Abstract
Bovine viral diarrhoea (BVD) is an economically important cattle disease with a world-wide distribution that is caused by BVD virus, a pestivirus of the flaviviridae family. BVD viruses are genetically highly variable. They are classified into two genetic species (BVDV-1 and -2) that are further divided into numerous subgroups, particularly for BVDV-1. The complexity of these viruses is also reflected in their interaction with the host animals. Infections are either transient or persistent and can cause a wide spectrum of clinical signs, from no or very mild disease to severe forms, reminiscent of viral haemorrhagic fevers. In this work, we have analysed the clinical signs and the pathology of BVD viral infections in a cattle population where different subgroups of BVDV-1 genotype viruses are endemic. In addition, we have examined potential virulence properties of BVDV-1 subgroups during persistent infection by comparing the viral subgroups present in clinical cases with those detected in persistently infected (PI) animals sampled for epidemiological criteria, irrespective of their health condition. Furthermore, the clinical and postmortem findings were compared with respect to genetic characteristics of the viruses isolated from these animals.
Our results indicate that the BVDV positive animals fall roughly into two categories, depending on the primary organ affected and the age, with lung-centred pathology occurring mainly in young animals and mucosal pathology predominantly in older animals. Furthermore, we found a markedly higher proportion of representatives of the BVDV-1e subgroup in stillborn calves and aborted foetuses originating from epidemically unrelated cattle herds, suggesting that BVDV-1e may play a special role in prenatal and perinatal losses.
Keywords: Bovine viral diarrhoea virus, Pestivirus, Clinic, Pathology, Genetic subgroup, Virulence
1. Introduction
Bovine viral diarrhoea virus (BVDV), classical swine fever virus and border disease virus form the genus pestivirus within the flaviviridae family. BVD viruses are divided into two species, BVDV-1 and -2. BVDV-1 strains are particularly diverse, with at least 13 different subgroups defined genetically (Pellerin et al., 1994, Becher et al., 1997, Becher et al., 2003, Vilček et al., 2001, Vilček et al., 2004, Falcone et al., 2003, Xu et al., 2006, Jacková et al., 2008) that differ also antigenically as shown by up to tenfold antibody titer differences between subgroups in crossed neutralization tests (Bachofen et al., 2008). Despite their diversity, all BVD viruses are either of the cytopathic (cp) or the non-cytopathic (ncp) biotype, as defined by the effect of viral growth in cultured cells. The two biotypes also differ with respect to their effects in infected animals. Only the ncp biotype can establish a persistent infection of the foetus if the dam is transiently infected between approximately days 40 and 120 of its development. Even though such foetuses may develop normally, they remain infected for life and shed large quantities of virus, thereby assuring the persistence of BVDV in the cattle population even in the absence of animals susceptible to transient infection. Different from other persistent viral infections in man and animals, BVDV persistently infected (PI) cattle are immunotolerant to the infecting viral strain (reviewed in Peterhans et al., 2006).
The clinical consequences of infection with BVD virus are as diverse as the genetic and antigenic properties of these viruses. Disease can result from different pathogenetical mechanisms, depending on the different types of infection. Most transient infections may take an inapparent or mild course, associated with low-grade fever, diarrhoea and coughing. Rarely, however, acutely infected animals may suffer from high grade fever and bleeding in internal organs. This often fatal form of acute infection was reported in outbreaks in the late 80s and early 90s, caused by highly virulent BVD virus-2 strains (Ridpath et al., 2000, Ridpath et al., 2006). Although generally considered to be of low virulence, BVDV-1, too, may cause similar severe disease signs in acutely infected animals, albeit only rarely (Liebler-Tenorio et al., 2000, Ridpath et al., 2007).
In the case of persistent infections, clinical signs in PI animals can be differentiated pathogenetically into mucosal disease (MD) and non-MD cases. The onset of MD is associated with the appearance of the cytopathic biotype of BVDV that arises as a result of mutations and/or recombination events from the persisting ncp virus (Kümmerer et al., 1998, Tautz et al., 1998, Becher et al., 2001). This lethal disease is characterised by mucosal lesions, destruction of the lymphoid tissue in the gastrointestinal tract (Liebler et al., 1995) and untreatable diarrhoea. The onset of the clinical signs is often acute but a more chronic form, called late onset MD, is also described (Liebler-Tenorio et al., 2000, Fritzemeier et al., 1997).
The clinical signs in PI animals that have not (yet) developed MD can encompass a wide spectrum of symptoms, ranging from normal health to subclinical disorders, ill-thrift and growth retardation. Chronic or recurrent intestinal and/or pulmonary symptoms are frequently observed but occasionally dermatological, neurological or haematological disorders are the only signs of a persistent infection (Braun et al., 1996, Taylor et al., 1997). In many cases the clinical and necropsy findings do not allow a clear differentiation between MD and non-MD cases. The isolation of both biotypes, ncp and cp, proves a PI animal to suffer from MD.
Possible reasons for the variable clinical signs seen in BVDV infections have been analysed repeatedly and in some cases an impact of the infecting virus strain was proven (Baule et al., 1997, Baule et al., 2001, Fulton et al., 2002, Jones et al., 2004, Ridpath et al., 2007). However, these studies focused on transient infections. Moreover, the analyses were restricted to single outbreaks of identical or very closely related viral strains that affected several animals within one herd or in different herds simultaneously, or to experimental infections. Hence, the observations may be representative for a given viral strain rather than for an entire subgroup of BVDV.
In contrast to transient infections, disease associations between individual BVDV strains or subgroups have not been investigated in persistent infection.
BVD is endemic in Switzerland, with roughly 60% of cattle seropositive and 0.7% persistently infected, with seemingly no change in prevalence over a time period of some 30 years (Bürki et al., 1964, Homberger et al., 1975, Rüfenacht et al., 2000, Stalder et al., 2005). In this work, we have analysed PI animals referred by private veterinary practitioners to the clinical and pathology departments of the veterinary hospital of the University of Zürich. Due to the endemic nature of BVD, these animals may represent the most severe clinical forms of persistent infection, comparable to the tip of the iceberg.
