, 23 G and 31 P genotypes have been described for GARVs of humans and animals (Abe et al., 2009, Ursu et al., 2009). As many more reassortant or new genotypes are predicted to appear, continuous monitoring of circulating rotaviruses is important for improving regional epidemiological information and updating the vaccine strains.
Porcine GARVs can cause enormous economic losses in the swine industry and are a potential source of heterologous GARV infections in humans (Jain et al., 2001, Leite et al., 1996, Martella et al., 2005, Timenetsky et al., 1994, Unicomb et al., 1999). Thus, molecular epidemiology on porcine GARVs in South Korea is needed to determine the prevalence, as well as the extent of diversity in the circulating strains to improve vaccination programs by updating the vaccine strains. This paper reports the prevalence of porcine GARVs in diarrheic piglets, along with the genetic diversity of the porcine GARVs based on the characterization of the G and P genotypes.
2. Materials and methods
2.1. Specimens
From 2006 to 2007, 475 fecal specimens from diarrheic pigs were obtained from 143 farms across 6 provinces in South Korea during the spring (215 samples/53 farms), summer (86 samples/17 farms), autumn (69 samples/20 farms), and winter (105 samples/53 farms). The ages of the pigs tested from these provinces ranged from 3 to 70 days old. The fecal samples were examined for common bacterial enteric pathogens including Escherichia coli (E. coli) and Salmonella spp. using specific agar media. Brachyspira hyodysenteriae was detected by PCR with the specific primers B.hyo nest3 (5′-CTGCTGCCTTCTTCATAAAT-3′) and B.hyo nest 5 (5′-AAGAATGGGTATTGTTGCTG-3′) (La et al., 2003). For the extraction of viral RNA, fecal suspensions of each sample were prepared immediately by diluting the feces 1:10 in 0.01 M phosphate-buffered saline (PBS), pH 7.2. The suspensions were then vortexed for 30 s, centrifuged (1200 × g for 20 min), and then the supernatants were collected and stored at −80 °C until needed.
2.2. RNA extraction
The RNA was extracted from a 200 μl starting volume of centrifuged 10% fecal suspensions and from the lysates of GARV-infected fetal rhesus monkey kidney (TF-104) cells using the SV Total RNA Isolation System reagent (Promega Corporation, Madison, WI) according to the manufacturer's instructions. The total RNA recovered was suspended in 50 μl of RNase free water and stored at −80 °C until used.
2.3. RT-PCR and nested PCR
RT-PCR assays with different primer sets (Table 1 ) for the detection of porcine groups A–C rotaviruses (GARVs-GCRVs), porcine sapovirus (PSaV), porcine norovirus (PNoV), porcine torovirus (PToV), transmissible gastroenteritis coronavirus (TGEV), and porcine epidemic diarrhea coronavirus (PEDV) were performed using a standard one-step RT-PCR as previously described (Jeong et al., 2007). In order to increase the sensitivity and specificity of RT-PCR, nested PCR assays with the primer pairs specific to porcine GARV, GCRV and PSaV (Table 1) were performed as previously described (Jeong et al., 2007). The amplification products were analyzed by 1.5 or 2% agarose gel electrophoresis and visualized by UV after ethidium bromide staining.
Table 1.
The list of the oligonucleotide primers designed for detecting and sequencing.
| Target virusesa | Target geneb | Primer sequence, 5′ to 3′c | Region (nt) | Size (bp) |
|---|---|---|---|---|
| GARV | VP6 | F: AAA GAT GCT AGG GAC AAA ATT G | 58–78 | 308 |
| R: TTC AGA TTG TGG AGC TAT TCC A | 344–365 | |||
| nF: GAC AAA ATT GTC GAA GGC ACA TTA TA | 69–94 | 121 | ||
| nR: TCG GTA GAT TAC CAA TTC CTC CAG | 166–189 | |||
| VP4 | F: GCT TCG CTC ATT TAT AGA CA | 12–31 | 877 | |
| R: ATT TCG GAC CAT TTA TAA CC | 868–887 | |||
| VP7 | F: GGC TTT AAA AGA GAG AAT TTC | 1–21 | 1062 | |
| R: GGT CAC ATC ATA CAA TTC TAA | 1042–1062 | |||
| GBRV | NSP2 | F: CTA TTC AGT GTG TCG TGA GAG G | 18–40 | 434 |
| R: GCA GAC AAG CTA GCC CGC TTC G | 429–451 | |||
| GCRV | VP6 | F: CTC GAT GCT ACT ACA GAA TCA G | 994–1018 | 366 |
| R: AGC CAC ATA GTT CAC ATT TCA TCC | 1339–1359 | |||
| nF: CTC GAT GCT ACT ACA GAA TCA G | 994–1018 | 328 | ||
| nR: GGG ATC ATC CAC GTC ATG CGT | 1300–1321 | |||
| PSaV | RdRp | F: GTG CTC TAT TGC CTG GAC TA | 4312–4331 | 572 |
| R: TCT GTG GTG CGG TTA GCC TT | 4864–4883 | |||
| nF: GTG GTA TGC TGA GGA CAC AC | 4392–4411 | 380 | ||
| nR: GAG TGT CTG TTG GCT CAA TG | 4752–4771 | |||
| PSaV& | RdRp | F: GAT TAC TCC AAG TGG GAC TCC AC | 4568–4590 | 319 |
| PNoV | R: TGACAA TGT AAT ATC ACC ATA | 4865–4886 | ||
| PToV | N | F: GTCAGAATAGATCACGCATT | 170–189 | 185 |
| R: CGCCAAACTCTGCAACTCAGGTGGA | 330–354 | |||
| TGEVd | ORF1b | F GGG TAA GTT GCT CAT TAG AAA TAA TGG | 7968–7994 | 1006 |
| Spike | R: CTT CTT CAA AGC TAG GGA CTG | 920–940 | ||
| PEDV | N | F: AGG AAC GTG ACC TCA AAG ACA TCC C | 812–836 | 540 |
| R: CCA GGA TAA GCC GGT CTA ACA TTG | 1328–1351 | |||
GARV: group A rotavirus; GBRV: group B rotavirus; GCRV: group C rotavirus; PSaV: porcine sapovirus; PNoV: porcine norovirus; PToV: porcine torovirus; TGE: transmissible gastroenteritis coronavirus; PED: porcine epidemic diarrhea coronavirus.
RdRp: RNA dependent RNA polymerase; ORF: open reading frame; N: nucleocapsid.
F: upstream primer for RT-PCR; R: downstream primer for RT-PCR; nF: upstream primer for nested PCR; nR: downstream primer for nested PCR.
TGEV: forward primer was designed from the portion of TGEV ORF1b; reverse primer was designed from the portion of TGEV spike gene.
2.4. Virus isolation
Monolayers of TF-104 cells (a cloned derivative of MA-104 monkey kidney cells) grown for 3 or 4 days in 6-well plates were used to isolate GARVs, as previously described (Bohl et al., 1984, Park et al., 2006). The isolated GARVs were confirmed by direct immunofluorescence (IF) tests and RT-PCR (Bohl et al., 1984, Park et al., 2006).
2.5. DNA sequencing
To obtain genomic data on the G and P genotypes of Korean porcine GARVs, porcine GARVs isolated from the diarrheic fecal samples were subjected to RT-PCR with primer pairs specific to each VP7 and VP4 gene of GARVs (Table 1). RT-PCR products amplified by each primer pair were selected based on the intensity of the bands shown by agarose gel electrophoresis and ethidium bromide visualization. Before sequencing, the RT-PCR products from each gene fragment were purified using a QIAEX II Gel Extraction kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. DNA sequencing was carried out using an ABI system 3700 automated DNA sequencer (Applied Biosystems, Foster City, CA).
2.6. Molecular analysis
Using the DNA Basic module (DNAsis MAX, Alameda, CA), the nucleotide and deduced amino acid sequences of the partial VP4 gene (834 bp, devoid of primer pair sequences) and VP7 gene (1020 bp, devoid of primer pair sequences) were compared with those selected from other known GARVs (Table 2 ). Phylogenetic analysis based on the nucleotide alignments was constructed using the neighbor-joining method and the UPGMA method of Molecular Evolutionary Genetics analysis (MEGA version 4.0) with a pair-wise distance comparison (Tamura et al., 2007). A sequence similarity search was performed for the GARV VP4 and VP7 genes using the LALIGN Query program of the GENESTREAM network server at Institut de Génétique Humaine, Montpellier, France (http://www.eng.uiowa.edu/∼tscheetz/sequence-analysis/examples/LALIGN/lalign-guess.html).
Table 2.
