Abstract
Two of the three adult dogs kept in a family developed severe gastroenteritis. From the feces of one of the affected dogs a minute virus of canines (MVC) was detected by PCR and virus isolation. That this virus had recently infected the dogs was indicated by high anti-MVC antibody titers of their sera. No other virus commonly associated with canine gastrointestinal disease was implicated. As no previous association of MVC infection and disease in aged dogs had been described, further characterization of the isolated virus was performed to determine if it had unique pathogenic or genetic properties. Experimental infection of adult dogs did not result in clinical disease and comparison of the viral genome with other MVCs did not reveal any novel elements. The American, Japanese and Korean MVC strains studied were closely related to bocaviruses of bovine and human origin, and appeared to have evolved uniquely in the dog population after dividing from the common ancestor of bocaviruses. Further detailed clinical and virological studies are warranted to define the role of MVCs in disease in adult dogs.
Keywords: Minute virus of canines, Canine parvovirus type 1, Canine bocavirus, Canine minute virus, Parvovirus, Dog
1. Introduction
Canine parvovirus type 2 (CPV-2), canine coronavirus (CCoV), canine adenoviruses (CAV), and canine distemper virus (CDV) are well-established pathogens of pups as well as adult dogs that possess either no or insufficient immunity. By contrast, another canine virus, minute virus of canines (MVC) (Binn et al., 1970) has been considered to be a “non-pathogenic orphan virus” (Carmichael, 1999). MVC, also known as canine parvovirus type 1 (Carmichael, 1987) or canine bocavirus (Manteufel and Truyen, 2008), and CPV-2 are unrelated parvovirus species belonging to different genera of the Parvoviridae (Macartney et al., 1988, Mochizuki et al., 2002, Schwartz et al., 2002). However, subsequent experimental studies revealed that MVC is a perinatal pathogen of dogs (Carmichael, 1987, Macartney et al., 1988). Clinical discrimination of the etiology of contagious pneumoenteric diseases of dogs is generally difficult, and at present, diagnosis for MVC infection is available only from particular research laboratories.
Although only a limited number of studies have been conducted, some features of MVC and its infection have been established. Thus, MVC is distributed worldwide among domestic dogs of all ages (Carmichael et al., 1994, Hashimoto et al., 2001, Jang et al., 2003, Järplid et al., 1996, Mochizuki et al., 2002, Pratelli et al., 1999, Truyen et al., 1996). The virus is pathogenic for the fetus and infection of pregnant bitches results in abortion (Carmichael et al., 1991, Truyen et al., 1996). MVC infection of young pups, typically less than 1-month old, may be either asymptomatic or cause a milder illness than that caused by CPV-2 (Carmichael et al., 1994, Macartney et al., 1988), although more serious disease may occur under certain field conditions (Harrison et al., 1992, Järplid et al., 1996, Pratelli et al., 1999). By contrast, the pathogenic potential of MVC for adult dogs has been considered to be minimal (Binn et al., 1970, Carmichael et al., 1991, Carmichael et al., 1994, Hashimoto et al., 2001, Mochizuki et al., 2002). Genetic analysis has shown that the genomic structure of MVC is similar to those of bovine parvoviruses (BPV) and human bocaviruses (HBoV), and the genome size of an American GA3 strain is 5402 nucleotides in length (Schwartz et al., 2002, Sun et al., 2009).
In this context, we had an opportunity to study a new strain of MVC. In a small group of dogs housed together an elderly dog was taken ill with signs of severe gastroenteritis, suspected initially to be due to CPV-2. Contrary to our expectations, a MVC was the only virus recovered in cell culture from a fecal specimen from the dog. Because to our knowledge this is the first time that MVC had been isolated from an old diseased dog, the isolate was subsequently examined for any unique features, focusing especially on its virulence for dogs and its genomic properties.
