Abstract
This paper describes the isolation of porcine epidemic diarrhea (PED) virus in Vero and porcine cell cultures, and the influence of age on disease in experimental infection. PED virus was isolated from the small intestine of piglets inoculated with PED samples and cultured in Vero, porcine bladder and kidney cells propagated in collagen-coated tissue culture plates in maintenance medium (MM) containing trypsin. In porcine bladder and kidney cell cultures inoculated with isolated PED virus, cytopathic effects (CPE) including cell fusion were detected. Specific brilliant fluorescence was observed in the cytoplasm of these cells. Two- and 7-day old, and 2-, 4-, 8- and 12-week old specific pathogen-free (SPF) pigs were orally inoculated with PED virus isolated from an outbreak. All 2- and 7-day old pigs inoculated developed severe watery diarrhea from post-inoculation day (PID) 1 and died between PID 3 and 4. Although three of five 2-week old pigs developed diarrhea on PID 1–4, they eventually recovered. In the 4-week old group, three of five pigs had mild diarrhea for 1–2 days. None of the 8- and 12-week old pigs showed any clinical signs. Antibodies against PED virus were detected in all surviving pigs by virus neutralization (VN) test and immunofluorescence assay (IFA). Therefore, there is an age-dependent resistance to pathogenic PED virus infection in pigs.
Keywords: Coronavirus, Diarrhea, PED virus, Pig
1. Introduction
Porcine epidemic diarrhea (PED) virus is a member of the Coronaviridae, and is antigenically distinguishable from the other porcine coronaviruses, transmissible gastroenteritis (TGE) virus and hemagglutinating encephalomyelitis virus (Pensaert et al., 1981, Cavanagh, 1991). Experimental infections with PED virus isolates have indicated that the virus is a primary etiologic agent of diarrhea in pigs (Debouck and Pensaert, 1980, Debouck et al., 1981, Coussement et al., 1982). The principal features of PED are watery diarrhea, dehydration and high mortality in suckling pigs.
In 1978, a coronavirus-like particle was first identified during episodes of epizootic diarrhea in pigs in Belgium (Pensaert and Debouck, 1978) and the UK (Wood, 1977, Chasey and Cartwright, 1978). PED has subsequently been reported in Canada (Turgeon et al., 1980), Hungary (Horvath and Mocsari, 1981), Germany (Pospischil et al., 1981) and Korea (Kweon et al., 1993). In Japan, there was an outbreak of PED-like disease from late 1982 to early 1983 (Takahashi et al., 1983, Kuwahara et al., 1988). Further outbreaks occurred between late 1993 and 1996 (Sueyoshi et al., 1995, Tsuda, 1997). In an acute outbreak of PED in 1996, more than the 39,000 suckling pigs died (Tsuda, 1997).
Attempts at propagating PED virus in porcine cell or organ cultures have been unsuccessful. The first adaptation of PED virus to Vero cell cultures using medium containing trypsin was reported in 1988 (Hofmann and Wyler, 1988). In Japan, PED virus was first isolated in Vero cell culture in the same manner (Kusanagi et al., 1992). This paper describes the isolation of PED virus not only in Vero cells but also in cultures derived from pig bladder and kidney cells by addition of trypsin to the medium. Furthermore, to determine the effect of pig age on disease, different age groups of pigs were inoculated with the Japanese isolate of PED virus.
2. Materials and methods
2.1. Medium and cells
Growth medium (GM) was Eagle’s minimum essential medium (MEM) containing 0.3% tryptose phosphate broth, 10% inactivated fetal calf serum (FCS) and antibiotics was used for the propagation of cells. Maintenance medium (MM) consisted of Eagle’s MEM with 0.3% tryptose phosphate broth, 0.02% yeast extract, and 10 μg/ml trypsin (Difco, USA), unless otherwise stated.
Established cell lines of Vero cells derived from African green monkey kidney (RCV0001) and MA104 from fetal macacus rhesus monkey kidney were used. SB1 and SB2 cells prepared from the bladder epithelial cells of cesarean-derived colostrum-deprived (CDCD) piglets were used for virus isolation at approximately 20–30 passages. SK cells prepared from the CDCD pig kidney were used at approximately 70–80 passages.
