Abstract
One hundred avian Pasteurella multocida isolates recovered from cases of fowl cholera and related infections in England and Wales over a 13-year period were characterised by capsular PCR typing and analysis of outer membrane protein (OMP) profiles. Sixty-eight percent of the strains were of capsular type A, 14% were type F, 5% were type D, 4% were type B and 9% were untypable. Nineteen distinct OMP profiles (OMP-types) were identified based mainly on molecular mass heterogeneity of the heat-modifiable (OmpA) and porin (OmpH) proteins. Fifty-six percent of the isolates were represented by 15 OMP-types, whereas 44% of the isolates were associated with four OMP-types. The extensive molecular mass heterogeneity of the OmpA and OmpH proteins supports previous findings that avian P. multocida strains are very diverse. Furthermore, the isolates studied were associated with different clinical symptoms and were recovered from a wide range of lesions and tissues. The high degree of strain diversity together with the wide variety of clinical symptoms suggest that certain avian strains of P. multocida are opportunistic pathogens of relatively low virulence. Strains of capsular types B, D and F, as well as the untypable isolates, were associated exclusively with specific OMP-types and represent distinct and widely disseminated clonal groups. These observations support the view that avian strains of P. multocida have a clonal population structure. Based on previous studies, the molecular mass heterogeneity of the OmpA and OmpH proteins might provide a selective advantage to P. multocida by generating antigenic variation.
Keywords: Pasteurella multocida, Avian isolates, Capsular PCR typing, OmpA, OmpH
1. Introduction
Pasteurella multocida is the aetiological agent of fowl cholera, a widely distributed and economically important disease of poultry, particularly chickens, turkeys, ducks and geese (Rhoades and Rimler, 1989, Rimler and Glisson, 1997). The organism is also responsible for disease in wild birds, commercially raised game birds and caged birds (Rhoades and Rimler, 1989). Four capsular serogroups are recognised among avian strains of P. multocida, namely A, B, D and F (Rhoades and Rimler, 1987, Rhoades and Rimler, 1989, Rimler and Rhoades, 1987). Strains of serogroup A are recognised as the primary cause of fowl cholera, whereas isolates of serogroups B, D and F are less frequently associated with disease (Rhoades and Rimler, 1987, Rhoades and Rimler, 1989, Wilson et al., 1993). In addition, some avian strains of P. multocida are non-encapsulated and are not serogroupable (Rhoades and Rimler, 1987, Wilson et al., 1993). Sixteen somatic serotypes (1–16) are also recognised in P. multocida (Rhoades and Rimler, 1987, Rhoades and Rimler, 1989, Rhoades and Rimler, 1990a) and most of these have been demonstrated in avian capsular serogroup A strains (Rhoades and Rimler, 1987).
There is considerable evidence, based on a wide range of molecular studies (Snipes et al., 1989, Carpenter et al., 1991, Christiansen et al., 1992, Wilson et al., 1993, Wilson et al., 1995, Blackall et al., 1995, Blackall et al., 1998, Gunawardana et al., 2000, Petersen et al., 2001), that avian strains of P. multocida are extremely diverse. In particular, a study of the population genetics of Australian strains using multilocus enzyme electrophoresis (MLEE) identified 56 electrophoretic types among only 81 field isolates (Blackall et al., 1998). Based on DNA–DNA hybridisation and sugar fermentation patterns P. multocida has been subdivided into three subspecies, subsp. multocida, subsp. gallicida, and subsp. septica (Mutters et al., 1985) and all of these have been isolated from birds (Snipes et al., 1989, Hirsh et al., 1990, Fegan et al., 1995). However, conflicting results from ribotyping and 16S rRNA sequence data (Petersen et al., 2001) suggest that the precise phylogenetic relationships of strains representing each of these subspecies is complex and has yet to be satisfactorily resolved.
Control of fowl cholera is primarily by good management practice and vaccination in areas where the disease is endemic (Rimler and Glisson, 1997). Both whole-cell bacterins and live vaccines composed of attenuated strains are currently available but neither is entirely satisfactory. Bacterins only induce serotype-specific protection, whereas live vaccines sometimes cause disease (Bierer and Derieux, 1975, Schlink and Olson, 1987, Prantner et al., 1990) and there is increasing interest in the development of sub-unit vaccines (Kasten et al., 1995, Luo et al., 1999). Outer membrane antigens that might be considered as potential vaccine candidates include the heat-modifiable or OmpA and the porin or OmpH proteins (Vasfi Marandi and Mittal, 1996, Vasfi Marandi and Mittal, 1997, Luo et al., 1997, Luo et al., 1999). Both of these proteins are expressed in high copy number, are surface exposed and immunogenic (Hancock, 1991, Tagawa et al., 1993, Yi and Murphy, 1997, Zeng et al., 1999, Neary et al., 2001). The OmpH protein has been shown to be heterogeneous in strains of P. multocida representing somatic serotypes 1–16 (Luo et al., 1999) and there is evidence that anti-OmpH antibodies are protective in chickens (Luo et al., 1999) and mice (Vasfi Marandi et al., 1996). There is less information available about the OmpA protein of P. multocida, but this protein also exhibits variation in other bacterial species (Duim et al., 1997, Webb and Cripps, 1998).