Genbank accession numbers of Korea strains and the reference group A rotavirus strains used in phylogenetic analysis.
| Genes | Strains | Type | Species | Accession number | Genes | Strains | Type | Species | Accession number |
|---|---|---|---|---|---|---|---|---|---|
| VP4 | BRV033 | P[1] | Bovine | U62155 | VP4 | 19 | P[7] | Porcine | FJ870331 |
| NCDV | P[1] | Bovine | M63267 | 24 | P[7] | Porcine | FJ870332 | ||
| C486 | P[1] | Bovine | Y00127 | 25-1 | P[7] | Porcine | FJ870333 | ||
| RF | P[1] | Bovine | U65924 | 25-2 | P[7] | Porcine | FJ870334 | ||
| 11-1 | P[1] | Porcine | FJ807880 | 42-1 | P[7] | Porcine | FJ870335 | ||
| 66-1 | P[1] | Porcine | FJ870285 | 47-1 | P[7] | Porcine | FJ870336 | ||
| SA11 | P[2] | Simian | M23188 | 47-2 | P[7] | Porcine | FJ870337 | ||
| RRV | P[3] | Simian | M18736 | 49 | P[7] | Porcine | FJ870338 | ||
| RV5 | P[4] | Human | M32559 | 52 | P[7] | Porcine | FJ870339 | ||
| CJN-M | P[5] | Bovine | D16351 | 53 | P[7] | Porcine | FJ870340 | ||
| Gotffried | P[6] | Porcine | M33516 | 57 | P[7] | Porcine | FJ870341 | ||
| OSU | P[7] | Porcine | X13190 | 57-1 | P[7] | Porcine | FJ870342 | ||
| JL94 | P[7] | Porcine | AY523636 | 61-1 | P[7] | Porcine | FJ870343 | ||
| SW20/21 | P[7] | Porcine | AF427125 | 63-1 | P[7] | Porcine | FJ870344 | ||
| PP-1 | P[7] | Bovine | AF427520 | 71 | P[7] | Porcine | FJ870345 | ||
| 06-6-1 | P[7] | Porcine | FJ870288 | 71-1 | P[7] | Porcine | FJ870346 | ||
| 06-10-1 | P[7] | Porcine | FJ870289 | 74-1 | P[7] | Porcine | FJ870347 | ||
| 06-12-1 | P[7] | Porcine | FJ870290 | 75-1 | P[7] | Porcine | FJ870348 | ||
| 06-14-1 | P[7] | Porcine | FJ870291 | 78-1 | P[7] | Porcine | FJ870349 | ||
| 06-22-1 | P[7] | Porcine | FJ870292 | 80-1 | P[7] | Porcine | FJ870350 | ||
| 06-42-2 | P[7] | Porcine | FJ870293 | 82-1 | P[7] | Porcine | FJ870351 | ||
| 06-44-2 | P[7] | Porcine | FJ870294 | 85 | P[7] | Porcine | FJ870352 | ||
| 06-46-2 | P[7] | Porcine | FJ870295 | 85-1 | P[7] | Porcine | FJ870353 | ||
| 06-54-1 | P[7] | Porcine | FJ870296 | 90-1 | P[7] | Porcine | FJ870354 | ||
| 06-61-3 | P[7] | Porcine | FJ870297 | 95-1 | P[7] | Porcine | FJ870355 | ||
| 06-121 | P[7] | Porcine | FJ870298 | 97-1 | P[7] | Porcine | FJ870356 | ||
| 06-176-10 | P[7] | Porcine | FJ870299 | 100-1 | P[7] | Porcine | FJ870357 | ||
| 06-235 | P[7] | Porcine | FJ870300 | 104-1 | P[7] | Porcine | FJ870358 | ||
| 06-258-1 | P[7] | Porcine | FJ870301 | 115-1 | P[7] | Porcine | FJ870359 | ||
| 06-261-4 | P[7] | Porcine | FJ870302 | 122-1 | P[7] | Porcine | FJ870360 | ||
| 07-2 | P[7] | Porcine | FJ870303 | 131 | P[7] | Porcine | FJ870361 | ||
| 07-08-1 | P[7] | Porcine | FJ870304 | 140-1 | P[7] | Porcine | FJ870362 | ||
| 07-10-1 | P[7] | Porcine | FJ870305 | 141-1 | P[7] | Porcine | FJ870363 | ||
| 07-12-3 | P[7] | Porcine | FJ870306 | 150-1 | P[7] | Porcine | FJ870364 | ||
| 07-13-2 | P[7] | Porcine | FJ870307 | 156-1 | P[7] | Porcine | FJ870365 | ||
| 07-14-1 | P[7] | Porcine | FJ870308 | 157-1 | P[7] | Porcine | FJ870366 | ||
| 07-15-1 | P[7] | Porcine | FJ870309 | 174-1 | P[7] | Porcine | FJ870367 | ||
| 07-16-1 | P[7] | Porcine | FJ870310 | 187-1 | P[7] | Porcine | FJ870368 | ||
| 07-17-1 | P[7] | Porcine | FJ870311 | 205-1 | P[7] | Porcine | FJ870369 | ||
| 07-17-2 | P[7] | Porcine | FJ870312 | 208-1 | P[7] | Porcine | FJ870370 | ||
| 07-25 | P[7] | Porcine | FJ870313 | 210-1 | P[7] | Porcine | FJ870371 | ||
| 07-26-1 | P[7] | Porcine | FJ870314 | A-1 | P[7] | Porcine | FJ870372 | ||
| 07-28-7 | P[7] | Porcine | FJ870315 | B-1 | P[7] | Porcine | FJ870373 | ||
| 07-33-2 | P[7] | Porcine | FJ870316 | C-1 | P[7] | Porcine | FJ870374 | ||
| 07-61-3 | P[7] | Porcine | FJ870317 | D-1 | P[7] | Porcine | FJ870375 | ||
| 07-74-1 | P[7] | Porcine | FJ870318 | E-1 | P[7] | Porcine | FJ870376 | ||
| 07-95-1 | P[7] | Porcine | FJ870319 | H-1 | P[7] | Porcine | FJ870377 | ||
| 07-109-8 | P[7] | Porcine | FJ870320 | I-1 | P[7] | Porcine | FJ870378 | ||
| 07-117-2 | P[7] | Porcine | FJ870321 | Wa | P[8] | Human | L34161 | ||
| 07-134-7 | P[7] | Porcine | FJ870322 | K8 | P[9] | Human | D90260 | ||
| 07-214-1 | P[7] | Porcine | FJ870323 | 69M | P10] | Human | M60600 | ||
| 1 | P[7] | Porcine | FJ870324 | KK3 | P11] | Bovine | D13393 | ||
| 2 | P[7] | Porcine | FJ870325 | FI23 | P[12] | Equine | D16342 | ||
| 3 | P[7] | Porcine | FJ870326 | MDR13 | P[13] | Porcine | L07886 | ||
| 4 | P[7] | Porcine | FJ870327 | Sun9 | P[14] | Bovine | AB158430 | ||
| 8-1 | P[7] | Porcine | FJ870328 | LP14 | P[15] | Ovine | L11599 | ||
| 8-2 | P[7] | Porcine | FJ870329 | Eb | P[16] | Murine | L18992 | ||
| 16 | P[7] | Porcine | FJ870330 | PO-13 | P[17] | Pigion | AB009632 | ||
| VP4 | L338 | P[18] | Equine | D13399 | VP7 | 74-1 | G[5] | Porcine | FJ807831 |
| MC345 | P[19] | Human | D38054 | 75-1 | G[5] | Porcine | FJ807832 | ||
| EHP | P[20] | Murine | U08424 | 78-1 | G[5] | Porcine | FJ807833 | ||
| Hg18 | P[21] | Bovine | AF237665 | 80-1 | G[5] | Porcine | FJ807834 | ||
| 160/01 | P[22] | Lapine | AF526374 | 82-1 | G[5] | Porcine | FJ807835 | ||
| A34 | P[23] | Porcine | AY174094 | 85 | G[5] | Porcine | FJ807836 | ||
| JP32-4 | P[23] | Porcine | AB176689 | 85-1 | G[5] | Porcine | FJ807837 | ||
| Hokkaido-14 | P[23] | Porcine | AB176684 | 95-1 | G[5] | Porcine | FJ807838 | ||
| 06-52-1 | P[23] | Porcine | FJ870286 | 97-1 | G[5] | Porcine | FJ807839 | ||
| 06-285 | P[23] | Porcine | FJ870287 | 100-1 | G[5] | Porcine | FJ807840 | ||
| TUCH | P[24] | Simian | AY596189 | 104-1 | G[5] | Porcine | FJ807841 | ||
| Dhaka6 | P[25] | Human | AY773004 | 110-1 | G[5] | Porcine | FJ807842 | ||
| 134/04-15 | P[26] | Porcine | DQ061053 | 115-1 | G[5] | Porcine | FJ807843 | ||
| CMP034 | P[27] | Porcine | DQ534016 | 122-1 | G[5] | Porcine | FJ807844 | ||
| ECU534 | P[28] | Bovine | EU805773 | 131 | G[5] | Porcine | FJ807845 | ||
| Azuk-1 | P[29] | Bovine | AB454420 | 140-1 | G[5] | Porcine | FJ807846 | ||
| Ch-02V0002G3 | P[30] | Chicken | EU486965 | 150-1 | G[5] | Porcine | FJ807847 | ||
| Ch-06V0661 | P[31] | Chicken | EU486962 | 187-1 | G[5] | Porcine | FJ807848 | ||