We have recorded and applied a scoring protocol for the clinical and postmortem findings of the diseased animals and analysed possible correlations between different organ manifestations and the subgroups of persisting BVDV. To obtain an insight into the possibility of virulence of BVDV in persistent infection, we have analysed the viral subgroups present in diseased PI animals as compared to subgroups present in animals diagnosed BVDV PI independently of the clinical status.
2. Animals, materials and methods
2.1. Patients and examinations
At the Department of Farm Animals of the University of Zurich, all calves less than 3 months of age and chronically diseased animals are routinely tested for the presence of BVDV. We have retrospectively analysed all 68 clinical reports of PI animals shown at the clinic for ruminants from first of January 1995 to 31st of December 2003 (group A, Table 1 ). In addition, the 18 prospective cases from the years 2004 and 2005 were included (group B, Table 1), yielding 86 clinical case reports. All animals had undergone the same thorough clinical examination following a standardised protocol as described previously (Braun et al., 1996). Haematological, biochemical, bacteriological and parasitological analyses were performed as described by Braun et al. (2007).
Table 1.
List of cases included in this study.
| Group | Clinical report | Necropsy report | Sequence analysis |
|---|---|---|---|
| (A) Retrospective cases 1995–2003 | 68 | 67 | 52 |
| (B) Prospective cases 2004 + 2005 | 18 | 18 | 18 |
| (C) Pathology onlya 1995–2005 | – | 60 (C.1b: 45; C.2c: 15) | 60 |
| (D) Abortions/neonatesd 1995–2005 | – | 25 | 25 |
aEuthanised or perished animals, referred directly to the Institute of Veterinary Pathology by private practitioners for postmortem analysis of the entire carcassb or of body parts/organs onlyc. There were no clinical reports available of these cases.
dAborted foetuses/foetal body parts and euthanised or perished neonatal calves (<3 days), referred directly to the Institute of Veterinary Pathology by private practitioners for postmortem analysis. There were no clinical reports available of these cases.
The BVDV status of 76 animals of groups A and B was determined intra vitam by immunohistochemistry (IHC) of skin biopsies (66 cases) and by antigen-capture ELISA of blood leukocytes (8 cases) (Strasser et al., 1995) or of skin biopsies (2 cases) (Idexx HerdCheck BVDV Ag/Serum Plus, Idexx Europe B.V., 1119 NE Schiphol-Rijk, The Netherlands). Some animals were repeatedly tested by multiple methods. Ten animals of groups A and B were only diagnosed after being euthanised. The BVDV status of all animals was confirmed postmortem by IHC of organ samples. All samples revealed the staining pattern typical for persistent infection, as described previously by Hilbe et al. (2007).
As a result of the unfavourable prognosis, all animals were euthanised within 1–22 days after the initial clinical investigation and sent to the Institute of Veterinary Pathology for necropsy. A necropsy report was available of 85 of the cases from groups A and B. In addition, we included in our study 60 necropsy reports of animals that had been euthanised or had perished and been submitted by private veterinarians for necropsy to the Institute of Veterinary Pathology where they were tested BVD virus-positive by IHC of organ samples (group C, Table 1). The reasons for the submission of these carcasses for postmortem analysis were variable and encompassed examination of herd problems, clarification of ambiguous clinical disease signs or suspicion of inherited disease such as spinal muscle atrophy of Swiss Brown calves. No information on intra vitam examinations and their results were available of the animals of group C.
All in all, we analysed 145 necropsy reports (groups A, B, C), 130 of which contained complete data sets (groups A, B, C.1). In 15 cases, postmortem reports were only available for single organs or body parts (group C.2, Table 1).
The cases analysed were extended by 25 cases of BVDV infected neonates (stillbirths and calves younger than 3 days) and aborted foetuses, tested positive by IHC (staining pattern typical for persistent infection (group D, Table 1)).
2.2. Recording of reports
Descriptive information from the standardised clinical and necropsy reports was transformed into ordinal data (e.g. general health condition, degree of lung alteration) or nominal data (e.g. gender) with the help of a specially created evaluation protocol. The evaluation protocol applied was created in collaboration with clinicians and pathologists and is based on clinical scoring protocols used previously (Braun et al., 1996, Braun et al., 2005). For the majority of findings we used a simple 4-level grading: nothing abnormal detected = 0, mild = 1, moderate = 2, severe alteration = 3. The evaluation of all reports was performed by the same person and without any information on the BVDV subgroup isolated from the corresponding PI animal. The scoring was based on the graduation of the clinical and postmortem findings made by the various clinicians and pathologists who had initially recorded the cases. Grading the findings is an essential part of the standardised protocols used for initial clinical examination and necropsy. In those cases where the organ was only partially affected (e.g. an abscess in a single lung lobe) we considered that the severity and spread of the lesion would serve to assess the general impairment of the organ.
2.3. Mucosal index
To compare the mucosal alterations between different PI animals, we used a standardised mucosal (mc) index calculated for each animal. This index considers the number of affected sections of the alimentary tract (from nose to large intestines = max. 7 organs; affected = any pathological change from the normal tissue integrity) and the severity of the alterations (0 = no alteration, 1 = mild, 2 = moderate, 3 = severe alteration). With respect to the multiple dispersed lesions typically seen in MD cases (Liebler-Tenorio et al., 2000) the calculation places more weight on the number of affected sections than on the severity of the alterations. The mc index may range from 0 to 21.
Example:
| Mucosal organ | Score | |
|---|---|---|
| Muzzle/nose | 3 | |
| Oral cavity | 2 | |
| Oesophagus | 1 | |
| Forestomach | 0 | |
| Abomasum | 2 | |
| Small intestines | 1 | |
| Large intestines | 0 | |
| ∑(mucosal scores) | 9 |
2.4. Viral genomic sequences
177 Swiss BVDV strains, isolated between 1990 and 2005 were sequenced and phylogenetically analysed by Stalder et al. (2005). These viruses originated from animals detected to be PI as a result of a cross-sectional epidemiological study to determine the prevalence of BVDV in Switzerland (Rüfenacht et al., 2000). In addition, viruses were derived from herd analyses performed in order to assess diagnostic tests or to apply molecular epidemiology.
The 155 BVDV isolates of the clinical cases described here were isolated, sequenced and phylogenetically analysed for a study published previously (Bachofen et al., 2008).
