Abstract
An isolated epizootic of a highly fatal feline calicivirus (FCV) infection, manifested in its severest form by a systemic hemorrhagic-like fever, occurred over a 1-month period among six cats owned by two different employees and a client of a private veterinary practice. The infection may have started with an unowned shelter kitten that was hospitalized during this same period for a severe atypical upper respiratory infection. The causative agent was isolated from blood and nasal swabs from two cats; the electron microscopic appearance was typical for FCV and capsid gene sequencing showed it to be genetically similar to other less pathogenic field strains. An identical disease syndrome was recreated in laboratory cats through oral inoculation with tissue culture grown virus. During the course of transmission studies in experimental cats, the agent was inadvertently spread by caretakers to an adjoining room containing a group of four normal adult cats. One of the four older cats was found dead and a second was moribund within 48–72 h in spite of symptomatic treatment; lesions in these animals were similar to those of the field cats but with the added feature of severe pancreatitis. The mortality in field cats, deliberately infected laboratory cats, and inadvertently infected laboratory cats ranged from 33–50%. This new isolate of calicivirus, named FCV-Ari, was neutralized at negligible to low titer by antiserum against the universal FCV-F9 vaccine strain. Cats orally immunized with FCV-F9, and then challenge-exposed shortly thereafter with FCV-Ari, developed a milder self-limiting form of disease, indicating partial protection. However, all of the field cats, including the three that died, had been previously immunized with parenteral FCV-F9 vaccine. FCV-Ari caused a disease that was reminiscent of Rabbit Hemorrhagic Disease, a highly fatal calicivirus infection of older rabbits.
Keywords: Cat, Feline calicivirus
1. Introduction
Feline calicivirus (FCV) is one of the most common viral pathogens of cats, especially in multi-cat environments such as shelters and catteries. FCV infection and disease occurs in acute and chronic forms (reviewed by Studdert, 1978, Reubel et al., 1992). The manifesting signs of acute disease depends on the route (oral versus aerosol) and strain of virus. Aerosol infection, which is commonly used in laboratory studies, produces a more serious disease than oral infection, with lesions extending further down the respiratory tract. Hoover and Kahn (1975) described the acute disease produced by 10 field strains of FCV. The disease differed only in severity, with the more virulent strains causing fever, depression, dyspnea, pneumonia, and vesicles/ulcers of the tongue, hard palate and nostrils. Lower virulence strains were less likely to affect the lungs, although other signs were similar. Transient mild conjunctivitis and rhinitis, has also been associated with acute FCV infection, but unlike pathogens such as feline herpesvirus, upper respiratory involvement is not the major feature of FCV infection. Pedersen and colleagues (1983) added the feature of ‘limping’ to the FCV disease syndrome. The lameness appears to be associated with acute viremia and localization of virus and/or immune complexes in the joints (Bennett et al., 1989, Dawson et al., 1994). Following recovery from acute disease, up to one-fourth of cats will shed the virus for a prolonged period of time from their oropharynx (Bennett et al., 1989, Harbour et al., 1991; Dawson et al., 1993a, Dawson et al., 1993b). Although most FCV carriers are asymptomatic, a small proportion will develop a distinct disease syndrome known as chronic plasmacytic/lymphocytic stomatitis or chronic ulceroproliferative stomatitis (Reubel et al., 1992). This chronic oral disease is progressive and extremely difficult to treat and is perhaps the single most important clinical manifestation of FCV infection today.
It is fortunate for cats that FCV is not more virulent. Related caliciviruses, such as rabbit hemorrhagic disease virus, have been associated with severe clinical disease and high mortality. Although recognized strains of FCV have not been associated with significant acute mortality, the calicivirus genome is highly mutable (Johnson, 1992, Pedersen and Hawkins, 1995, Geissler et al., 1997, Kreutz et al., 1998, Radford et al., 1998) and more highly virulent strains could arise at any time. The present report describes the sudden appearance and disappearance of a novel strain of FCV, which induced a severe systemic hemorrhagic-like fever and high mortality. The new strain, named FCV-Ari, has weak to negligible cross-reactivity to current vaccine-induced immunity and would pose considerable risk to cats if it were to spread widely among either vaccinated or unvaccinated animals.
2. Materials and methods
2.1. Experimental animals and animal care
Specific pathogen free (SPF) cats, of mixed gender, were obtained from the breeding colony of the Veterinary Nutrition Laboratory, School of Veterinary Medicine, UC Davis (compliments of Dr. James Morris and Dr. Quinton Rogers). Cats were from 12 to 16 weeks of age and housed in the experimental animal facilities of the Center for Companion Animal Health and cared for under the oversight of the Animal Resources Services, UC Davis.
2.2. Clinicopathologic procedures
Complete blood counts, 20 parameter serum chemistries, and various clotting studies were done by either IDEXX Veterinary Services, Western Region, West Sacramento, CA, or by the Clinical Pathology Service of the Veterinary Medical Teaching Hospital (VMTH), School of Veterinary Medicine, UC Davis. Gross and/or histopathologic studies were done by either IDEXX Veterinary Services or the Pathology Service of the VMTH, UC Davis.
2.3. Virus isolation
Initial virus isolation were from cats VMTH-308887 (Ari) and -308877 (Ian). Swabs of nasal exudate, and herparinized whole blood, were taken from both animals on 23 October 1998. Blood (1.5 ml) was diluted with an equal volume of Hank’s buffered salt solution (HBSS) and centrifuged at 300×g for 30 min. The buffy coat was suspended in 100 μl of HBSS; the suspended cells were overlayered onto subconfluent monolayers of Crandell feline kidney (CrFK) and Felis catus whole fetus-4 (Fcwf-4) cells grown in 25 cm2 flasks. Nasal swabs were placed in 5 ml snap-top tubes containing 1 ml of HBSS with 10× gentamicin and penicillin/streptomycin and allowed to sit for at least 2 h at room temperature. Supernatant (100 μl) was placed into 25 cm2 flasks containing subconfluent monolayers of CrFK or Fcwf-4 cells. Cell cultures were observed every 12 h for cytopathic effect (CPE).
2.4. Experimental infection studies
Three 14-week old SPF kittens were infected oronasally with 0.5 ml of tissue culture fluid containing approximately 2×106 TCID100 of FCV-Ari. One half of the inoculum was instilled up the nostrils and one half placed in back of the throat. Complete blood counts and serum chemistries were taken 24–48 h prior to infection and every 3–7 days thereafter. Rectal temperatures were recorded starting 2 days prior to infection and daily thereafter. Cats were observed for clinical signs of depression, anorexia, limping, subcutaneous swelling and edema, skin lesions, nasal discharge/nasal congestion/sneezing, diarrhea, and vesicles and/or ulcers of the palate, tongue, gums, cheeks, lips, or skin.
For vaccine studies, four 12–16 weeks old SPF kittens were immunized oronasally, as described earlier, with 1 ml of tissue culture fluid containing approximately 5×106 TCID100 of the universal vaccine strain FCV-F9 (Heska subisolate). Two sibling kittens were kept strictly isolated and used as unimmunized controls. Kittens were monitored for clinical signs of infection as outlined earlier.
2.5. Virologic studies
2.5.1. FCV strains
A number of FCV strains, of vaccine and field origin, were used in this study. The origin and serologic relationships of most of these strains has been previously reported (Pedersen and Hawkins, 1995). Vaccine strains, which were genetically identical to each other and to the original FCV-F9 (Kahn et al., 1975), were re-isolated from several commercial brands of vaccine (Pedersen and Hawkins, 1995).