| VP7 | Wa | G[1] | Human | M21843 | 205-1 | G[5] | Porcine | FJ807849 | |
| S2 | G[2] | Human | M11164 | 210-1 | G[5] | Porcine | FJ807850 | ||
| RRV | G[3] | Simian | Z32535 | B-1 | G[5] | Porcine | FJ807851 | ||
| Gotffried | G[4] | Porcine | X06386 | E-1 | G[5] | Porcine | FJ807852 | ||
| OSU | G[5] | Porcine | X04613 | I-1 | G[5] | Porcine | FJ807853 | ||
| JL94 | G[5] | Porcine | AY538665 | NCDV | G[6] | Bovine | M12394 | ||
| KJ44 | G[5] | Bovine | DQ494393 | Erv99 | G[6] | Equine | DQ981478 | ||
| 06-6-1 | G[5] | Porcine | FJ807788 | Ch2 | G[7] | Avian | X56784 | ||
| 06-10-1 | G[5] | Porcine | FJ807789 | BRV16 | G[8] | Bovine | AB077058 | ||
| 06-12-1 | G[5] | Porcine | FJ807790 | Sun9 | G[8] | Bovine | AB158431 | ||
| 06-61-3 | G[5] | Porcine | FJ807791 | KAG80 | G[8] | Bovine | AB077055 | ||
| 06-258-1 | G[5] | Porcine | FJ807792 | NGRBg8 | G[8] | Bovine | AF361439 | ||
| 07-08-1 | G[5] | Porcine | FJ807793 | 06-46-2 | G[8] | Porcine | FJ807854 | ||
| 07-10-1 | G[5] | Porcine | FJ807794 | 06-54-1 | G[8] | Porcine | FJ807879 | ||
| 07-12-3 | G[5] | Porcine | FJ807795 | 06-176-10 | G[8] | Porcine | FJ807855 | ||
| 07-14-1 | G[5] | Porcine | FJ807796 | 06-261-4 | G[8] | Porcine | FJ807856 | ||
| 07-15-1 | G[5] | Porcine | FJ807797 | 07-28-7 | G[8] | Porcine | FJ807857 | ||
| 07-16-1 | G[5] | Porcine | FJ807798 | 07-109-8 | G[8] | Porcine | FJ807858 | ||
| 07-17-1 | G[5] | Porcine | FJ807799 | 07-134-7 | G[8] | Porcine | FJ807859 | ||
| 07-17-2 | G[5] | Porcine | FJ807800 | 11-1 | G[8] | Porcine | FJ807860 | ||
| 07-20-1 | G[5] | Porcine | FJ807801 | 42-1 | G[8] | Porcine | FJ807861 | ||
| 07-25 | G[5] | Porcine | FJ807802 | 141-1 | G[8] | Porcine | FJ807862 | ||
| 07-26-1 | G[5] | Porcine | FJ807803 | 156-1 | G[8] | Porcine | FJ807863 | ||
| 07-33-2 | G[5] | Porcine | FJ807804 | 157-1 | G[8] | Porcine | FJ807864 | ||
| 07-61-3 | G[5] | Porcine | FJ807805 | 174-1 | G[8] | Porcine | FJ807865 | ||
| 07-74-1 | G[5] | Porcine | FJ807806 | 208-1 | G[8] | Porcine | FJ807866 | ||
| 07-95-1 | G[5] | Porcine | FJ807807 | A-1 | G[8] | Porcine | FJ807867 | ||
| 07-95-3 | G[5] | Porcine | FJ807808 | C-1 | G[8] | Porcine | FJ807868 | ||
| 07-117-2 | G[5] | Porcine | FJ807809 | D-1 | G[8] | Porcine | FJ807869 | ||
| 07-214-1 | G[5] | Porcine | FJ807810 | W161 | G[9] | Human | EF672623 | ||
| 3 | G[5] | Porcine | FJ807811 | Au32 | G[9] | Human | AB045372 | ||
| 4 | G[5] | Porcine | FJ807812 | F45 | G[9] | Human | AB180970 | ||
| 8-1 | G[5] | Porcine | FJ807813 | 116E | G[9] | Human | L14072 | ||
| 8-2 | G[5] | Porcine | FJ807814 | 95H115 | G[9] | Human | AB045373 | ||
| 16 | G[5] | Porcine | FJ807815 | 97CM108 | G[9] | Human | AY866504 | ||
| 19 | G[5] | Porcine | FJ807816 | MW69 | G[9] | Human | AJ250545 | ||
| 24 | G[5] | Porcine | FJ807817 | N23 | G[9] | Human | AJ491177 | ||
| 25-1 | G[5] | Porcine | FJ807818 | 3710CM | G[9] | Human | AY816184 | ||
| 25-2 | G[5] | Porcine | FJ807819 | US1205 | G[9] | Human | AF060487 | ||
| 47-1 | G[5] | Porcine | FJ807820 | US321 | G[9] | Human | AJ250275 | ||
| 47-2 | G[5] | Porcine | FJ807821 | BS1414/02 | G[9] | Human | DQ822599 | ||
| 49 | G[5] | Porcine | FJ807822 | 6222LP | G[9] | Human | AF529871 | ||
| 52 | G[5] | Porcine | FJ807823 | PH301 | G[9] | Human | AJ491184 | ||
| 53 | G[5] | Porcine | FJ807824 | BD524 | G[9] | Human | AJ250543 | ||
| 57 | G[5] | Porcine | FJ807825 | R136 | G[9] | Human | AF438228 | ||
| 57-1 | G[5] | Porcine | FJ807826 | Bulumkutu | G[9] | Human | AF359358 | ||
| 61-1 | G[5] | Porcine | FJ807827 | 3298CM | G[9] | Human | DQ647423 | ||
| 63-1 | G[5] | Porcine | FJ807828 | MD28 | G[9] | Human | AB297791 | ||
| 71 | G[5] | Porcine | FJ807829 | CAU202 | G[9] | Human | EF059922 | ||
| 71-1 | G[5] | Porcine | FJ807830 | KNIH-13 | G[9] | Human | DQ990319 | ||
| VP7 | KUMS04-102 | G[9] | Human | DQ056300 | VP7 | 06-44-2 | G[9] | Porcine | FJ807874 |
| E192 | G[9] | Human | EU708592 | 06-52-1 | G[9] | Porcine | FJ807875 | ||
| E205 | G[9] | Human | EU708591 | 06-121 | G[9] | Porcine | FJ807876 | ||
| L865 | G[9] | Human | EU708599 | 06-235 | G[9] | Porcine | FJ807877 | ||
| L880 | G[9] | Human | EU708601 | 06-285 | G[9] | Porcine | FJ807878 | ||
| CMP003 | G[9] | Porcine | AY707787 | 1 | G[9] | Porcine | FJ807870 | ||
| 97'SZ | G[9] | Human | EU486975 | 2 | G[9] | Porcine | FJ807871 | ||
| OM46 | G[9] | Human | AJ491181 | B223 | G[10] | Bovine | X57852 | ||
| OM67 | G[9] | Human | AJ491179 | YM | G[11] | Porcine | M23194 | ||
| Hokkaido-14 | G[9] | Porcine | AB176677 | L26 | G[12] | Human | M58290 | ||
| JP3-6 | G[9] | Porcine | AB176678 | L338 | G[13] | Equine | D13549 | ||
| JP13-3 | G[9] | Porcine | AB176679 | FI23 | G[14] | Equine | M61876 | ||
| JP16-3 | G[9] | Porcine | AB176680 | Hg18 | G[15] | Bovine | AF237666 | ||
| JP29-6 | G[9] | Porcine | AB176681 | EW | G[16] | Murine | U08430 | ||
| JP32-4 | G[9] | Porcine | AB176682 | Ty1 | G[17] | Turkey | D82980 | ||
| JP35-7 | G[9] | Porcine | AB176683 | PO-13 | G[18] | Pigion | D82979 | ||
| T203 | G[9] | Human | AY003871 | 02V0002G3 | G[19] | Chicken | FJ169859 | ||
| K-1 | G[9] | Human | AB045374 | Ecu534 | G[20] | Bovine | Ecu805775 | ||
| 99-Sp1904 | G[9] | Human | AB091754 | Azuk-1 | G[21] | Bovine | AB454421 | ||
| 06-22-1 | G[9] | Porcine | FJ807872 | Tu-03V0002E10 | G[22] | Turkey | EU486973 | ||
| 06-42-2 | G[9] | Porcine | FJ807873 | HUN | G[23] | Pheasant | FN393056 |
3. Results
3.1. Prevalence of porcine GARVs in pigs with diarrhea in South Korea
In order to determine the prevalence of porcine GARVs in diarrheic Korean piglets, a total of 475 fecal samples from diarrheic pigs in 143 farms across South Korea were screened by RT-PCR and nested PCR using two sets of primer pairs (Table 1). By RT-PCR, 106 out of 475 diarrheic fecal samples tested positive for porcine GARVs. In nested PCR, an additional 76 samples were found to be positive for porcine GARVs. Overall, 182 (38.3%) out of 475 diarrheic fecal samples were positive for porcine GARVs (Table 3 ).