2.5. Statistical analysis
For all statistical calculations the NCSS/PASS software (Kaysville, UT, USA) was used. As the data were not normally distributed, we used the non-parametric one-way ANOVA test (Kruskal–Wallis) to compare medians of three or more groups of patients. If this test revealed a significant over-all difference, pair-wise comparisons were performed using the Wilcoxon Rank-Sum test. To compare proportions we applied the Chi-square or Fisher's exact test. The probability level was set to 95%. The displayed p-values represent the results of the two-tailed hypothesis testing.
2.6. Determination of biotypes
We tested serum of 14 PI animals from the prospective cases for the existence of cytopathic BVDV virus as a hallmark for the onset of MD. The sera were diluted seven times in tenfold steps in cell culture medium (Earle's minimal essential medium (MEM)) enriched with 2% foetal bovine serum (FBS). MEM was purchased from Seromed (Biochrom, Munich, Germany) and FBS from Sigma or Oxoid GmbH (Wesel, Germany). FBS was free of BVDV and antibody to BVDV as tested by virus isolation and serum neutralization test, respectively. Each serum dilution step was distributed to six wells (100 μl) of a 96-well microtiter plate, seeded with primary bovine turbinate cells. After 5 days of incubation at 37 °C and 5% CO2, 20 μl of supernatant was transferred to a fresh 96-well microtiter plate, pre-seeded with bovine turbinate cells. After addition of 80 μl of fresh MEM, the microtiter plate was incubated as before and the passaging procedure repeated once more. After each passage, the cells were fixed and stained for viral protein as described by Adler et al. (1997). The cells were then microscopically controlled for the presence of a cytopathic effect.
3. Results
In order to investigate differences between BVDV subgroups regarding virulence during persistent infection, clinical recordings and necropsy reports of PI animals infected with viruses of different BVDV-1 subgroups were analysed. We included patients from the clinic for ruminants of the Department of Farm Animals (University Zürich) and additional necropsy reports of animals referred by private veterinarians directly to the Institute of Veterinary Pathology (University Zürich) for postmortem analysis.
3.1. Groups of patients and anamneses
As shown in Table 1, the cases could be separated into four groups: retrospective cases (group A, n = 68) and prospective cases (group B, n = 18) with both a clinical and a necropsy report, cases directly referred for necropsy by private practitioners without clinical report (group C, n = 60) and postmortem analyses of abortions and neonates (animals ≤3 days of age) without clinical report (group D, n = 25).
Of the 146 cases of groups A, B and C (abortions and neonates not included since the gender is often not stated) 78% were females, while for the animals with a clinical report (groups A and B, n = 86) the proportion of females was 90%. Animals of five different breeds and diverse crossbreeds were analysed. The majority of cases (n = 146) were of the Swiss Brown (67%), Holstein-Frisian (12%) and Swiss Fleckvieh (11%) breeds. These numbers reflect the over-all breed proportions of the patient population of the clinic. Single cases of Rhätisches Grauvieh, Dexter, Limousin and beef crossbreeds were also represented. The median age of the animals (n = 146) was 7 months, with a range from 2 days to 6 years. Animals referred to the clinic (groups A and B, n = 86) were significantly older (median = 12 months) than those submitted directly for necropsy (group C, n = 60, median age = 2 months). Of the animals with a clinical record (n = 86), 26% had an age of 24 months or over and four animals were older than 3 years (48, 52, 60 and 72 months). The cases originated from 17 of the 26 Swiss cantons. Most of the cases (n = 146) had a history of recurrent or untreatable diarrhoea (41%), pneumonia (20%) or both conditions together (9%). Neurological symptoms (10%) were often anamnestically described in necropsy reports of young calves, directly referred for postmortem examination (group C). Other anamnestic signs were abortion, lameness due to claw problems, anaemia and ill-thrift. In 45% of the cases (n = 146) the anamnestic report indicated concomitant health problems in the herd. Growth retardation was indicated in 30% of the cases.
3.2. Clinical and postmortem findings
The 12 most frequently observed clinical and postmortem manifestations are shown in Fig. 1 . Nearly all animals showed unspecific signs like loss of appetite (94%), reduction of the general condition (93%) and ruminal hypoperistalsis (92%), followed by intestinal disorders like diarrhoea (69%) and the resulting dehydration (63%). Respiratory sounds of different degree were present in 63% of the cases. Mucosal erosions as a more specific manifestation were found in the oral cavity and on the muzzle of 45% and 36% of the animals, respectively (Fig. 1A). For the determination of the most frequent postmortem findings, only cases with a complete necropsy report were included, whereas partial necropsies (e.g. single organs or body parts) were excluded (group C.2). Group D was excluded, because the abortions were often in a condition that would no longer allow clear postmortem diagnosis or consisted only of foetal body parts. The most frequent necropsy findings differed between animals with clinical reports (groups A and B) and those referred directly for necropsy (group C.1) (Fig. 1B). In the latter, these were pathological changes in the lung, while alterations in one or more mucosal organs (from muzzle to large intestines) were the primary findings in the clinical cases. Generally, except for the lung, a higher proportion of cases referred to the clinic (groups A and B) showed macroscopic lesions than animals directly referred for a postmortem examination (group C.1).
Fig. 1.
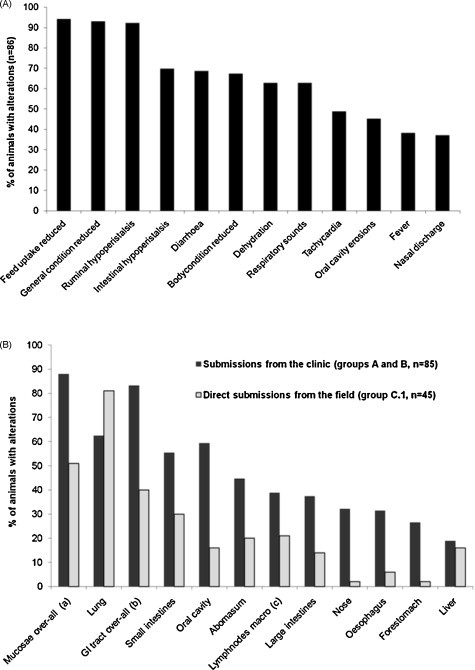
(A) The 12 most frequently observed clinical findings in 86 clinically examined BVDV positive animals (68 of group A and 18 of group B, Table 1). (B) The 12 most frequently observed necropsy findings from PI animals submitted for postmortem examination from the clinic (black bars) or directly from private practitioners (grey bars). Only necropsies of entire carcasses were included. The animal groups indicated refer to Table 1. aOne or multiple pathological alteration(s) of the mucosa of the whole alimentary tract, including nose and muzzle. bOne or multiple pathological alteration(s) of the mucosa of the gastrointestinal tract. cMacroscopic alteration of the mediastinal or mesenterial lymph nodes.