2. Materials and methods
2.1. Clinical episode of the case and specimens obtained
Three dogs, one male Yorkshire terrier (11 years and 2 months old) and two female Chihuahuas (1- and 6-year old), had been kept together for more than 1 year. The dogs had been vaccinated annually with combined canine vaccines that had no CCoV component. The Yorkshire terrier was taken to a veterinary clinic because of vomiting in November, 2008. On the third day of hospitalization, the dog had bloody diarrhea in addition to vomiting. CPV-2 was considered as a possible cause but since a fecal specimen was found to be negative by a commercial test kit for CPV-2, it was submitted to our diagnostic laboratory for further virological examination. The dog received symptomatic therapy and subsequently recovered. The older Chihuahua also had similar signs just before the present case; however, the cause could not be established because no specimen was available for examination. The younger Chihuahua did not show any clinical signs throughout the period. Serum samples were taken from all three dogs in December, 2008, about 1 month after the incident.
2.2. Virological examination of the specimen
The fecal swab specimen obtained from the Yorkshire terrier was placed in 2 ml of Eagle's minimal essential medium and the extract was clarified by centrifugation at 15,000 rpm for 20 min. The resulting supernatant fluid was examined for viruses. For virus isolation, the supernatant was inoculated into both Madin–Darby canine kidney (MDCK) and Felis catus whole fetus-4 (fcwf-4) cell (Pedersen et al., 1981) cultures. The cultures were examined for cytopathic effect (CPE) and were subsequently blind-passaged twice when no CPE was apparent. Detection of CCoV, CDV, CPV-2, canine respiratory coronavirus (CRCoV), and MVC was attempted by the molecular methods described previously (Mochizuki et al., 2002, Mochizuki et al., 2008, Ohshima et al., 2008, Yachi and Mochizuki, 2006).
2.3. MVC isolation in cell culture
Since the fecal specimen was found to contain either MVC or a related gene fragment by the PCR test, MVC isolation in the MDCK cell culture and subsequent specific virus identification were performed by the methods described previously (Mochizuki et al., 2002).
2.4. Serology
Anti-MVC neutralization (NT) antibody in the dog serum samples was determined by the method described previously (Mochizuki et al., 2002). The serum samples were also examined for antibodies against CAV-2, CDV, CPV-2, canine parainfluenza virus (CPIV), and CCoV which are the components of commonly employed combined canine vaccines by the routine methods in our laboratory (see legend to Table 1 ).
Table 1.
Antibody titers of serum samples obtained after recovery from disease.
| Dog | Age | Clinical signs | Antibody titer against |
|||||
|---|---|---|---|---|---|---|---|---|
| CAV-2a | CCoV | CDV | CPIV | CPV-2 | MVC | |||
| Yorkshire terrier | 11 years and 2 months | Vomiting, bloody stool | 32 | 4 | 11 | 8 | 512 | 2,048 |
| Chihuahua | 6 years | Vomiting, bloody stool | 256 | <2 | 96 | 8 | 32 | 1,024 |
| Chihuahua | 1 year | None | 2048 | <2 | 280 | 4 | 32 | 512 |
CAV-2, canine adenovirus type 2; CCoV, group 1 canine coronavirus; CDV, canine distemper virus; CPIV, canine parainfluenza virus; CPV-2, canine parvovirus type 2; MVC, minute virus of canines. Neutralization antibodies for CAV-2, CCoV, CDV, CPIV, and MVC, and HI antibody for CPV-2.
2.5. Experimental infection
Three 7-month-old SPF Beagles were used. The dogs had been vaccinated with a canine combined vaccine twice when 8 and 11 weeks old. They did not possess anti-MVC NT antibody before challenge. The present MVC isolate 08-017, passaged 5 times in MDCK cell culture, was used as inoculum. Each dog received 3 ml of the culture fluid containing 105 TCID50 of virus by intraoronasal instillation. The clinical condition of each dog was observed for 2 weeks after challenge. Rectal swab samples were taken periodically (before inoculation, and on days 3, 5, 7, 10, and 14 after inoculation) to detect virus excretion by PCR. Blood samples were obtained before inoculation and on day 14 after inoculation to determine serum anti-MVC NT titers.