The aim of the study was to investigate capsular and outer membrane protein (OMP) diversity among avian P. multocida strains isolated from diseased poultry in England and Wales. In particular, heterogeneity of the OmpA and OmpH proteins was examined and used as the basis for an OMP classification scheme. Since OmpA and OmpH are important surface-exposed components of the outer membrane, analysis of their diversity in avian P. multocida strains will contribute to our understanding of host–pathogen interactions in fowl cholera, including the role of these proteins in immune evasion, and to the development of improved vaccines against this pathogen.
2. Materials and methods
2.1. Bacterial strains and growth conditions
One hundred avian field isolates of P. multocida were investigated. These were obtained from regional laboratories of the Veterinary Laboratories Agency (VLA) and originated from widespread geographic locations within England and Wales over a 13-year period (1987–1999). The isolates were recovered predominantly from cases of fowl cholera and related acute disease conditions such as septicaemia and pneumonia. However, some isolates were associated with chronic conditions such as conjunctivitis, sinusitis, swollen head, arthritis, etc. Properties of the isolates and details of the clinical symptoms of the birds of origin are provided in Table 1 . The capsular reference strains X73 (A), M1404 (B), P3881 (D), P1235 (E) and P4679 (F) were kindly provided by Dr. R. Rimler, National Animal Disease Center, Ames, IA.
Table 1.
Properties of avian P. multocida isolates
| OMP-type | Serotype | No. of isolates | Clinical symptoms |
| 1.1 | A | 7 | Septicaemia (3); arthritis (1); sinusitis (1); other (2) |
| 1.2 | A | 9 | Pneumonia (9) |
| 1.3 | F | 2 | Sinusitis (2) |
| 2.1 | A | 5 | Pneumonia (2); fowl cholera (1); scour (1); other (1) |
| 2.2 | A | 4 | Septicaemia (2); pericarditis (1); not known (1) |
| F | 11 | Septicaemia (6); conjunctivitis (2); pneumonia (1); death (2) | |
| 3.1 | A | 7 | Fowl cholera (2); septicaemia (2); death (2); pneumonia (1) |
| 4.1 | A | 9 | Septicaemia (3); oedema (1); peritonitis (1); conjunctivitis (1); pneumonia (1); other (2) |
| 5.1 | D | 1 | Septicaemia (1) |
| UT | 2 | Septicaemia (1); fowl cholera (1) | |
| 6.1 | A | 4 | Septicaemia (1); swollen joints (1); synovitis (1); swollen head (1) |
| F | 1 | Conjunctivitis (1) | |
| 7.1 | A | 11 | Swollen head (4); septicaemia (3); respiratory infection (2); arthritis (1); death (1) |
| 8.1 | A | 3 | Septicaemia (1); pneumonia (1); other (1) |
| 9.1 | A | 3 | Septicaemia (3) |
| 10.1 | UT | 3 | Septicaemia (2); fowl cholera (1) |
| 10.2 | UT | 3 | Septicaemia (1); fowl cholera (1); death (1) |
| 10.3 | UT | 1 | Respiratory infection (1) |
| 11.1 | A | 3 | Septicaemia (2); fowl cholera (1) |
| 12.1 | B | 4 | Septicaemia (1); respiratory infection (1); air saculitis (1); sinusitis (1) |
| 13.1 | D | 4 | Pneumonia (3); sinusitis (1) |
| 14.1 | A | 3 | Swollen head (3) |
The isolates were stored at −85 °C in 50% (v/v) glycerol in brain heart infusion broth (BHIB). From −85 °C stock cultures, bacteria were streaked onto blood agar (brain heart infusion agar containing 5% (v/v) defibrinated sheep’s blood) and incubated overnight at 37 °C. For preparation of DNA, a few colonies were inoculated into 10 ml volumes of BHIB and grown overnight at 37 °C at 120 rpm. For preparation of outer membranes, 0.4 ml of overnight growth in BHIB was inoculated into 400 ml volumes of BHIB in 2 l Erlenmeyer flasks and incubated for 7 h at 37 °C at 120 rpm.
2.2. Preparation of chromosomal DNA
Cells from 1.0 ml of overnight cultures were harvested by centrifugation for 1 min at 13,000×g and washed once in sterile, distilled H2O. DNA was prepared with the InstaGene Matrix (Bio-Rad) according to the manufacturers’ instructions and stored at −20 °C.
2.3. Capsular PCR typing
The capsular types were determined by multiplex capsular PCR typing with the capsule-specific primer pairs (CAPA, CAPB, CAPD, CAPE and CAPF) described by Townsend et al. (2001). Isolates that were negative for all five capsular types were confirmed as P. multocida with a P. multocida-specific primer set (KMT1T7 and KMT1SP6) (Townsend et al., 2001) in separate PCR reactions (see Section 3) and classified as untypable. All primers were synthesised by Sigma-GenoSys (Cambridge, UK) and the capsular gene fragments were amplified with a TaqDNA polymerase kit (Boehringer Mannheim) according to the manufacturers instructions. PCRs were carried out in a GeneAmp PCR System 9700 (Applied Biosystems) thermal cycler using the following amplification parameters: denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 1 min. Thirty cycles were performed and a final elongation step of 72 °C for 10 min was used. Production of PCR amplicons of the expected size was confirmed by electrophoresis in 2% agarose gels. Pooled PCR amplicons of capsular type A, B, D, E and F reference strains were used as standards in each gel.