3.3. Haematological and microbiological findings
Haematological data were available from 82 of the 86 cases with a clinical report (groups A and B). The most frequently observed changes were elevated bilirubin levels (89%) and monocytosis (88%), neutrophilia (in 74%) and leukocytosis (in 59% of the animals). Decreased leukocyte counts were found in 11% of the cases. Alterations in erythrocyte parameters were frequent and were displayed as polycythemia (73% of the animals), decreased cellular haemoglobin content (61%) and a decreased erythrocyte volume (59%).
For groups A and B, intra vitam parasitological and bacteriological analyses were not performed routinely but rather upon clinical suspicion. However, with the exception of gastrointestinal nematodes that were present in 62% of the 47 cases analysed, parasites and bacteria such as liver fluke, lung worm, Salmonella, Campylobacter and enterotoxic Escherichia coli were only occasionally confirmed (0–10% of analysed cases). Furthermore, all 30 animals analysed for the presence of ovine herpesvirus-2, the causative agent of malignant catarrhal fever in cattle, were negative. Four and five animals were analysed for the presence of rota- and coronavirus, respectively. For each virus, one animal tested positive. Bovine herpesvirus-1 that can cause symptoms similar to BVD/MD is eradicated in Switzerland and can be excluded as contributing factor to the symptoms observed. For all groups, postmortem parasitological and bacteriological analyses were performed sporadically, depending on demand and suspicion and were not included to the study.
3.4. Comparison of mucosal lesions
In many cases, mucosal lesions were observed at multiple locations of the gastrointestinal tract. To be able to compare the general degree of mucosal lesions between groups of animals, it was necessary to condense the multiple mucosal alterations to one parameter. We used the number of affected organs and the severity of the single alterations to calculate an index for each animal, referred to as mucosal (mc) index (see Section 2 for details). The highest value that is theoretically possible (= all analysed organs are severely affected) is 21. The maximal and minimal mc index values observed from the 130 cases analysed (complete necropsy reports only: groups A, B and C.1) was 16 and 0, respectively, with an arithmetic mean of 1.9.
Because intestinal and pulmonary symptoms were the most frequent findings observed in the BVDV infected animals, we investigated whether these findings were correlated. Interestingly, these organ manifestations tended to exclude each other. As shown in Fig. 2A, animals with a high mucosal index had significantly less severe lung alterations than those with a low mucosal index. Moreover, animals with severe pulmonary manifestations were significantly younger than those with less severe pathological changes in the lungs (Fig. 2B). By contrast, the mucosal index was significantly higher in older animals than in young ones (Fig. 2C). Linear regression analysis (Spearman Rank) demonstrated a weak (ρ = 0.46) but statistically significant (p = 0.0003) correlation between the mc index and the age of the animals.
Fig. 2.
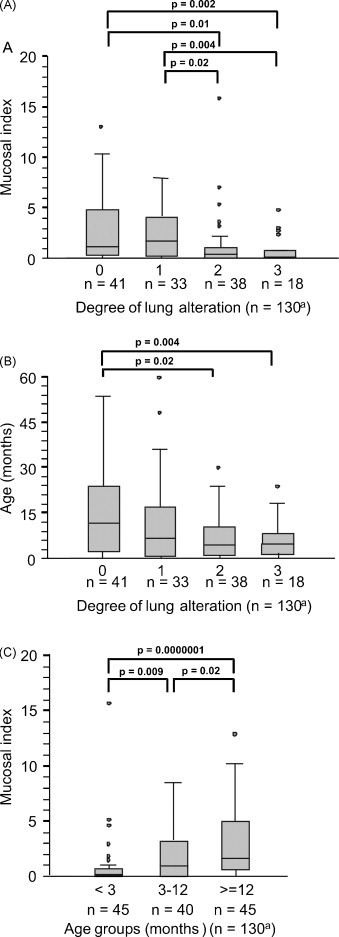
(A) Comparison of medians of BVDV positive animals showing different degrees of postmortem lung alterations (0 = no alteration, 1 = mild, 2 = moderate, 3 = severe alteration) in regard of the severity and spread of mucosal lesions (displayed as mucosal index) and (B) with respect to the age of the animals. (C) Comparison of different age groups of animals examined in relation to the mucosal index. a67 cases of group A, 18 cases of group B and 45 cases of group C (Table 1).
3.5. Correlation between postmortem findings and presence of cp biotype
The dichotomy between lung and mucosal lesions pointed to roughly two groups of BVDV infected patients. In order to investigate if this correlated with the presence or absence of mucosal disease, we tested serum samples of 14 PI animals of group B for the presence of cytopathic (cp) virus by passaging different dilutions of the sera three times on cultured bovine turbinate cells. This analysis was restricted to 14 animals, since blood samples were only available from the prospective cases (group B) and young calves with maternal antibodies had to be excluded due to inhibition of virus isolation. The results are shown in Table 2 . With one exception (04-06), the cp biotype was only present in sera of animals with an mc index of ≥1.7, suggesting that these patients had suffered from MD. No cp biotype was observed in animals with an index of ≤0.9. Therefore, we decided to set the threshold between MD- and non-MD cases to 1.0. Extrapolating this finding to the mucosal-indices of all cases, 40% of the animals (52/130) had suffered from MD.
Table 2.
