Highlights
-
•
First detection of PEDV in wild boar population.
-
•
PEDV positive samples were spread throughout the mainland of South Korea.
-
•
Our results provide novel insight into the epidemiology of PEDV infection.
Keywords: Wild boar, Porcine epidemic diarrhea virus, PEDV, South Korea, Sus scrofa
Abstract
Porcine epidemic diarrhea virus (PEDV) is a burdensome pathogen in the swine industry. Wild boar population poses a high risk for reservoir of viral pathogen. Two hundred eighty seven samples from wild boar (Sus scrofa) collected in South Korea during 2010/11 were analyzed using RT-PCR, revealing a PEDV infection rate of 9.75% (28/287). PEDV positive samples were distributed throughout the mainland of South Korea, clustering at the northern border adjacent to the Demilitarized Zone (DMZ) and in mountainous regions. PEDV in wild boar was genetically similar to Chinese PEDV strains in phylogenetic investigations. Our results indicated that PEDV is circulating in the wild boar and provided a novel knowledge into epidemiology of PEDV infection.
1. Introduction
Porcine epidemic diarrhea virus (PEDV; Order Nidovirales, family Coronaviridae, subfamily Coronavirinae, genus Alphacoronavirus) is one of the most devastating diseases in the swine industry worldwide (Schulz and Tonsor, 2015). Clinical symptoms of PEDV include severe diarrhea, dehydration, and vomiting, with high mortality in suckling piglets (Pensaert and de Bouck, 1978, Pijpers et al., 1993). Since its discovery in the UK and Belgium in 1978, PEDV has spread throughout Europe and Asia (Jung and Saif, 2015, Pensaert and de Bouck, 1978). In the United States, a PEDV epidemic occurred in May 2013 and rapidly disseminated throughout North America (Huang et al., 2013). During the North American outbreak, US-related PEDV strains was emerged in Asia, subsequently causing an outbreak (Lee and Lee, 2014).
Wild animals have been regarded as a deleterious factor to the domestic livestock industry because they are reservoirs for a variety of infectious agents that can infect livestock animals (Wiethoelter et al., 2015). Of them, wild boar (Sus scrofa) had been considered a carrier for various porcine viral pathogens, primarily CSFV (Classical swine fever virus) and ASFV (African swine fever virus) (Costard et al., 2013, Ruiz-Fons et al., 2008, Seo et al., 2012). Several studies of Coronaviridae infection did not find transmissible gastroenteritis virus (TGEV) in wild boar, whereas 3% of anti-porcine respiratory coronavirus (PRCV) antibodies were detected in Slovenian wild boar (Ruiz-Fons et al., 2008, Vengust et al., 2006). However, there were no reported occurrences of PEDV in wild pigs according to the World Organisation for Animal Health (OIE, 2014).
In this study, fecal samples from South Korean wild boar collected during 2010/2011 were analyzed for molecular evidence of PEDV infection, and the overall prevalence of PEDV was estimated. Geographical distribution of PEDV was assessed, and partial spike sequences of isolates were subjected to phylogenetic analysis.
2. Materials and methods
To detect PEDV in wild boar, 287 fecal samples of wild boar were collected at random by hunters in 2010/2011. Those samples were verified as wild boar feces in a previous study (Seo et al., 2012). The fecal samples were diluted with phosphate-buffered saline to 20% suspensions and centrifuged at 1500g for 20 min. The supernatants were centrifuged at 16,000g for 15 min and filtered through a 0.45-μm filter (Pall Corporation, Port Washington, New York, USA).