2.6. Genomic analyses of MVC strains
In addition to the present isolate 08-017, two previous MVC isolates 97-047 and 97-114 from Japanese dogs (Mochizuki et al., 2002) were used for genome sequencing. Genome DNA was amplified from the infected-MDCK cell culture fluid by the method described previously (Mochizuki et al., 2002, Ohshima et al., 2004) and an almost full-length nucleotide sequence, excepting the 5′- and 3′-terminal palindromes, was obtained for each isolate. The PCR product was purified and sequenced directly with an ABI PRISM BigDye Terminator version 3.0 cycle sequencing kit on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems Inc.). The nucleotide sequence was analyzed by the methods described previously (Ohshima et al., 2004).
2.7. Nucleotide sequence accession numbers
MVC strains reported thus far were the American GA3 (AF495467 and FJ214110) and Korean HM-6 (AB158475) strains. The nucleotide sequence data of MVC strains 08-017, 97-047, and 97-114 first described in the present study are available in the DDBJ/EMBL/GenBank databases with accession nos. AB518882, AB518883, and AB518884, respectively. The other parvoviruses used in the phylogeny study are specified in the legend to Fig. 1 .
Fig. 1.
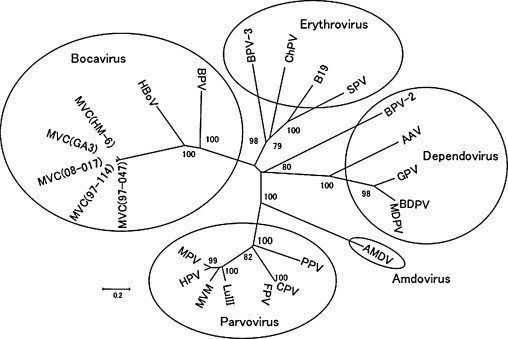
Phylogenetic relationship of MVC strains, including the present isolate 08-017, to other parvovirus species of subfamily Parvovirinae. A number at the node indicates the value of 100 bootstrap analyses. The horizontal bar indicates the number of base substitutions per site. The reference viruses and their accession numbers used in the phylogeny are as follows: AAV, adeno-associated virus (NC_002077); AMDV, Aleutian mink disease virus (NC_001662); B19, B19 virus (NC_000883); BDPV, Barbarie duck parvovirus (U22967); BPV, bovine parvovirus (NC_001540); BPV-2, bovine parvovirus 2 (AF406966); BPV-3, bovine parvovirus 3 (AF406967); CPV-2, canine parvovirus type 2 (D26079); ChPV, Chipmunk parvovirus (U86868); FPV, feline panleukopenia virus (M38246); GPV, goose parvovirus (NC_001701); HPV, hamster parvovirus (U34255); HBoV, human bocavirus (DQ000495); LuIII, LuIII virus (M81888); MVC GA3 (FJ214110); MVC HM-6 (AB158475); MVM, minute virus of mice (NC_001510); MPV, mouse parvovirus (NC_001630); MDPV, Muscovy duck parvovirus (X75093); PPV, porcine parvovirus (NC_001718); SPV, simian parvovirus (U26342).
3. Results
3.1. Isolation of a MVC strain
A fecal swab was obtained from the affected dog and PCR was carried out on an extract. Although no positive PCR amplification was obtained for CCoV, CDV, CPV-2 or CRCoV, a product specific for MVC was generated (data not shown). Subsequently MVC was isolated in MDCK cell culture accompanied by the formation of typical intranuclear inclusion bodies that contained MVC-specific antigen (data not shown). This isolate was designated as MVC strain 08-017 and was characterized further. No other viral agent was recovered from the specimen in either MDCK or fcwf-4 cell cultures.
3.2. Serological analysis of the dogs in the affected household
The serum samples obtained from the three dogs contained antibodies at various titers against CAV-2, CDV, CPIV, and CPV-2, but relatively low or no antibody titer was found against CCoV (Table 1). The titers were not sufficiently high to suggest recent exposure to these viruses. In contrast, the antibody titers against MVC were high enough to indicate a recent infection.
3.3. Pathogenicity of the new MVC isolate for experimental dogs
None of the experimental dogs showed any clinical signs or shed virus in the feces during the 2 weeks following challenge. However, anti-MVC NT titers of the serum samples taken 2 weeks after challenge were elevated between 64 and 256 indicating that the virus had replicated in vivo.