2.4. Preparation of OMPs
OMPs were prepared by Sarkosyl extraction as previously described (Davies et al., 1992, Davies and Donachie, 1996). Protein concentrations were determined by the modified Lowry procedure (Markwell et al., 1978) and adjusted to 2.0 mg/ml.
2.5. SDS-PAGE
OMPs were separated by SDS-PAGE in 12% (w/v) resolving gels (Hoefer SE600 electrophoresis apparatus) using the SDS discontinuous system of Laemmli (Laemmli, 1970) as previously described (Davies et al., 1992, Davies and Donachie, 1996). Unless otherwise stated all samples were heated at 100 °C for 5 min prior to electrophoresis. Twenty micrograms of protein were loaded per lane and the proteins were visualised by staining with Coomassie brilliant blue. Protein molecular mass standards (Pharmacia) consisted of phosphorylase b (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa) and α-lactalbumin (14.4 kDa). The molecular masses of individual proteins were calculated with the Labworks™ image acquisition and analysis computer software.
3. Results
3.1. Capsular PCR typing
The capsular types of the 100 avian P. multocida isolates were determined by capsular PCR typing and typical results are shown in Fig. 1 . The distribution of capsular types among the 100 isolates is summarised in Table 1. Sixty-eight (68%) isolates were of capsular type A, 14 (14%) were of type F, five (5%) were of type D, four (4%) were of type B and nine (9%) isolates were untypable. Capsular type E was not detected among the population sampled. The P. multocida-specific primers were omitted from the capsular primer mixture because they interfered with the capsule-specific primers and resulted in a reduction of capsular PCR product (i.e. reduced band intensity). Therefore, isolates that were negative for capsular typing were confirmed as untypable P. multocida in separate PCR assays with the P. multocida-specific primers (Townsend et al., 2001). Microscopic examination of the untypable isolates after Indian ink staining (Hansen and Hirsh, 1989) indicated that they were non-encapsulated.
Fig. 1.
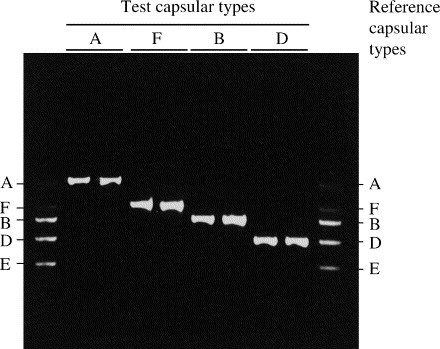
Agarose gel showing results of capsular PCR typing for eight avian P. multocida isolates of serotypes A, F, B and D. Pooled amplification products representing reference capular types A, F, B, D and E are also shown.
3.2. Analysis of OMP profiles
The stability of the OMP profiles was examined by comparing the profiles of two isolates after repeated subculture and at different stages of the growth cycle. The profiles of these isolates were identical after 5, 10, 15 and 20 rounds of subculture on blood agar and after 6, 8, 12 and 24 h of growth in BHIB (results not shown). The OMP profiles of the 100 isolates were analysed by SDS-PAGE and provisionally assigned to OMP-types based on profile similarity (described below). Isolates assigned to the same OMP-type were subsequently re-run on up to three or four occasions such that isolates of the same OMP-type were directly compared on the same gel. An OMP classification scheme was devised-based, firstly, on molecular mass variation of the two major proteins, OmpA and OmpH (OMP-type 1, 2, etc.), and, secondly, on variation of minor protein patterns (OMP-type 1.1, 1.2, etc.).
The OmpA and OmpH proteins have overlapping molecular mass ranges (33–39 kDa) and were distinguished on the basis of their different behaviours in SDS-PAGE gels after heat-treatment. The OmpH porin protein is tightly associated with peptidoglycan and is not released unless heated at a temperature of approximately 60 °C or higher (Rosenbusch, 1974). Therefore, the OmpH protein does not migrate into the gel unless heated at 60 °C or higher prior to SDS-PAGE. In contrast, the OmpA protein is not associated with peptidoglycan and freely migrates into the gel after heat-treatment at temperatures below 60 °C prior to SDS-PAGE. However, the OmpA protein undergoes a characteristic conformational change when heated at 100 °C that results in an increase in its apparent molecular mass in SDS-PAGE gels (Beher et al., 1980). Therefore, to identify OmpA and OmpH, one isolate representing each OMP-type was subjected to heat-treatment at 50, 60, 70, 80, 90 and 100 °C prior to SDS-PAGE. The results for two isolates of OMP-types 3.1 and 10.2 are shown in Fig. 2 . The OmpA (A) and OmpH (H) proteins for each OMP-type are indicated in Fig. 3 and the results are described below.
Fig. 2.
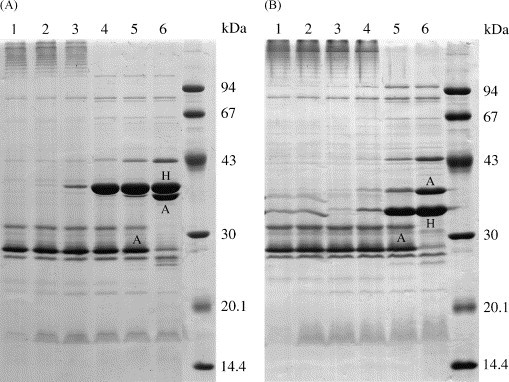
Coomassie blue-stained SDS-PAGE gels showing the OMP profiles of two P. multocida isolates of OMP-types 3.1 (A) and 10.2 (B). The OMP samples were heated at 50, 60, 70, 80, 90 and 100 °C (lanes 1–6, respectively) prior to SDS-PAGE. The effect of heat-treatment on the major OmpA (A) and OmpH (H) proteins is clearly seen (see text).