2.2. Virus samples
The small intestines obtained from four piglets with naturally acquired infection showing severe diarrhea were homogenized with MM. PED virus antigens were detected in the enterocytes of these pigs by immunohistochemistry. The 10% pooled homogenates were centrifuged and the resulting supernatants were passed through a 450 nm membrane filter. Six 1-day old CDCD piglets were orally inoculated with 2 ml of the homogenized samples and sacrificed on post-inoculation days (PID) 1–3. The small intestines collected from these infected pigs were treated as described above and the pooled homogenates were used as virus stock. The virus stock was stored at −80°C until experimental inoculation of pigs. The virus stock was approximately 105.0 LD50/2 ml for a 3-day old pig. For virus isolation, three 7-day old CDCD piglets were inoculated with the virus stock and sacrificed on PID 1 (second passage in pig). Homogenized samples were prepared for virus isolation according to the method described previously (Hofmann and Wyler, 1988).
2.3. Virus isolation
After removal of GM, confluent cell cultures in collagen-coated 12-well tissue culture plates (Iwaki glass, Japan) were washed once with MEM. Then, the cells were inoculated with 0.2 ml per well of the clarified homogenate. After adsorption at 37°C for 1 h, the inoculum was removed and the monolayers were washed three times with 2 ml of MEM. The cell cultures were fed with 2 ml per well of MM. Control cultures were mock-inoculated with the same volume of MM instead of viral inoculum. If no cytopathic effect (CPE) was detected within 7 days, five blind passages were performed using supernatant fluids of cell culture in the same manner as the original samples.
2.4. Experimental design
Twenty eight specific pathogen-free (SPF) pigs in different age groups (six at 2 days old, two at 7 days old, five each at 2, 4, 8 and 12 weeks old) were obtained from an SPF pig herd. Each group of pigs was housed separately in a containment room and fed a commercial milk or solid diet. The ambient room temperature was maintained at 29–30°C for 2- and 7-day old pigs and 23–24°C for the other pigs. Two-day old pigs were divided into uninoculated control or infection groups. Two-day old and all other pigs were orally inoculated with 2 ml of 100- and 2-fold diluted virus stock, respectively. Blood samples were collected weekly from each pig. Clinical signs were recorded twice a day until slaughter. Surviving pigs were euthanized and necropsied at post-inoculation week (PIW) 4 or 6.
2.4.1. Immunofluorescence assay (IFA)
Vero cell cultures on coverslips were fixed in acetone for 10 min, about 24 h after virus inoculation. They were then stained with the experimentally inoculated pig serum at 37°C for 50 min. After incubation, they were washed with phosphate buffered saline (PBS) three times and stained with protein A conjugated with fluorescein isothiocyanate (Zymed, USA) at 37°C for 50 min. After washing, the cells were mounted with buffered glycerol and examined under a fluorescence microscope.
2.5. Virus neutralization (VN) test
The VN test was carried out by the microtiter method using Vero cells. Sera were heated at 56°C for 30 min before use. Serial two-fold dilutions of serum were mixed with an equal volume of Z94P5 strain virus suspension containing 200 TCID50/0.1 ml. The mixture were incubated at 37°C for 1 h and 0.1 ml of virus–serum mixture was inoculated into each of the two wells. Following adsorption at 37°C for 1 h, the inocula were removed and the monolayers were washed three times with MEM. Then, 0.1 ml of MM containing 5 μg/ml trypsin was added to each well and the cultures were incubated at 37°C for 7 days. The antibody titer was expressed as the reciprocal of the highest serum dilution inhibiting CPE in at least one of the two wells.
2.6. Immunohistochemical examination
The avidin–biotin (AB) technique was used for the detection of PED virus antigen in tissues. An ABC kit (Vector Laboratories, USA) was used to examine formalin-fixed, paraffin wax-embedded sections of the gastrointestinal tract. Sections were counterstained with methyl green. Rabbit antiserum against PED virus (Tsuda, 1997) was used as primary antibody.
2.7. Pathological examination
Histopathological examination was performed according to routine procedures. In brief, tissue samples were fixed in 20% neutral phosphate-buffered formalin. Thin sections of paraffin-embedded samples were stained by hematoxylin and eosin.