Table 3.
Summary of enteric pathogens present in the fecal samples obtained from pigs with diarrhea (2006–2007).
| Enteric pathogens presenta | No. of samples (%)b |
|---|---|
| GARV alone | 58 (12.21) |
| GARV plus GBRV | 5 (1.05) |
| GARV plus GCRV | 49 (10.32) |
| GARV plus PSaV | 2 (0.42) |
| GARV plus PToV | 2 (0.42) |
| GARV plus E. coli | 11 (2.32) |
| GARV plus Salmonella | 7 (1.47) |
| GARV, GCRV plus PSaV | 11 (2.32) |
| GARV, GCRV plus PToV | 7 (1.47) |
| GARV, GBRV plus E. coli | 1 (0.21) |
| GARV, GCRV plus E. coli | 8 (1.68) |
| GARV, GCRV plus Salmonella | 12 (2.53) |
| GARV, PSaV plus E. coli | 1 (0.21) |
| GARV, GCRV plus Brachyspira hyodysenteriae | 1 (0.21) |
| GARV, PSaV plus Brachyspira hyodysenteriae | 1 (0.21) |
| GARV, GBRV, GCRV plus PSaV | 1 (0.21) |
| GARV, GCRV, PSaV plus E. coli | 1 (0.21) |
| GARV, GCRV, PSaV plus Salmonella | 2 (0.42) |
| GARV, GCRV, PToV plus Salmonella | 1 (0.21) |
| GARV, GBRV, GCRV, PToV plus PSaV | 1 (0.21) |
| Other enteric pathogens detected | 168 (35.37) |
| No enteric pathogens detected | 125 (26.32) |
| Total | 475 (100) |
GARV: group A rotavirus; GBRV: group B rotavirus; GCRV: group C rotavirus; PSaV: porcine sapovirus; PToV: porcine torovirus.
Number of positive fecal samples.
3.2. Other enteric pathogens
Of the 182 porcine GARV-positive diarrheic fecal specimens, 58 fecal samples (12.2%) tested positive only for the porcine GARVs, while the other 124 fecal samples (26.1%) were also positive for other enteric pathogens, including GBRV, GCRV, PSaV, PToV, E. coli, Salmonella and B. hyodysenteriae (Table 3). In addition, 168 fecal specimens (35.4%) that tested negative for porcine GARVs were positive for other enteric pathogens (Table 3). No enteric pathogens were detected in the remaining 125 fecal samples (26.3%).
3.3. Seasonal distribution of porcine GARVs in diarrheic piglets in South Korea
Porcine GARV infections were more prevalent in fecal samples of pigs in summer than in the other seasons: 87 (40.5%) out of 215 fecal samples were positive in spring; 43 (50.0%) out of 86 fecal samples were positive in summer; 17 (24.6%) out of 69 fecal samples were positive in autumn; and 35 (33.3%) out of 105 fecal samples were positive in winter.
3.4. Virus isolation in TF-104 cells
Of the 182 porcine GARV-positive fecal samples by RT-PCR or nested PCR, porcine GARVs were isolated from 98 fecal samples. After the second or third passage, cytopathic effect (CPE), characterized by rounded and detached cells in clusters, was observed in the cultures inoculated with each fecal sample from diarrheic piglets at post inoculation days 1–2. No differences in CPEs were observed among the isolates. CPE was not observed in the mock-infected TF-104 cells. The direct IF test detected GARV-specific cytoplasmic fluorescence in the TF-104 cells inoculated with each of these samples at the second or third passage. A specific band was detected after amplification of all isolates using a RT-PCR assay targeting a 308 bp fragment of the VP6 gene of GARVs.
3.5. Sequence and phylogenetic analysis of VP7 gene
Using RT-PCR to amplify full length sequence (1062 nucleotides in length) of the VP7 gene, amplicons could be achieved for 92 out of 98 strains and could be sequenced. A comparison of the nucleotide and deduced amino acid sequences of the VP7 gene between all Korean porcine GARV strains and the GARV strains representing all 23 G genotypes was performed with a 1020 bp fragment (excluding the primer sequences) (Table 4, Table 5 ).
Table 4.
Nucleotide and deduced amino acid sequences comparison of the VP7 of 83 Korean porcine rotavirus strains with those of the G5 and G8 serotypes.
| Strain | G type | Origin | % identity with strains: 66 Korea strains |
% identity with strains: 17 Korea strains |
||
|---|---|---|---|---|---|---|
| nt | aa | nt | aa | |||
| OSU | G5 | Porcine | 98.2–99.8 | 95.4–99.9 | 75.4–77.7 | 78.5–82.4 |
| JL94 | G5 | Porcine | 98.5–99.7 | 96.6–100 | 75.5–77.8 | 78.8–82.7 |
| KJ44 | G5 | Bovine | 97.6–98.9 | 94.5–97.9 | 74.6–77.9 | 77.2–80.1 |
| BRV16 | G8 | Bovine | 75.8–76.9 | 77.7–80.8 | 87.7–97.8 | 90.9–98.2 |
| Sun9 | G8 | Bovine | 76.0–77.1 | 79.4–81.6 | 87.7–95.1 | 93.5–97.7 |
| KAG80 | G8 | Bovine | 74.80–75.9 | 77.9–80.1 | 87.2–96.1 | 91.7–96.6 |
| NGRBg8 | G8 | Bovine | 75.9–76.7 | 78.8–81.3 | 84.7–86.1 | 91.7–96.9 |
Table 5.
Nucleotide and deduced amino acid sequences comparison of the G9 of 9 Korean porcine rotavirus strains with those of the other lineages.