Comparison of mucosal-indices and BVDV biotypes isolated from sera of 14 PI animals of group A (Table 1).
| PI animal | mc index | BVDV biotype |
|---|---|---|
| 04-06 | 0.14 | Ncp + cp |
| 05-13 | 0.14 | Ncp |
| 05-14 | 0.14 | Ncp |
| 04-05 | 0.57 | Ncp |
| 05-05 | 0.86 | Ncp |
| 04-07 | 1.71 | Ncp + cp |
| 05-04 | 1.71 | Ncp + cp |
| 05-01 | 2.14 | Ncp + cp |
| 05-03 | 2.86 | Ncp + cp |
| 04-01 | 3.43 | Ncp + cp |
| 05-12 | 4.57 | Ncp + cp |
| 04-04 | 5.14 | Ncp + cp |
| 05-10 | 5.14 | Ncp + cp |
| 05-11 | 8.57 | Ncp + cp |
These 14 animals were chosen due to availability of serum and absence of maternal antibodies.
3.6. BVDV genetic subgroup analysis
Of the 171 cases analysed in this work (groups A, B, C and D), the partial genomic sequence of the persisting virus was known from 155 cases (Bachofen et al., 2008). For the 18 cases of group B, viral RNA was isolated from blood, from the other cases from frozen tissue samples. Phylogenetic subgroup assessment was performed based of the 5′untranslated region and in some cases additionally on the Npro coding region. The subgroups present are shown in Fig. 3B. The majority of the animals harboured viruses of the subgroups BVDV-1e (32%) and 1h (34%), followed by 1k (24%) and 1b (9%). From one single animal we isolated the BVD-1x virus as described previously (Bachofen et al., 2008). These are (with exception of 1x) the same subgroups as described by Stalder et al. (2005) (Fig. 3A). The subgroup 1e was most prominent in the latter (43%) while 1h was the most frequent subgroup in clinical cases (34%) (Fig. 3). However, statistical analysis revealed no significant differences of the proportions of BVDV subgroups present in the diseased PI's and animals detected persistently infected independent of their health condition.
Fig. 3.
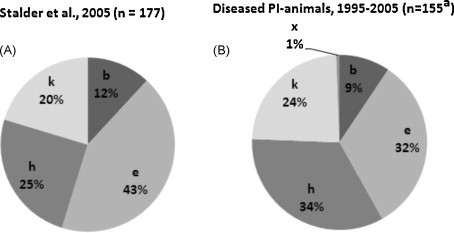
Comparison of the proportions of BVDV-1 subgroups isolated from PI animals diagnosed for epidemiological studies and independent of the health status (A) and from clinical cases (B). aAll cases of subgroups A, B, C and D where the viral genomic sequence was available.
3.7. Comparison of BVDV subgroups and manifestations
114 cases with a complete necropsy report and a known BVDV sequence could be included in this part of the study. This number is composed of 52 animals of group A, 18 of group B and the 45 cases of group C.1. The single animal harbouring BVDV 1x was excluded from the analysis, resulting in 114 PI animals. Of these, 7% had harboured viruses belonging to subgroup 1b, 34% to 1e and 1h each, and 25% to 1k (Fig. 4 ).
Fig. 4.
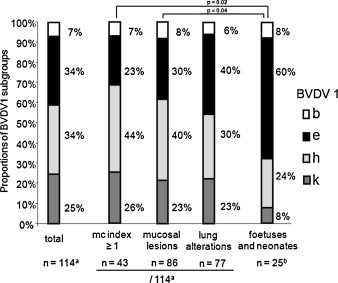
Proportions of the four main Swiss BVDV subgroups isolated from different groups of PI animals. aOnly complete necropsy reports were included: 52 cases of group A, 18 of group B and 45 cases of group C (Table 1) = 115. However, one single animal harboured a virus that did not belong to any of the four subgroups analysed and was therefore not included (n = 114). b25 cases of group D (Table 1).
We observed no statistically significant differences in the associations of viruses of a given subgroup with mucosal or pulmonary alterations or suspected MD cases (Fig. 4), nor did we see differences with regard to different age groups, breeds or other clinical, postmortem or haematological findings (data not shown). However, there was a tendency for 1h viruses to be more frequently isolated from animals with mucosal problems, presumable MD cases (mucosal index ≥ 1), and thus older animals, while 1e was the predominant subgroup of BVD virus in patients with pulmonary alterations and thus younger animals (Fig. 4). To broaden the age range of the patients, we additionally analysed the proportions of the different BVDV-1 subgroups in the 25 cases of group D (abortions, stillbirths and neonates). Interestingly, we observed a clear predominance of representatives of subgroup 1e BVD viruses in these animals (Fig. 4). The subgroup proportions in group D were significantly different from those observed in animals with mucosal lesions and suspected MD cases (mc index ≥ 1).
4. Discussion
The spectrum and severity of clinical signs caused by BVDV infection are highly variable. Factors contributing to this complexity include two types of infection with entirely different involvement of the immune system (immune response in transient versus immunotolerance in persistent infection), two different biotypes of virus (non-cytopathic versus cytopathic) and the diverse genetic spectrum of BVDV as well as that of the host animals. Studies on the virulence of individual strains of BVDV focus on transient infections. Experimental infections and analysis of disease outbreaks in transiently infected animals characterised by particular signs and severity of disease clearly suggested an influence of the infecting viral strain on disease manifestation. For example, Baule et al. (2001) as well as Jones et al. (2004), have shown BVDV-1d strains to be associated with respiratory disease in acutely infected calves. Furthermore, Ridpath et al. (2007) could experimentally reproduce the clinical symptoms caused by virulent BVDV-1 strains in acutely infected heifers.
In persistent infection, in contrast, analysis of a potential impact of the viral strain on the clinical picture is, for a number of reasons, more difficult. Among them is the existence of disease signs associated with persistent infection as such, and the occurrence of MD as a form of infection associated with the emergence of a cytopathic biotype of BVDV in these immunotolerant animals. Furthermore, some of the signs of persistent infection unrelated to MD may be of chronic nature, such as growth retardation and general ill-thrift. However, as shown by pluriparous PI cows and PI bulls detected among the candidates for artificial insemination stations, clinical signs of persistent infection can be absent in some animals. Experimental generation of a sufficient number of PI animals with different strains of BVDV would be laborious and require long-term follow-up. In addition, many different factors such as time point of maternal infection, genetic background of the animals and environmental factors, e.g. the presence of various other cattle pathogens, may modulate the outcome. Moreover, the epidemiological situation – endemic versus non-endemic – may influence the clinical appearance of infections. It is not surprising, therefore, that questions such as the impact of BVDV genetic properties on the health status of a PI animal, or possible associations between disease signs and a given subgroup of BVDV during this type of infection, have to date not been addressed.