3. Results
3.1. Isolation of PED virus in cell cultures
As shown in Table 1 , cytopathic agents were isolated in Vero, SK and SB1 cell cultures inoculated with second passage intestinal samples. At first passage, CPE was inapparent because of the cytotoxic effect of the inoculum. On passage 2 in Vero cell culture, CPE characterized by cell fusion and syncytial formation was detected microscopically after an incubation period of 1–2 days. CPE progressed more slowly in SK and SB1 cell cultures than in Vero cell culture. The number of cells which were rounded, detached and fused were increased after 3–4 days of incubation (Fig. 1 ). Although syncytial formation was not apparent under microscope in non-stained cultured cells, fused cells were obvious in SB1 cell culture samples stained with Wright Giemsa’s solution (Fig. 1). Vero cells inoculated with viruses isolated in SB1 or SK cells showed the same CPE with syncytial formation. By IFA, specific brilliant fluorescence were observed in the cytoplasm of these cells (Fig. 1) and the isolates were identified as PED virus. No fluorescing cells were observed in uninoculated control cells. The isolated PED virus from pig No. 5 in Vero cell culture was clone-purified three times by plaque selection and designated Z94P5. CPE was not detected microscopically in SB2 and MA104 cells at the fifth passage.
Table 1.
Virus isolation in cell cultures from small intestines of experimentally inoculated piglets
| Cells | Pig No. |
||
| 1 | 2 | 3 | |
| Vero | − | +a | + |
| SK | − | + | − |
| SB1 | + | + | − |
| SB2 | − | − | − |
| MA104 | − | − | − |
CPE positive.
Fig. 1.
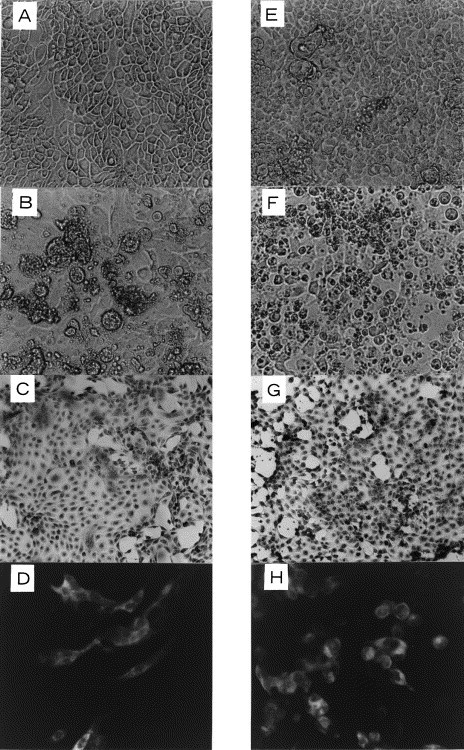
Cytopathic effect in SB1(A–D) and SK(E–H) cell cultures induced by cell culture-adapted PED virus. A and E: uninfected cells (×100), B and F: infected cells 3 days after inoculation (×100), C and G: infected cells stained with Wright Giemsa’s solution 3 days after inoculation (×40), D and H: immunofluorescence of infected cells 26 h after inoculation (×100).
3.2. Experimental infection
All 2- and 7-day old pigs inoculated with PED virus developed severe watery diarrhea from PID 1 and died between PID 3 and 4 (Table 2 ). PED virus antigen was detected by the AB technique in the small intestine of dead pigs. Vomiting was occasionally seen on PID 1–3. Non-inoculated control 2-day old pigs kept under the same conditions remained healthy. Three of five 2-week old pigs developed diarrhea on PID 1–4 which lasted 2–4 days. Although all these pigs were slightly depressed and anorectic after inoculation, they recovered after 1 week. PED virus antigen was detected by the AB technique in the jejunum and ileum of pig No. 9 which was euthanized on PID 5. In the 4-week old group, three of five pigs had mild diarrhea which started on PID 3–5 and continued for 1–2 days. In this group, other clinical signs such as depression and anorexia were not observed. All of 8- and 12-week old pigs showed no clinical signs including diarrhea throughout the experiment. All the pigs in the 2-, 4-, 8- and 12-week old groups survived.
Table 2.
Clinical signs of pigs in different age groups orally inoculated with PED virus
| Pig No. | No. of pigs inoculated | Days or weeks of age | Incubation period of infection (day) | No. of pigs |
|||||
| Fecal scorea (days of duration) |
Developed clinical signsb | Died (days after inoculation) | Survived | ||||||
| − | + | ++ | |||||||
| 1–4 | 4 | 2 days | 1 | 0 | 0 | 4 (>3) | 4 | 4 (3–4) | 0 |
| 5 and 6 | 2 (control) | 2 days (not inoculated) | 2 | 0 | 0 | 0 | 0 | 2 | |
| 7 and 8 | 2 | 7 days | 1 | 0 | 0 | 2 (>3) | 2 | 2 (4) | 0 |
| 9–13 | 5 | 2 weeks | 1–4 | 2 | 1 (2) | 2 (4) | 5 | 0 | 5c |
| 14–18 | 5 | 4 weeks | 3–5 | 2 | 3 (1–2) | 0 | 0 | 0 | 5 |
| 19–23 | 5 | 8 weeks | – | 5 | 0 | 0 | 0 | 0 | 5 |
| 24–28 | 5 | 12 weeks | – | 5 | 0 | 0 | 0 | 0 | 5 |
−=Normal; +=light diarrhea, pasty feces; ++=severe diarrhea, watery feces.