| Strain | Lineage | Origin | % identity with strains: 9 Korea strains |
Strain | Lineage | Origin | % identity with strains: 9 Korea strains |
||
|---|---|---|---|---|---|---|---|---|---|
| nt | aa | nt | aa | ||||||
| W161 | L1 | Human | 87.0–89.7 | 89.9–96.0 | KNIH-13 | L3 | Human | 90.1–93.0 | 91.1–97.0 |
| Au32 | L1 | Human | 87.0–89.9 | 89.3–95.4 | KUMS04-102 | L3 | Human | 90.0–92.9 | 90.8–96.7 |
| F45 | L1 | Human | 87.3–90.1 | 89.9–95.7 | E192 | L3 | Human | 89.8–92.7 | 94.8–96.0 |
| 116E | L2 | Human | 85.3–88.1 | 87.1–93.3 | E205 | L3 | Human | 89.8–92.7 | 90.8–95.4 |
| 95H115 | L3 | Human | 90.1–92.9 | 91.4–97.2 | L865 | L3 | Human | 89.9–92.8 | 91.1–96.9 |
| 97CM108 | L3 | Human | 89.3–92.1 | 90.8–96.6 | L880 | L3 | Human | 90.1–93.0 | 91.1–96.9 |
| MW69 | L3 | Human | 90.2–93.0 | 91.4–97.2 | CMP003 | L3 | Porcine | 89.1–91.8 | 90.2–95.7 |
| N23 | L3 | Human | 90.0–92.8 | 91.1–96.9 | 97'SZ | L4 | Human | 87.7–89.9 | 90.5–96.1 |
| 3710CM | L3 | Human | 90.0–92.8 | 91.1–96.9 | OM46 | L5 | Human | 86.5–89.1 | 90.5–96.3 |
| US1205 | L3 | Human | 89.9–92.8 | 91.4–97.2 | OM67 | L5 | Human | 86.7–89.7 | 90.5–96.3 |
| US321 | L3 | Human | 89.9–92.7 | 91.4–97.2 | Hokkaido-14 | L6 | Porcine | 90.4–93.1 | 91.7–97.5 |
| BS1414/02 | L3 | Human | 90.0–92.6 | 90.6–96.4 | JP3-6 | L6 | Porcine | 89.8–92.6 | 90.8–96.6 |
| 6222LP | L3 | Human | 89.8–92.4 | 91.1–96.0 | JP13-3 | L6 | Porcine | 90.1–92.8 | 90.2–96.3 |
| PH301 | L3 | Human | 90.0–92.8 | 91.1–96.0 | JP16-3 | L6 | Porcine | 94.1–97.2 | 93.3–99.1 |
| BD524 | L3 | Human | 88.7–91.5 | 89.0–94.5 | JP29-6 | L6 | Porcine | 89.9–92.6 | 90.8–96.6 |
| R136 | L3 | Human | 90.3–93.2 | 91.4–97.2 | JP32-4 | L6 | Porcine | 89.3–92.1 | 90.5–96.0 |
| Bulumkutu | L3 | Human | 89.9–92.6 | 90.8–96.6 | JP35-7 | L6 | Porcine | 89.9–92.6 | 90.2–96.3 |
| 3298CM | L3 | Human | 89.9–92.8 | 90.2–96.0 | T203 | L6 | Human | 91.7–94.6 | 90.5–96.7 |
| MD28 | L3 | Human | 89.7–92.7 | 89.9–95.7 | K-1 | L6 | Human | 91.1–94.1 | 90.8–96.7 |
| CAU202 | L3 | Human | 90.0–92.9 | 92.0–97.9 | 99-Sp1904 | L6 | Human | 91.3–94.3 | 91.4–97.3 |
Sixty-six Korean strains showed high nucleotide (97.6–99.8%) and deduced amino acid (94.5–100%) identities with the G5 strains, which include the porcine OSU and JL94 strains, and the bovine KJ44 strains. On the other hand, these strains had comparatively lower nucleotide (63.4–83.2%) and deduced amino acid (54.2–89.8%) identities with the other G genotypes (data not shown). Phylogenetic analysis also confirmed that the VP7 gene of 66 Korean porcine GARV strains was closely related to the G5 strains and clustered with the porcine G5 strains (Fig. 1A). Seventeen of the 92 Korean porcine GARV strains had 84.7–97.8% nucleotide and 90.9–98.2% deduced amino acid identities to the G8 GARVs including the bovine BRV16, Sun9, KAG80, and NGRBg8 strains (Table 4), whereas they showed relatively lower nucleotide (61.8–78.7%) and deduced amino acid (54.4–84.7%) identities with other G genotypes (data not shown). Phylogenetically, these strains are grouped with G8 strains, including the bovine BRV16, Sun9, KAG80, and NGRBg8 strains. The remaining nine Korean porcine GARV strains showed high nucleotide (85.3–97.2%) and deduced amino acid (87.1–99.1%) identities with the G9 strains (Table 5). In contrast, these strains shared lower nucleotide (60.7–80.4%) and deduced amino acid (53.9–87.2%) identities with the other G genotypes (data not shown). Phylogenetic analysis showed that all G9 Korean porcine strains clustered with those of lineage VI. In addition, all Korean human G9 strains were found to be grouped with those of lineage III (Fig. 1B).
Fig. 1.
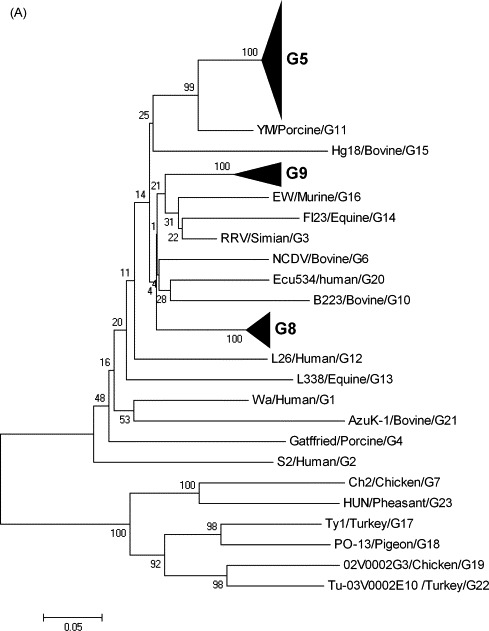
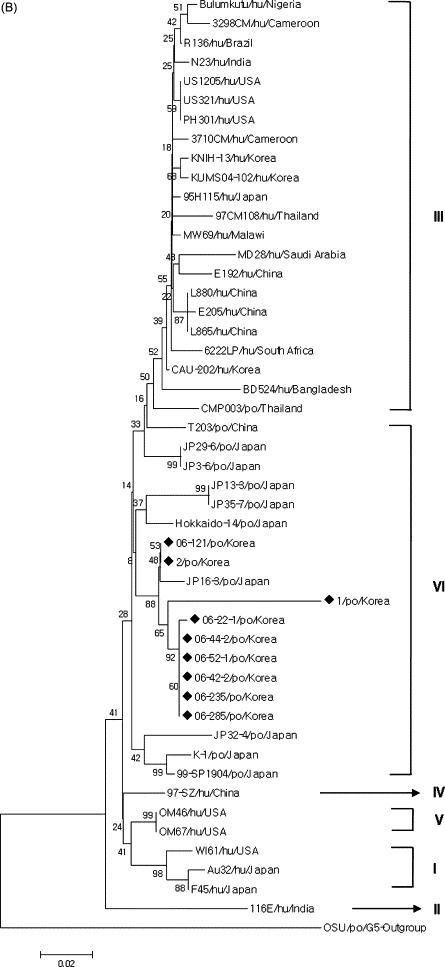
(A) Phylogenetic tree of the complete VP7 genes of the sixty-six G5, seventeen G8, and nine G9 strains of Korean porcine GARVs indicating their genetic relationships with other G genotypes. Black triangles contain rotavirus G5, G8, and G9 strains. (B) A detailed phylogenetic tree of the complete VP7 genes of the nine Korean porcine G9 strains with other known G9 strains indicating their genetic relationships with other known VI lineages of G9 genotype. Reference sequences used in the analysis (A and B) were obtained from the GenBank database (Table 2).
3.6. Sequence and phylogenetic analysis of VP4 gene
A part of the VP4 gene (874 nucleotides in length) was able to be amplified in 95 out of 98 isolated strains. The nucleotide and deduced amino acid sequences encoding 290 amino acids representing VP8* and the amino terminus of VP5* of the 95 strains were compared with GARV strains representing all the 31 P genotypes. Of the 95 Korean strains, 91 had high nucleotide (88.7–99.8%) and deduced amino acid (91.0–99.3%) identities with the P[7] GARVs including the porcine OSU, JL94, and SW20/21 strains, and the bovine PP-1 strain (Table 6 ), but less than 73.8% nucleotide and 79.4% deduced amino acid identities with the other P genotypes (data not shown). Phylogenetic analysis of the VP4 gene provided a molecular basis for their similarity to the P[7] genotype strains (Fig. 2 ). The sequences of the Korean strains, 11-1 and 66-1, were most closely related to the bovine BRV033, NCDV, C486, and RF strains, representing the P[1] genotype with 93.0–99.1% nucleotide and 94.5–99.3% deduced amino acid identities (Table 6). In contrast, these strains showed lower nucleotide (53.0–76.1%) and deduced amino acid (43.6–80.4%) identities to the representatives of other P genotypes (data not shown). Phylogenetically, these two strains clustered with those of the P[1] genotype (Fig. 2). The remaining two strains, 06-52-1 and 06-285, shared high nucleotide (84.5–89.7%) and deduced amino acid (94.1–95.1%) identities to the P[23] strains (A34, JP32-4, and Hokkaido-14 strains) (Table 6), but less than 73.9% nucleotide and 82.1% deduced amino acid identities compared to representatives of the other P genotypes (data not shown). Phylogenetic analysis of the VP4 gene showed that these strains were grouped with those of the P[23] genotype (Fig. 2).
Table 6.