Viral RNA was extracted from filtered fecal suspensions using Viral Gene Spin (Intron Biotechnology, Seongnam, Gyeonggi, Korea) as per the manufacturer’s manual. For obtaining sequences of partial spike gene, the fecal samples were analyzed by reverse transcription PCR (RT-PCR) and sequencing methods. The cDNA was constructed using SuPrimeScript RT Premix (GENETBIO Inc., Daejeon, Korea) with gene-specific reverse primers (5′-GGGTGAGTAATTGTTTACAACG-3′). The PCR amplification of the partial spike gene was conducted by HS Prime Taq premix (GENETBIO Inc., Daejeon, Korea) with the following primers as per the previous publication: [P1: 5′-TTCTGAGTCACGAACAGCCA -3′, P2: 5′‐CATATGCAGCCTGCTCTGAA-3′] (Kim et al., 2001). The thermal cycling program for PCR was as follows: 10 min at 94° C (initial denaturation), 35 cycles with 30 s at 94° C, 30 s at 55° C, and 30 s at 72° C (denaturation, annealing, and extension), 5 min at 72° C (final extension), and a final hold temperature of 4° C. The amplicons were separated by gel electrophoresis and target DNA was extracted using a Dokdo-prep gel extraction kit (ELPIS Biotech, Inc., Daejeon, Korea). Macrogen Inc. (Seoul, Korea) sequenced purified DNA fragments using a 3730xl DNA analyzer (Applied Biosystems, Poster city, CA, USA).
Reference sequences of PEDV from domestic boar were obtained from the GenBank database. Sequence alignment of PEDV from wild boar and livestock pigs was performed using MUSCLE, and sequence identities were estimated in BioEdit 7.0 (Ibis Biosciences, Carlsbad, CA, USA). A neighbor-joining unrooted phylogenetic tree was constructed by MEGA 6 with the Kimura-2 parameter method with uniform rates variation among sites, and test of phylogeny was conducted with 1000 replications of bootstrapping (Tamura et al., 2013). All positions which contain gaps and missing data were deleted.
3. Result and discussion
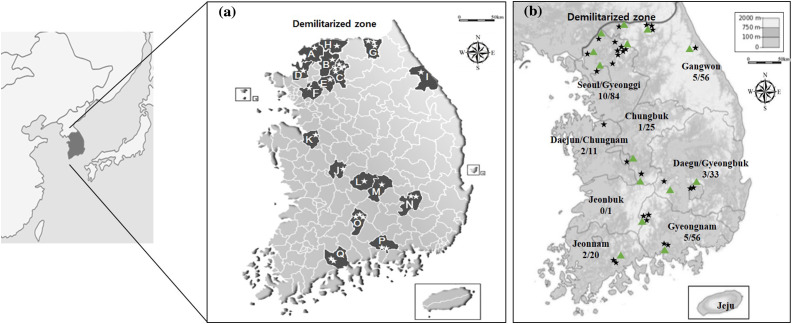
It was revealed that 9.75% (28/287) of fecal samples contained PEDV. Geographical distribution of PEDV in wild boar was observed in mainland South Korea (Fig. 1 ). About half of the positive samples were intensively detected in Northern districts near the DMZ such as Seoul/Gyeonggi (10/84) and Gangwon Province (5/56) (Fig. 1). Almost all of the detected samples were adjacent to mountainous regions (Fig. 1).
Fig. 1.
Geographical distribution of porcine epidemic diarrhea virus (PEDV) in wild boar in South Korea (a) and topographic relation with location of PEDV positive wild boar in Korea (b). Regions of PEDV positive samples are indicated as stars. The major mountains near where positive samples were collected are indicated as green triangles. Each province where PEDV were detected is labeled as follows: A: Yeoncheon-gun, Gyeonggi Province; B: Pocheon-si, Gyeonggi Province; C: Gapyeong-gun, Gyeonggi Province; D: Paju-si, Gyeonggi Province; E: Namyangju-si, Gyeonggi Province; F: Eunpyeong-gu, Seoul; G: Yanggu-gun, Gangwon Province; H: Cheorwon-gun, Gangwon Province; I: Gangneung-si, Gangwon Province; J: Seo-gu, Daejun; K: Asan-si, Chungnam Province; L: Youngdong-gun, Chungbuk Province; M: Kimcheon-si, Gyeongbuk Province; N: Buk-gu, Daegu; O: Hamyang-gun, Gyeongnam Province; P: Jinju-si, Gyeongnam Province; Q: Suncheon-si, Jeonnam Province.
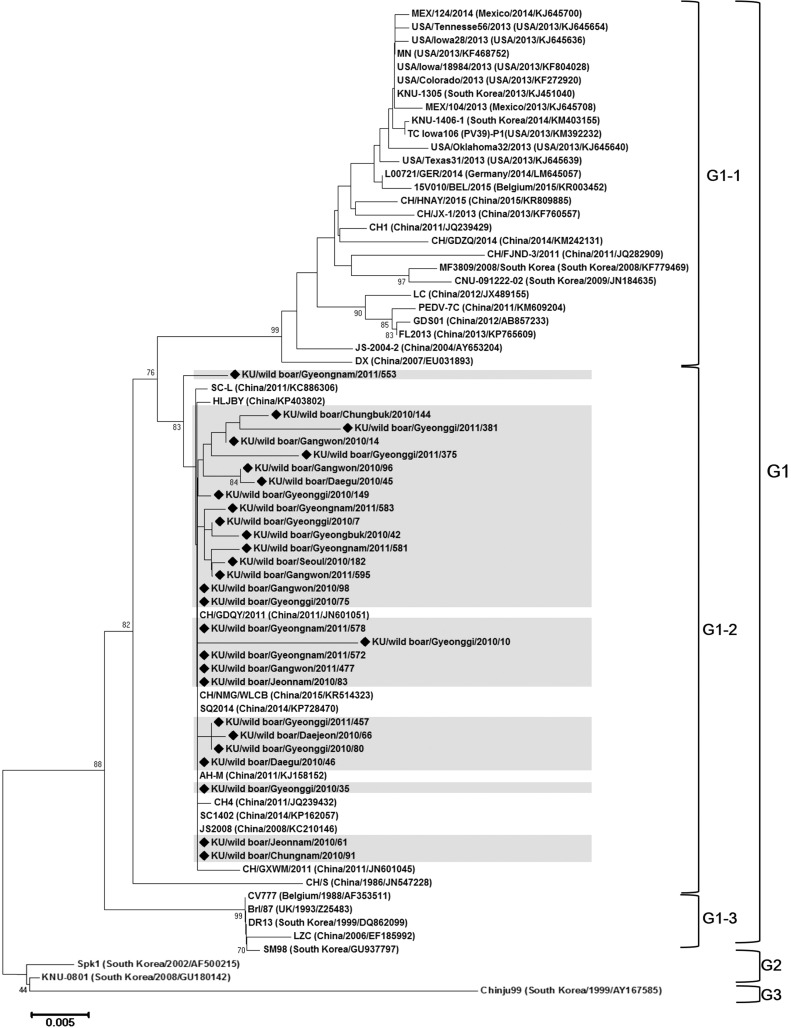
The 612 bp of partial spike genomes (S1 C-terminal domain: 1486–2096 nt of the S gene) were obtained from wild boar samples (primer sequences were subtracted). Accession numbers were KU529655- KU529682 (Table 1 ). In previous studies, a phylogenetic tree corresponding to a partial spike gene was divided into G1 (subdivided into G1-1, G1-2, and G1-3), G2, and G3 (Park et al., 2007, Puranaveja et al., 2009). Phylogenetic analysis of PEDV in this study showed that all wild boar strains belong to G1-2 (Fig. 2 ). The pairwise sequence identities between the Korean wild boar strains shared 96.5-100% sequence identity. Those strains were closely related to Chinese strains (Accession no.: KP728470, KP162057, KJ158152, KC210146, JN601051, KR514323, JQ239432, KP403802, JN601045, and KC886306), showing 97.7-100% homology. Since 2004, there have been no reports of domestic pig field strains belonging to the G1-2 group in Korea. Several wild boar PEDV strains found in Korea, including KU529669/KU529676 (Gangwon province), KU529662/KU529666 (Jeonnam province), KU529678/KU529679 (Gyeongnam province), KU529667 (Chungnam province), KU529658 (Gyeonggi province), and KU529661 (Daegu/Gyeongbuk province), showed 100% homology with each other despite the distant geographical locations (Table 1 and Fig. 2).
Table 1.
Summary of the wild boar strains used in this study. Sequences 1–28 are PEDV sequences obtained from wild boar in this study.
| No. | Virus strains | Country | Collected area | Date | Host/Source | Accession numbers |
|---|---|---|---|---|---|---|
| 1 | KU/wild_boar/Gyeonggi/2010/7 | South Korea | Gyeonggi | December 2010 | Wild boar/feces | KU529655 |
| 2 | KU/wild_boar/Gyeonggi/2010/10 | South Korea | Gyeonggi/ | December 2010 | Wild boar/feces | KU529656 |
| 3 | KU/wild_boar/Gangwon/2010/14 | South Korea | Gangwon | December 2010 | Wild boar/feces | KU529657 |
| 4 | KU/wild_boar/Gyeonggi/2010/35 | South Korea | Gyeonggi | December 2010 | Wild boar/feces | KU529658 |
| 5 | KU/wild_boar/Gyeongbuk/2010/42 | South Korea | Gyeongbuk | December 2010 | Wild boar/feces | KU529659 |
| 6 | KU/wild_boar/Daegu/2010/45 | South Korea | Daegu | December 2010 | Wild boar/feces | KU529660 |
| 7 | KU/wild_boar/Daegu/2010/46 | South Korea | Daegu | December 2010 | Wild boar/feces | KU529661 |
| 8 | KU/wild_boar/Jeonnam/2010/61 | South Korea | Jeonnam | December 2010 | Wild boar/feces | KU529662 |
| 9 | KU/wild_boar/Daejeon/2010/66 | South Korea | Daejeon | December 2010 | Wild boar/feces | KU529663 |
| 10 | KU/wild_boar/Gyeonggi/2010/75 | South Korea | Gyeonggi | December 2010 | Wild boar/feces | KU529664 |
| 11 | KU/wild_boar/Gyeonggi/2010/80 | South Korea | Gyeonggi | December 2010 | Wild boar/feces | KU529665 |
| 12 | KU/wild_boar/Jeonnam/2010/83 | South Korea | Jeonnam | December 2010 | Wild boar/feces | KU529666 |
| 13 | KU/wild_boar/Chungnam/2010/91 | South Korea | Chungnam | December 2010 | Wild boar/feces | KU529667 |
| 14 | KU/wild_boar/Gangwon/2010/96 | South Korea | Gangwon | December 2010 | Wild boar/feces | KU529668 |
| 15 | KU/wild_boar/Gangwon/2010/98 | South Korea | Gangwon | December 2010 | Wild boar/feces | KU529669 |
| 16 | KU/wild_boar/Chungbuk/2010/144 | South Korea | Chungbuk | December 2010 | Wild boar/feces | KU529670 |
| 17 | KU/wild_boar/Gyeonggi/2010/149 | South Korea | Gyeonggi | December 2010 | Wild boar/feces | KU529671 |
| 18 | KU/wild_boar/Seoul/2010/182 | South Korea | Seoul | December 2010 | Wild boar/feces | KU529672 |
| 19 | KU/wild_boar/Gyeonggi/2011/375 | South Korea | Gyeonggi | December 2011 | Wild boar/feces | KU529673 |
| 20 | KU/wild_boar/Gyeonggi/2011/381 | South Korea | Gyeonggi | December 2011 | Wild boar/feces | KU529674 |
| 21 | KU/wild_boar/Gyeonggi/2011/457 | South Korea | Gyeonggi | December 2011 | Wild boar/feces | KU529675 |
| 22 | KU/wild_boar/Gangwon/2011/477 | South Korea | Gangwon | December 2011 | Wild boar/feces | KU529676 |
| 23 | KU/wild_boar/Gyeongnam/2011/553 | South Korea | Gyeongnam | December 2011 | Wild boar/feces | KU529677 |
| 24 | KU/wild_boar/Gyeongnam/2011/572 | South Korea | Gyeongnam | December 2011 | Wild boar/feces | KU529678 |
| 25 | KU/wild_boar/Gyeongnam/2011/578 | South Korea | Gyeongnam | December 2011 | Wild boar/feces | KU529679 |
| 26 | KU/wild_boar/Gyeongnam/2011/581 | South Korea | Gyeongnam | December 2011 | Wild boar/feces | KU529680 |
| 27 | KU/wild_boar/Gyeongnam/2011/583 | South Korea | Gyeongnam | December 2011 | Wild boar/feces | KU529681 |
| 28 | KU/wild_boar/Gangwon/2011/595 | South Korea | Gangwon | December 2011 | Wild boar/feces | KU529682 |
Fig. 2.
Phylogenetic tree of PEDV partial spike sequences of wild boar strains (gray shadow and diamonds) and GenBank available sequences. Reference strains were indicated as follows; strain (country/year/accession number). Bootstrap replication values using from 1000 replicates are listed as percentages at branch nodes. Bootstrap support values above 70% are shown. Subgroups of the phylogenetic tree were divided as G1, G2, and G3. G1 was further subdivided into G1-1, G1-2, and G1-3 (Park et al., 2007).
Recently, the population of wild boar in the Korean peninsula was expected to increase because of the extinction of predators such as Panthera tigris and Canis lupus chanco (Choi et al., 2012). Nevertheless, the accurate estimation of population size and movement of wild boar is virtually impossible because of the existence of the demilitarized zone (DMZ), which works as a physical limitation. Since the DMZ has been restricted from human disturbance since the 1953 ceasefire, the undeveloped forest remains an untapped resource for the study of wild life disease and ecology (Yoon et al., 2010). Since wild boars prefer forest habitats, the DMZ and other forest regions of the Korean peninsula may be densely populated with wild boar. Furthermore, about 70% of the Korean peninsula landscape comprises hill and mountain ranges, with pig farms primarily in rural valleys at the foot of mountain ranges. Collectively, these circumstances connoted a high risk for wild boar acting as reservoirs of infectious diseases that threaten the livestock industry.
Since the first isolation of PEDV in Korea (Kweon et al., 1993), PEDV has been considered one of a most contagious viruses in the Korean swine industry due to rapid onset of the disease and tremendous losses of piglets. Although vaccination and preventive measures have been used to control endemics, outbreaks of this disease have consistently emerged. However, the pig population in Korea has plummeted due to a foot-and-mouth disease (FMD) outbreak in 2010–2011. As a consequence of strict biosecurity measures and a decreased pig population, outbreaks of PEDV have been attenuated (Lee et al., 2015). Even with the reduction of PEDV outbreaks in domestic pigs in this period, wild boar strains of PEDV were still detected. This study demonstrated that the PEDV is distributed in wild boar populations around the mainland of South Korea. The geographical distribution of PEDV, focused on Northern districts and mountainous area, provides new information for PEDV epidemiology and disease control strategies. Several studies suggested that highly contagious pathogens in wild boar, such as CSFV and ASFV, are transmitted by the direct or indirect contact between wild boar and domestic swine (Costard et al., 2013, Penrith et al., 2011, Seo et al., 2012). However, it is unclear because there is not sufficient evidence of direct transmission of PEDV between domestic pigs and wild boars. Our retrospective data, which is obtained from wild boar feces during 2010/2011, merely demonstrated the previous existence of PEDV in wild boar and the role of wild boar as conceivable host of PEDV. Moreover, we hypothesized that the possibility of PEDV cross-over in Korea between wild boar and domestic pig populations may be low, based on the fact that the domestic strains were rarely found in the G1-2 including wild boar strains. Hence, further studies will be necessary to substantiate the possibility of transmission between domestic and wild pigs.
Given the phylogenetic analysis result, the wild boar PEDV strains had close genetic similarity with Chinese strains. Among the Korean wild boar strains, we found 100% homology between strains from distant geographical locations (Fig. 2). This result is unexpected considering the geographical barriers that isolated the populations of wild boars. However, the genetic analysis of this study was limited to a partial sequence, and the phylogenetic relationship of partial sequences does not always correspond to full spike gene analysis. Moreover, the sample distribution and collections were not performed uniformly over the collection years. Therefore, in order to explore the accurate origin and evolutionary history of wild boar PEDV, further studies of extended gene sequences in wild boar populations are needed on an annual basis with attention to broader geographical distributions.
Although the origin of PEDV in wild boars may be controversial, we assumed that the ancestral PEDV strains in wild boars had evolved consistently until detection. Moreover, Coronaviruses undergo RNA homologous recombination events with high frequency (He et al., 2014, Woo et al., 2010). Several studies determined that the recombination events of PEDV can occur among other PEDV strains as well as between other Coronaviridae (Boniotti et al., 2016). In particular, in the case of wild animal viral hosts, the introduction of novel PEDV strains can more increase the diversity of viral genetics and induce recombination events (Cadar et al., 2012).
In the case of CSFV, wild boar have a role as a viral reservoir by self-limiting infection despite the high mortality in domestic pigs (Rossi et al., 2005, Ruiz-Fons et al., 2008). The moderate prevalence of PEDV in wild boar implies that PEDV may not be fatal to wild boar. Hence, the wild boar may undergo persistent infection with PEDV. However, in order to test this hypothesis further studies are necessary, such as analysis of pathogenesis in tagged wild boar challenged with a known strain of PEDV followed by long term monitoring.
In summary, our findings demonstrated that PEDV in wild boar was distributed in the Korean peninsula. Furthermore, the current study emphasized the necessity for comprehensive epidemiological and pathological studies of PEDV infection in wild boar to determine if wild boar do act as a PEDV reservoir. Therefore, the current study provides a novel insight into potential epidemiology and controls of PEDV infection and reveals a hitherto-undiscovered potential host of PEDV.
References
- Boniotti M.B., Papetti A., Lavazza A., Alborali G., Sozzi E., Chiapponi C., Faccini S., Bonilauri P., Cordioli P., Marthaler D. Porcine epidemic diarrhea virus and discovery of a recombinant swine enteric coronavirus, Italy. Emerg. Infect. Dis. 2016;22:83–87. doi: 10.3201/eid2201.150544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadar D., Cságola A., Lőrincz M., Tombácz K., Spînu M., Tuboly T. Detection of natural inter- and intra-genotype recombination events revealed by cap gene analysis and decreasing prevalence of PCV2 in wild boars. Infect. Genet. Evol. 2012;12:420–427. doi: 10.1016/j.meegid.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Choi E.-J., Lee C.-H., Hyun B.-H., Kim J.-J., Lim S.-I., Song J.-Y., Shin Y.-K. A survey of porcine reproductive and respiratory syndrome among wild boar populations in Korea. J. Vet. Sci. 2012;13:377. doi: 10.4142/jvs.2012.13.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costard S., Mur L., Lubroth J., Sanchez-Vizcaino J.M., Pfeiffer D.U. Epidemiology of African swine fever virus. Virus Res. 2013;173:191–197. doi: 10.1016/j.virusres.2012.10.030. [DOI] [PubMed] [Google Scholar]
- He B., Zhang Y., Xu L., Yang W., Yang F., Feng Y., Xia L., Zhou J., Zhen W., Feng Y., Guo H., Zhang H., Tu C. Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in China. J. Virol. 2014;88:7070–7082. doi: 10.1128/JVI.00631-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.W., Dickerman A.W., Pineyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio. 2013;4 doi: 10.1128/mBio.00737-13. e00737–00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204:134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Song D.S., Park B.K. Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J. Vet. Diagn. Invest. 2001;13:516–520. doi: 10.1177/104063870101300611. [DOI] [PubMed] [Google Scholar]
- Kweon C.H., Kwon B.J., Jung T.S., Kee Y.J., Hur D.H., Hwang E.K., Rhee J.C., An S.H. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J. Vet. Res. 1993;33:249–254. [Google Scholar]
- Lee S., Lee C. Outbreak-related porcine epidemic diarrhea virus strains similar to US strains, South Korea, 2013. Emerg. Infect. Dis. 2014;20:1223–1226. doi: 10.3201/eid2007.140294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kim Y., Lee C. Isolation and characterization of a Korean porcine epidemic diarrhea virus strain KNU-141112. Virus Res. 2015;208:215–224. doi: 10.1016/j.virusres.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE, 2014. OIE TECHNICAL FACTSHEET, http://www.oie.int/fileadmin/Home/fr/Media_Center/docs/pdf/factsheet_PEDV.pdf.
- Park S.J., Moon H.J., Yang J.S., Lee C.S., Song D.S., Kang B.K., Park B.K. Sequence analysis of the partial spike glycoprotein gene of porcine epidemic diarrhea viruses isolated in Korea. Virus Genes. 2007;35:321–332. doi: 10.1007/s11262-007-0096-x. [DOI] [PubMed] [Google Scholar]
- Penrith M.L., Vosloo W., Mather C. Classical swine fever (hog cholera): review of aspects relevant to control. Transbound. Emerg. Dis. 2011;58:187–196. doi: 10.1111/j.1865-1682.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpers A., van Nieuwstadt A., Terpstra C., Verheijden J. Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. Vet. Rec. 1993;132:129–131. doi: 10.1136/vr.132.6.129. [DOI] [PubMed] [Google Scholar]
- Puranaveja S., Poolperm P., Lertwatcharasarakul P., Kesdaengsakonwut S., Boonsoongnern A., Urairong K., Kitikoon P., Choojai P., Kedkovid R., Teankum K., Thanawongnuwech R. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Infect. Dis. 2009;15:1112–1115. doi: 10.3201/eid1507.081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Artois M., Pontier D., Cruciere C., Hars J., Barrat J., Pacholek X., Fromont E. Long-term monitoring of classical swine fever in wild boar (Sus scrofa sp.) using serological data. Vet. Res. 2005;36:27–42. doi: 10.1051/vetres:2004050. [DOI] [PubMed] [Google Scholar]
- Ruiz-Fons F., Segales J., Gortazar C. A review of viral diseases of the European wild boar: effects of population dynamics and reservoir role. Vet. J. 2008;176:158–169. doi: 10.1016/j.tvjl.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz L.L., Tonsor G.T. Assessment of the economic impacts of porcine epidemic diarrhea virus in the United States. J. Anim. Sci. 2015;93:5111–5118. doi: 10.2527/jas.2015-9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S., Sunwoo S., Hyun B., Lyoo Y.S. Detection of antibodies against classical swine fever virus in fecal samples from wild boar. Vet. Microbiol. 2012;161:218–221. doi: 10.1016/j.vetmic.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengust G., Valencak Z., Bidovec A. A serological survey of selected pathogens in wild boar in Slovenia. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2006;53:24–27. doi: 10.1111/j.1439-0450.2006.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoelter A.K., Beltran-Alcrudo D., Kock R., Mor S.M. Global trends in infectious diseases at the wildlife-livestock interface. Proc. Natl. Acad. Sci. U. S. A. 2015;112:9662–9667. doi: 10.1073/pnas.1422741112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Huang Y., Lau S.K., Yuen K.Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B.I., Kim H.C., Kim J.T. Lung worm (Metastrongylus elongatus) infection in wild boars (Sus scrofa) of the demilitarized zone, Korea. J. Wildl. Dis. 2010;46:1052–1054. doi: 10.7589/0090-3558-46.3.1052. [DOI] [PubMed] [Google Scholar]