3.4. Genomic structure of Japanese MVC isolates
Nucleotide sequences composed of 5020 and 5019 bases were obtained for isolates 08-017 and 97-047, and isolate 97-114, respectively, which represented about 92.9% of the MVC genome. In the case of isolate 97-114, one base deletion was found in the 5′ untranslated region. The same three open reading frames (ORFs) were predicted from the genome sequence of each isolate. The left-hand ORF was predicted to encode the non-structural protein NS1 composed of 774 amino acid (aa) residues. The right-hand ORF was predicted to encode 703 aa residues, encoding the overlapping capsid proteins VP1 (703 aa residues) and VP2 (571 aa residues). The central ORF that overlapped with the NS1 and VP1/2 ORFs was predicted to encode the non-structural protein NP1 and consisted of 186 aa residues. The length of each ORF of the present MVC isolate 08-017 was the same as those of other MVC isolates including the American GA3 (Schwartz et al., 2002) and the Korean HM-6 (Ohshima et al., 2004) strains. In addition, neither base insertion nor deletion was detected in the genome sequence of isolate 08-017.
Among all MVC strains, including the American and Korean strains, the predicted amino acid sequence identities ranged from 96.8% to 99.7% for NS1, from 97.4% to 99.4% for VP1/2 and from 92.5% to 100% for NP1. A noteworthy difference was found in the NP1 identities among MVC strains: complete sequence identity was obtained for those between Japanese and American strains, while the sequence identity between Korean and Japanese/American strains was 92.5%.
3.5. Phylogenetic analysis of MVC strains
Two kinds of unrooted phylogenetic tree were made based on the nucleotide sequence alignments of the full genome size data that were available for each parvovirus species. The newly characterized Japanese MVC strains together with the previous American and Korean MVC strains belonged to the branch of the genus Bocavirus with BPV and HBoV (Fig. 1); and MVC strains showed a close mutual evolutionary relationship that was distant from different bocavirus species. On the other hand, as shown in Fig. 2 , there was a mutual relationship among MVC strains isolated from American, Japanese and Korean dogs. Although the available data may not be sufficient to make a definite conclusion, they suggested that each branch was based on their geographical origin.
Fig. 2.
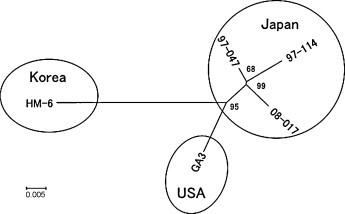
Phylogeny showing a mutual relationship of MVC strains. A number at the node indicates the value of 100 bootstrap analyses. The horizontal bar indicates the number of base substitutions per site.
4. Discussion
The genus bocavirus was originally assigned to a small virus group comprising BPV and MVC. Subsequently HBoV was added as the third member and more recently evidence of porcine bocavirus was found associated with postweaning multisystemic wasting syndrome of pigs (Blomström et al., 2009). Characteristically the bocaviruses appear to be pathogenic in very young animals, and MVC is no exception. Both experimental and spontaneous field case studies suggest that MVC causes mild to severe pneumonitis and/or enteritis in newborn pups (Carmichael et al., 1994, Harrison et al., 1992, Järplid et al., 1996, Pratelli et al., 1999) but is relatively non-pathogenic in older dogs. Therefore the association of MVC with an old diseased dog, as described here, is unusual.
In addition to the recovery of MVC from the sick dog, the blood samples taken after recovery from the disease were found to possess anti-MVC NT antibody titers between 512 and 2048 (Table 1). Considering the serological data reported previously (Carmichael et al., 1994, Hashimoto et al., 2001, Mochizuki et al., 2002), higher antibody titers, in the hundreds for example, may be used as grounds for indicating recent exposure to MVC. In this context, MVC was strongly suspected of being associated with the clinical signs of gastroenteritis. In the household, the two older dogs recovered from the illness and in the youngest dog the infection may have taken a subclinical course. This outcome is, however, contrary to the current concept of canine MVC infection (Carmichael, 1987, Carmichael, 1999) and suggested that the present MVC isolate 08-017 might be a novel strain which is pathogenic for adult dogs. Subsequently this was not confirmed by the experimental infection of the adolescent SPF dogs. In simple terms, the present animal experiment results may reflect the most probable relationship that occurs between MVC and aged dogs in the field: MVC infection of aged dogs results in antibody production but is accompanied only rarely by virus shedding so that MVC has not been found in most field cases. If this is true, the present field case with disease signs and virus shedding is a peculiar clinical experience.