2.5.2. FCV genetic analyses
A nested RT-PCR reaction was used to detect FCV-RNA in blood (buffy coat), oral swabs, and feces. Forward and reverse primers amplifying a 235 bp region of the capsid gene corresponding to nucleotides 6533–6767 of the FCV genome were used, following the protocol of Radford et al. (1998). PCR products were run through Microcon-50 columns (Millipore, Burlington, MA) to remove primers and salts and then submitted to a commercial sequencing laboratory (Davis Sequencing, Davis, CA) for automated sequencing analysis. Sequence data were analyzed on a VAX computer using the University of Wisconsin’s Genetics Computer Group (GCG) sequence analysis software package (GCG Program manual for the Wisconsin package, Version 8, 1994). A dendrogram (cladogram) was generated by sequence comparison using the Cantor-Jukes correction, followed by distance calculations using the UPGMA algorithm.
2.6. Virus neutralizing antibody assay
Virus neutralizing (VN) antibodies to FCV were measured by a residual virus infectivity assay using constant amounts of virus and antiserum. A diluted tissue culture fluid stock of FCV-F9 or FCV-Ari (100 μl), containing approximately 1000 TCID100, was incubated at 37°C for 1 h with either 100 μl of a 1:20 dilution of tissue culture medium (negative control) or a 1:20 dilution in tissue culture medium of the cat serum to be tested. Ninety-six well microtiter plates containing a monolayer of subconfluent CrFK cells were used for the titration; each well contained 100 μl of tissue culture fluid. The virus/antiserum mixture of 50 μl was added to the first well, mixed well, and 50 μl carried serially to each of the next 11 wells in the same manner. This procedure was repeated in triplicate. A similar titration was done using the same virus stock and an equal volume of tissue culture medium alone. The plates were incubated for 24 h and observed under an inverted microscope for typical FCV CPE; the last well containing any detectable CPE was read as endpoint. The VN antibody titer of the serum was calculated from the difference in the average virus titer with and without serum treatment, i.e. if the average virus titer of a sample of virus+antiserum was 1:27, and the titer of the corresponding virus+tissue culture fluid 1:19,629, the difference was six wells (1/36) or a VN titer of 1:729.
2.7. Electron microscopy
FCV-Ari was infected onto fresh CrFK in a 25 cm2 flask, and the cultures observed closely for CPE. About 24 h after infection, when cytopathic effect was noticeable but most cells were still attached, the tissue culture medium was decanted and the monolayer overlayered with cold 2.0% paraformaldehyde/2.5% glutaraldehyde in 0.06 M Sorenson’s phosphate buffer, pH 7.2. The flasks were held for several hours at room temperature, and then placed into the refrigerator for several days. Post-fixation was in 1% osmium tetroxide in 0.1 M Sorenson’s phosphate buffer at 4°C. The samples were processed by the Electron Microscopy Service of the Dept. of Pathology, School of Medicine (with special assistance from Mr. Bob Munn). Grid staining was with 2% aqueous uranyl acetate and lead citrate. Grids were examined and photographed using a Philips EM400 electron microscope.
2.8. Necropsy and histopathology
The first tissue analyzed were three full-thickness skin biopsies from the face, nose and ear of the cat named Ria. The samples were fixed in formol-saline and hematoxylin and eosin stained tissue sections prepared by a private laboratory (IDEXX Veterinary Services, Inc., West Sacramento, CA 95605). Subsequent necropsies and gross and microscopic pathologic analyses were done by the Veterinary Pathology Service of the VMTH, Department of Pathology, School of Veterinary Medicine, University of California, Davis, CA 95616.
3. Results
3.1. Case histories
3.1.1. Naturally acquired infections of pet cats
The index case in this outbreak of atypical FCV infection was probably an orphaned, fully vaccinated, 4-month old, female domestic kitten from a local animal shelter. One of the authors (JBE), noticed that the kitten had a severe upper respiratory infection. Therefore, the kitten was brought from the shelter into the clinic on 17 September 1998 for hospitalization and treatment. The kitten subsequently developed crusty, ulcerative lesions on her face and oral vesicles on the margins of the tongue and soft palate. The kitten was intensively treated for 40 days before being well enough to be adopted. The kitten intussuscepted 1 week later and died.
The second cat in the outbreak was Ria, a 6-year old, spayed female, domestic, strictly-indoors cat owned by a veterinary assistant in the same veterinary clinic. The cat received her last feline panleukopenia-/herpes-/calici-virus (FPHCV) immunization on 10 April 1997. The only exposure outside the home was a brief hospitalization for dental prophylaxis on 8 October 1998. Ria presented on 12 October 1998 with a primary complaint of 24 h of lethargy, anorexia, and fever. Physical examination was uninformative except for fever. Tests for FeLV and FIV infections were negative and CBC and blood chemistry analyses were unremarkable. A slight swelling of the dorsal muzzle, extending from one canthus to the other, was observed that afternoon. Shortly thereafter, hair over the right side of the muzzle epilated, revealing a 5 mm diameter erythematous patch with a dark necrotic center. Ria was sent home for observation, but returned on 14 October 1998 still lethargic, anorectic and febrile. Additional lesions, with focal crusting, erythema and epilation, were present on the right and left medial canthi, commissures of the mouth, and on the margins of both pinnae. The patient also demonstrated diffuse cutaneous edema of the face and submandibular area and extending down the limbs. Biopsies were taken from the muzzle and pinnae lesions and swabs of several sores were collected for routine aerobic bacterial culture. Ria was still febrile on 15 October 1998, but did not appear to be in discomfort and was beginning to eat and groom. Skin biopsies taken on 14 October 1998 revealed multifocal moderate to severe neutrophilic/lymphoplasmacytic perivascular and periadnexal dermatitis, superficial pyoderma, and extensive ulceration and superficial dermal necrosis with underlying vasculitis. No infectious organisms were observed in routine or special stained tissues sections and no aerobic bacteria grew in culture. Ria was treated with prednisolone and antibiotics; within the next 2 days she was eating well and actively grooming, the cutaneous edema was resolving, and the skin lesions were healing.
The third affected cat, Indy, was a 4-month old, domestic, indoor/outdoor cat. He was brought to the hospital on 12 October 1998 for castration and his final parenteral FPHCV immunization. Indy returned to the hospital on 17 October 1998 with depression and fever. Abnormalities on a standard blood chemistry panel included a mild increase in creatinine phosphokinase (CPK) and total bilirubin. FeLV and FIV tests were negative and a coronavirus IFA antibody titer was 1:100. Indy was treated with IV electrolyte solution and antibiotics. The fever was resolved by 19 October 1998, but ulcerative lesions appeared on the margins of both pinnae on 20 October. The cat made an uneventful recovery.
The fourth cat, Garth, was a 5-year old, castrated male, domestic, indoor/outdoor cat and the housemate of Indy. The cat had received the recommended series of parenteral FPHCV immunizations as a kitten and yearly boosters. Garth was seen on 16 October 1998 for mild conjunctivitis and squinting and sent home on ophthalmic antibiotic ointment. The cat appeared to make an uneventful recovery, but 2 weeks later the owner noticed focal areas of alopecia and encrustation on the muzzle, base of the right pinna, and bilaterally on both flanks, compatible with old healing ulcers.
The fifth cat in this focal epidemic was Aristotle (Ari), a 3.5-year old, neutered male, domestic, indoors-only cat and the son of Ria. There were no significant past health problems; he tested negative for FeLV and FIV infections and had been parenterally vaccinated against rabies and FPHCV on 10 April 1997 Ari presented on 19 October 1998 with a history of an acute onset of lethargy and anorexia of 2 days duration. A slight lameness in the right foreleg was noted 2 days prior but had spontaneously resolved. The patient had vomited a bile tinged fluid with flecks of red blood. Physical examination revealed fever and a small crusty lesion on the lower lip. Ari returned to the hospital on 21 October 1998 afebrile and alert, but moderately dehydrated and anorectic. Diffuse facial edema with central ulceration were noted, primarily over the muzzle and left pinna; focal crusty and erythematous lesions were also present on the upper and lower lips. Intravenous fluids, metoclopramide and antibiotic therapy were instituted. Ari was afebrile on 22 October 1998 but anorectic. The facial swelling was mildly increased but the central ulcerated areas seemed to be less exudative. The patient’s right forepaw was slightly edematous. Ari was still afebrile on 23 October 1998 but remained anorectic; the edema of the right forepaw remained unchanged. The patient was transported to the UC Davis VMTH for additional testing, including blood and nasal swabs for virus isolation and blood for chemistry and coagulation profiles. Blood chemistries revealed moderate elevation of CPK, mild elevation of total bilirubin, and moderate hypoproteinemia. There was evidence of a coagulopathy with moderately increased prothrombin and partial thromboplastin times and a slightly prolonged activated clotting time (ACT). A FCV was isolated in cell cultures exposed to both blood and nasal exudate. Ari was still euthermic on 24 October 1998; however, he was anorectic and his entire face was edematous. The facial lesions had coalesced to form large irregular crusts on both sides of the muzzle (Fig. 1 ), and the mucus membranes were slightly icteric. A nasoesophogeal feeding tube was positioned and oral hyperalimentation instituted; this therapy that would be continued until his death. Ari seemed more responsive on 25 October 1998, although still anorectic and hypocoagulable. The patient appeared comfortable on 26 October 1998; there was no more vomiting, but the subcutaneous edema of the right foreleg was persisting. Ari was transfused with one unit of whole blood and low level heparin therapy was instituted to counteract a suspected disseminated intravascular coagulopathy (DIC). Dextran 70 was given initially as an IV bolus and then maintained as a continuous infusion. Laboratory tests taken in the evening of 26 October indicated a persisting coagulopathy, hypoproteinemia, and hyperbilibubinemia. Ari was alert, responsive, and afebrile on the morning of 27 October; however, he was diffusely edematous, had mild watery diarrhea and exhibited a slightly increased respiratory rate and effort. Laboratory work revealed a decreased hematocrit, hypoproteinemia and greatly elevated ACT. The patient was then given a second unit of cross matched whole blood. The respiratory rate continued to increase throughout the day and a thoracocentesis was performed late that evening and a yellowish-transudate withdrawn. Ari was alert and responsive on 28 October 1998, but still anorectic. The patient received a third unit of whole blood at mid-day and more pleural fluid was removed. On 29 October 1998, Ari was quiet, responsive and vocal when handled, but polypneic and totally anorectic. A chest tube was placed to facilitate thoracic drainage and the dextran 70 replaced with hetastarch. Ari was still anorectic and lethargic on the morning of 30 October; he was diffusely edematous with small areas of miliary crusting noted in areas of greatest swelling, especially on the distal limbs. Despite treatment, Ari’s condition slowly deteriorated and he died on 8 November 1998 from complications of pneumothorax, shock and cardiac arrest. A FCV, genetically indistinguishable from the earlier isolate, was recovered from blood and tissues at post-mortem examination.
Fig. 1.
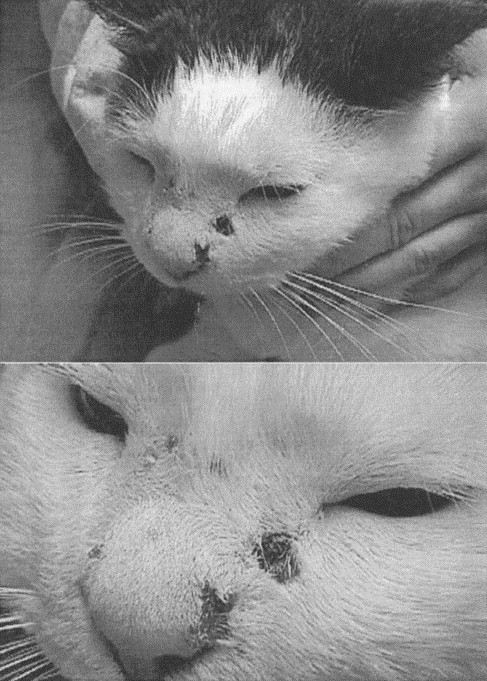
(Upper) Digital photographs of the facial lesions of Ari, showing diffuse facial edema and cutaneous ulceration. (Lower) A close up view showing the deep, encrusted nature of the facial ulcers. The facial ulcers extended from the subcutis and tended to occur at sites of most intense edema.
Ian, a 3.5-year old, neutered male, domestic, indoors-only cat, also a son of Ria, was the sixth cat to be affected. On 21 October 1998, the owner observed that Ian was febrile and manifesting signs similar to those shown by his mother. Previous clinical history included a negative FeLV/FIV test on August 1998 and routine parenteral vaccination for rabies and FPHCV on 2 June 1998. Ian became febrile on 22 October 1998 and was lethargic and sneezing occassionally. Physical examination revealed mild edema of his muzzle with patchy erythematous macules and miliary crusts on margins of both pinnae. Ian was transported to UC Davis, VMTH for consultations with one of the authors (NCP) and a veterinary dermatolgist (Dr. Peter Ihrke). Blood and nasal swabs were collected and the cat returned home. A FCV, which was genetically identical to the isolate from Ari, was obtained in tissue cultures from both blood and nasal exudate. On 23 October 1998, Ian was still febrile at home but was eating and drinking. The facial and pinna swellings were increasing and new crusts were present on his pinnae and around his nares. Ian continued to do well at home on 24 October, although the owner reported sneezing and nasal congestion. Ian was still sneezing and congested on 28 October and the nasal and ear lesions had begun to coalesce. His appetite and attitude remained very good and he made an uneventful recovery.
The seventh cat in the outbreak was Emma, a 2.5-year old spayed female, domestic, who was kept strictly indoors. Emma tested negative for FeLV and FIV as a kitten and was last vaccinated for FeLV and FPHCV on 29 June 1996. Emma was owned by a second technician who worked at the same practice. Emma presented on 4 November 1998 with a history of 2 days of lethargy and anorexia. Her physical examination was unremarkable except for fever, moderate dehydration and possible abdominal tenderness. The patient was hospitalized and started on intravenous fluids and antibiotics. Presenting lab work revealed a slightly elevated total blood bilirubin and glucose. FeLV and FIV tests were negative. On 5 November 1998, Emma was depressed, anorectic, febrile and hyperirritable. The fever increased by evening and her left hind paw appeared swollen. Emma was still anorectic on 6 November 1998 but was much more alert; rectal temperatures continued to be elevated and her right hind paw was swollen. Emma remained anorectic on 7 November 1998, her hind paws remained swollen and slight swelling was observed on the muzzle. Small areas of ecchymosis were noted on the right caudal abdomen. A naso-esophageal feeding tube was inserted and the patient was started on a full strength hyperalimentation solution. The next morning Emma became restless and mildly dyspneic, with pronounced decreases in the hematocrit and total plasma proteins. Emma was started on vigorous treatment for a developing hypoproteinemia and DIC; therapy included intravenous Hetastarch, subcutaneous heparin, intravenous antibiotics, and nasoesophageal tube feeding. The patient’s respiratory rate continued to increase throughout the day even though her fever abated. A chest tube was placed but only small amounts of a transudate were withdrawn. She became progressively more dypneic and depressed throughout the day and was hypothermic by midnight. Results of laboratory tests taken in the evening revealed a moderate hypoproteinemia, hyperbilirubinemia and thrombocytopenia. Blood tests taken on 8 November 1998 showed an elevated glucose, decreased hematocrit and total protein, and a prolonged ACT. Emma’s condition continued to decline throughout the day, with increasing respiratory difficulty. Lasix and dobutamine were administered by intravenous drip and intravenous Hetastarch and nasoesophageal tube feeding discontinued. On 9 November 1998, Emma’s condition continued to decline overnight and the owner elected to have the cat euthanatized.
3.1.2. Naturally acquired infections of laboratory cats
Affected cats eight through 11 (96-101, 96-179, 97-689 and 97-680) were 2.5–3.5 years old, neutered male, moderately obese, laboratory cats that were housed two rooms away from kittens that had been experimentally infected with an FCV isolate obtained from the blood of Ari (see Section 3.3). The four cats had not been exposed to other agents and were quarantined from other cats through separate ante-rooms, and were cared for by different caretakers. The caretakers took extra precautions in terms of disposable overalls, gloves, boots, and foot baths, as well as separate food and litter sources, to prevent inadvertent infections from the outside. On the morning of 13 November 1998, one of the four cats (97-659) in this group was found dead and the other three animals were depressed and febrile. Gross necropsy findings showed a reddened and somewhat swollen pancreas with areas of hemorrhage and saponification in peripancreatic mesenteric fat. Several large areas of yellow-tinged subcutaneous edema were observed in the groin and trunks. The lungs appeared grossly congested with many areas of collapse, and there was a small amount of free fluid in the abdomen and chest cavities. The condition of the remaining three cats rapidly deteriorated, in spite of antibiotic, fluid and whole blood therapy. Cat 96-101 was in shock on 18 November 1998 and euthanatized. The remaining two cats (96-179 and 97-680) were still febrile on 19 November 1998 but were becoming rapidly more depressed; a decision was made to euthanatize them rather than to continue symptomatic treatment. Blood chemistry panels taken near the time of death from all four animals revealed mild to moderate elevations in blood glucose, moderately decreased serum total proteins, and mild to moderate hyperbilirubinemia. Gross necropsy findings in the three latter cats were identical to those observed in the first cat. All three cats had reddened and swollen-appearing pancreases with hemorrhage and saponification of peripancreatic fat. In addition to these findings, cat 97-680 had an edematous omentum, ulceration of the skin on the phalanges of the right hindleg, sloughing of the claw on second digit of the right hindleg, an ulcer of the skin under the jaw, a yellowish edema under the tongue and icteric appearing nictitating membranes. Cat 96-179, in addition to pancreatic lesions, had congestion and consolidation of the right lung lobes, an edematous omentum, and splenomegaly. Cat 96-101 had identical lesions on the pancreas and surrounding tissues, but also had subcutaneous edema with yellowing of the tissues of the hind limbs and edema of all four paws. CrFK and Fcwf-4 cell cultures of blood and pancreatic tissue from all four cats yielded FCV. Genetic sequencing of FCV isolates from all four cats showed them to be identical to FCV-Ari (data not shown). Maximum quarantine was initiated at the time of the secondary outbreak; the virus was only handled under the strictest of quarantine conditions, with special isolation facilities, separate caretakers, complete changes of disposable coveralls, gloves, and foot coverings. No additional cases were observed in either the clinic of origin or in the experimental animal facilities after this time.
3.2. Virus isolation and characterization
Cytopathic effect, typical of FCV, was evident within 24 h on both CrFK and Fcwf-4 cells cultures exposed to blood and nasal secretions of Ari. Transmission electron micrographs of the cells demonstrated characteristic FCV capsids, 25–30 nm in diameter, within paracrystalline viral arrays in the nuclei (not shown) and parallel linear viral arrays in the cytoplasm (Fig. 2 ). RNA was extracted from infected cultures, reverse transcribed, and subjected to PCR. The PCR product was sequenced (Table 1 ) and proved to be identical, within the range of strain variation, of a number of vaccine- and field-origin FCVs (Fig. 3 ). The virus isolate was designated FCV-Ari.
Fig. 2.
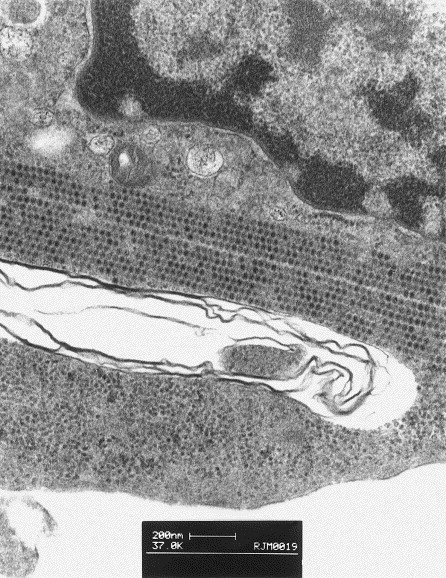
Transmission electron micrograph of Crandell feline kidney cells infected with FCV-Ari. Parallel stacked linear arrays made up of numerous calicivirus virions 25–30 nm in diameter are present in the cytoplasm of infected cells.
Table 1.
A comparison of sequenced PCR products from the capsid gene of a vaccine strain of FCV (-F9), FCV-Ari and three unrelated field isolates of normal virulence FCV-2-93, FCV-256660 and FCV-254075a
| 1 | 50 | ||||
| FCV-F9 | ∼∼∼TCCCTGA | TGGCTGGCCT | GACACCACAA | TTCCTGGGGA | GTTGATACCT |
| FCV-Ari | ∼∼TACCAGGA | TGGTTGGCCT | GACACCACAA | TCCCTGAGAA | GCTGACACCT |
| FCV-2-93 | AGCCCGTAA | AATATCCAAC | ACTGCACCAA | TTCCTGGGGA | GTTGATACCA |
| FCV-256660 | ∼GATTCCTGA | CGGATGGCCA | GACACTACTA | TCCCTGAGAA | GCTAATCCCT |
| FCV-254075 | ∼AATACCTGA | TGGGTGGCCG | GACACAACAA | TTCCAAGCAA | GCTCACGCCT |
| 51 | 100 | ||||
| FCV-F9 | GCTGGCGATT | ACGCAATCAC | CAATGGTACT | GGCAATGACA | TCACCACGGC |
| FCV-Ari | GCTGGCGATT | ACGCCATCGT | AGATGGATCA | GGCAATGACA | TCACAACTAA |
| FCV-2-93 | GCTGGCGATT | ACGCAATCAC | CAATGGTACT | GGCAATGACA | TCACCACGGC |
| FCV-256660 | GCTGGTGATT | ACGCAATCAC | AACATTAAGT | GGTTCTGACA | TCACAACTCC |
| FCV-254075 | GCCGGCAACT | ATGCCATTAC | CAACGGAAGT | GGTAGCGACA | TTGTGACGCC |
| 101 | 150 | ||||
| FCV-F9 | TACAGGATAT | GACACTGCTG | ATATAATTAA | GAACAATACC | AACTTTAGGG |
| FCV-Ari | GGATAAATAT | GAAAGTGCTG | ATGTGATCAA | GAATAACACC | AATTTCAGGG |
| FCV-2-93 | TACAGGATAT | GACACTGCTG | ATATAATTAA | GAACAATACC | AACTTTAGGG |
| FCV-256660 | CCAAGGGTAT | GATAATGCAG | ATGTAATTAA | GAATAATACA | AACTTTAAAG |
| FCV-254075 | TGCTGGGTAC | GACTCTGCTG | ATGTCATCCT | GAACAATACA | AACTTTAAGG |
| 151 | 200 | ||||
| Fcv-F9 | GCATGTACAT | ATGTGGTTCG | CTCCAGCGTG | CCTGGGGTGA | CAAGAAAATA |
| Fcv-Ari | GCATGTACAT | TTGTGGCTCA | CTTCAAAGAG | CATGGGGTGA | CAAGAAAATA |
| Fcv2-93 | GCATGTACAT | ATGTGGTTCG | CTCCAGCGTG | CCTGGGGTGA | CAAGAAAATA |
| Fcv-256660 | GAATGTACAT | TTGTGGATCT | TTACAACGAG | CATGGGGTGA | CAAGAAAATA |
| Fcv-254075 | GCATGTACAT | CTGTGGTTCC | CTTCAAAGAG | CCTGGGGTGA | CAAGAAAATA |
| 201 | 213 | ||||
| Fcv-F9 | TCCAACACTG | CAT | |||
| Fcv-Ari | TCCAACNGGC | A∼∼ | |||
| Fcv2-93 | TCCAACACTG | CAA | |||
| Fcv-256660 | TCCAACACTG | A∼∼ | |||
| Fcv-254075 | TCCAACTGCA | ∼∼∼ |
FCV-Ari falls within the predicted range of genetic variability of both vaccine and field strains.
Fig. 3.
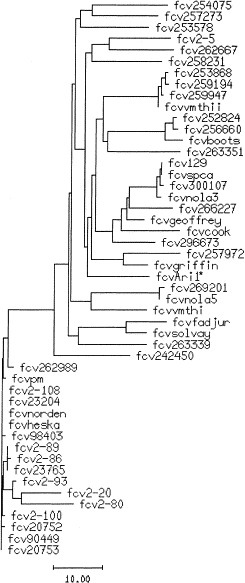
A cladogram showing the genetic relationship of FCV-Ari (FCV-Ari1)* to other field and vaccine strains of FCV. FCV-Ari was clearly within the genetic range of a large number of other field and vaccine (FCV-Solvay, FCV-Heska and FCV-Norden) isolates. Horizontal bar indicates substitutions per 100 residues.
3.3. Experimental transmission studies
Three 14-week old SPF kittens, 98-401, 98-432, and 98-442, were infected oronasally with FCV-Ari on 30 October 1998. The kittens were febrile by 24 h post-inoculation. The fever continued unabated for the course of the study, while other signs appeared in rapid progression. Evidence of nasal congestion with occasional sneezing and slight ocular discharge were noticeable by the second day. Nasal congestion was associated in all three cats with subcutaneous edema over the bridge or side of the nose. In one cat, a shallow ulcer appeared over the site of edema. The feet and lower limbs of the kittens also became edematous. All of the infected cats were anorectic after Day 1 and required subcutaneous fluid therapy to maintain hydration. Cat 98-401 was in shock on 6 November 1998 and was given 40 ml of fresh whole blood intravenously. He failed to rally and was euthanatized on 17 November 1998. The remaining two cats, 98-432 and 98-442, began to show progressive improvement from 7 November 1998 onward and were essentially normal by 17 November 1998. Significant abnormalities, including hypoproteinemia with hypoalbuminemia and hypoglobulinemia, hyperglucosemia, and hyperbilibubinemia were seen at the peak of illness in cat 98-401 on 5 November 1998. Hyperbilibubinemia was the only significant finding on the remaining two animals.
Virus was readily isolated from both blood and nasal secretions of the experimentally infected cats. The genetic sequence of PCR products from the two isolates that were tested was identical to FCV-Ari isolates obtained from the field cases.
3.4. Pathologic features
Detailed necropsy and histopathologic studies were done on Ari, four laboratory cats inadvertently infected with FCV-Ari, and on four laboratory cats deliberately infected with FCV-Ari. Gross lesions present on Ari included alopecia of the rear limbs, inguinal and perineal regions and the dorsal aspects of the forelimbs, with dozens of ulcers ranging from 0.2–3 cm in diameter within alopecic foci. A small vesicle was present on the left carpus and all of the digital pads were ulcerated. Small ulcers were also present on the lateral aspects of the gum and medial buccal mucosa. Subcutaneous edema was prominent on the lateral body wall, and numerous irregular chalky, white foci were widely disseminated in the subcutaneous fat. The lungs were reddened and atelectatic and 30 ml of a pinkish milky fluid was present in the pleural cavity. Fifty or more miliary grey foci were randomly distributed on the surface of all lung lobes. The cranial mediastinum was thickened and a thrombus was present in the right jugular vein. The abdomen contained about 80 ml of serosanguinous fluid with fibrin clots and fibrin tags adherent to the surface of the viscera. A 0.7 cm×0.8 cm ulcer was present in the gastric cardia, and smaller ulcer was also observed on the mucosa of the trigone of the bladder. Histopathologic examination showed a severe multifocal epidermal necrosis with ulceration. There was a chronic suppurative necrotizing bronchopneumonia with squamous metaplasia and intralesional fungal hyphae consistent with Aspergillus sp. The pulmonary aspergillosis was presumed to be a terminal sequelae of the persistent calicivirus infection, debilitation of the immune system, and prolonged antibiotic therapy. The lung tissue was severely atelectatic, with intraalveolar histiocytic exudate and multifocal vascular thrombosis. A moderately severe and chronic pleuritis was present. Tracheobronchial lymph nodes manifested widespread fibrinous sinus thrombi and moderate lymphoid depletion. A severe diffuse and chronic thrombosis was evident in subcutaneous vessels with associated fat necrosis. The spleen showed marked red-pulp histiocytosis.
Cat 97-659, which was representative of the four laboratory animals that were inadvertently infected with FCV-Ari, demonstrated several large areas of subcutaneous yellowish edema, diffuse consolidation of the lungs, swelling and reddening of the pancreas, and numerous chalk-like plaques with surrounding hemorrhage were present in the peripancreatic fat. Histopathologic findings included a moderate to severe multifocal pancreatitis with a mixed inflammatory infiltrate, peripancreatic fat necrosis, mild to moderately severe multifocal crypt necrosis in the duodenum and colon, moderately severe and diffuse individual hepatocellular necrosis with mild periportal lymphocytic/histiocytic infiltrate, and acute multifocal interstitial pneumonia.
Gross findings in laboratory cats (96-101, 96-179, 97-680, 97-689), which were purposefully infected with FCV-Ari, were similar, and included patchy to diffuse consolidation of the lungs, small amounts of free pleural fluid, and areas of subcutaneous edema, especially around the face. Intestinal crypt lesions were apparent in all four animals; crypts were lined with large pleomorphic, or flattened, epithelial cells and necrotic cells and cellular debris often filled the crypt lumens. Peyer’s patches were hyperplastic, and the sinuses of lymph nodes were expanded by numerous foamy macrophages and subcapsular neutrophilic infiltrate. Paracortical lymphoid hyperplasia with abundant apoptotic cells were also evident. The medullary centers of the thymus were often expanded by fibrin and eosinophilic cellular debris. A foci of pancreatic necrosis with inflammatory infiltrate was noted in one animal. Pulmonary changes included a mild multifocal atelectasis with a few neutrophils and lymphocytes present in the interstitium of capillaries and small airways. Rare inflammatory cells were present in the lumen of pulmonary capillaries and bronchioles. Multiple randomly situated foci of necrotic hepatocytes, with or without a small number of neutrophils, were observed. Marked diffuse edema and epidermal necrosis were present in sections of diseased appearing skin.
3.5. Serologic studies and vaccine protection
Four 16-week old SPF cats (98-382, 98-394, 98-431 and 98-444) were immunized orally with FCV-F9 and serum collected 5 weeks later. These sera were then tested in a virus neutralization assay against FCV-F9 and FCV-Ari (Table 2 ). There was low to negligible cross-reactivity between FCV-F9 immune sera and FCV-Ari.
Table 2.
Virus neutralizing antibody titers of sera from FCV-F9 immunized cats against FCV-F9 and FCV-Ari
| Virus strain | Cat source of serum and virus neutralizing antibody titer |
|||
| 98-382 | 98-394 | 98-431 | 98-444 | |
| FCV-F9 | 18638 | 243 | 2282 | 6546 |
| FCV-Ari | 9 | 0 | 3 | 0 |
Serum collected from cat 98-442 3 weeks following FCV-Ari infection was titrated for virus neutralizing activity against a number of vaccine and field strains of FCV (Table 3 ). FCV-Ari antiserum neutralized all four vaccine strains (three containing FCV-F9), but failed to neutralize 5/8 field isolates.
Table 3.
Virus neutralizing titer of serum from FCV-Ari recovered cat 98-442 against several vaccine and field strains of FCV
| Virus strain | Virus neutralizing titer of anti-FCV-Ari serum |
| FCV-Ari | 7500 |
| FCV-Nordena,b | ≥270 |
| FCV-Heskaa,b | ≥270 |
| FCV-PMa,b | 270 |
| FCV-Solvaya | 270 |
| FCV-258231 | 0 |
| FCV-257972 | ≥270 |
| FCV-SPCA | 0 |
| FCV-VMTH-I | ≥270 |
| FCV-FI2-5 | 0 |
| FCV-Cook | 0 |
| FCV-25914 | 0 |
Isolated from a commercial vaccine.
FCV-F9 by gene sequence analysis.
Six SPF kittens were divided into vaccine- (cats 98-382, 98-394, 98-431 and 98-444) and control-groups (cats 98-516, 98-537). The vaccine group was inoculated orally on 11 January 1999 with 0.5 ml of a tissue culture supernatant containing approximately 5×106 TCID100 of FCV-F9, the common vaccine strain used in virtually all attenuated live FCV vaccines. Oral swabs were collected weekly and cultured for FCV. All four vaccinated cats shed vaccine virus through Week 2, three through Week 4, but only two animals (cats 98-382 and 98-431) were still shedding at Week 6 (time of challenge-exposure) (Table 4 ).
Table 4.
The oral shedding (culture/PCR) of feline calicivirus by FCV-F9 vaccinated cats, following vaccination and after challenge exposure to FCV-Ari at Week 6 post-immunizationa
| Weeks post oral vaccination with FCV-F9 |
|||||||||||
| Cat # | Group | 1 | 2 | 3 | 4 | 5. | 6b | 7 | 8 | 9 | 10 |
| 98-382 | Vaccinate | +/−c | +/+ | +/+ | −/− | −/+ | +/− | −/− | +/+ | −/+ | −/− |
| 98-394 | Vaccinate | +/+ | +/− | +/+ | +/+ | +/− | −/− | +/+ | −/+ | −/+ | −/− |
| 98-431 | Vaccinate | +/+ | +/+ | −/+ | −/+ | +/− | +/+ | +/+ | −/+ | −/+ | −/− |
| 98-444 | Vaccinate | +/+ | +/+ | +/− | +/− | −/− | −/+ | −/+ | −/+ | −/+ | −/− |
| 98-516 | Nonvaccinate | −/− | −/− | −/− | −/− | −/− | −/− | +/+ | −/+ | +/+ | −/− |
| 98-537 | Nonvaccinate | −/− | −/− | −/− | −/− | −/− | −/− | +/+ | −/+ | −/+ | −/− |
FCV isolates prior to challenge-exposure were uniformly of the FCV-F9 strain by sequence analysis, while isolates after challenge-exposure were identical to FCV-Ari.
Challenge-exposed with FCV-Ari immediately after oral swabbing.
Culture/PCR.
The four recently vaccinated cats, along with two additional unvaccinated animals, were challenge-exposed to FCV-Ari on 22 February 1999, which was 6 weeks post-vaccination. The route and dosage were identical to the initial experimental infection study. Both vaccinated and unvaccinated cats became febrile within 24 h, and the fever persisted for 5–6 days (Fig. 4 ). The febrile responses were slightly lower and about 1 day shorter in duration in vaccinated versus unvaccinated cats. Clinical signs were similar in both groups, but noticeably less severe in vaccinates than nonvaccinates. Clinical signs included transient anorexia, dehydration, lameness, slight ocular and nasal discharge, mild sneezing, swelling of the face with occasional ulceration, edema of the pinnae with ulceration or crusting of the edges, and variable swelling of the lower limbs. There appeared to be no relationship between whether the cats were still shedding FCV-F9 vaccine virus at the time of challenge-exposure and subsequent disease severity; cat 98-382, a vaccine virus carrier, was almost as symptomatic as the nonvaccinates. Both vaccinated and unvaccinated cats were treated with subcutaneous lactated ringers solution for several days, but all animals made a rapid uncomplicated recovery starting at Days 5–7 post-infection. Virus shedding, as measured by both tissue culture isolation and PCR, from the oral cavity was observed in both nonvaccinates following FCV-Ari challenge-exposure and in 3/4 vaccinates (Table 4). Virus shedding lasted from 1–3 weeks; no cats were still shedding calicivirus by culture or PCR after Week 10. Virus shed at the time of FCV-Ari challenge-exposure and earlier was of the F9 strain, while virus shed after challenge was entirely of the FCV-Ari strain as determined by sequence analysis (data not shown).
Fig. 4.
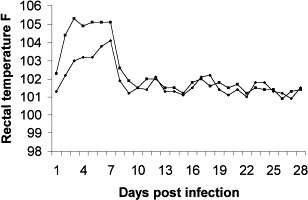
Rectal temperatures of previously FCV-F9 immunized (♦------♦) and nonimmunized (■------■) cats that were challenge-exposed to FCV-Ari. The febrile responses of vaccinates developed more slowly and were somewhat less severe than those of the unvaccinated cats.
4. Discussion
FCV is endemic in most catteries, shelters and large multiple cat households, where up to one-fourth of cats may be orally shedding the virus at any given time (Bennett et al., 1989, Harbour et al., 1991; Dawson et al., 1993a, Dawson et al., 1993b). FCV exists in numerous overlapping serotypes but is basically a single species (Gillespie and Scott, 1973). Different strains vary somewhat in the main types of disease signs that they cause (Hoover and Kahn, 1975, Pedersen et al., 1983, Pedersen and Hawkins, 1995), but these differences are hard to discern from genetic or serologic comparisons (Dawson et al., 1993a, Dawson et al., 1993b; Pedersen and Hawkins, 1995, Geissler et al., 1997, TeeWee et al., 1997). Although original descriptions of FCV infection suggested that it might be a major cause of mortality in young cats, this has not been borne out by subsequent experimental studies. So-called highly virulent strains of FCV, such as FCV-255 (Gillespie and Scott, 1973), can exist in breeding populations of cats without significant disease; kittens being infected from 3–9 weeks of age when they still have significant levels of passive immunity (Johnson and Povey, 1984). Clinical disease can be of acute or chronic form. Acute disease, when it is seen, is generally systemic with vesicular involvement of the oral cavity (ulcers on palate, tongue, inflammation of gums and oral fauces), arthropathy (limping) and mild interstitial pneumonia (Hoover and Kahn, 1975, Pedersen et al., 1983, Pedersen and Hawkins, 1995, TeeWee et al., 1997). Upper respiratory disease is relatively uncommon, associated with certain strains much more than others, and manifested as a mild and transient bilateral conjunctivitis and rhinitis (compare FCV-263351 and FCV-Cook in Pedersen and Hawkins, 1995). A viremia is detectable during the first week of infection (Pedersen et al., 1983), and oral shedding occurs for several weeks to months (reviewed by Pedersen and Hawkins, 1995). The source of viral carriage and shedding appears to be the tonsils and surrounding mucosa (Dick et al., 1989).
There seemed to be little doubt that the infection in the first six client-owned cats was nosocomial. However, catteries and shelters are more common sources of infection than veterinary clinics, an observation which may have been borne out by the theorized index case in this outbreak, a 4-month old female domestic kitten that had been brought into the hospital from a local shelter and treated for a severe atypical viral infection during the period of 17 September 1998 to 26 October 1998. The dates that this particular animal was in the clinic both preceded and overlapped the dates when client-owned cats were exposed. This kitten was receiving almost constant medical attention and contamination of hands, clothes, instruments, etc., would have been difficult to prevent under the strictest of conditions.
The disease caused by FCV-Ari in both natural and experimental infections appeared to target blood vessels, as evidenced by the severe edema (sometimes with hemorrhage) in subcutaneous tissues and lungs and local necrosis of skin and adipose tissues. Although vasculitis was difficult to discern as a distinct lesion in tissues of laboratory infected cats, it was definitely a feature of the early skin lesions that were first observed on the cat Ria. Loss of vascular integrity was also the best explanation for the significant drop in serum proteins, icteric serum (from breakdown of extravasated red blood cells), variable thrombocytopenia, and coagulopathies. Elevations in CPK also indicated myonecrosis. The noteworthy feature of the infection was its persistence in the blood of those cases that went on to die; virus was still present in the blood of Ari at the time of his death, almost 3 weeks after first clinical presentation. Persistence of viremia may also have explained the death of the index kitten after a 6-week course of illness.
The type of disease caused by FCV-Ari is highly reminiscent of Rabbit Hemorrhagic Disease, also caused by a calicivirus and first reported in China (Liu et al., 1984). Similarities included the high mortality, acute nature, the tendency to cause more severe disease in older animals, the ease of spread, hepatocyte tropism, and widespread vascular disease. Following its apperance in China, RHD spread throughout Europe, where it caused severe disease in rabbits but mild to unapparent infections in hares (Mitro and Krauss, 1993). A closely related calicivirus, the European Brown Hare Syndrome Virus (EBHS virus) (Bascuñana et al., 1997), affects both wild and farmed hares, and was also first reported in the 1980s in Europe. Both viruses cause severe necrotizing hepatitis and widespread hemorrhaging. The disease caused by the RHDV is particularly severe, with mortalities approaching 100% in rabbits older than 8 weeks of age (Nowotny et al., 1990, Nowotny et al., 1991). The high species specificity and virulence of RHDV led the Australian government to experiment on the virus as a means to control wild rabbits (Kovaliski, 1998). Experiments conducted on an offshore island showed the potential of the virus as a biologic agent, but before a final decision for controlled release could be made, the RHDV appeared in wild rabbits on the mainland. The virus spread rapidly, with over 97% mortality on wild rabbit populations recorded for one region of South Australia (Mutze et al., 1998).
Reasons for the high mortality associated with FCV-Ari were undetermined. The virus is definitely more virulent than any FCV strain yet tested by one of the authors (NCP) or reported in literature. It was noteworthy that the cats that became the most ill were also among the oldest. RHDV causes a mild self-limiting infection in rabbits less than 8 weeks of age, while the mortality approaches 100% in older animals (Mutze et al., 1998). Inherent resistance factors also seemed to play a role; some individual cats developed mild self-limiting disease, while others were devastated by the infection. The close genetic relationship of Ria, Ari and Ian could have contributed to the severity of their illness. At least one of the cats (Ari) was also treated with prednisolone early in the course of his infection, in the belief that the disease was some sort of immune vasculitis. However, another cat (Emma) in the initial outbreak, and several laboratory cats, were not treated with prednisolone and had a similar fatal disease course.
Even though many field strains of FCV do not cross-react with the almost universal FCV-F9 vaccine strain (Pedersen et al., 1983, Knowles et al., 1990, Pedersen and Hawkins, 1995, Laruritzen et al., 1997, Hohdatsu et al., 1999), none of these strains have been of this virulence and the consequences have therefore been small. Antibodies against the universal FCV-F9 vaccine strain also did not significantly cross-react with FCV-Ari, and laboratory cats immunized by the oral route with FCV-F9 6 weeks prior to challenge-exposure possessed only a small measure of immunity. Two field cats that died of the infection (Ari and Emma) had been previously immunized for FCV also indicating that current FCV vaccines, given by prescribed parenteral routes, will not protect against FCV-Ari.
Why such a virulent and obviously contagious strain of FCV would appear and disappear so suddenly is unknown. The virus was spread readily from one group of cats to another both within the practice of origin and in the laboratory setting with ease, and it is fortunate that this spread was contained in both areas. Unfortunately, highly virulent, vaccine resistant, viruses like FCV-Ari may arise again in the future and vigilance is required.
Acknowledgements
Funding for this study was provided by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis, CA 95616. We wish to acknowledge the special contributions of Dr. Kelly Byam and Dr. Katja Hermann and Ms. Karla Klayer of the Mueller Animal Hospital, Sacramento, CA for their care of cats initially involved in this outbreak. Special thanks for similar duties goes to Mr. David Weber and his animal care staff of the Center for Companion Animal Health. The authors also acknowledge the contribution of M. Rebecca McElhaney, DVM, Diplomate, American College of Veterinary Pathologists, IDEXX Veterinary Services, for her interpretation of biopsies of abnormal skin.
References
- Bascuñana C.R, Nowotny N, Belák S. Detection and differentiation of rabbit hemorrhagic disease and European brown hare syndrome viruses by amplification of vp60 genomic sequences from fresh and fixed tissue specimens. J. Clin. Microbiol. 1997;35:2492–2495. doi: 10.1128/jcm.35.10.2492-2495.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Gaskell R.M, Mills A, Knowles J, Carter S, McArdle F. Detection of feline calicivirus antigens in the joints of infected cats. Vet. Rec. 1989;124:329–332. doi: 10.1136/vr.124.13.329. [DOI] [PubMed] [Google Scholar]
- Dawson S, McArdle F, Bennett M, Carter M, Milton I.P, Turner P, Meanger J, Gaskell R.M. Typing of feline calicivirus isolates from different clinical groups by virus neutralization tests. Vet. Rec. 1993;133:13–17. doi: 10.1136/vr.133.1.13. [DOI] [PubMed] [Google Scholar]
- Dawson S, McArdle F, Bennett D, Carter S.D, Bennett M, Ryvar R, Gaskell R.M. Investigation of vaccine reactions and breakdowns after feline calicivirus vaccination. Vet. Rec. 1993;132:346–350. doi: 10.1136/vr.132.14.346. [DOI] [PubMed] [Google Scholar]
- Dawson S, Bennett D, Carter S.D, Bennett M, Meanger J, Turner P.C, Carter M.J, Milton I, Gaskell R.M. Acute arthritis of cats associated with feline calicivirus infection. Res. Vet. Sci. 1994;56:133–143. doi: 10.1016/0034-5288(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Dick C.P, Johnson R.P, Yamashiro S. Sites of persistence of feline calicivirus. Res. Vet. Sci. 1989;47:367–373. [PubMed] [Google Scholar]
- Geissler K, Schneider K, Platzer G, Truyen B, Kaaden O.R. Genetic and antigenic heterogeneity among feline calicivirus isolates from distinct disease manifestations. Virus Res. 1997;48:193–206. doi: 10.1016/s0168-1702(97)01440-8. [DOI] [PubMed] [Google Scholar]
- Gillespie J.H, Scott F.W. Feline viral infections. Adv. Vet. Sci. 1973;17:163–200. [PubMed] [Google Scholar]
- Harbour D.A, Howard P.E, Gaskell R.M. Isolation of feline calicivirus and feline herpesvirus from domestic cats 1980 to 1989. Vet. Rec. 1991;128:77–80. doi: 10.1136/vr.128.4.77. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T, Sato K, Tajima T, Koyama H. Neutralizing feature of commercially available feline calicivirus (FCV) vaccine immune sera against FCV field isolates. J. Vet. Med. Sci. 1999;61:299–301. doi: 10.1292/jvms.61.299. [DOI] [PubMed] [Google Scholar]
- Hoover E.A, Kahn D.E. Experimentally induced feline calicivirus infection: clinical signs and lesions. J. Am. Vet. Med. Assoc. 1975;166:463–468. [PubMed] [Google Scholar]
- Johnson R.P. Antigenic change in feline calicivirus during presistent infection. Can. J. Vet. Res. 1992;56:326–330. [PMC free article] [PubMed] [Google Scholar]
- Johnson R.P, Povey R.C. Feline calicivirus infection in kittens born by cats persistently infected with the virus. Res. Vet. Sci. 1984;37:114–119. [PubMed] [Google Scholar]
- Kahn D.E, Hoover E.A, Bittle J.L. Induction of immunity to feline caliciviral disease. Infect. Immun. 1975;11:1003–1009. doi: 10.1128/iai.11.5.1003-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J.O, Dawson S, Gaskell R.M, Gaskell C.J, Harvey C.E. Neutralisation patterns among recent British and North American feline calicivirus isolates from different clinical origins. Vet. Rec. 1990;127:125–127. [PubMed] [Google Scholar]
- Kreutz L.C, Johnson R.P, Seal B.S. Phenotypic and genotypic variation of feline calicivirus during persistent infection of cats. Vet. Microbiol. 1998;59:229–236. doi: 10.1016/s0378-1135(97)00158-2. [DOI] [PubMed] [Google Scholar]
- Kovaliski J. Monitoring the spread of rabbit hemorrhagic disease virus as a new biological agent for control of wild European rabbits in Australia. J. Wildlife Dis. 1998;34:421–428. doi: 10.7589/0090-3558-34.3.421. [DOI] [PubMed] [Google Scholar]
- Laruritzen A, Jarrett O, Sabara M. Serological analysis of feline calicivirus isolates from the United States and United Kingdom. Vet. Microbiol. 1997;56:55–63. doi: 10.1016/S0378-1135(96)01252-7. [DOI] [PubMed] [Google Scholar]
- Liu S.J, Xue H.P, Pu P.Q, Qian N.H. A new viral disease in rabbits. Anim. Husbandry Vet. Med. 1984;16:253–255. [Google Scholar]
- Mitro S, Krauss H. Rabbit hemorrhagic disease: a review with special reference to its epizootiology. Eur. J. Epidemiol. 1993;9:70–78. doi: 10.1007/BF00463093. [DOI] [PubMed] [Google Scholar]
- Mutze G, Cooke B, Alexander P. The initial impact of rabbit hemorrhagic disease on European rabbit populations in South Australia. J. Wildlife Dis. 1998;34:221–227. doi: 10.7589/0090-3558-34.2.221. [DOI] [PubMed] [Google Scholar]
- Nowotny N, Fuchs A, Schilcher F, Loupal G. Zum Auftreten der rabbit hemorrhagic disease (RHD) in Österreich. I. Pathomophologische und virologische Untersuchungen. Wien. Tieraerztl. Monschr. 1990;77:19–23. [Google Scholar]
- Nowotny N, Steineck T, Tatrruch F, Schilcher F, Weissenböck H. European brown hare syndrome (EPHS) — Experimentelle Untersuchungen. Wien. Tierareztl. Monschr. 1991;78:370–378. [Google Scholar]
- Pedersen N.C, Hawkins K.F. Mechanisms for persistence of acute and chronic feline calicivirus infections in the face of vaccination. Vet. Microbiol. 1995;47:141–156. doi: 10.1016/0378-1135(95)00101-f. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C, Laliberte L, Ekman S. A transient febrile limping syndrome of kittens caused by two different strains of feline calicivirus. Feline Practice. 1983;13(1):26–35. [Google Scholar]
- Radford A.D, Turner P.C, Bennett M, McArdle F, Dawson S, Glenn M.A, Williams R.A, Gaskell R.M. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats. J. Gen. Virol. 1998;79:1–10. doi: 10.1099/0022-1317-79-1-1. [DOI] [PubMed] [Google Scholar]
- Reubel G.H, Hoffmann D.E, Pedersen N.C. Acute and chronic faucitis of domestic cats. A feline calicivirus-induced disease. Vet. Clin. No. Am. Small Anim. Pract. 1992;22:1347–1360. doi: 10.1016/s0195-5616(92)50131-0. [DOI] [PubMed] [Google Scholar]
- Studdert M.J. Caliciviruses. Brief review. Arch. Virol. 1978;58:157–191. doi: 10.1007/BF01317600. [DOI] [PubMed] [Google Scholar]
- TeeWee J, Lauritzen A.Y, Sabara M, Dreier K.J, Kokjohn K. Comparison of the primary signs induced by experimental exposure to either a pneumotrophic or a limping strain of feline calicivirus. Vet. Microbiol. 1997;56:33–45. doi: 10.1016/S0378-1135(96)01344-2. [DOI] [PubMed] [Google Scholar]