Nucleotide and deduced amino acid sequences similarities of the VP4 of 95 Korean porcine rotavirus strains with those of the P[1], P[7] and P[23] genotypes.
| Strain | P type | Origin | % identity with strains: 91 Korea strains |
% identity with strains: 2 Korea strains |
% identity with strains: 2 Korea strains |
|||
|---|---|---|---|---|---|---|---|---|
| nt | aa | nt | aa | nt | aa | |||
| BRV033 | P[1] | Bovine | 69.5–69.8 | 73.1–73.9 | 93.0–93.4 | 94.5–94.9 | 70.1 | 74.3 |
| NCDV | P[1] | Bovine | 71.2–73.1 | 73.9–79.0 | 98.1–99.1 | 96.2–99.3 | 72.3 | 79.7 |
| C486 | P[1] | Bovine | 72.2–73.0 | 75.9–78.7 | 97.4–98.2 | 95.2–97.9 | 71.9 | 79.0 |
| RF | P[1] | Bovine | 72.5–73.3 | 75.5–78.4 | 97.6–98.6 | 95.2–98.3 | 72.3 | 79.4 |
| OSU | P[7] | Porcine | 92.4–99.8 | 93.1–99.3 | 71.7–73.0 | 74.9–77.7 | 71.7–71.9 | 78.0 |
| JL94 | P[7] | Porcine | 92.4–99.7 | 92.8–99.3 | 72.1–73.3 | 75.6–78.4 | 71.7–71.9 | 78.0 |
| SW20/21 | P[7] | Porcine | 92.2–98.2 | 92.9–98.1 | 72.7–73.1 | 77.8 | 72.0–72.1 | 77.1 |
| PP-1 | P[7] | Bovine | 88.7–93.0 | 91.0–96.6 | 73.0–73.1 | 78.9–79.3 | 69.8–70.0 | 77.4 |
| A34 | P[23] | Porcine | 69.6–71.2 | 70.3–74.1 | 73.5–73.7 | 76.6–77.0 | 89.6–89.7 | 94.1 |
| JP32-4 | P[23] | Porcine | 70.8–71.5 | 72.2–75.6 | 72.5 | 78.2 | 89.5–89.6 | 95.1 |
| Hokkaido-14 | P[23] | Porcine | 70.1–71.3 | 72.6–75.9 | 71.9–72.1 | 77.4 | 84.5–84.6 | 94.7 |
Fig. 2.
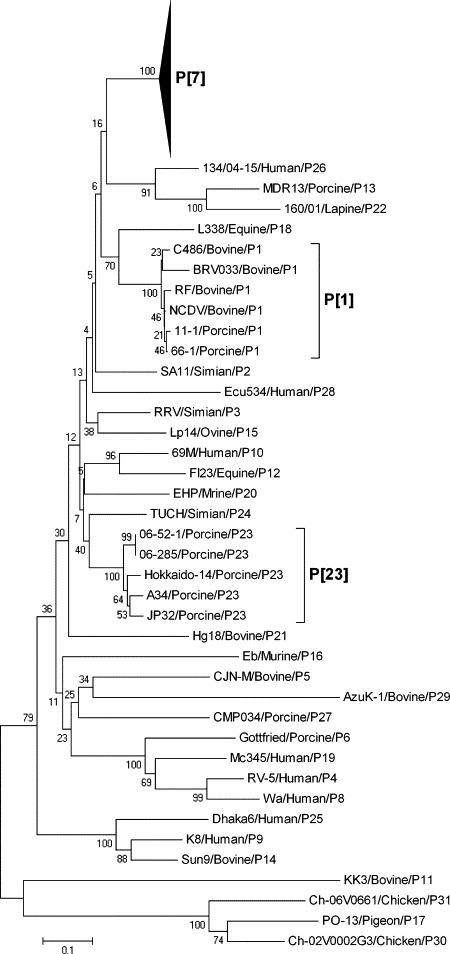
Phylogenetic tree of the VP4 gene of the ninety-five porcine rotavirus strains indicating their genetic relationships with other known P genotypes. Reference sequences used in the analysis were obtained from the GenBank database (Table 2).
3.7. Combinations of G and P genotypes
Based on the sequence and phylogenetic analyses of 98 Korean porcine GARVs, G and P genotype combinations were determined in the Korean porcine GARVs (Table 7 ). The most common combination of G and P genotypes was G5P[7], which was detected in 63 GARVs. Sixteen GARVs had the G8P[7] combination, while G9P[7] GARVs were detected in 7 strains. Two GARVs showed the G9P[23] combination, and one strain had the G8P[1] combination. In addition, the counterparts of G and P genotypes were not determined in three G5, five P[7], and one P[1] GARV strains (Table 7).
Table 7.
Combinations of G and P genotypes of 98 Korean porcine rotaviruses.
| Genotypes | G5 | G8 | G9 | Unknown |
|---|---|---|---|---|
| P[1] | 0 | 1 | 0 | 1 |
| P[7] | 63 | 16 | 7 | 5 |
| P[23] | 0 | 0 | 2 | 0 |
| Unknown | 3 | 0 | 0 | 0 |
4. Discussion
Epidemiological information related to the prevalence and genotype specificities of porcine GARVs are beneficial for the development of effective vaccines (Rosen et al., 1994). Therefore, we investigated the prevalence of porcine GARV infections as well as their genotype diversities in South Korea. The fecal prevalence of porcine GARV infections in diarrheic piglets has been reported to be 3.3% in Argentina (Parra et al., 2008), 4% in Southern Germany (Wieler et al., 2001), 9.2% in Canada (Morin et al., 1983), 22.3% in Thailand (Khamrin et al., 2007) and 35.3% in Brazil (Rácz et al., 2000). In this study, porcine GARV infections in South Korea were found widespread and highly prevalent at 38.3%, similar to Brazil at 35.3% (Rácz et al., 2000). This suggests that porcine GARV infections are epidemic in diarrheic piglets in South Korea. This is the first large-scale, epidemiological study on the prevalence of porcine GARV infections in diarrheic piglets in South Korea.
Epidemiological studies have demonstrated that five G genotypes (G3, G4, G5, G9, and G11) in combination with six dominant P genotypes (P[6], P[7], P[13], P[19], P[23], and P[26]) are the most frequent VP7 and VP4 types associated with porcine GARV infections (Kobayashi et al., 2007). In this study, two-thirds of the VP7 and VP4 genotypes were comprised of G5 and P[7] genotypes, respectively. The other G and P genotypes including G8, G9, P[1], and P[23] were a minority of the VP7 and VP4 genotypes. However, G3, G4, G11, P[6], P[13], P[19], and P[26] genotypes, which were known to be common, were not detected in this study. It is unclear whether the data in this study exactly reflected the true prevalence of G and P genotypes in the field farms due to the difficulty in cultivating some rotaviruses in cell culture (Zaberezhny et al., 1994). For example, P[6] porcine GARVs are quite common in nature, but are not usually cultivatable (Martella et al., 2006, Zaberezhny et al., 1994). Since we analyzed only cell culture cultivated porcine GARV strains, future studies should use the fecal samples for G and P genotyping of porcine GARVs to generate a more accurate picture of GARV genotypes in South Korea (Zaberezhny et al., 1994).
In this study, we isolated 17 G8 GARVs (17.3%) in combination with P[1] and P[7] across South Korea, which were ranked the second most frequently detected G and P types, indicating that these strains may be prevalent throughout South Korea. The discovery of these G8 GARVs is important to the swine industry, veterinary practitioners, and GARV vaccine producers in South Korea. It should be noted that the serotype G8 is one of the major bovine serotypes in combination with P[5] and P[1] genotypes (Alfieri et al., 2004, Chang et al., 1996, Fukai et al., 2004, Gentsch et al., 1992). In addition, G8 GAVR serotype has been detected in rare cases in humans (Adah et al., 2001, Cunliffe et al., 1999, Fischer et al., 2003, Matthijnssens et al., 2006, Palombo et al., 2000, Steele et al., 1999), and pigs (Gouvea et al., 1994). Among the G8 GARVs, one strain contained bovine-like P[1]-VP4 gene, indicating bovine-like G8P[1] strains can infect heterologous species in nature, such as pigs. The remaining 16 G8 strains contained the porcine-like P[7]-VP4 gene. This result implies that reassortant events between porcine and bovine GARVs occur in nature. In previous reports (Ha et al., 2009; Park et al., unpublished data), we demonstrated that reassortant GARVs between bovine and porcine, and heterologous GARVs whose 11 genome segments are of pig origin infect calves and induce diarrhea. Therefore, interspecies transmission of GARVs between bovine and porcine, either as whole virions or by gene segment reassortment, appear to occur in nature at a relatively high frequency in South Korea.
Since G9 GARV was first detected in a child with gastroenteritis in the United States in 1983 (Clark et al., 1987) and subsequently in other countries (Das et al., 1993, Nakagomi et al., 1990, Urasawa et al., 1992, Zizdić et al., 1992), G9 GARVs have not been reported in humans for a decade. From mid-1990s, G9 GARVs reemerged and efficiently spread throughout the world as the fifth globally important serotype (Ramachandran et al., 2000, Santos and Hoshino, 2005). Recently, G9 GARVs have been classified into I–VI lineages, with I–II consisting of strains isolated in the 1980s, and III–VI composing of strains isolated from the mid-1990s (Phan et al., 2007). Of these, lineages III and VI were found in both humans and pigs (Phan et al., 2007). In the present study, G9 GARVs were isolated and identified as the third most important genotype in the diarrheic pigs. All Korean strains were clustered in lineage VI of known porcine and human G9 GARVs. Thus, continuous genotypic characterization of the GARVs and cautions against the increase of the G9 is necessary in South Korea. Moreover, human G9 GARV infections belonging to lineage III have been emerging in South Korea since 2002 (Kang et al., 2005), meaning that Korean porcine G9 GARVs are different from Korean human G9 GARVs.