As described above, we could not identify any particular pathological characteristics of the present MVC isolate 08-017 to explain the present field case of disease. In addition, it had neither insertion nor deletion in the genome sequence and was not a recombinant between other parvoviruses including CPV-2. That is, the present MVC isolate 08-017 seems to be molecularly very similar to previous MVC strains (Schwartz et al., 2002, Ohshima et al., 2004). Therefore, it is uncertain why the clinical signs suspected of CPV-2 infection appeared in this case and how MVC played a part in the development of the illness. There is a possibility that some host factors and/or accidental secondary pathogens influenced the disease course, and it may be worthwhile to perform experimental infection of immunosuppressed dogs, for example, to determine if MVC is pathogenic in these conditions. In any event, it would be useful to carry out more field case studies, with MVC included in the list of viruses for routine examination in small animal veterinary clinics.
Because only a few MVC strains were available (Binn et al., 1970, Mochizuki et al., 2002), the genetic relation between MVC strains of different origin has not been studied previously. In the present study, the genomes of five MVC strains were compared. All MVCs have diverged from the common ancestor of bocaviruses and have evolved uniquely in the dog population worldwide (Fig. 1). A Korean MVC is genetically distant from American and Japanese MVCs especially when the NP1 genes were compared. MVCs may have evolved further within each geographical region (Fig. 2), but the analysis of more MVC isolates would be necessary to confirm such a conclusion.
In conclusion, a MVC strain was the only virus recovered from an elderly dog that showed severe signs of gastroenteritis. Serological analysis showed that the dogs in the same house had high NT antibodies against MVC, suggesting a close association of MVC with the clinical signs. However the MVC isolate was not pathogenic in aged dogs infected experimentally. It will be necessary to accumulate further field cases to elucidate the clinical significance of MVC as a canine pathogen. Phylogenetic analysis of MVC strains including the present MVC isolate suggested a possibility of geographically dependent evolution pattern of MVC.
Acknowledgment
We are much obliged to Oswald Jarrett of the University of Glasgow, Institute of Comparative Medicine, for valuable discussions in the preparation of the manuscript.
References
- Binn L.N., Lazar E.C., Eddy G.A., Kajima M. Recovery and characterization of a minute virus of canines. Infect. Immunol. 1970;1:503–508. doi: 10.1128/iai.1.5.503-508.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomström A.-L., Belák S., Fossum C., McKillen J., Allan G., Wallgren P., Berg M. Detection of a novel porcine boca-like virus in the background of porcine circovirus type 2 induced postweaning multisystemic wasting syndrome. Virus Res. 2009;146:125–129. doi: 10.1016/j.virusres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Carmichael L.E. Canine parvovirus type 1 (minute virus of canines) In: Appel M.J.G., editor. Virus Infections of Carnivores. Elsevier Science Publications; Amsterdam: 1987. pp. 63–67. [Google Scholar]
- Carmichael L.E. Neonatal pup diseases. Current status of canine herpesvirus (CHV) and minute virus of canines (MVC, canine parvovirus-type 1, CPV-1) In: Carmichael L.E., editor. Document no. A0102. 1199. Recent Advances in Canine Infectious Diseases. International Veterinary Information Service; Ithaca, New York: 1999. www.ivis.org [Google Scholar]
- Carmichael L.E., Schlafer D.H., Hashimoto A. Pathogenicity of minute virus of canines (MVC) for the canine fetus. Cornell Vet. 1991;81:151–171. [PubMed] [Google Scholar]