Depression and/or anorexia.
Pig No. 9 was necropsied for pathological examination on post-inoculation day 5.
Before infection, all of the pigs were negative for antibody against PED virus by both VN test and IFA. The antibodies were first detected on PIW 1 or 2 and they peaked between PIW 4 and 5 on VN test and on PIW 3 on IFA (Fig. 2 ). IFA titers were higher than those of VN antibody. All of the non-inoculated control pigs were negative for both antibodies throughout the study.
Fig. 2.
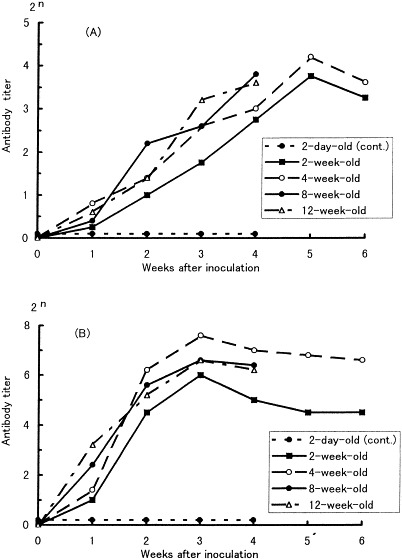
Antibody response in pigs orally inoculated with a small intestine homogenate containing PED virus. Mean antibody titers by VN test (A) and IFA (B).
4. Discussion
In the present study, PED virus was successfully propagated not only in Vero cells but also in SB1 and SK cell cultures. Hofmann and Wyler (1988) first isolated and adapted PED virus to serial propagation in Vero cells, which are derived from African green monkeys, by adding trypsin to the MM. However, attempts at isolating PED virus in porcine cell cultures in the presence or absence of trypsin have been unsuccessful until now. Previous reports suggested that porcine cell cultures were damaged by trypsin, which then might not be able to support virus replication (Hofmann and Wyler, 1988). In the present study, collagen-coated plates were used; cells cultured on collagen-coated plates are more resistant to trypsin (data not shown). This might be the main reason for the successful isolation of PED virus in porcine cell cultures in the present study. This method may be suitable for cell culture in medium containing trypsin or other proteinases.
PED virus was isolated in SB1 cells but not in SB2 cells. Although PED virus propagated in SB1 cells showed CPE in SB2 cells, the titer in these cells was lower than in SB1 cells (data not shown); the cells had been derived from the bladder of different pigs and were used at the same passage numbers. The reason for the difference in susceptibility for viral propagation between these cells is not known.
Upon experimental infection, 2- and 7-day old pigs inoculated with PED virus developed severe diarrhea and died. The 2- and 4-week old pigs showed mild diarrhea after inoculation and survived. The 8- and 12-week old pigs did not show any clinical signs, but developed antibody against PED virus. Coussement et al. (1982) described that 2- or 3-day old cesarian-derived piglets infected oronasally with the CV777 strain of PED virus showed severe diarrhea after an incubation period of 22–36 h. Debouck and Pensaert (1980) reported that pigs between 1 and 20 days old experimentally inoculated with PED virus developed diarrhea and some pigs younger than 19 days old died. These results are similar to those of the present study.
In several outbreaks, PED has caused diarrhea in pigs of all ages (Pensaert and Debouck, 1978, Takahashi et al., 1983). In the present study, however, severe diarrhea was only observed in 2- and 7-day old pigs. The difference in the clinical signs between field cases and the present examination may reflect differences in the infectious dose, the susceptibility of pigs, the environmental conditions or the virulence of the strains. The majority of herd outbreaks of PED occur during the colder months, especially between January and April (Tsuda, 1997). In TGE virus infection, (Shimizu et al., 1978, Shimizu and Shimizu, 1979) showed that a high ambient temperature resulted in increased disease resistance, while a low ambient temperature or temperature changes caused a dramatic enhancement in clinical signs. In the present study, 2–12-week old pigs were kept at 23–24°C throughout the study. Consequently, a stable comfortable ambient temperature might induce resistance to clinical disease after PED virus infection.
References
- Cavanagh, D., 1991. Classification and nomenclature of virus. Fifth report of the International Committee on Taxonomy of Viruses. In: Francki, R.I.B., Fauquet, C.M., Knudson, D.L., Brown, F. (Eds.). Arch. Virol., Suppl. 2.
- Chasey D, Cartwright S.F. Virus-like particles associated with porcine epidemic diarrhea. Res. Vet. Sci. 1978;25:255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussement W, Ducatelle R, Debouck P, Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets. Vet. Pathol. 1982;19:46–56. doi: 10.1177/030098588201900108. [DOI] [PubMed] [Google Scholar]
- Debouck P, Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus CV777. Am. J. Vet. Res. 1980;41:219–223. [PubMed] [Google Scholar]
- Debouck P, Pensaert M, Coussement W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV777. Vet. Microbiol. 1981;6:157–167. [Google Scholar]
- Hofmann M, Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath I, Mocsari E. Ultrastructural changes in the small intestinal epithelium of suckling pigs affected with a transmissible gastroenteritis (TGE)-like disease. Arch. Virol. 1981;68:103–113. doi: 10.1007/BF01314440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusanagi K, Kuwahara H, Katoh T, Nunoya T, Ishikawa Y, Samejima T, Tajima M. Isolation and serial propagation of porcine epidemic diarrhea virus in cell cultures and partial characterization of the isolate. J. Vet. Med. Sci. 1992;54:313–318. doi: 10.1292/jvms.54.313. [DOI] [PubMed] [Google Scholar]
- Kuwahara, H., Nunoya, T., Samejima, T., Tajima, M., 1988. Passage in piglets of a coronavirus associated with porcine epidemic diarrhea. J. Jpn. Vet. Med. Assoc. 41, 169–173 (in Japanese with English summary).
- Kweon C.H, Kwon B.J, Jung T.S, Kee Y.J, Hur D.H, Hwang E.K, Rhee J.C, An S.H. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Kor. J. Vet. Res. 1993;33:249–254. [Google Scholar]
- Pensaert M, Debouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B, Debouck P, Reynolds D.J. An immunoelectron microscopic and immunofluorescent study on the antigenic relationship between the coronavirus-like agent, CV777, and several coronaviruses. Arch. Virol. 1981;68:45–52. doi: 10.1007/BF01315166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil A, Hess R.G, Bachmann P.A. Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic diarrhoea virus (EVD): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infection. Zentralbl. Veterinaermed. B. 1981;28:564–577. doi: 10.1111/j.1439-0450.1981.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Shimizu Y. Effects of ambient temperatures on clinical and immune responses of pigs infected with transmissible gastroenteritis virus. Vet. Microbiol. 1979;4:109–116. [Google Scholar]
- Shimizu M, Shimizu Y, Kodama Y. Effects of ambient temperatures on induction of transmissible gastroenteritis in feeder pigs. Infect. Immun. 1978;21:747–752. doi: 10.1128/iai.21.3.747-752.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi M, Tsuda T, Yamazaki K, Yoshida K, Nakazawa M, Sato K, Minami T, Iwashita K, Watanabe M, Suzuki Y, Mori M. An immunohistochemical investigation of porcine epidemic diarrhoea. J. Comp. Pathol. 1995;113:59–67. doi: 10.1016/S0021-9975(05)80069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Okada K, Ohshima K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Jpn. J. Vet. Sci. 1983;45:829–832. doi: 10.1292/jvms1939.45.829. [DOI] [PubMed] [Google Scholar]
- Tsuda, T., 1997. Porcine epidemic diarrhea: its diagnosis and control. Proc. Jpn. Pig. Vet. Soc. 31, 21–28 (in Japanese).
- Turgeon D.C, Morin M, Jolette J, Higgins R, Marsolais G, Difranco E. Coronavirus-like particles associated with diarrhea in baby pigs in Quebec. Can. Vet. J. 1980;21:100–101. [PMC free article] [PubMed] [Google Scholar]
- Wood E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977;100:243–244. doi: 10.1136/vr.100.12.243. [DOI] [PubMed] [Google Scholar]