We have investigated these questions in an endemic situation that was virtually undisturbed by vaccination or other control measures, both of which might influence the outcome of infection. In order to obtain information on the pathogenic potential of BVDV in persistent infection, we analysed clinical and necropsy findings of diseased PI animals and compared the BVDV subgroups present in these animals those isolated from animals diagnosed as PI independently of the health status.
Since we included prospective as well as retrospective cases examined over a period of 10 years, the long time range and the fact that different clinicians and pathologists examined the animals might influence the comparability of the raw data. However, the protocol applied to both the clinical and pathological investigations did not change over this time. In addition, the fact that the initial examinations were performed by many different clinicians and pathologists decreases the risk of a systematic bias. Moreover, the transformation of nominal into numerical data was done by one person, using a standardised protocol.
To obtain a larger number of cases, we analysed different groups of patients. While the retro- and prospective cases from the ruminants’ clinic (groups A and B) showed similar demographic parameters and postmortem findings (data not shown), we observed significant differences to the animals of group C. Animals of groups A and B were significantly older and generally more severely diseased than the cases directly referred for postmortem analysis (group C) (Fig. 1B). This might be due to the fact that, generally, the more valuable and thus primarily older animals with complex or unsuccessfully pre-treated health problems are referred for a stay at the clinic. The high proportion of females in groups A and B simply reflects the fact that most of these animals originate from dairy farms.
Interestingly, also the main postmortem findings differed between groups A and B versus group C, with mucosal alterations playing a prominent role in groups A and B and pulmonary lesions in group C (Fig. 1B). Keeping in mind the age difference between the groups, we tested the statistical correlation between lung and mucosal manifestations and the age of the animals. Mucosal lesions were more frequent in older and lung lesions in younger animals (Fig. 2); that held also true when the two animal groups (A + B and C) were tested separately (data not shown). The reason for the correlation between age and the postmortem finding may be related to the higher risk of MD onset with increasing age. MD is correlated to the appearance of the cp virus that arises as a result of mutations or recombination during viral RNA replication from the persisting ncp virus (Deregt and Loewen, 1995, Neill and Ridpath, 2001, Lackner et al., 2004). Statistically, a longer time period of viral replication may increase the risk of generating mutations leading to a cp biotype. Due to the retrospective nature of the study, we were not in a position to fulfill the virological criterion for diagnosing MD by showing the presence of cytopathic and non-cytopathic BVDV in all cases. To overcome this problem we combined a virological determination of MD of selected cases (namely the isolation of both biotypes) with a standardised pathological investigation (Table 2). The latter was achieved by creating a “mucosal (mc) index”, comparable to other clinical or pathological indices, e.g. the psoriasis area severity index (PASI) in human dermatology. Typically, MD leads to mucosal lesions at multiple locations of the digestive tract. Therefore, the mc index places more weight on the number of affected organs than on the severity of single lesions. This approach also minimizes individual differences in rating the severity of lesions and favours completeness of the necropsy reports. The results of the comparison of the biotype analysis with the mc index (Table 2) support the validity of the concept.
Taking all BVD viral sequences of groups A, B, C and D together, we did not observe statistically significant differences to the BVDV subgroup distribution previously described by Stalder et al., 2005 (Fig. 3). With the exception of the single “orphan” BVD 1x strain, we found the same four BVD-1 subgroups in the diseased animals and in the PI animals sampled in the course of an epidemiological survey. Although sampled in the same time period, the two large groups of PI animals are not matched with respect to breed, age or gender. On the one hand, using unmatched hosts, only major differences in tropism and virulence would show up. The results of our study argue against such major differences between entire subgroups. On the other hand, this observation does not exclude that individual strains may differ in virulence and tropism, as shown for transient infections.
A different aspect concerns possible BVDV subgroup affiliations with the two main clinical and pathological patterns observed, i.e. lung- versus gastrointestinal tract-centred. In this context, the possibility that these patterns might be influenced by the presence (or absence) of other pathogens needs to be considered. Such pathogens could include other viruses, bacteria and parasites. Viruses known to induce similar clinical signs and pathology, i.e. bovine herpesvirus-1 and ovine herpesvirus-2, can be excluded. The former is eradicated in Switzerland, and all PI animals suspected of suffering from malignant catarrhal fever tested negative for this lethal form of infection caused by ovine herpes virus-2 (Ackermann, 2005). Where data were available, we recorded additional bacteriological and parasitological examinations. However, in most cases the suspected infections were not confirmed.
We did not find a statistically significant association between a given BVD viral subgroup and the two major disease manifestations (Fig. 4), nor to any other clinical or mortem finding (data not shown). However, we observed a tendency for viruses of subgroup 1h to be more frequently isolated from animals with mucosal lesions, while 1e was the predominant subgroup in animals with lung alterations (Fig. 4). Regarding the age dependency of the mucosal and pulmonary manifestations (Fig. 2), the observed subgroup tendencies may well correlate to different age groups of PI animals rather than reflect a selective organ tropism. Indeed, 38% of the animals younger than 7 months (7 months = median age of all animals analysed) harboured BVDV-1e, while this proportion was only 29% in older animals (≥7 months). Since all PI animals are infected as foetuses, animals infected with BVDV-1e may die younger than animals infected with other subgroups and are therefore less frequently observed among older animals. This is supported by the observation of a surprisingly high proportion of BVDV-1e strains in abortions, stillbirths and neonates (Fig. 4), which clearly encourages additional investigations. We can exclude that a 1e live vaccine may be responsible, since, as indicated above, vaccines are used only very infrequently, and a 1e vaccine is not licensed in Switzerland. Moreover, we can also exclude that a particular strain of 1e may cause these abortions because the sequence data indicate that genetically different strains were present in these animals (Bachofen et al., 2008).