Human GARVs showed a striking seasonal pattern of infection in developed countries, with epidemic peaks occurring in the cooler months of each year (Estes and Kapikian, 2007). This may be related to the influence of low relative humidity as a factor facilitating the survival of GARVs on surfaces (Brandt et al., 1982). Studies describing the seasonal pattern of porcine GARVs in diarrheic piglets have rarely been published, and those published data varied widely (Will et al., 1994, Svensmark et al., 1989). In one of the few comparable studies, the seasonal curves of porcine GARV infections were highest in winter and the slightly higher in late summer and early autumn in Iowa, USA (Will et al., 1994). In contrast, Danish porcine GARV infections showed a slight increase during the autumn (Svensmark et al., 1989). In this study, however, porcine GARVs occurred throughout the year with the highest prevalence during the summer months. The reason for the seasonal pattern variations around the world is not yet known. Therefore, more intensified epidemiological studies throughout the world will be needed to fully understand the seasonal pattern of porcine GARV infections and to establish porcine GARV surveillance programs to prevent infections.
In summary, this study demonstrates that porcine GARV infections are epidemic and widespread in diarrheic piglets in South Korea. The infecting strains are genetically diverse, and include homologous (G5P[7]), heterologous (G8P[1]), and reassortant (G8P[7]), as well as emerging G9 GARV strains.
Acknowledgments
This study was supported by the National Veterinary Research and Quarantine Services (NVRQS), Ministry of Agriculture and Forestry, the Korea Science and Engineering Foundation (KOSEF) grant (2009-0081752), and the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE), Republic of Korea. The authors would like to acknowledge a graduate fellowship from the Korean Ministry of Education and Human Resources Development through the Brain Korea 21 project.
Contributor Information
Kyoung-Oh Cho, Email: choko@chonnam.ac.kr.
Su-Jin Park, Email: sjpark@kribb.re.kr.
References
- Abe M., Ito N., Morikawa S., Takasu M., Murase T., Kawashima T., Kawai Y., Kohara J., Sugiyama M. Molecular epidemiology of rotaviruses among healthy calves in Japan: isolation of a novel rotavirus bearing new P and G genotypes. Virus Res. 2009;144:250–257. doi: 10.1016/j.virusres.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Adah M.I., Wade A., Taniguchi K. Molecular epidemiology of rotaviruses in Nigeria: detection of unusual strains with G2P[6] and G8P[1] specificities. J. Clin. Microbiol. 2001;39:3969–3975. doi: 10.1128/JCM.39.11.3969-3975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A.F., Alfieri A.A., Barreiros M.A., Leite J.P., Richtzenhain L.J. G and P genotypes of group A rotavirus strains circulating in calves in Brazil, 1996–1999. Vet. Microbiol. 2004;99:167–173. doi: 10.1016/j.vetmic.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Bohl E.H., Theil K.W., Saif L.J. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J. Clin. Microbiol. 1984;19:105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C.D., Kim H.W., Rodriguez W.J., Arrobio J.O., Jeffries B.C., Parrott R.H. Rotavirus gastroenteritis and weather. J. Clin. Microbiol. 1982;16:478–482. doi: 10.1128/jcm.16.3.478-482.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.O., Parwani A.V., Saif L.J. The characterization of VP7 (G type) and VP4 (P type) genes of bovine group A rotaviruses from field samples using RT-PCR and RFLP analysis. Arch. Virol. 1996;141:1727–1739. doi: 10.1007/BF01718295. [DOI] [PubMed] [Google Scholar]
- Ciarlet M., Estes M.K. Rotaviruses: basic biology, epidemiology and methodologies. In: Bitton G., editor. Encyclopedia of Environmental Microbiology. John Wiley and Sons; New York: 2002. pp. 2753–2773. [Google Scholar]
- Clark H.F., Hoshino Y., Bell L.M., Groff J., Hess G., Bachman P., Offit P.A. Rotavirus isolate WI61 representing a presumptive new human serotype. J. Clin. Microbiol. 1987;25:1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe N.A., Gondwe J.S., Broadhead R.L., Molyneux M.E., Woods P.A., Bresee J.S., Glass R.I., Gentsch J.R., Hart C.A. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: predominance of novel P[6]G8 strains. J. Med. Virol. 1999;57:308–312. [PubMed] [Google Scholar]
- Das B.K., Gentsch J.R., Hoshino Y., Ishida S., Nakagomi O., Bhan M.K., Kumar R., Glass R.I. Characterization of the G serotype and genogroups of New Delhi newborn rotavirus strain 116E. Vrology. 1993;197:99–107. doi: 10.1006/viro.1993.1570. [DOI] [PubMed] [Google Scholar]
- Estes M.K., Kapikian A.Z. Rotaviruses. In: Knipe D.M., Griffin D.E., Lamb R.A., Straus S.E., Howley P.M., Martin M.A., Roizman B., editors. Fields Virology. fifth edition. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 1917–1974. [Google Scholar]
- Fischer T.K., Page N.A., Griffin D.D., Eugen-Olsen J., Pedersen A.G., Valentiner-Branth P., Mølbak K., Sommerfelt H., Nielsen N.M. Characterization of incompletely typed rotavirus strains from Guinea-Bissau: identification of G8 and G9 types and a high frequency of mixed infections. Virology. 2003;311:125–133. doi: 10.1016/s0042-6822(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Fukai K., Saito T., Inoue K., Sato M. Molecular characterization of novel P[14],G8 bovine group A rotavirus, Sun9, isolated in Japan. Virus Res. 2004;105:101–106. doi: 10.1016/j.virusres.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Gentsch J.R., Laird A.R., Bielfelt B., Griffin D.D., Bányai K., Ramachandran M., Jain V., Cunliffe N.A., Nakagomi O., Kirkwood C.D., Fischer T.K., Parashar U.D., Bresee J.S., Jiang B., Glass R.I. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J. Infect. Dis. 2005;192(Suppl. 1):S146–S159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- Gentsch J.R., Glass R.I., Woods P., Gouvea V., Gorziglia M., Flores J., Das B.K., Bhan M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R.I., Bressee J.S., Parashar U., Miller M., Gentsch J.R. Rotavirus vaccines at the threshold. Nat. Med. 1997;3:1324–1325. doi: 10.1038/nm1297-1324. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Santos N., Timenetsky, Mdo C. VP4 typing of bovine and porcine group A rotaviruses by PCR. J. Clin. Microbiol. 1994;32:1333–1337. doi: 10.1128/jcm.32.5.1333-1337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T.P., Kim H.J., Saif L.J., Jeong Y.J., Kim H.H., Kwon H.J., Park S.J., Cho K.O. Sequence analysis of unusual P[7]G5 bovine rotavirus strains reveals evidence of interspecies transmission. J. Clin. Microbiol. 2009;47:3329–3332. doi: 10.1128/JCM.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V., Das B.K., Bhan M.K., Glass R.I., Gentsch J.R. Great diversity of group A rotavirus strains and high prevalence of mixed rotavirus infections in India. J. Clin. Microbiol. 2001;39:3524–3529. doi: 10.1128/JCM.39.10.3524-3529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C., Park S.I., Park S.H., Kim H.H., Park S.J., Jeong J.H., Choy H.E., Saif L.J., Kim S.K., Kang M.I., Hyun B.H., Cho K.O. Genetic diversity of porcine sapoviruses. Vet. Microbiol. 2007;122:246–257. doi: 10.1016/j.vetmic.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.O., Kilgore P., Kim J.S., Nyambat B., Kim J., Suh H.S., Yoon Y., Jang S., Chang C., Choi S., Kim M.N., Gentsch J., Bresee J., Glass R. Molecular epidemiological profile of rotavirus in South Korea, July 2002 through June 2003: emergence of G4P[6] and G9P[8] strains. J. Infect. Dis. 2005;192(Suppl. 1):S57–S63. doi: 10.1086/431502. [DOI] [PubMed] [Google Scholar]
- Khamrin P., Maneekarn N., Peerakome S., Chan-it W., Yagyu F., Okitsu S., Ushijima H. Novel porcine rotavirus of genotype P[27] shares new phylogenetic lineage with G2 porcine rotavirus strain. Virology. 2007;361:243–252. doi: 10.1016/j.virol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Ishino M., Wang Y.-H., Chawla-Sarkar M., Krishnan T., Naik T.N. Diversity of G-type and P-type of human and animal rotaviruses and its genetic background. In: Mendez-Vilas A., editor. Communicating Current Research and Educational Topics and Trends in Applied Microbiology. second edition. FORMATEX; Badajoz, Spain: 2007. pp. 847–858. [Google Scholar]
- La T., Phillips N.D., Hampson D.J. Development of a duplex PCR assay for detection of Brachyspira hyodysenteriae and Brachyspira pilosicoli in pig feces. J. Clin. Microbiol. 2003;41:3372–3375. doi: 10.1128/JCM.41.7.3372-3375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J.P., Alfieri A.A., Woods P.A., Glass R.I., Gentsch J.R. Rotavirus G and P types circulating in Brazil: characterization by RTPCR, probe hybridization, and sequence analysis. Arch. Virol. 1996;141:2365–2374. doi: 10.1007/BF01718637. [DOI] [PubMed] [Google Scholar]
- Martella V., Bányai K., Ciarlet M., Iturriza-Gómara M., Lorusso E., De Grazia S., Arista S., Decaro N., Elia G., Cavalli A., Corrente M., Lavazza A., Baselga R., Buonavoglia C. Relationship among procine and human P[6] rotaviruses: evidence that the different human P[6] lineages have originated from multiple interspecies transmission events. Virology. 2006;344:509–519. doi: 10.1016/j.virol.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Martella V., Ciarlet M., Bányai K., Lorusso E., Cavalli A., Corrente M., Elia G., Arista S., Camero M., Desario C., Decaro N., Lavazza A., Buonavoglia C. Identification of a novel VP4 genotype carried by a serotype G5 porcine rotavirus strain. Virology. 2005;346:301–311. doi: 10.1016/j.virol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Ciarlet M., Rahman M., Attoui H., Bányai K., Estes M.K., Gentsch J.R., Iturriza-Gómara M., Kirkwood C.D., Martell V., Mertens P.P., Nakagomi O., Patton J.T., Ruggeri F.M., Saif L.J., Santos N., Steyer A., Taniguchi K., Desselberger U., Van Ranst M. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008;153:1621–1629. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J., Rahman M., Yang X., Delbeke T., Arijs I., Kabue J.P., Muyembe J.J., Van Ranst M. G8 rotavirus strains isolated in the Democratic Republic of Congo belong to the DS-1-like genogroup. J. Clin. Microbiol. 2006;44:1801–1809. doi: 10.1128/JCM.44.5.1801-1809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin M., Turgeon D., Jolette J., Robinson Y., Phaneuf J.B., Sauvageau R., Beauregard M., Teuscher E., Higgins R., Larivière S. Neonatal diarrhea of pigs in Quebec: infectious causes of significant outbreaks. Can. J. Comp. Med. 1983;47:11–17. [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T., Ohshima A., Akatani K., Ikegami N., Katsushima N., Nakagomi O. Isolation and molecular characterization of a serotype 9 human rotavirus strain. Microbiol. Immunol. 1990;34:77–82. doi: 10.1111/j.1348-0421.1990.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Palombo E.A., Clark R., Bishop R.F. Characterization of a “European-like” serotype G8 human rotavirus isolated in Australia. J. Med. Virol. 2000;60:56–62. [PubMed] [Google Scholar]
- Parashar U.D., Gibson C.J., Bresse J.S., Glass R.I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Saif L.J., Jeong C., Lim G.K., Park S.I., Kim H.H., Park S.J., Kim Y.J., Jeong J.H., Kang M.I., Cho K.O. Molecular characterization of novel G5 bovine rotavirus strains. J. Clin. Microbiol. 2006;44:4101–4112. doi: 10.1128/JCM.01196-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra G.I., Vidales G., Gomez J.A., Fernandez F.M., Parreño V., Bok K. Phylogenetic analysis of porcine rotavirus in Argentina: increasing diversity of G4 strains and evidence of interspecies transmission. Vet. Microbiol. 2008;126:243–250. doi: 10.1016/j.vetmic.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Phan T.G., Okitsu S., Maneekarn N., Ushijima H. Genetic heterogeneity, evolution and recombination in emerging G9 rotaviruses. Infect. Genet. Evol. 2007;7:656–663. doi: 10.1016/j.meegid.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Rácz M.L., Kroeff S.S., Munford V., Caruzo T.A., Durigon E.L., Hayashi Y., Gouvea V., Palombo E.A. Molecular characterization of porcine rotaviruses from the southern region of Brazil: characterization of an atypical genotype G[9] strain. J. Clin. Microbiol. 2000;38:2443–2446. doi: 10.1128/jcm.38.6.2443-2446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran M., Kirkwood C.D., Unicomb L., Cunliffe N.A., Ward R.L., Bhan M.K., Clark H.F., Glass R.I., Gentsch J.R. Molecular characterization of serotype G9 rotavirus strains from a global collection. Virology. 2000;278:436–444. doi: 10.1006/viro.2000.0682. [DOI] [PubMed] [Google Scholar]
- Rosen B.I., Parwani A.V., Lopez S., Flores J., Saif L.J. Serotypic differentiation of rotaviruses in field samples from diarrheic pigs by using nucleic acid probes specific for porcine VP4 and human and porcine VP7 genes. J. Clin. Microbiol. 1994;32:311–317. doi: 10.1128/jcm.32.2.311-317.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos N., Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- Steele A.D., Parker S.P., Peenze I., Pager C.T., Taylor M.B., Cubitt W.D. Comparative studies of human rotavirus serotype G8 strains recovered in South Africa and the United Kingdom. J. Gen. Virol. 1999;80:3029–3034. doi: 10.1099/0022-1317-80-11-3029. [DOI] [PubMed] [Google Scholar]
- Svensmark B., Jorsal S.E., Nielsen K., Willeberg P. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. I. Pre-weaning diarrhoea. Acta Vet. Scand. 1989;30:43–53. doi: 10.1186/BF03548067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Timenetsky, Mdo C., Santos N., Gouvea V. Survey of rotavirus G and P types associated with human gastroenteritis in Sao Paulo, Brazil, from 1986 to 1992. J. Clin. Microbiol. 1994;32:2622–2624. doi: 10.1128/jcm.32.10.2622-2624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicomb L.E., Podder G., Gentsch J.R., Woods P.A., Hasan K.Z., Faruque A.S., Albert M.J., Glass R.I. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J. Clin. Microbiol. 1999;37:1885–1891. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa S., Hasegawa A., Urasawa T., Taniguchi K., Wakasugi F., Suzuki H., Inouye S., Pongprot B., Supawadee J., Suprasert S. Antigenic and genetic analysis of human rotaviruses in Chiang Mai, Thailand: evidence for a close relationship between human and animal rotaviruses. J. Infect. Dis. 1992;166:227–234. doi: 10.1093/infdis/166.2.227. [DOI] [PubMed] [Google Scholar]
- Ursu K., Kisfali P., Rigó D., Ivanics É., Erdélyi K., Dán Á., Melegh B., Martella V., Bányai K. Molecular analysis of the VP7 gene of pheasant rotaviruses identifies a new genotype, designated G23. Arch. Virol. 2009;154:1365–1369. doi: 10.1007/s00705-009-0439-0. [DOI] [PubMed] [Google Scholar]
- Wieler L.H., Ilieff A., Herbst W., Bauer C., Vieler E., Bauerfeind R., Failing K., Klös H., Wengert D., Baljer G., Zahner H. Prevalence of enteropathogens in suckling and weaned piglets with diarrhoea in Southern Germany. J. Vet. Med. B: Infect. Dis. Vet. Public Health. 2001;48:151–159. doi: 10.1111/j.1439-0450.2001.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will L.A., Paul P.S., Proescholdt T.A., Aktar S.N., Flaming K.P., Janke B.H., Sacks J., Lyoo Y.S., Hill H.T., Hoffman L.J., Wu L.L. Evaluation of rotavirus infection and diarrhea in Iowa commercial pigs based on an epidemiologic study of a population represented by diagnostic laboratory cases. J. Vet. Diagn. Invest. 1994;6:416–422. doi: 10.1177/104063879400600403. [DOI] [PubMed] [Google Scholar]
- Zaberezhny A.D., Lyoo Y.S., Paul P.S. Prevalence of P types among porcine rotaviruses using subgenomic VP4 gene probes. Vet. Microbiol. 1994;39:97–110. doi: 10.1016/0378-1135(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Zizdić S., Ridjanović Z., Masić I. Newly discovered rotavirus serotypes in 4 years of collecting samples from children with diarrheal syncromes. Med. Arh. 1992;46:15–18. [PubMed] [Google Scholar]