Fig. 3.
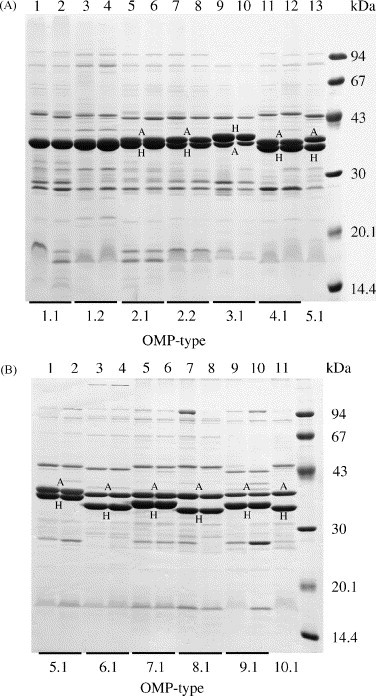
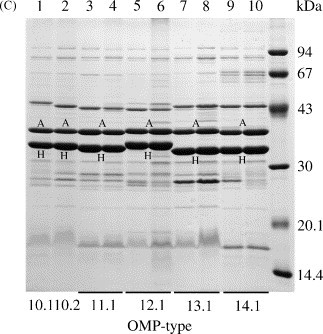
Representative OMP profiles of avian P. multocida isolates in Coomassie blue-stained SDS-PAGE gels. The OMP-types are based on differences in the electrophoretic mobility of the major OmpA (A) and OmpH (H) proteins as well as differences in the banding patterns of the minor proteins. The OMP profiles of two isolates of each OMP-type are shown to demonstrate reproducibility. Molecular mass standards (kDa) are shown in the right-hand lane of each gel.
The 100 isolates consisted of 14 major OMP groups that were classified as OMP-types 1–14 based on variation of OmpA and OmpH (described above). Based on variation of minor proteins isolates of OMP-types 1, 2 and 10 could be further subdivided into OMP-types 1.1–1.3, 2.1 and 2.2, and 10.1–10.3, respectively. Profiles representing each of these OMP-types (with the exception of OMP-types 1.3 and 10.3) are shown in Fig. 3. The molecular mass of OmpA (A) varied from 36.9 to 37.9 kDa and that of OmpH (H) varied from 33.1 to 38.3 kDa. The distribution of OMP-types among the avian isolates is shown in Table 1. Isolates of OMP-types 2.2 (15%), 7.1 (11%), 1.2 (9%) and 4.1 (9%) were the most numerous and accounted for 44% of the total. A smaller number of isolates, ranging from 1 to 7, were associated with each of the other 15 OMP-types but these accounted for 56% of the total number of isolates.
3.3. Relationship between capsular types and OMP-types
There was a strong correlation between certain capsular types and specific OMP-types (Table 1). The frequently occurring capsular type A was associated with 68 isolates representing 12 of the 19 OMP-types. In contrast, capsular type B was associated exclusively with the four isolates of OMP-type 12.1; these isolates originated from four different regional laboratories. Capsular type D was associated with 1/3 isolates of OMP-type 5.1 and with the four isolates of OMP-type 13.1; three of the four isolates of OMP-type 13.1 originated from different regional laboratories and the single OMP-type 5.1 isolate came from a fourth laboratory. Capsular type F was associated with the two isolates of OMP-type 1.3, with 11/15 isolates of OMP-type 2.2 and with 1/5 isolates of OMP-type 6.1. All seven of the isolates representing OMP-types 10.1–10.3 were untypable, as were 2/3 isolates of OMP-type 5.1. The seven untypable/OMP-type 10 isolates originated from six different regional laboratories; the two OMP-type 5.1 isolates also came from different laboratories. Overall, the majority of OMP-types were represented by a single capsular type, but isolates of OMP-types 2.2 and 6.1 were associated with capsular types A and F, and isolates of OMP-type 5.1 were either untypable or possessed capsular type D.
4. Discussion
There are a number of difficulties associated with conventional capsular serotyping of P. multocida (Chengappa et al., 1986, Rimler and Rhoades, 1987, Rimler and Rhoades, 1989). However, Townsend et al. (2001) described an alternative and highly specific multiplex capsular PCR assay that is based on nucleotide sequence variation within the five capsular biosynthetic loci. This PCR-based capsular typing method was used in the present study and found to be a reliable and rapid method for capsular typing large numbers of P. multocida isolates. Reference strains were used as internal standards and no cases of ambiguity occurred. The observed incidence of capsular serotypes in our sample was very similar to that described in the study of 246 isolates by Rhoades and Rimler (1987). In the latter investigation, capsular types A, F, B and D were associated with 67, 5, 2 and 2% of isolates, respectively, whereas 24% of strains were untypable. The significantly higher incidence of serotype A strains with respect to isolates of serotypes B, D and F in this and previous studies (Rhoades and Rimler, 1987, Wilson et al., 1993) suggests that the various serotypes differ in their virulence characteristics. Although virulence studies have shown that strains of serotypes B, D and F are potentially pathogenic (Rimler and Rhoades, 1987, Rhoades and Rimler, 1988, Rhoades and Rimler, 1990b), there is very little information about the comparative virulence of strains representing the different serotypes.
The OMP profiles of the avian P. multocida isolates were very diverse. The isolates could be classified into 19 distinct OMP-types based on variation of OmpA and OmpH and, to a lesser extent, of the minor proteins. Fifty-six percent of the isolates were represented by 15 OMP-types, whereas 44% of the isolates were associated with four OMP-types. The high degree of heterogeneity observed in the OMP profiles, and of OmpA and OmpH in particular, was not unexpected because previous studies have shown that avian P. multocida strains are extremely diverse (Snipes et al., 1989, Christiansen et al., 1992, Wilson et al., 1993, Wilson et al., 1995, Blackall et al., 1995, Blackall et al., 1998, Petersen et al., 2001). In particular, Blackall et al. (1998) identified 56 electrophoretic types among only 81 P. multocida isolates from Australian poultry by MLEE. In a previous study of Mannheimia haemolytica (Davies and Donachie, 1996), 184 strains were sub-divided into three distinct groups based on their OMP profiles and these were subsequently shown to represent phylogenetically distinct lineages by MLEE (Davies et al., 1997). However, no such demarcation was apparent among the OMP profiles of the avian P. multocida isolates.
OMP patterns have been shown to be closely associated with electrophoretic types and clones identified by MLEE in other species (Achtman et al., 1983, Musser et al., 1985, Musser et al., 1988, Achtman and Pluschke, 1986, Kapur et al., 1992, Davies et al., 1997). The exclusive association of isolates of the less common capsular types with specific OMP-types provided evidence that OMP-types mark individual clonal groups of P. multocida (Achtman and Pluschke, 1986). For example, isolates of OMP-type 1.3 were associated with capsular type F, isolates of OMP-type 10 were untypable, isolates of OMP-type 12.1 were associated with capsular type B and isolates of OMP-type 13.1 were associated with capsular type D (Table 1). Furthermore, almost all of the isolates representing each of these groups originated from a different regional laboratory. This is significant because a characteristic feature of clonal bacterial populations is that strains representing the same clone originate from widespread geographic origins (Selander and Musser, 1990). The association of capsular types B and D and certain untypable isolates, with specific OMP-types is also important because it demonstrates for the first time that strains of these uncommon capsular types, together with untypable isolates, probably represent specific clones of P. multocida. In contrast, Dziva et al. (2001) were unable to demonstrate a relationship between RAPD patterns and capsular serogroups in their study of Zimbabwean isolates of P. multocida (Dziva et al., 2001). Isolates of OMP-types 2.2 and 6.1 were associated with capsular types A and F. This observation is probably due to the close relationships of these two capsular types (Townsend et al., 2001).
In many pathogenic bacterial species, the majority of cases of infectious disease are often caused by a small proportion of the total number of extant clones (Selander and Musser, 1990). In this respect, avian P. multocida strains differ from many other pathogens because the majority of cases of disease were associated with a relatively large number of OMP-types/clones. A possible reason for this is that the isolates were recovered from a diverse range of lesions and tissues and were associated with different types of infection ranging from pneumonia and septicaemia to sinusitis, conjunctivitis and swollen head. High levels of diversity were also observed among Eschericha coli strains isolated from chickens with swollen-head syndrome and from birds with colibacillosis (White et al., 1990). It was suggested that the large number of clonal genotypes associated with these avian diseases was due either to the opportunistic nature of the infections or to the widespread occurrence of unknown virulence factors (White et al., 1990, Whittam, 1995). Swollen-head syndrome associated with E. coli is thought to be the result of a secondary infection subsequent to an initial viral infection caused by paramyxovirus, coronavirus, or pneumovirus. The high level of diversity observed among avian P. multocida isolates, together with the wide range of clinical symptoms and tissues of origin, similarly suggests that a high proportion of the isolates might represent opportunistic pathogens of relatively low virulence. In particular, isolates associated with conjunctivitis, sinusitis and swollen head could potentially be secondary pathogens following initial viral infection. Confirmation of this hypothesis will require the comparison of bacterial isolates from diseased birds with the normal avian flora.
The OmpA and OmpH proteins of avian isolates of P. multocida were shown to be heterogeneous since numerous molecular mass variants were identified (Fig. 3). However, the OmpH protein (33.1–38.3 kDa) is clearly more heterogeneous than the OmpA protein (36.9–37.9 kDa). Comparative nucleotide sequence analysis of the OmpH proteins representing the 16 somatic serotypes of P. multocida has shown that the molecular mass heterogeneity of this protein is due to variation in the number of amino acids (318–333) in the protein (Luo et al., 1999). However, most of this variation is confined to two discrete hypervariable regions (amino acids 60–80 and 200–220) which are thought to correspond to external surface-exposed loops (Luo et al., 1999). Similar heterogeneity occurs in the corresponding P2 (OmpH) and P5 (OmpA) proteins of Haemophilus influenzae, and has also been shown to be due to differences in the size of hypervariable surface-exposed loop regions (Forbes et al., 1992, Sikkema and Murphy, 1992, Duim et al., 1997, Webb and Cripps, 1998). These surface-exposed loops are thought to interact with the host immune system and, by undergoing antigenic variation, provide the bacterium with an important defence mechanism (Yi and Murphy, 1994, Yi and Murphy, 1997, Neary et al., 2001). Furthering knowledge of the molecular basis of this diversity in P. multocida will lead to a better understanding of the role of these proteins in avian disease and contribute to the development of improved vaccines.
In summary, this investigation of capsule and OMP variation has confirmed the view that avian P. multocida isolates are very diverse. A possible explanation for the high level of strain diversity observed in the study is that many of the isolates were associated with chronic infections, were recovered from a wide range of lesions and tissues, and represent opportunistic pathogens. The association of certain capsular types with specific OMP-types suggests that OMP profiles mark individual clones of P. multocida. In particular, isolates of the uncommon capsular types B and D and certain untypable isolates, represent distinct clonal groups. The OmpA and OmpH proteins exhibit extensive molecular mass heterogeneity that might provide a selective advantage to the pathogen by generating antigenic variation.
Acknowledgements
This study was supported by a Wellcome Trust University Award to R.L. Davies (053669/Z/98/Z). We are extremely grateful to staff of the Veterinary Laboratories Agency (VLA) for the provision of isolates, including those at Bury St. Edmunds for making available strains of their P. multocida collection.
References
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Ann. Rev. Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder W., Aaronson W., Sutton A., Silver R.P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect. Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M.G., Schnaitman C.A., Pugsley A.P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the OmpA protein of Escherichia coli. J. Bacteriol. 1980;143:906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B.W., Derieux W.T. Immunologic response to turkey poults of various ages to an avirulent Pasteurella multocida vaccine in the drinking water. Poult. Sci. 1975;54:784–787. doi: 10.3382/ps.0540784. [DOI] [PubMed] [Google Scholar]
- Blackall P.J., Pahoff J.L., Marks D., Fegan N., Morrow C.J. Characterisation of Pasteurella multocida isolated from fowl cholera outbreaks on turkey farms. Aust. Vet. J. 1995;72:135–138. doi: 10.1111/j.1751-0813.1995.tb15033.x. [DOI] [PubMed] [Google Scholar]
- Blackall P.J., Fegan N., Chew G.T.I., Hampson D.J. Population structure and diversity of avian isolates of Pasteurella multocida from Australia. Microbiology. 1998;144:279–289. doi: 10.1099/00221287-144-2-279. [DOI] [PubMed] [Google Scholar]
- Carpenter T.E., Snipes K.P., Kasten R.W., Hird D.W., Hirsh D.C. Molecular epidemiology of Pasteurella multocida in turkeys. Am. J. Vet. Res. 1991;52:1345–1349. [PubMed] [Google Scholar]
- Chengappa M.M., Carter G.R., Bailie W.E. Identification of type D Pasteurella multocida by counterimmunoelectrophoresis. J. Clin. Microbiol. 1986;24:721–723. doi: 10.1128/jcm.24.5.721-723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen K.H., Carpenter T.E., Snipes K.P., Hird D.W. Transmission of Pasteurella multocida on California turkey premises in 1988–1989. Avian Dis. 1992;36:262–271. [PubMed] [Google Scholar]
- Davies R.L., Donachie W. Intra-specific diversity and host specificity within Pasteurella haemolytica based on variation of capsular polysaccharide, lipopolysaccharide and outer-membrane proteins. Microbiology. 1996;142:1895–1907. doi: 10.1099/13500872-142-7-1895. [DOI] [PubMed] [Google Scholar]
- Davies R.L., Parton R., Coote J.G., Gibbs H.A., Freer J.H. Outer membrane protein and lipopolysaccharide variation in Pasteurella haemolytica A1 under different growth conditions. J. Gen. Microbiol. 1992;138:909–922. doi: 10.1099/00221287-138-5-909. [DOI] [PubMed] [Google Scholar]
- Davies R.L., Arkinsaw S., Selander R.K. Evolutionary genetics of Pasteurella haemolytica isolates recovered from cattle and sheep. Infect. Immun. 1997;65:3585–3593. doi: 10.1128/iai.65.9.3585-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duim B., Bowler L.D., Eijk P.P., Jansen H.M., Dankert J., Van Alphen L. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect. Immun. 1997;65:1351–1356. doi: 10.1128/iai.65.4.1351-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziva F., Christensen H., Olsen J.E., Mohan K. Random amplification of polymorphic DNA and phenotypic typing of Zimbabwean isolates of Pasteurella multocida. Vet. Microbiol. 2001;82:361–372. doi: 10.1016/s0378-1135(01)00406-0. [DOI] [PubMed] [Google Scholar]
- Fegan N., Blackall P.J., Pahoff J.L. Phenotypic characterisation of Pasteurella multocida isolates from Australian poultry. Vet. Microbiol. 1995;47:281–286. doi: 10.1016/0378-1135(95)00119-0. [DOI] [PubMed] [Google Scholar]
- Forbes K.J., Bruce K.D., Ball A., Pennington T.H. Variation in length and sequence of porin (ompP2) alleles of non-capsulate Haemophilus influenzae. Mol. Microbiol. 1992;6:2107–2112. doi: 10.1111/j.1365-2958.1992.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Gunawardana G.A., Townsend K.M., Frost A.J. Molecular characterisation of avian Pasteurella multocida isolates from Australia and Vietnam by REP-PCR and PFGE. Vet. Microbiol. 2000;72:97–109. doi: 10.1016/s0378-1135(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Hancock R.E.W. Bacterial outer membranes: evolving concepts. ASM News. 1991;57:175–182. [Google Scholar]
- Hansen L.M., Hirsh D.C. Serum resistance is correlated with encapsulation of avian strains of Pasteurella multocida. Vet. Microbiol. 1989;21:177–184. doi: 10.1016/0378-1135(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Hirsh D.C., Jessup D.A., Snipes K.P., Carpenter T.E., Hird D.W., McCapes R.H. Characteristics of Pasteurella multocida isolated from waterfowl and associated avian species in California. J. Wildl. Dis. 1990;26:204–209. doi: 10.7589/0090-3558-26.2.204. [DOI] [PubMed] [Google Scholar]
- Kapur V., White D.G., Wilson R.A., Whittam T.S. Outer membrane protein patterns mark clones of Escherichia coli O2 and O78 strains that cause avian septicemia. Infect. Immun. 1992;60:1687–1691. doi: 10.1128/iai.60.4.1687-1691.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten R.W., Hansen L.M., Hinojoza J., Bieber D., Ruehl W.W., Hirsh D.C. Pasteurella multocida produces a protein with homology to the P6 outer membrane protein of Haemophilus influenzae. Infect. Immun. 1995;63:989–993. doi: 10.1128/iai.63.3.989-993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luo Y., Glisson J.R., Jackwood M.W., Hancock R.E.W., Bains M., Cheng I.H., Wang C. Cloning and characterization of the major outer membrane protein gene (ompH) of Pasteurella multocida X-73. J. Bacteriol. 1997;179:7856–7864. doi: 10.1128/jb.179.24.7856-7864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Zeng Q., Glisson J.R., Jackwood M.W., Cheng I.H., Wang C. Sequence analysis of Pasteurella multocida major outer membrane protein (OmpH) and application of synthetic peptides in vaccination of chickens against homologous strain challenge. Vaccine. 1999;17:821–831. doi: 10.1016/s0264-410x(98)00266-7. [DOI] [PubMed] [Google Scholar]
- Markwell M.A.K., Haas S.M., Bieber L.L., Tolbert N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Musser J.M., Granoff D.M., Pattison P.E., Selander R.K. A population genetic framework for the study of invasive diseases caused by serotype b strains of Haemophilus influenzae. Proc. Natl. Acad. Sci. USA. 1985;82:5078–5082. doi: 10.1073/pnas.82.15.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J.M., Kroll J.S., Moxon E.R., Selander R.K. Clonal population structure of encapsulated Haemophilus influenzae. Infect. Immun. 1988;56:1837–1845. doi: 10.1128/iai.56.8.1837-1845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutters R., Ihm P., Pohl S. Reclassification of the genus Pasteurella Trevisan 1887 on the basis of deoxyribonucleic acid homology, with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaa. Int. J. Syst. Bacteriol. 1985;35:309–322. [Google Scholar]
- Neary J.M., Yi K., Karalus R.J., Murphy T.F. Antibodies to loop 6 of the P2 porin protein of nontypeable Haemophilus influenzae are bactericidal against multiple strains. Infect. Immun. 2001;69:773–778. doi: 10.1128/IAI.69.2.773-778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K.D., Christensen H., Bisgaard M., Olsen J.E. Genetic diversity of Pasteurella multocida fowl cholera isolates as demonstrated by ribotyping and 16S rRNA and partial atpD sequence comparisons. Microbiology. 2001;147:2739–2748. doi: 10.1099/00221287-147-10-2739. [DOI] [PubMed] [Google Scholar]
- Prantner M.M., Harmon B.G., Glisson J.R., Mahaffey E.A. The pathogenesis of Pasteurella multocida serotype A:3,4 infection in turkeys: a comparison of two vaccine strains and a field isolate. Avian Dis. 1990;34:260–266. [PubMed] [Google Scholar]
- Rhoades K.R., Rimler R.B. Capsular groups of Pasteurella multocida isolated from avian hosts. Avian Dis. 1987;31:895–898. [PubMed] [Google Scholar]
- Rhoades K.R., Rimler R.B. Virulence of avian capsular serogroup B Pasteurella multocida for turkey poults. Avian Dis. 1988;32:121–123. [PubMed] [Google Scholar]
- Rhoades, K.R., Rimler, R.B., 1989. Fowl cholera. In: Adlam, C.F., Rutter, J.M. (Eds.), Pasteurella and Pasteurellosis. Academic Press, London, pp. 95–113.
- Rhoades K.R., Rimler R.B. Somatic serotypes of Pasteurella multocida strains isolated from avian hosts (1976–1988) Avian Dis. 1990;34:193–195. [PubMed] [Google Scholar]
- Rhoades K.R., Rimler R.B. Virulence and toxigenicity of capsular serogroup D Pasteurella multocida strains isolated from avian hosts. Avian Dis. 1990;34:384–388. [PubMed] [Google Scholar]
- Rimler, R.B., Glisson, J.R., 1997. Fowl cholera. In: Calnek, B.W., Barnes, H.J., Beard, C.W., McDougald, L.R., Saif, Y.M. (Eds.), Diseases of Poultry. Iowa State University Press, Ames, IA, pp. 143–159.
- Rimler R.B., Rhoades K.R. Serogroup F, a new capsule serogroup of Pasteurella multocida. J. Clin. Microbiol. 1987;25:615–618. doi: 10.1128/jcm.25.4.615-618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler, R.B., Rhoades, K.R., 1989. Pasteurella multocida. In: Adlam, C.F., Rutter, J.M. (Eds.), Pasteurella and Pasteurellosis. Academic Press, London, pp. 37–73.
- Rosenbusch J.P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulphate binding. J. Biol. Chem. 1974;249:8019–8029. [PubMed] [Google Scholar]
- Schlink G.T., Olson L.D. Vaccination of turkey breeder hens and toms for fowl cholera with CU strain. Avian Dis. 1987;31:29–38. [PubMed] [Google Scholar]
- Selander, R.K., Musser, J.M., 1990. Population genetics of bacterial pathogenesis. In: Iglewski, B.H., Clark, V.L. (Eds.), Molecular Basis of Bacterial Pathogenesis. Academic Press, San Diego, pp. 11–36.
- Sikkema D.J., Murphy T.F. Molecular analysis of the P2 porin protein of nontypeable Haemophilus influenzae. Infect. Immun. 1992;60:5204–5211. doi: 10.1128/iai.60.12.5204-5211.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes K.P., Hirsh D.C., Kasten R.W., Hansen L.M., Hird D.W., Carpenter T.E., McCapes R.H. Use of an rRNA probe and restriction endonuclease analysis to fingerprint Pasteurella multocida isolated from turkeys and wildlife. J. Clin. Microbiol. 1989;27:1847–1853. doi: 10.1128/jcm.27.8.1847-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y., Haritani M., Ishikawa H., Yuasa N. Characterization of a heat-modifiable outer membrane protein of Haemophilus somnus. Infect. Immun. 1993;61:1750–1755. doi: 10.1128/iai.61.5.1750-1755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K.M., Boyce J.D., Chung J.Y., Frost A.J., Adler B. Genetic organization of Pasteurella multocidacap loci and development of a multiplex capsular PCR typing system. J. Clin. Microbiol. 2001;39:924–929. doi: 10.1128/JCM.39.3.924-929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasfi Marandi M.V., Mittal K.R. Characterization of an outer membrane protein of Pasteurella multocida belonging to the OmpA family. Vet. Microbiol. 1996;53:303–314. doi: 10.1016/s0378-1135(96)01219-9. [DOI] [PubMed] [Google Scholar]
- Vasfi Marandi M.V., Mittal K.R. Role of outer membrane protein H (OmpH)- and OmpA-specific monoclonal antibodies from hybridoma tumors in protection of mice against Pasteurella multocida. Infect. Immun. 1997;65:4502–4508. doi: 10.1128/iai.65.11.4502-4508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasfi Marandi M.V., Dubreuil J.D., Mittal K.R. The 32 kDa major outer-membrane protein of Pasteurella multocida capsular serotype D. Microbiology. 1996;142:199–206. doi: 10.1099/13500872-142-1-199. [DOI] [PubMed] [Google Scholar]
- Webb D.C., Cripps A.W. Secondary structure and molecular analysis of interstrain variability in the P5 outer-membrane protein of non-typable Haemophilus influenzae isolated from diverse anatomical sites. J. Med. Microbiol. 1998;47:1059–1067. doi: 10.1099/00222615-47-12-1059. [DOI] [PubMed] [Google Scholar]
- White D.G., Wilson R.A., San Gabriel A., Saco M., Whittam T.S. Genetic relationships among strains of avian Escherichia coli associated with swollen-head syndrome. Infect. Immun. 1990;58:3613–3620. doi: 10.1128/iai.58.11.3613-3620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam, T.S., 1995. Genetic population structure and pathogenicity in enteric bacteria. In: Baumberg, S., Young, J.P.W., Wellington, E.M.H., Saunders, J.R. (Eds.), Population Genetics of Bacteria. Cambridge University Press, Cambridge, pp. 217–245.
- Wilson M.A., Morgan M.J., Barger G.E. Comparison of DNA fingerprinting and serotyping for identification of avian Pasteurella multocida isolates. J. Clin. Microbiol. 1993;31:255–259. doi: 10.1128/jcm.31.2.255-259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.A., Duncan R.M., Nordholm G.E., Berlowski B.M. Pasteurella multocida isolated from wild birds of North America: a serotype and DNA fingerprint study of isolates from 1978 to 1993. Avian Dis. 1995;39:587–593. [PubMed] [Google Scholar]
- Yi K., Murphy T.F. Mapping of a strain-specific bactericidal epitope to the surface-exposed loop 5 on the P2 porin protein of non-typeable Haemophilus influenzae. Microbiol. Pathog. 1994;17:277–282. doi: 10.1006/mpat.1994.1073. [DOI] [PubMed] [Google Scholar]
- Yi K., Murphy T.F. Importance of an immunodominant surface-exposed loop on outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect. Immun. 1997;65:150–155. doi: 10.1128/iai.65.1.150-155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Pandher K., Murphy G.L. Molecular cloning of the Pasteurella haemolytica PomA gene and identification of bovine antibodies against PomA surface domains. Infect. Immun. 1999;67:4968–4973. doi: 10.1128/iai.67.9.4968-4973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