In summary, our study showed that, in an endemic situation, clinical cases of BVDV PI animals fall roughly in two distinct categories, with lung-centred pathology occurring mainly in young animals and mucosal pathology mainly in older animals. Moreover, even though we did not find evidence for one BVDV subgroup being generally more virulent during persistent infection, the epidemiologically unrelated concentration of 1e subgroup BVD viruses in stillborn calves and aborted foetuses provides preliminary evidence that viruses of this subgroup may play a special role in prenatal and perinatal losses due to BVD virus.
Acknowledgments
This work was supported by the Swiss National Science Foundation and a grant from Vetsuisse. We thank Matthias Schweizer and Reto Zanoni for helpful discussions, the team of the diagnostic unit for technical help, Marcus Doherr for statistical support and Ruth Parham for linguistic assistance.
References
- Ackermann M. Virus in sheep's skin. Schweiz. Arch. Tierheilkd. 2005;147:155–164. doi: 10.1024/0036-7281.147.4.155. [DOI] [PubMed] [Google Scholar]
- Adler B., Adler H., Pfister H., Jungi T.W., Peterhans E. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 1997;71:3255–3258. doi: 10.1128/jvi.71.4.3255-3258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachofen C., Stalder H., Braun U., Hilbe M., Ehrensperger F., Peterhans E. Co-existence of genetically and antigenically diverse bovine viral diarrhoea viruses in an endemic situation. Vet. Microbiol. 2008;131:93–102. doi: 10.1016/j.vetmic.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Baule C., van Vuuren M., Lowings J.P., Belak S. Genetic heterogeneity of bovine viral diarrhoea viruses isolated in Southern Africa. Virus Res. 1997;52:205–220. doi: 10.1016/s0168-1702(97)00119-6. [DOI] [PubMed] [Google Scholar]
- Baule C., Kulcsár G., Belak K., Albert M., Mittelholzer C., Soós T., Kucsera L., Belak S. Pathogenesis of primary respiratory disease induced by isolates from a new genetic cluster of bovine viral diarrhea virus type I. J. Clin. Microbiol. 2001;39:146–153. doi: 10.1128/JCM.39.1.146-153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher P., Orlich M., Shannon A.D., Horner G., König M., Thiel H.J. Phylogenetic analysis of pestiviruses from domestic and wild ruminants. J. Gen. Virol. 1997;78:1357–1366. doi: 10.1099/0022-1317-78-6-1357. [DOI] [PubMed] [Google Scholar]
- Becher P., Orlich M., Thiel H.J. RNA recombination between persisting pestivirus and a vaccine strain: generation of cytopathogenic virus and induction of lethal disease. J. Virol. 2001;75:6256–6264. doi: 10.1128/JVI.75.14.6256-6264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher P., Avalos-Ramirez R., Orlich M., Cedillo R.S., König M., Schweizer M., Stalder H., Schirrmeier H., Thiel H.J. Genetic and antigenic characterization of novel pestivirus genotypes: implications for classification. Virology. 2003;311:96–104. doi: 10.1016/s0042-6822(03)00192-2. [DOI] [PubMed] [Google Scholar]
- Braun U., Thür B., Weiss M., Giger T. Bovine Virus Diarrhea Mucosal Disease: clinical findings in 103 calves and heifers. Schweiz. Arch. Tierheilkd. 1996;138:465–475. [PubMed] [Google Scholar]
- Braun U., Feige K., Schweizer G., Pospischil A. Clinical findings and treatment of 30 cattle with botulism. Vet. Rec. 2005;156:438–441. doi: 10.1136/vr.156.14.438. [DOI] [PubMed] [Google Scholar]
- Braun U., Lejeune B., Schweizer G., Puorger M., Ehrensperger F. Clinical findings in 28 cattle with traumatic pericarditis. Vet. Rec. 2007;161:558–563. doi: 10.1136/vr.161.16.558. [DOI] [PubMed] [Google Scholar]
- Bürki F., König H., Schmid H.R. Kasuistischer Beitrag zur Mucosal Disease. Schweiz. Arch. Tierheilkd. 1964:473–477. [Google Scholar]
- Deregt D., Loewen K.G. Bovine viral diarrhea virus: biotypes and disease. Can. Vet. J. 1995;36:371–378. [PMC free article] [PubMed] [Google Scholar]
- Falcone E., Cordioli P., Tarantino M., Muscillo M., La Rosa G., Tollis M. Genetic heterogeneity of bovine viral diarrhoea virus in Italy. Vet. Res. Commun. 2003;27:485–494. doi: 10.1023/a:1025793708771. [DOI] [PubMed] [Google Scholar]
- Fritzemeier J., Haas L., Liebler E., Moennig V., Greiser-Wilke I. The development of early vs. late onset mucosal disease is a consequence of two different pathogenic mechanisms. Arch. Virol. 1997;142:1335–1350. doi: 10.1007/s007050050164. [DOI] [PubMed] [Google Scholar]
- Fulton R.W., Ridpath J.F., Saliki J.T., Briggs R.E., Confer A.W., Burge L.J., Purdy C.W., Loan R.W., Duff G.C., Payton M.E. Bovine viral diarrhea virus (BVDV) 1b: predominant BVDV subtype in calves with respiratory disease. Can. J. Vet. Res. 2002;66:181–190. [PMC free article] [PubMed] [Google Scholar]
- Hilbe M., Arquint A., Schaller P., Zlinszky K., Braun U., Peterhans E., Ehrensperger F. Immunohistochemical diagnosis of persistent infection with Bovine Viral Diarrhea Virus (BVDV) on skin biopsies. Schweiz. Arch. Tierheilkd. 2007;149:337–344. doi: 10.1024/0036-7281.149.8.337. [DOI] [PubMed] [Google Scholar]
- Homberger F., Schneider F., Dossenbach P. Zur epizootologischen Bedeutung der Sömmerung bei der Mucosal Disease/Virusdiarrhöe des Rindes. Schweiz. Arch. Tierheilkd. 1975;117:145–152. [PubMed] [Google Scholar]
- Jacková A., Novácková M., Pelletier C., Audeval C., Gueneau E., Haffar A., Petit E., Rehby L., Vilček S. The extended genetic diversity of BVDV-1: typing of BVDV isolates from France. Vet. Res. Commun. 2008;32:7–11. doi: 10.1007/s11259-007-9012-z. [DOI] [PubMed] [Google Scholar]
- Jones L.R., Cigliano M.M., Zandomeni R.O., Weber E.L. Phylogenetic analysis of bovine pestiviruses: testing the evolution of clinical symptoms. Cladistics. 2004;20:443–453. doi: 10.1111/j.1096-0031.2004.00030.x. [DOI] [PubMed] [Google Scholar]
- Kümmerer B.M., Stoll D., Meyers G. Bovine viral diarrhea virus strain Oregon: a novel mechanism for processing of NS2-3 based on point mutations. J. Virol. 1998;72:4127–4138. doi: 10.1128/jvi.72.5.4127-4138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner T., Müller A., Pankraz A., Becher P., Thiel H.J., Gorbalenya A.E., Tautz N. Temporal modulation of an autoprotease is crucial for replication and pathogenicity of an RNA virus. J. Virol. 2004;78:10765–10775. doi: 10.1128/JVI.78.19.10765-10775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler E.M., Küsters C., Pohlenz J.F. Experimental mucosal disease in cattle: changes of lymphocyte subpopulations in Peyer's patches and in lymphoid nodules of large intestine. Vet. Immunol. Immunopathol. 1995;48:233–248. doi: 10.1016/0165-2427(95)05440-h. [DOI] [PubMed] [Google Scholar]
- Liebler-Tenorio E.M., Lanwehr A., Greiser-Wilke I., Loehr B.I., Pohlenz J. Comparative investigation of tissue alterations and distribution of BVD-viral antigen in cattle with early onset versus late onset mucosal disease. Vet. Microbiol. 2000;77:163–174. doi: 10.1016/s0378-1135(00)00273-x. [DOI] [PubMed] [Google Scholar]
- Neill J.D., Ridpath J.F. Recombination with a cellular mRNA encoding a novel DnaJ protein results in biotype conversion in genotype 2 bovine viral diarrhea viruses. Virus Res. 2001;79:59–69. doi: 10.1016/s0168-1702(01)00298-2. [DOI] [PubMed] [Google Scholar]
- Pellerin C., van den Hurk J., Lecomte J., Tijssen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994;203:260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- Peterhans E., Jungi T.W., Schweizer M. How the bovine viral diarrhea virus outwits the immune system. Dtsch. Tierarztl. Wochenschr. 2006;113:124–129. [PubMed] [Google Scholar]
- Ridpath J.F., Neill J.D., Frey M., Landgraf J.G. Phylogenetic, antigenic and clinical characterization of type 2 BVDV from North America. Vet. Microbiol. 2000;77:145–155. doi: 10.1016/s0378-1135(00)00271-6. [DOI] [PubMed] [Google Scholar]
- Ridpath J.F., Neill J.D., Peterhans E. Impact of variation in acute virulence of BVDV1 strains on design of better vaccine efficacy challenge models. Vaccine. 2007;25:8058–8066. doi: 10.1016/j.vaccine.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Ridpath J.F., Neill J.D., Vilček S., Dubovi E.J., Carman S. Multiple outbreaks of severe acute BVDV in North America occurring between 1993 and 1995 linked to the same BVDV2 strain. Vet. Microbiol. 2006;114:196–204. doi: 10.1016/j.vetmic.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Rüfenacht J., Schaller P., Audigé L., Strasser M., Peterhans E. Prevalence of cattle infected with bovine viral diarrhoea virus in Switzerland. Vet. Rec. 2000;147:413–417. doi: 10.1136/vr.147.15.413. [DOI] [PubMed] [Google Scholar]
- Stalder H.P., Meier P., Pfaffen G., Wageck-Canal C., Rüfenacht J., Schaller P., Bachofen C., Marti S., Vogt H.R., Peterhans E. Genetic heterogeneity of pestiviruses of ruminants in Switzerland. Prev. Vet. Med. 2005;72:37–41. doi: 10.1016/j.prevetmed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Strasser M., Vogt H.R., Pfister H., Gerber H., Peterhans E. Proc. 3rd Congress Europ. Soc. Vet. Virol., Immunobiology of Viral Infections. 1995. Detection of Bovine Virus Diarrhea Virus (BVDV) in peripheral blood, cell cultures and tissue using a monoclonal antigen-capture ELISA; pp. 311–316. [Google Scholar]
- Tautz N., Meyers G., Thiel H.J. Pathogenesis of mucosal disease, a deadly disease of cattle caused by a pestivirus. Clin. Diagn. Virol. 1998;10:121–127. doi: 10.1016/s0928-0197(98)00037-3. [DOI] [PubMed] [Google Scholar]
- Taylor L.F., Janzen E.D., Ellis J.A., van den Hurk J.V., Ward P. Performance, survival, necropsy, and virological findings from calves persistently infected with the bovine viral diarrhea virus originating from a single Saskatchewan beef herd. Can. Vet. J. 1997;38:29–37. [PMC free article] [PubMed] [Google Scholar]
- Vilček S., Ďurkovič B., Kolesárová M., Greiser-Wilke I., Paton D. Genetic diversity of international bovine viral diarrhoea virus (BVDV) isolates: identification of a new BVDV-1 genetic group. Vet. Res. 2004;35:609–615. doi: 10.1051/vetres:2004036. [DOI] [PubMed] [Google Scholar]
- Vilček S., Paton D.J., Ďurkovič B., Strojny L., Ibata G., Moussa A., Loitsch A., Rossmanith W., Vega S., Scicluna M.T., Paifi V. Bovine viral diarrhoea virus genotype 1 can be separated into at least eleven genetic groups. Arch. Virol. 2001;146:99–115. doi: 10.1007/s007050170194. [DOI] [PubMed] [Google Scholar]
- Xu X., Zhang Q., Yu X., Liang L., Xiao C., Xiang H., Tu C. Sequencing and comparative analysis of a pig bovine viral diarrhea virus genome. Virus Res. 2006;122:164–170. doi: 10.1016/j.virusres.2006.05.005. [DOI] [PubMed] [Google Scholar]