- Carmichael L.E., Schlafer D.H., Hashimoto A. Minute virus of canines (MVC, canine parvovirus type-1): pathogenicity for pups and seroprevalence estimate. J. Vet. Diagn. Invest. 1994;6:165–174. doi: 10.1177/104063879400600206. [DOI] [PubMed] [Google Scholar]
- Hashimoto A., Takiguchi M., Hirai K., Kida H., Carmichael L.E. A serological survey of minute virus of canines (MVC; canine parvovirus type-1) in dogs in the Tokai area of Japan. Jpn. J. Vet. Res. 2001;49:249–253. [PubMed] [Google Scholar]
- Jang H.-K., Tohya Y., Han K.-Y., Kim T.-J., Song C.-S., Mochizuki M. Seroprevalence of canine calicivirus and canine minute virus in the Republic of Korea. Vet. Rec. 2003;153:150–152. doi: 10.1136/vr.153.5.150. [DOI] [PubMed] [Google Scholar]
- Järplid B., Johansson H., Carmichael L.E. A fatal case of pup infection with minute virus of canines (MVC) J. Vet. Diagn. Invest. 1996;8:484–487. doi: 10.1177/104063879600800415. [DOI] [PubMed] [Google Scholar]
- Harrison L.R., Styer E.L., Pursell A.R., Carmichael L.E., Nietfeld J.C. Fatal disease in nursing puppies associated with minute virus of canines. J. Vet. Diagn. Invest. 1992;4:19–22. doi: 10.1177/104063879200400105. [DOI] [PubMed] [Google Scholar]
- Macartney L., Parrish C.R., Binn L.N., Carmichael L.E. Characterization of minute virus of canines (MVC) and its pathogenicity for pups. Cornell Vet. 1988;78:131–145. [PubMed] [Google Scholar]
- Manteufel J., Truyen U. Animal bocaviruses: a brief review. Intervirology. 2008;51:328–334. doi: 10.1159/000173734. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Hashimoto M., Hajima T., Takiguchi M., Hashimoto A., Une Y., Roerink F., Ohshima T., Parrish C.R., Carmichael L.E. Virologic and serologic identification of minute virus of canines (canine parvovirus type 1) from dogs in Japan. J. Clin. Microbiol. 2002;40:3993–3998. doi: 10.1128/JCM.40.11.3993-3998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Yachi A., Ohshima T., Ohuchi A., Ishida T. Etiologic study of upper respiratory infections of household dogs. J. Vet. Med. Sci. 2008;70:563–569. doi: 10.1292/jvms.70.563. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Hisaka M., Kawakami K., Kishi M., Tohya Y., Mochizuki M. Chronological analysis of canine parvovirus type 2 isolates in Japan. J. Vet. Med. Sci. 2008;70:769–775. doi: 10.1292/jvms.70.769. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Kishi M., Mochizuki M. Sequence analysis of an Asian isolate of minute virus of canines (canine parvovirus type 1) Virus Genes. 2004;29:291–296. doi: 10.1007/s11262-004-7430-3. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F., Floyd K. Infection studies in kittens, using feline infectious peritonitis virus propagated in cell culture. Am. J. Vet. Res. 1981;42:363–367. [PubMed] [Google Scholar]
- Pratelli A., Buonavoglia D., Tempesta M., Guarda F., Carmichael L., Buonavoglia C. Fatal canine parvovirus type-1 infection in pups from Italy. J. Vet. Diagn. Invest. 1999;11:365–367. doi: 10.1177/104063879901100413. [DOI] [PubMed] [Google Scholar]
- Schwartz D., Green B., Carmichael L.E., Parrish C.R. The canine minute virus (minute virus of canines) is a distinct parvovirus that is most similar to bovine parvovirus. Virology. 2002;302:219–223. doi: 10.1006/viro.2002.1674. [DOI] [PubMed] [Google Scholar]
- Sun Y., Chen A.Y., Cheng F., Guan W., Johnson F.B., Qiu J. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J. Virol. 2009;83:3956–3967. doi: 10.1128/JVI.02569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen U., Wolf G., Carmichael L.E. Das andere Parvovirus: Erstbeschreibung des Minute Virus of Canines (canine parvovirus type 1) in Deutschland. Tierarztl. Prax. 1996;24:511–513. (in German, with English summary) [PubMed] [Google Scholar]
- Yachi A., Mochizuki M. Survey of dogs in Japan for group 2 canine coronavirus infection. J. Clin. Microbiol. 2006;44:2615–2618. doi: 10.1128/JCM.02397-05. [DOI] [PMC free article] [PubMed] [Google Scholar]


