Abstract
Equine rotaviruses were first detected in foals over 30 years ago and remain a major cause of infectious diarrhoea in foals. During this time, there has been substantial progress in the development of sensitive methods to detect rotaviruses in foals, enabling surveillance of the genotypes present in various horse populations. However, there has been limited epidemiological investigation into the significance of these circulating genotypes, their correlation with disease and the use of vaccination in these animal populations. Our knowledge of the pathogenesis of rotavirus infection in foals is based on a limited number of studies on a small number of foals and, therefore, most of our understanding in this area has been extrapolated from studies in other species. Questions such as the concentrations of rotavirus particles shed in the faeces of infected foals, both with and without diarrhoea, and factors determining the presence or absence of clinical disease remain to be investigated, as does the relative and absolute efficacy of currently available vaccines. The answer to these questions may help direct research into the development of more effective control measures.
Abbreviations: G, glycoprotein; P, protease sensitive protein; EM, electron microscopy; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction; RT-LAMP, reverse transcription loop-mediated isothermal amplification; TLPs, triple-layered particles; DLPs, double-layered particles; SLPs, single-layered particles; BLS, Brucella spp. lumazine synthase
Keywords: Equine rotavirus, Foal, Diarrhoea, Virus, Review
1. Introduction
Rotaviruses were first observed in the faeces of a foal with diarrhoea in 1975 in Great Britain (Flewett et al., 1975). They had been detected previously in faeces from a vervet monkey in 1958, but it was not until rotaviruses were recognised as a major cause of neonatal diarrhoea in calves in 1969 and children in 1973 that significant research into this pathogen in other species commenced (Bishop and Davidson, 1973, Malherbe and Strickla, 1967, Mebus et al., 1969). Initially referred to as a reovirus-like agent, the name rotavirus was later adopted from the Latin “rota” (wheel), because of the wheel-like appearance of virions by electron microscopy (EM) (Fig. 1 ) (Flewett et al., 1974).
Fig. 1.
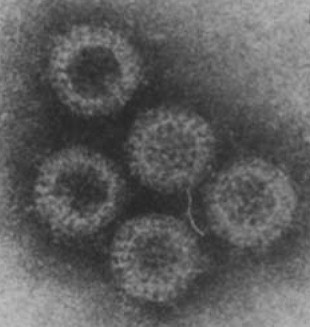
Rotavirus particles as seen by negatively stained electron microscopy.
From Rodger et al. (1980).
Rotaviruses are the most prevalent viral pathogens identified in the faeces of foals with diarrhoea. The frequency of detection of rotaviruses in clinical cases varies from 20 to 77% and they appear to be endemic in most, if not all, horse populations (Browning et al., 1991c, Conner and Darlington, 1980, Dwyer et al., 1990, Netherwood et al., 1996). Diarrhoea in young foals is a labour intensive disease that is costly to manage. An inactivated maternal vaccine has been available commercially since the mid 1990s, but despite this rotaviruses are still a major cause of diarrhoea in foals.
2. Rotavirus classification
Rotaviruses belong to the family Reoviridae, subfamily Sedoreovirinae, genus Rotavirus (Carstens, 2010). They are icosahedral, non-enveloped viruses that have a segmented, double stranded RNA genome (Newman et al., 1975, Welch and Thompson, 1973). The genome consists of 11 segments (Rodger et al., 1975) encoding six virion proteins (VP1-4, 6 & 7) and six non-structural proteins (NSP 1-6). The eleventh gene segment codes for both NSP5 and NSP6.
The virions have a triple capsid (Labbé et al., 1991, Rothnagel et al., 1994). The outer capsid is composed of the glycoprotein VP7, with spikes composed of VP4, the intermediate capsid is formed by VP6, and the inner capsid is enclosed by VP2 and contains VP1 and VP3 (Fig. 2 ). When visualised by EM, the infectious triple-layered particles (TLPs) have a diameter of 100 nm. Double-layered particles (DLPs) that have lost the outer capsid are 70 nm in diameter, and the less frequently identified single-layered particles (SLPs) have only the inner core remaining (Ciarlet and Estes, 2003). Neither DLPs nor SLPs are infectious. Loss of the outer capsid is promoted by chelation of Ca2+.
Fig. 2.
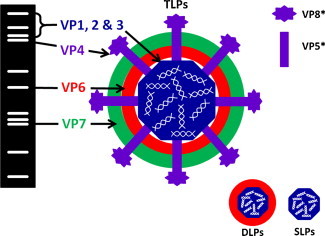
Schematic representation of the equine rotavirus genomic electrophoretic pattern and the virion morphology including triple-layered particles (TLPs), double-layered particles (DLPs) and single-layered particles (SLPs).
The intermediate capsid protein VP6 is used to classify rotaviruses into groups A–H (Matthijnssens et al., 2012b). Group A rotaviruses are the major cause of diarrhoea in humans and animals. Group B rotaviruses have been detected in calves, lambs, piglets and humans and group C rotaviruses in calves, piglets and humans (Ghosh et al., 2007, Medici et al., 2011, Park et al., 2011, Theil et al., 1995). Only group A rotaviruses have been detected in horses (Browning et al., 1991c, Dwyer, 2007).
The VP7 glycoprotein in the outer capsid is the major neutralisation antigen. It is encoded by the ninth genomic segment and is used to classify group A rotaviruses into G types, 27 of which are currently recognised (Matthijnssens et al., 2011). There are 6 G types reported in equine rotaviruses (Browning et al., 1991a, Browning et al., 1991d, Hoshino et al., 1983a, Hoshino et al., 1983b, Imagawa et al., 1994, Isa et al., 1996).
The protease sensitive VP4 is a minor neutralisation antigen encoded by the fourth genomic segment, and determines the P type. There are 35 different P types currently recognised (Matthijnssens et al., 2011), 6 in equine rotaviruses (Garaicoechea et al., 2011, Hardy et al., 1993, Isa and Snodgrass, 1994, Isa et al., 1996, Taniguchi et al., 1994). VP4 is also associated with haemagglutination, infectivity and virus attachment. Infectivity is enhanced by proteolytic cleavage of VP4 into two fragments, VP5* and VP8*, by trypsin in the small intestine (Graham and Estes, 1980).
Initially, the classification of rotaviruses into G types required cross-neutralisation assays with panels of hyperimmune sera (Hoshino et al., 1984, Kalica et al., 1981, Offit and Blavat, 1986). Classification into P types was more complex and generally required either P type specific monoclonal antibodies or the generation of reassortant viruses with identical G serotype genes but distinct P type genes. Serotyping has been largely replaced by genotyping (Gentsch et al., 1992, Gouvea et al., 1990). The nucleotide sequence of the VP7 gene correlates closely with G serotype designations. As VP4 is the minor neutralisation antigen, it is difficult to raise P serotype specific antibodies, making P serotyping much more demanding. As a result, fewer P serotypes have been definitively characterised, and P genotyping has been much more commonly used in epidemiological studies. When known, the P serotype is denoted by a number, while the P genotype is denoted by a number within square brackets.
A uniform scheme for the nomenclature of group A rotaviruses based on full genome sequencing has been adopted by the Rotavirus Classification Working Group. The nomenclature is shown in Table 1 (Matthijnssens et al., 2008). Full genome comparisons suggest that rotaviruses are generally host specific and that interspecies genomic reassortment is uncommon.
Table 1.
Rotavirus full genome nomenclature system.
| Gene product | RNA segment | Genotype | Description of gene product |
|---|---|---|---|
| VP7 | 9 | G | Glycosylated |
| VP4 | 4 | P[] | Protease sensitive |
| VP6 | 6 | I | Intermediate capsid shell |
| VP1 | 1 | R | RNA-dependent RNA polymerase |
| VP2 | 2 | C | Core shell protein |
| VP3 | 3 | M | Methyltransferase |
| NSP1 | 5 | A | Interferon antagonist |
| NSP2 | 8 | N | NTPase |
| NSP3 | 7 | T | Translation enhancer |
| NSP4 | 10 | E | Enterotoxin |
| NSP5 | 11 | H | Phosphoprotein |
Adapted from Matthijnssens et al. (2008).
3. Epidemiology and aetiology
Equine rotaviruses are ubiquitous in horse populations. The evidence for the widespread nature of rotavirus infection includes the high prevalence of rotavirus antibodies in adult horses (Conner and Darlington, 1980, Eichhorn and Huan-Chun, 1987, Goto et al., 1981, Pearson et al., 1982) and the detection of rotaviruses in horse populations from many countries, including the United Kingdom (Flewett et al., 1975, Strickland et al., 1982), the USA (Kanitz, 1976), Japan (Imagawa et al., 1984a), Australia (Studdert et al., 1978, Tzipori and Walker, 1978), New Zealand (Durham et al., 1979, Schroeder et al., 1983), Germany (Elschner et al., 2005), Italy (Monini et al., 2011), Greece (Ntafis et al., 2010), France (Puyalto-Moussu and Taouji, 2002), the Netherlands (van der Heide et al., 2005), Venezuela (Ciarlet et al., 1994), Argentina (Barrandeguy et al., 1998) and India (Gulati et al., 2009).
Transmission is by the faeco-oral route via contaminated faeces or fomites. Rotaviruses are highly contagious, replicate rapidly and are found in high titres in the faeces of infected animals. The minimum infective dose for foals has not been published. Studies in pigs demonstrate that infection can occur with as few as 90 viral particles, while one gram of faeces from an infected animal can contain up to 1010 rotavirus particles (Payment and Morin, 1990). Equine rotaviruses can be detected in the faeces of dams of infected foals. Whether this is just transit of viral particles through the mare's intestinal tract or subclinical infection is not known, but seroconversion of the dam of an infected foal has been reported (Conner and Darlington, 1980, Higgins et al., 1987, Powell et al., 1997).
Six G types and 6 P types have been described among equine rotaviruses to date (Table 2 ). However, the majority of circulating equine rotaviruses are G3P[12] and G14P[12] (Browning et al., 1992a, Browning and Begg, 1996, Ciarlet et al., 1994, Collins et al., 2008, Elschner et al., 2005, Garaicoechea et al., 2011, Hardy et al., 1991, Isa et al., 1996, Monini et al., 2011, Nemoto et al., 2011, Ntafis et al., 2010, Ohta et al., 1990, Tsunemitsu et al., 2001, van der Heide et al., 2005).
Table 2.
Reported equine rotavirus G and P types.
G3 equine rotaviruses have been further classified into two subtypes, G3A and G3B, based on cross neutralisation assays, correlating with 5 amino acid polymorphisms in three VP7 antigenic regions (Browning et al., 1992b, Dyall-Smith et al., 1986, Tsunemitsu et al., 2001). Of the two subtypes, G3A predominates (Browning et al., 1992a, Browning et al., 1992b) in Australia, Argentina, the UK, Germany, Greece and Ireland (Collins et al., 2008, Elschner et al., 2005, Garaicoechea et al., 2011, Ntafis et al., 2010), but not in Japan (Browning et al., 1992b, Nemoto et al., 2011, Tsunemitsu et al., 2001).
The other G and P types have only been detected either in a single foal, or from limited numbers of foals, and probably represent cross infection of foals from other species. The G8P[1] and G10P[11] rotavirus isolates are thought to be of bovine origin (Imagawa et al., 1994, Isa et al., 1996), while G5P[7] rotavirus isolates from foals are likely to be porcine in origin (Ciarlet et al., 2001). The G13P[18] equine rotavirus is unique and has only ever been isolated from a single, very young animal. All G3 and G14 equine rotaviruses that have been P genotyped have been P[12], except for one virus isolated in Argentina that was G3P[3] (Garaicoechea et al., 2011).
Full genome sequencing of 9 rotavirus isolates from horses further supports the theory that G3P[12] and G14P[12] are the typical equine rotaviruses and that the G5P[7] and G3P[3] isolates are most probably rare cases of cross-species transmission (Table 3 ). The G5P[7] strain shares 10 of 11 gene segments with typical porcine rotavirus strains and none with typical equine rotavirus strains, supporting its porcine origin (Ghosh et al., 2012), while the unusual G3P[3] isolate from a foal in Argentina appears to be most closely related to a cluster of canine and feline rotaviruses (Miño et al., 2013). The other G3 and G14 strains show a high level of conservation across 8 of the 11 gene segments, falling into 4 different patterns. In contrast, the unique nature of the G13 strain is highlighted when its full genome is compared to those of other rotavirus strains from all species, with the only similarity being the VP6 genotype I6, which is shared with typical equine rotaviruses (Matthijnssens et al., 2012a).
Table 3.
Equine rotavirus full genome genotype assortants.
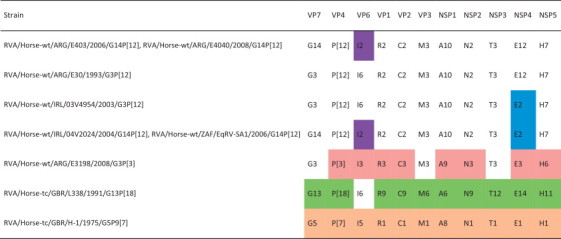
Adapted from Matthijnssens et al. (2012a), Ghosh et al. (2012) and Miño et al. (2013).
Dual infections with more than one strain of rotavirus, including with viruses of different G types, are possible and have been reported in large scale surveillance projects, but the clinical significance of this is poorly understood (Collins et al., 2008, Garaicoechea et al., 2011). Co-infections with other pathogens, including Salmonella spp., Cryptosporidium spp. and equine coronavirus have also been observed, although the significance of and association between pathogens in co-infections has not been established (Browning et al., 1991c, Eugster et al., 1978, Slovis et al., 2010).
4. Clinical disease
Rotaviruses are a significant cause of diarrhoea in foals. Rotaviral diarrhoea has a high morbidity in foals and, although clinical disease is usually self-limiting, dehydration may lead to mortalities. Clinical disease presents as reluctance to nurse, depression, diarrhoea, dehydration, pyrexia and recumbency and has been reported in foals from 3 days to 5 months of age (Conner and Darlington, 1980, Dickson et al., 1979, Kanitz, 1976, Strickland et al., 1982, Tzipori and Walker, 1978), with younger foals generally showing more severe signs of disease (Dwyer et al., 1990). The incubation period is generally short, with the onset of diarrhoea usually within 1–4 days of infection (Higgins et al., 1987, Imagawa et al., 1984a, Kanitz, 1976). Virus can be shed in faeces before the onset of diarrhoea, during the clinical phase of disease, which may persist for 1–12 days, and after resolution of diarrhoea (Conner and Darlington, 1980, Dickson et al., 1979, Dwyer et al., 1990, Higgins et al., 1987, Imagawa et al., 1984a, Strickland et al., 1982, Tzipori and Walker, 1978). Subclinical infections can also occur, contributing to environmental contamination with rotavirus and the infection of other foals (Dwyer et al., 1990, Tzipori et al., 1982).
5. Pathogenesis
There have been limited experimental infection studies in foals (Conner and Darlington, 1980, Imagawa et al., 1984b, Kanitz, 1976, Wada et al., 1984), so the pathogenesis of rotaviral infection has generally been extrapolated from studies in other species, such as piglets, calves and laboratory animals, or from in vitro studies.
The pathogenesis of rotaviral diarrhoea is considered to be multifactorial. Rotaviruses infect the mature absorptive epithelial cells of tips of the villi of the duodenum, jejunum and ileum, but not the crypt cells. The virus replicates in the cytoplasm of the epithelial cells and virions are released by lysis of the infected cells. Enterocyte destruction results in desquamation of epithelial cells, shortening of the absorptive villi and a malabsorptive diarrhoea (Woode and Crouch, 1978). Other reported histological changes include oedema, mononuclear cell infiltration, vacuolation and the presence of viral particles in the epithelial cell cytoplasm (Conner and Darlington, 1980).
However, the severity of diarrhoea does not always correlate with histological lesions, suggesting other mechanisms of diarrhoeal pathogenesis. NSP4 has been identified as a viral enterotoxin, acting via a number of mechanisms, including: inhibition of sodium-glucose co-transport, thus impairing solute and water uptake; reduction of disaccharidase enzymatic activity causing accumulation of disaccharides in the gut lumen and osmotic diarrhoea; and dysregulation of Ca2+ homeostasis, affecting chloride secretion by crypt cells and epithelial cell cytoskeletal integrity (Beau et al., 2007, Halaihel et al., 2000, Jourdan et al., 1998, Morris et al., 1999). Activation of the enteric nervous system also plays a role in the pathogenesis of rotavirus diarrhoea, as shown by the attenuation of diarrhoea in mice using drugs that block neurotransmitters, although the exact mechanism has not been determined (Lundgren et al., 2000).
While it has been suggested that rotaviruses may induce gastric ulceration and intussusception in foals, no published studies have clearly demonstrated an association in the field and there have not been any experimental studies demonstrating these outcomes from infection (Baldwin et al., 1990, Dwyer et al., 1990, Strickland et al., 1982, Studdert et al., 1978).
6. Diagnosis
Numerous techniques are used to detect rotaviruses in the faeces of foals. The initial discovery of rotaviruses in foals was by EM examination of faecal samples, but this requires specialised equipment and expertise. The diagnostic sensitivity of EM is low, with the lower threshold for detection exceeding 107 viral particles per ml of faeces (McIntosh, 1996).
Virus isolation in cell culture was initially challenging until the discovery that proteolytic enzymes, such as trypsin, enhance viral infectivity, replication and cell-to-cell spread. Prior to this, serial passage of equine rotaviruses in cell culture was not possible. The first equine rotavirus to be serially cultured in cells was from a UK foal faecal sample in 1981, using MA-104 cells (Imagawa et al., 1981).
Polyacrylamide gel electrophoresis has been used to detect RNA in faeces, with the advantage that the electrophoretic migration pattern of the 11 segments of double stranded RNA can be used to distinguish between groups of rotaviruses, as well as different strains. In a typical equine group A rotavirus pattern (Fig. 2), gene segments 3 and 4 migrate close together, and segments 7, 8, and 9 form a well spaced triplet (Hardy et al., 1991, Ohta et al., 1990, Snodgrass and Browning, 1991).
The development of enzyme-linked immunosorbent assays (ELISA) to detect rotaviruses in faeces has superseded the use of EM due to the greatly improved sensitivity and the accessibility of these techniques for routine diagnostic laboratories. Many immunoassays are based on the VP6 protein of the intermediate capsid. This protein is the most abundant rotaviral protein, is present in particles that have lost the outer capsid and is highly conserved in group A rotaviruses from a number of species. This is advantageous, as commercial assays developed for detection of rotaviruses in humans can also be used to detect equine rotaviruses. There are a number of rapid antigen detection kits, based on immunochromatography or latex agglutination, for detection of rotavirus in humans that are rapid, simple and can be performed on the farm. Some of these kits have been evaluated for use in horses and, while the sensitivity of rapid antigen detection kits is not as high as that of ELISAs, these kits can be useful for veterinarians in the field (Dwyer et al., 1988, Imagawa et al., 1989, Nemoto et al., 2010a).
Reverse transcription polymerase chain reaction (RT-PCR) assays can be used to detect rotaviruses, but are more frequently used in a research setting for G and P genotyping of rotaviruses, thus providing information on the molecular epidemiology of outbreaks (Fukai et al., 2006, Garaicoechea et al., 2011, Gentsch et al., 1992, Gouvea et al., 1994, Tsunemitsu et al., 2001).
An alternative, sensitive and rapid molecular detection method is reverse transcription loop-mediated isothermal amplification (RT-LAMP) targeting the VP4 gene, in particular the P[12] genotype. This method has the advantage that specialist equipment, such as thermocyclers, and post-PCR processing, such as electrophoresis, are not required, making it more accessible to diagnostic laboratories (Nemoto et al., 2010b).
More recently, real time RT-PCR assays have become commercially available in the USA for rapid, sensitive, quantitative detection of rotaviruses in faecal samples (Slovis et al., 2010).
7. Immunity and vaccination
Production of an effective vaccine to control rotaviral diarrhoea in young animals remains a challenge. Live attenuated vaccines delivered orally to the neonate potentially stimulate a local IgA response, but the balance between attenuation and adequate immunogenicity is difficult to achieve. In addition, live vaccines are often inactivated by maternally derived immunoglobulins when administered to neonates. It is because of these difficulties that prevention of equine rotaviral diarrhoea is currently dependent on inactivated, parenterally delivered vaccines administered to the dam to enhance levels of colostral and lactogenic immunity.
There are three licensed inactivated equine rotavirus vaccines currently in use. These vaccines were developed in the USA, Japan and Argentina. The first two of these are monovalent, one using the G3AP[12] strain H-2, the other using the G3BP[12] strain HO-5, and have been commercially available since 1998 (Fort Dodge Animal Health, USA) and 2001 (Nisseiken Co., Ltd., Japan), respectively (Imagawa et al., 1998, Imagawa et al., 2005, Powell et al., 1997). The third vaccine is a trivalent vaccine that contains the equine rotavirus H-2 strain, the simian rotavirus G3P[2] strain SA11 and the bovine rotavirus G6P[1] strain NCDV Lincoln, and has been in use in Argentina since 1996 (Barrandeguy et al., 1998, Garaicoechea et al., 2011). All three vaccines have been shown to significantly increase concentrations of circulating serum neutralising antibodies in mares and foals (Barrandeguy et al., 1998, Imagawa et al., 2005, Powell et al., 1997). However, experimental infection studies and field studies have shown that foals can acquire rotavirus infection despite having substantial amounts of circulating antibody (Conner and Darlington, 1980, Higgins et al., 1987). Results from field vaccination studies have been equivocal, with some studies reporting a reduction in the incidence and severity of diarrhoea and the shedding of virus in faeces (Barrandeguy et al., 1998, Imagawa et al., 2005), while another study found no significant reduction in the incidence of diarrhoea (Powell et al., 1997). However, as foals from vaccinated mares in all of these studies were still affected by rotavirus diarrhoea, at best these vaccines can only be considered partially protective.
Studies conducted in calves and lambs have shown that, while colostral antibody absorbed prior to gut closure may have a limited protective effect against rotaviral diarrhoea, maximal protection is achieved by the continued presence of neutralising antibodies in ingested milk, indicating that maximal protection from dam vaccination depends on induction of lactogenic immunity (Fahey et al., 1981, Parreno et al., 2010, Saif et al., 1983, Snodgrass et al., 1980). It is not clear from studies reported to date how effective any of the commercial vaccines are in inducing lactogenic immunity in mares.
An ideal rotavirus vaccine would provide heterotypic immunity against a wide variety of rotavirus serotypes. Experimental studies on specific pathogen free foals infected with G3 rotavirus at 10 weeks of age found that although a serotype specific antibody response was detectable at day 6 after infection, a heterotypic response peaked later, at 32 days after infection (Browning et al., 1991b). This suggests that, while heterotypic immunity is possible with a monovalent vaccine, it is likely to be delayed in onset compared to homotypic immunity. Currently, there is a multivalent vaccine available that aims to induce heterotypic immunity, however, of all the G genotypes in group A rotaviruses, the two G types most prevalent in horses, G3 and G14, are also the most genetically similar (Browning et al., 1991d), which raises questions about the benefit of using a multivalent vaccine in horses. Furthermore, studies on vaccination of dams with monovalent inactivated rotavirus vaccines have shown that the response generated is typically pan-serotypic, generating neutralising antibodies against rotavirus serotypes that are unlikely to have infected the dam previously (Browning et al., 1991b, Snodgrass et al., 1991).
Further evidence for this was found in a more recent study in which sera from mares vaccinated with a monovalent G3BP[12] vaccine were shown to have increased concentrations of virus-neutralising antibodies to both homologous G3BP[12] rotavirus strains and heterologous G14P[12] strains, although the increase in heterologous neutralising antibodies was less than the increase in homologous antibodies (Nemoto et al., 2012).
8. Disinfection
Environmental contamination is a major route of rotavirus transmission. While the minimal dose required to infect naïve foals and the titres of rotavirus excreted in foal faeces have not been reported, studies in other species suggest the faeces from one infected foal can rapidly contribute to infection of many in-contact susceptible foals. To further compound the problem of environmental contamination, rotaviruses are quite resilient outside the host, surviving for 9 months in faeces at room temperature and an hour at 60 °C (Woode, 1978).
In addition, rotaviruses are also resistant to a number of antiseptics and disinfectants. It is beyond the scope of this paper to review all products available globally, so those products commonly used in veterinary practice are considered below. The majority of disinfectant studies have been performed on human and simian rotaviruses in conditions containing much less organic matter than a typical equine environment would contain. Some studies conflict with others on efficacy, so when interpreting disinfectant efficacy data it is important to consider the type of surface disinfected, the concentration and contact time, the diluents used (hard or distilled water), the type of rotavirus used (field strain or cell culture adapted strain) and the effect of organic matter and how this was tested (faeces/intestinal contents, foetal bovine serum or tryptose phosphate broth).
For hand disinfection, chlorhexidine alone is ineffective. The efficacy of 1.5% chlorhexidine is improved by addition of 15% cetrimide, but not in the presence of organic matter (Sattar et al., 1983). Preparations containing alcohol are generally more effective disinfectants, although the type of alcohol and the concentration is important. Methanol is less active than either ethanol or isopropanol (Kurtz et al., 1980). Solutions of 70% ethanol or isopropanol provide effective disinfection in the presence of organic matter, while 35% solutions do not (Springthorpe et al., 1986). A combination of 1.5% chlorhexidine and 15% cetrimide in 70% ethanol is effective, even in the presence of organic matter (Sattar et al., 1983, Springthorpe et al., 1986). Topical application of a 10% povidone iodine preparation is also efficacious in the presence of organic matter (Sattar et al., 1983, Springthorpe et al., 1986).
A variety of disinfectant preparations have been used to disinfect equine facilities. Although sodium hypochlorite is commonly used because of its wide spectrum of action against bacteria and viruses, including spores of Clostridium spp., it is readily inactivated by organic matter and, therefore, ineffective against rotaviruses (Lloyd-Evans et al., 1986, Snodgrass and Herring, 1977, Springthorpe et al., 1986). Similarly, quaternary ammonium compounds, such as ammonium chloride, are also ineffective against rotaviruses (Springthorpe et al., 1986). The majority of phenolic compounds were ineffective in inactivating human rotaviruses in a study of the efficacy of commercial disinfectants. One product containing o-phenylphenol, o-benzyl-chlorophenol and p-tertiary-amyl phenol had a high rate of efficacy in the presence of high loads of organic matter, but only when tested on viral suspensions. This same product was not effective on a contaminated inanimate surface (Lloyd-Evans et al., 1986, Springthorpe et al., 1986).
Other commonly used disinfectants such as glutaraldehyde-based disinfectants and hydrogen peroxide have also been tested against rotavirus. A 2% glutaraldehyde solution is effective for sterilisation of instruments, but not appropriate for environmental or topical disinfection (Sattar et al., 1983, Springthorpe et al., 1986). Hydrogen peroxide at 0.3% has been shown to be ineffective in inactivating human rotavirus on a faecally contaminated inanimate surface, while a 6% hydrogen peroxide −0.85% phosphoric acid preparation inactivated a cell culture adapted simian rotavirus, but was not tested in the presence of organic matter (Ojeh et al., 1995). A newer disinfectant compound, potassium peroxymonosulfate, is commonly used in the field, but published data to support its use against rotaviruses is not available.
The resistance of rotaviruses to environmental factors and disinfectants has important implications in the field for decontamination of hands, equipment and the environment. Disinfection of some environments, such as paddocks and yards, is not achievable. When cleaning stables, removal and disposal of stable bedding requires careful consideration, as well as the potential generation of aerosols in the process. Once the stable is emptied, it is advisable to remove as much organic matter as possible with a detergent, followed by thorough rinsing and drying, prior to the application of a disinfectant.
9. Future directions
The equine rotavirus vaccines currently in use may play a role in reducing the incidence and severity of rotaviral diarrhoea, but outbreaks still occur on properties that vaccinate. It is known that local mucosal immunity is more important than systemic immunity for protection against gastrointestinal viral diseases. While the current equine rotavirus vaccines stimulate high serum antibody levels, further investigation of the antibody levels in milk in vaccinated mares and the correlation between these and protection in foals is needed. If these studies revealed adequate IgA levels in milk, then research into other aspects of foal susceptibility needs to be conducted. These areas may include the use of quantitative PCR to investigate if there is a correlation between faecal pathogen load and development of clinical disease, and the effect of vaccination on faecal shedding by infected foals and their dams. The role of cell mediated immunity in foal susceptibility to rotaviruses also remains to be explored.
Recombinant sub-unit and vectored vaccines are being explored in other domestic animal species as alternatives to live and attenuated whole virus vaccines. These include the use of plants to express immunogenic antigens, such as a subunit vaccine incorporating Vp8*and Brucella spp. lumazine synthase (BLS), with the BLS acting as an antigen delivery system (Bellido et al., 2009, Garaicoechea et al., 2011).
Other areas of vaccine development that could be explored are the effect of including both G3 and G14 rotavirus antigens in a vaccine, subunit vaccines containing the P[12] VP4 or the NSP4 enterotoxin and new targets for cell mediated immune responses that may be identified with the increase in full genome sequencing of rotaviruses.
There appears to be considerable scope for the future development and application of vectored vaccines to induce active immunity in neonates and thus improve control of this important equine pathogen.
Conflict of interest statement
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Baldwin J.L., Cooper W.L., Higgins W.P. An outbreak of rotavirus-associated disease in foals on a large horse breeding farm. Proceedings of the 36th Annual Convention of the American Association of Equine Practitioners; Lexington, Kentucky, 2–5 December, 1990; 1990. p. 325. [Google Scholar]
- Barrandeguy M., Parreño V., Lagos Marmol M., Pont Lezica F., Rivas C., Valle C., Fernandez F. Prevention of rotavirus diarrhoea in foals by parenteral vaccination of the mares: field trial. Dev. Biol. Stand. 1998;92:253–257. [PubMed] [Google Scholar]
- Beau I., Cotte-Laffitte J., Geniteau-Legendre M., Estes M.K., Servin A.L. An NSP4-dependant mechanism by which rotavirus impairs lactase enzymatic activity in brush border of human enterocyte-like Caco-2 cells. Cell. Microbiol. 2007;9:2254–2266. doi: 10.1111/j.1462-5822.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- Bellido D., Craig P.O., Mozgovoj M.V., Gonzalez D.D., Wigdorovitz A., Goldbaum F.A., Santos M.J.D. Brucella spp. lumazine synthase as a bovine rotavirus antigen delivery system. Vaccine. 2009;27:136–145. doi: 10.1016/j.vaccine.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Bishop R.F., Davidson G.P. Virus-particles in epithelial-cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;2:1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Browning G.F., Chalmers R.M., Fitzgerald T.A., Snodgrass D.R. Serological and genomic characterization of L338, a novel equine group A rotavirus G serotype. J. Gen. Virol. 1991;72:1059–1064. doi: 10.1099/0022-1317-72-5-1059. [DOI] [PubMed] [Google Scholar]
- Browning G.F., Chalmers R.M., Sale C.S.H., Fitzgerald T.A., Snodgrass D.R. Homotypic and heterotypic serum and milk antibody to rotavirus in normal, infected and vaccinated horses. Vet. Microbiol. 1991;27:231–244. doi: 10.1016/0378-1135(91)90150-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G.F., Chalmers R.M., Snodgrass D.R., Batt R.M., Hart C.A., Ormarod S.E., Leadon D., Stoneham S.J., Rossdale P.D. The prevalence of enteric pathogens in diarrheic thoroughbred foals in Britain and Ireland. Equine Vet. J. 1991;23:405–409. doi: 10.1111/j.2042-3306.1991.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G.F., Fitzgerald T.A., Chalmers R.M., Snodgrass D.R. A novel group A rotavirus G serotype: serological and genomic characterization of equine isolate F123. J. Clin. Microbiol. 1991;29:2043–2046. doi: 10.1128/jcm.29.9.2043-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G.F., Chalmers R.M., Fitzgerald T.A., Corley K.T.T., Campbell I., Snodgrass D.R. Rotavirus serotype G3 predominates in horses. J. Clin. Microbiol. 1992;30:59–62. doi: 10.1128/jcm.30.1.59-62.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G.F., Chalmers R.M., