Abstract
We have previously described a synthetic T7-driven cDNA minigenome containing the antisense sequence of luciferase gene and internal ribosome entry site of encephalomyocarditis virus flanked by 5′- and 3′-end sequences of hepatitis C virus (HCV) that contain cis-acting replication elements. Synthesis of minus-strand RNA from the artificial minigenome was determined by using Huh-7 cells harboring autonomously replicating HCV subgenome as a helper for provision of functional replication components. To further confirm and extend these studies, we investigated here whether the minigenome replication system could be reconstituted by transfection of naïve Huh-7 cells with plasmid expressing nonstructural (NS) proteins. Reporter assay and Northern blot analysis revealed that trans-expression of NS proteins from 3 to 5 resulted in high level of luciferase activity and synthesized minus-strand RNA. The analogous result was also obtained with the minigenome derived from HCV 2a, and both HCV 1b- and 2a-derived NS protein were able to support the chimeric minigenomes whose 5′- or 3′-end was replaced by the respective region of the heterologous virus. These results provide a basis for establishing the reverse genetic system that is helpful to study cis- and trans-acting factors involved in HCV RNA replication.
Keywords: Hepatitis C virus, Minigenome, trans-Replication
Hepatitis C virus (HCV) is an important human pathogen with an estimated 170 million chronic carriers throughout the world, and many of them are at a high risk for developing liver cirrhosis and hepatocellular carcinoma [1]. HCV is a member of the Flaviviridae family with a positive-sense RNA genome of ∼9600 nucleotides in length. The genome is flanked by highly structured nontranslated regions (NTRs) important for both RNA translation and replication. The viral genome encodes a polyprotein precursor of approximately 3010 amino acids, which is processed by viral and cellular protease to produce the structural proteins (core, E1, and E2) and nonstructural (NS) proteins (p7 and NS2 to NS5B).
Like other plus-stranded RNA viruses, HCV genomic RNA is first transcribed into a minus-strand intermediate, which in turn serves as the template for production of progeny plus-strand RNA. Although the basic steps in replication have been well established, little is known about the detail of these processes. Studies of HCV replication have been hampered by the lack of an efficient tissue culture system. Although the development of subgenomic replicon has facilitated the investigation of viral RNA replication in cell culture [2], culture-adaptive mutations within the NS proteins are required for efficient replication [3], [4], and full-length genomes carrying such mutations do not produce infectious virus particles [5], [6]. More recently, it was reported that genotype 2a JFH1 genome replicates efficiently independent of the culture-adaptive mutations and supports production of viral particles [7]. This in vitro system, together with the later-developed JFH1-based chimeras [8], [9], are an important progress in HCV research, allowing the study of unknown aspects of HCV life cycle. However, a comparison study showed that JFH1 differs from the earlier-generated HCV 1b replicon in independence of the cellular cofactor (cyclophilin B) for the replication and less sensitivity to antiviral reagent [10], suggesting that the strain- or genotype-specific properties may exist and the observation obtained with JFH1 cannot be simply extrapolated to other isolates.
Synthetic minigenomes have been described in a number of minus- and plus-stranded RNA viruses, which has contributed greatly to the analysis of cis-acting sequences and trans-acting proteins required for viral replication, maturation, and packaging [11], [12]. We previously established a helper virus-dependent expression system utilizing HCV-derived minigenome, and Huh-7 cells harboring autonomously replicating HCV subgenome [13]. In this study, we further investigated whether the minigenome replication system could be reconstituted by transfection of naïve Huh-7 cells with plasmid expressing NS proteins. It was shown that synthesis of minus-strand RNA from HCV minigenome can be supported by trans-expressed polyprotein NS3 to NS5B, and the NS proteins were able to replicate not only the homologous minigenome but the heterologous and chimeric minigenome as well.
Materials and methods
Plasmids. HCV 1b-derived minigenome p1b-1b was previously referred to as pT7cRLNS5B1 [13]. For construction of chimeric minigenome p2a-1b, the first 376 nucelotides of HCV 2a cDNA with the T7 promoter directly coupled at the 5′-end were amplified by PCR with primers 5′-tataagcttTAATACGACTCACTATAACCTGCCCCTAATAGGGGC-3′ and 5′-tgcgcatgcTTTGGTTTTTCTTTGAGGTT-3′. The Renilla luciferase gene was amplified from pRL-TK (Promega) using primers 5′-ctctctagaATGACTTCGAAAGTTTATGA-3′ and 5′- tgcgcatgcTTATTGTTCATTTTTGAGAA-3′. The resulting PCR products were digested with HindIII–SphI and SphI–XbaI, respectively, and inserted into the HindIII/XbaI sites of p1b-1b. The 3′- part of the NS5B coding region fused 3′ UTR of HCV 2a cDNA was amplified by PCR using primers 5′-ataggatccCCTCAGAAAACTTGGGG-3′ and 5′-ataggcgccagcgaggaggctgggaccatgccggccACATGATCTGCAGAGAGACC-3′, digested with BamHI and NarI, and cloned, along with the annealed oligonucleotides containing partial sequence of the HDV ribozyme [13], into BamHI/EcoRI-cut p1b-1b or p2a-1b, creating p1b-2a and p2a-2a, respectively.
To construct plasmid pNS3-51b expressing polyprotein from HCV 1b, a cDNA containing the ORF of NS3 to NS5B was amplified with primers 5′-atatctagaATGGGCCCATCACGGCTTA-3′ and 5′-ataggcgcgccTCACCGGTTGGGGAGCAGG-3′, digested with XbaI and AscI, and cloned, along with HindIII/XbaI-cut HCV sequence (1--341 nt), into pGEMEX-1 vector (Promega) which was modified by deletion of all of the T7 gene 10 and introduction of HindIII and AscI sites between T7 promoter and terminator [14]. pNS3-52a, which expresses the polyprotein from HCV 2a, was constructed similarly except with the primers of corresponding sequence from genotype 2a. The sequences of these constructs were confirmed by nucleotide sequencing.
Cells. The cell line Huh-7 was purchased from the American Type Culture Collection (ATCC) and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 10% fetal calf serum and 50 U/ml penicillin and streptomycin in a 5% CO2 humidified atmosphere. A Huh-7-derived cell line (Huh–NNRZ) stably replicating HCV subgenomic replicon was grown in DMEM containing 300 μg/ml G418 (Geneticin, Invitrogen) [15], [16].
Transfection. Huh-7 cells were seeded at 1 × 105 per well of 12-well plates. Twenty-four hours later, 0.5 μg EcoRI-linearized minigenome (p1b-1b, p1b-2a, p2a-1b, or p2a-2a), 0.5 μg pGEMEX-1, pNS3-51b, or pNS3-52a, 0.5 μg pAM8-1, and 0.1 μg pGL3-Control vector were cotransfected into cells with Fugene HD Transfection Reagent (Roche). The cells were harvested at the indicated time points, and cell lysates were assayed for luciferase activity as described below.
Luciferase assay. Cell lysates were prepared from transfected cells, centrifuged briefly, and 20 μl of the supernatants was used for luciferase assays with Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. Luciferase activities were measured using a TD-20/20 Luminometer (Promega).
Western blot analysis. Protein was electrophoresed on a sodium dodecyl sulfate–polyacrylamide gel, transferred to Hybond-P PVDV Membrane (Amersham). The blots were probed with Antiserum Product 2871 and 2881 (ViroStat) for detection of NS3 and NS4, rabbit polyclonal antibody (ab2594, Abcam Limited) for NS5A, and goat polyclonal antibody (sc-17532, Santa Cruz Biotechnology, Inc.) for NS5B. Signals were visualized with ECL Plus Western Blotting Detection Reagents (Amersham).
Northern blot analysis. RNAs were isolated from transfected cells with Trizol reagent (Invitrogen) and treated with RNase-free DNase (Promega). The purified RNAs were separated by denaturing agarose gel electrophoresis and analyzed by Northern blot using digoxigenin-labeled antisense Renilla luciferase sequence.
Results
Synthetic minigenome derived from HCV
The minigenome construct derived from HCV 1b consists of the antisense sequence of the Renilla luciferase gene and internal ribosome entry site (IRES) of encephalomyocarditis virus (EMCV) flanked upstream by 5′-end (nucleotides 1–377) and downstream by 3′-end sequence containing NS5B coding region from nucleotides 9067 to 9371 plus 3′-UTR of HCV cDNA. The cassette was positioned precisely at the T7 transcription start site followed by self-cleaving HDV ribozyme to ensure authentic 5′- and 3′-ends (Fig. 1 A, p1b-1b). If the minigenome could be accepted as a template by the replication complex provided in trans, the luciferase gene, which is encoded by synthesized minus-strand RNA, would express in HCV-infected cells. Fully consistent with this hypothesis, luciferase activity was selectively detected in Huh-7 cells harboring an autonomously replicating HCV subgenome (Huh–NNRZ) [13].
Fig. 1.

Schematic diagrams of T7-based minigenomes derived from HCV 1b (A), HCV 2a (B), chimeric minigenomes p1b-2a consisting of 5′-end of HCV 1b and 3′-end of HCV 2a (E) and p2a-1b consisting of 5′-end of HCV 2a and 3′-end of HCV 1b (F). HCV minigenome containing the antisense sequence of the Renilla luciferase gene and EMCV IRES flanked by the 5′-end and 3′-partial NS5B coding sequence-connected 3′-UTR was juxtaposed precisely at the T7 transcription start site and followed by the HDV ribozyme sequence. pnu-1b (C) and pnu-2a (D) were identical to p1b-1b and p2a-2a except for the 5′-end sequences deleted.
Replication of HCV minigenome in Huh-7 cells expressing polyprotein NS3 to NS5B
The ability of the synthetic minigenome to replicate in replicon cells prompted us to investigate whether the replication of minigenome could be supported by HCV proteins expressed in trans. For this purpose, Huh-7 cells were transfected with the minigenomic construct p1b-1b, plasmid encoding a polyprotein encompassing NS3 to NS5B under the control of T7 RNA polymerase promoter, pAM8-1 plasmid expressing T7 RNA polymerase [14], and pGL3-Control vector. The cells were harvested at 3 days posttransfection, protein expression was verified by Western blot analysis (Fig. 2 ) and the replication of minigenome was determined by luciferase assay and Northern blot analysis. The firefly luciferase activity from cotransfected pGL3-Control vector was simultaneously measured to normalize the transfection efficiency. As shown in Fig. 3 A, only background level of Renilla luciferase (Rluc) activity was detected in cells transfected with the empty vector. Cotransfection of the plasmid encoding the polyprotein NS3 to NS5B (pNS3-51b) resulted in significant Renilla luciferase expression. Omission of pAM8-1 in the transfection mixture completely abrogated Renilla luciferase activity, largely ruling out the possibility that the minus-strand RNA used here as the mRNA for reporter gene expression was synthesized as a consequence of the transcription by a cryptic promoter. Consistent with the results reported previously [13], Renilla luciferase activity was also detected in Huh–NNRZ cells stably replicating the HCV subgenomic replicon, although it was lower than that in cells trans-expressing the polyprotein. The fact that the replicase complex reconstituted by trans-expressed polyprotein could support more efficient replication of the minigenome may be attributable to the higher expression level of plasmid-encoded protein on a per-transfected-cell basis. Alternatively, the recruitment of the replication complex to the minigenome may be competed by the subgenomic replicon, because both of these share the replication machinery in replicon cells.
Fig. 2.

Expression of NS proteins in Huh-7 cells transfected with plasmid encoding a polyprotein encompassing NS3 to NS5B. Huh-7 cells were transfected with pNS3-51b together with pAM8-1 plasmid expressing T7 RNA polymerase and harvested at day 3 posttransfection. Cell lysates of the transfected cells were analyzed by Western blot using antibodies against each NS protein. Protein standard is shown on the right, and the band corresponding to each NS protein is indicated by an arrowhead. Huh-7 cells transfected with the empty vector served as a negative control (NC).
Fig. 3.
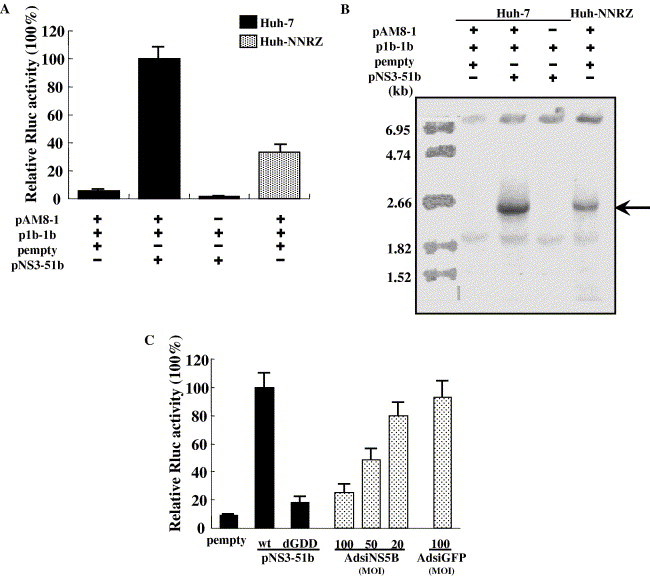
Replication of HCV minigenome in Huh-7 cells expressing polyprotein NS3 to NS5B. (A) Huh-7 or Huh–NNRZ cells were transfected with p1b-1b, pNS3-51b expressing polyprotein NS3 to NS5B, together with or without pAM8-1. Relative Renilla luciferase activities in the lysates were determined at 72 h posttransfection. The columns and bars represent mean and standard deviation of four independent experiments. (B) Northern blot was performed on 8 μg of extracted RNA using digoxigenin-labeled antisense Renilla luciferase RNA probe to detect minus-strand transcripts. RNA size markers are shown on the left, and the bands corresponding to minus-strand RNA are indicated on the right. (C) Huh-7 cells were transfected with p1b-1b, pAM8-1, and pempty, pNS3-51b or pNS3-51b/dGDD (column 1–3), or infected with AdsiNS5B at an MOI of 100, 50, and 20 (column 4–6) before transfection, and relative Renilla luciferase activities in the lysates were determined as described above.
To further confirm the result of reporter assay, RNA was extracted from transfected cells and subjected to Northern blot analysis using digoxigenin-labeled antisense Renilla luciferase probes. Also, minus-strand RNA transcripts of the expected size were specifically detected in Huh-7 cells expressing NS 3–5 protein and Huh–NNRZ cells replicating HCV subgenomic replicon (Fig. 3B, lanes 2 and 4). These data demonstrate that trans-replication of HCV minigenome does not require replication of the helper viral RNA.
To document that the reporter gene expression detected above was dependent on HCV replicase reconstituted by trans-expressed NS proteins, we employed inactive mutant pNS3-51b/dGDD (in which the GDD motif of NS5B was deleted) and AdsiNS5B expressing siRNA directed against NS5B [13] in the reporter assay. As shown in Fig. 3C, the deletion of GDD motif significantly attenuated the ability of NS proteins to support minigenome replication, and transduction with AdsiNS5B resulted in a substantial and dose-dependent reduction in luciferase expression. These results provide further evidence that the reporter gene was expressed as a result of replication of HCV minigenome by trans-supplied NS proteins.
Chimeric minigenomes as templates for HCV replication complex
Next, we were interested in investigating whether the replicase of HCV can recognize the heterologous signals for synthesis of minus-strand RNA. For this purpose, HCV minigenome from distantly related genotype 2a (Fig. 1B, p2a-2a), minigenomes with 5′-end deleted (Fig. 1C and D, pnu-1b and pnu-2a), and chimeric minigenomes whose 5′- or 3′-end was replaced by the respective region of the heterologous virus (Fig. 1E and F, p1b-2a and p2a-1b) were constructed. Huh-7 cells were transfected with these minigenomes together with the plasmid expressing HCV 1b- or 2a-derived NS proteins, pAM8-1, and Renilla luciferase activities were measured as fore-mentioned. Consistent with the results described above, replicase of HCV 1b and 2a accepted its respective minigenome as the template for synthesis of minus-strand RNA, and exchange of NS proteins between HCV1b- and 2a-derived minigenome systems also led to reporter gene expression (Fig. 4 ), implying that the replication complex is not strictly specific for the homologous RNA template. Deletion of the 5′-end region in the minigenome fully abrogated its replication, both NS proteins from HCV 1b and 2a, however, could support the replication of chimeric minigenomes, suggesting that both RNA–protein interaction between replicase and viral genome and long range RNA–RNA interaction between 5′- and 3′-terminal sequence involved in HCV minus-strand RNA synthesis are functionally conserved between genotype 1b and 2a. Additionally, in all tested minigenomes, the NS proteins originated from HCV 1b constantly yielded higher levels of luciferase expression than that from 2a, suggesting that intrinsic differences in the replication capabilities of the replicase complex from different strains may exist. More likely, the superior capability of pNS3-51b in supporting the minigenomes replication may be attributable to the fact that the coding sequence in pNS3-51b was amplified from the replicon which harbors the adaptive mutations due to long-term culture, whereas the coding region in pNS3-52a was directly amplified from HCV 2a-infected serum.
Fig. 4.

Replication of chimeric HCV minigenomes. Huh-7 cells were transfected with each indicated minigenome, pAM8-1, and pNS3-51b or pNS3-52a expressing polyprotein NS3 to NS5B derived from genotype 1b or 2a. Relative Renilla luciferase activities in the lysates were determined as described for Fig. 3A. The columns and bars represent means and standard deviations of three independent transfections.
Discussion
Successful establishment of the minigenome system has been described in a number of minus-stranded RNA viruses from different families and plus-stranded RNA viruses belonging to the Coronaviridae family, which has contributed greatly to the analysis of cis-acting sequences and trans-acting proteins essential for viral replication [11], [12]. The rescue of synthetic minigenomes was achieved either through helper virus infection of minigenome-transfected cells with virus particles or through co-transfection of plasmids expressing viral proteins. For viruses of Flaviviridae family, however, a little has been reported in the development of similar approach except an in vitro replication system which utilizes cytoplasmic extracts from viral-infected cells and exogenous RNA template containing 5′- and 3′-terminal regions was described for dengue virus [17]. Together with those reported previously [13], the data shown here represent the first example of minigenome system for HCV, indicating that both the replicase complex supplied from replicating subgenomic replicon and that reconstituted by plasmid-encoded NS proteins are capable of supporting the replication of HCV minigenome.
The data shown here further confirm that the viral 5′- and 3′-end sequence together with the 3′-partial NS5B coding region represent sufficient cis-acting signals for minus-strand RNA synthesis. These results, however, do not rule out the possibility for the existence of cis-acting elements in other coding region, which may act as regulatory elements (either enhancers or silencers) in RNA synthesis. The presence of noncontiguous cis-acting signals involved in viral RNA replication has been reported in the viral genome of the brome mosaic virus [18], tobacco mosaic virus [19], and the double-stranded RNA virus of yeast [20].
Similar to that found in dengue virus, it was shown that deletion of the 5′-end region in the minigenome fully abrogated its replication, but substitution of the 5′-end with the respective sequence from heterologous virus (p1b-2a or p2a-1b) did not significantly affect its template ability, suggesting that the long range RNA–RNA interaction between 5′- and 3′-ends essential for RNA replication is functionally conserved between HCV 1b and 2a. In addition to homologous minigenome, both HCV 1b- and 2a-derived replicase were able to accept the heterologous and chimeric minigenomes as the templates for synthesis of minus-strand RNA, indicating that the replicase-catalyzed RNA synthesis is not strictly strain- or genotype-specific.
Using replicon system, Bartenschlager’s group obtained the evidence showing that only mutations in NS5A, but not mutations in NS3, NS4B, and NS5B, could be rescued by trans-complementation [21]. Our data presented here indicate that replication of the minigenome can be supported by trans-expressed NS proteins. One scenario may make these two different findings compatible: the cis-expressed, lethally mutated NS proteins may exert dominant negative effect in reconstituting replication complex, and thus interfere with the incorporation of trans-supplied NS proteins into a functional replication complex, which may account for the failure of NS proteins (other than NS5A) to trans-complement HCV RNA replication; however, such a dominant negative effect does not exist in the minigenome system described here because there is no NS protein expressed in cis, and trans-expressed NS proteins might be able to reconstitute the functional replication complex to support minigenome replication. Further experiments are now in progress to substantiate this assumption.
It is generally believed that the HCV replication follows the pathway used by other plus-strand RNA viruses: the input RNA is first transcribed into a minus strand, which in turn serves as the template for production of progeny plus strand. The negative strand intermediates are postulated to exist as a dsRNA form. However, there is no direct evidence demonstrating this postulation in HCV, and whether there is free HCV-specific RNA of negative polarity in infected cells is still an issue to be elucidated. On the other hand, increasing evidence showed that the RNA in native replication intermediates of some positive strand RNA viruses is single-stranded. For example, in polio virus-infected cells, a careful electron microscope analysis using a membrane-permeable cross-linking reagent demonstrated that the native replication intermediate in vivo has a predominantly single-stranded backbone attached to several nascent RNA chains with few or no regions of extensive base-pairing, although deproteined (phenol-extracted) replication intermediate has a backbone mostly double-stranded [22]. More recently, Fujimura et al., reported that native replication intermediates of 20 S RNA virus have a single-stranded RNA backbone [23]. After completion of product-strand elongation, both the product and template strands are released from the replication complex as single-stranded RNA. The data presented here indicate that the minus strand RNA could serve as the mRNA for transgene expression, implying a similar scenario may also occur in HCV replication and minus strand RNA may be dissociated and present as a free single-strand form after RNA synthesis is completed.
One issue of concern in using minigenome to study the molecular mechanism of viral replication is whether the elements controlling viral replication in the context of minigenome could authentically reflect those that occurred in the context of full-length genome. Recently, differential effect of a point mutation in the replicase gene on genome and minigenome replication was reported in coronavirus, emphasizing the need to use full-length genome to validate the replication signals obtained from minigenome system [24]. Nonetheless, the HCV minigenome system described here represents a useful tool for identification of cis- and trans-acting factors involved in viral replication while eliminating biosafety constraints required for work with infectious systems. Additionally, it will be of interest to explore whether the HCV minigenome can be packaged by additional provision of the viral structural protein in trans, and its success will not only further broaden the application of the HCV minigenome, but also facilitate the development of HCV-based gene delivery system.
We describe here a reverse genetic system for HCV that is based on T7-driven minigenome coupled with plasmid-encoded NS proteins. This system opens the possibility of manipulation of cis-acting signals and trans-acting factors involved in the control of HCV RNA synthesis, which may facilitate future studies aimed at investigation of the mechanisms involved in the replication of viral RNA.
References
- 1.Alter M.J. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 2.Lohmann V., Korner F., Koch J.O., Herian U., Theilmann L., Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 3.Blight K.J., Kolykhalov A.A., Rice C.M. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 4.Krieger N., Lohmann V., Bartenschlager R. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 2001;75:4614–4624. doi: 10.1128/JVI.75.10.4614-4624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukh J., Pietschmann T., Lohmann V., Krieger N., Faulk K., Engle R.E., Govindarajan S., Shapiro M., St Claire M., Bartenschlager R. Mutations that permit efficient replication of hepatitis C virus RNA in Huh-7 cells prevent productive replication in chimpanzees. Proc. Natl. Acad. Sci. USA. 2002;99:14416–14421. doi: 10.1073/pnas.212532699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietschmann T., Lohmann V., Kaul A., Krieger N., Rinck G., Rutter G., Strand D., Bartenschlager R. Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. J. Virol. 2002;76:4008–4021. doi: 10.1128/JVI.76.8.4008-4021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakita T., Pietschmann T., Kato T., Date T., Miyamoto M., Zhao Z., Murthy K., Habermann A., Krausslich H.G., Mizokami M., Bartenschlager R., Liang T.J. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindenbach B.D., Evans M.J., Syder A.J., Wolk B., Tellinghuisen T.L., Liu C.C., Maruyama T., Hynes R.O., Burton D.R., McKeating J.A., Rice C.M. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 9.Pietschmann T., Kaul A., Koutsoudakis G., Shavinskaya A., Kallis S., Steinmann E., Abid K., Negro F., Dreux M., Cosset F.L., Bartenschlager R. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. USA. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii N., Watashi K., Hishiki T., Goto K., Inoue D., Hijikata M., Wakita T., Kato N., Shimotohno K. Diverse effects of cyclosporine on hepatitis C virus strain replication. J. Virol. 2006;80:4510–4520. doi: 10.1128/JVI.80.9.4510-4520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izeta A., Smerdou C., Alonso S., Penzes Z., Mendez A., Plana-Duran J., Enjuanes L. Replication and packaging of transmissible gastroenteritis coronavirus-derived synthetic minigenomes. J. Virol. 1999;73:1535–1545. doi: 10.1128/jvi.73.2.1535-1545.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez M., Sanchez A., Cubitt B., Rosario D., de la Torre J.C. A reverse genetic system for Borna disease virus. J. Gen. Virol. 2003;84:3099–3104. doi: 10.1099/vir.0.19467-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Yamada O., Sakamoto T., Yoshida H., Araki H., Shimotohno K. Exploiting cis-acting replication elements to direct hepatitis C virus-dependent transgene expression. J. Virol. 2005;79:5923–5932. doi: 10.1128/JVI.79.10.5923-5932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Yamada O., Ito T., Akiyama M., Hashimoto Y., Yoshida H., Makino R., Masago A., Uemura H., Araki H. A single nucleotide insertion in the 5′-untranslated region of hepatitis C virus leads to enhanced cap-independent translation. Virology. 1999;261:263–270. doi: 10.1006/viro.1999.9879. [DOI] [PubMed] [Google Scholar]
- 15.Kishine H., Sugiyama K., Hijikata M., Kato N., Takahashi H., Noshi T., Nio Y., Hosaka M., Miyanari Y., Shimotohno K. Subgenomic replicon derived from a cell line infected with the hepatitis C virus. Biochem. Biophys. Res. Commun. 2002;293:993–999. doi: 10.1016/S0006-291X(02)00342-X. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Yamada O., Sakamoto T., Yoshida H., Iwai T., Matsushita Y., Shimamura H., Araki H., Shimotohno K. Down-regulation of viral replication by adenoviral-mediated expression of siRNA against cellular cofactors for hepatitis C virus. Virology. 2004;320:135–143. doi: 10.1016/j.virol.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 17.You S., Padmanabhan R. A novel in vitro replication system for Dengue virus. J. Biol. Chem. 1999;274:33714–33722. doi: 10.1074/jbc.274.47.33714. [DOI] [PubMed] [Google Scholar]
- 18.Lahser F.C., Marsh L.E., Hall T.C. Contributions of the brome mosaic virus RNA-3 3′-nontranslated region to replication and translation. J. Virol. 1993;67:3295–3303. doi: 10.1128/jvi.67.6.3295-3303.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leathers V., Tanguay R., Kobayashi M., Gallie D.R. A phylogenetically conserved sequence within viral 3′ untranslated RNA pseudoknots regulates translation. Mol. Cell. Biol. 1993;13:5331–5347. doi: 10.1128/mcb.13.9.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteban R., Fujimura T., Wickner R.B. Internal and terminal cis-acting sites are necessary for in vitro replication of the L-A double-stranded RNA virus of yeast. EMBO J. 1989;8:947–954. doi: 10.1002/j.1460-2075.1989.tb03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appel N., Herian U., Bartenschlager R. Efficient rescue of hepatitis C virus RNA replication by trans-complementation with nonstructural protein 5A. J. Virol. 2005;79:896–909. doi: 10.1128/JVI.79.2.896-909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards O.C., Martin S.C., Jense H.G., Ehrenfeld E. Structure of poliovirus replicative intermediate RNA. Electron microscope analysis of RNA cross-linked in vivo with psoralen derivative. J. Mol. Biol. 1984;173:325–340. doi: 10.1016/0022-2836(84)90124-4. [DOI] [PubMed] [Google Scholar]
- 23.Fujimura T., Solorzano A., Esteban R. Native replication intermediates of the yeast 20 S RNA virus have a single-stranded RNA backbone. J. Biol. Chem. 2005;280:7398–7406. doi: 10.1074/jbc.M412048200. [DOI] [PubMed] [Google Scholar]
- 24.Galan C., Enjuanes L., Almazan F. A point mutation within the replicase gene differentially affects coronavirus genome versus minigenome replication. J. Virol. 2005;79:15016–15026. doi: 10.1128/JVI.79.24.15016-15026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


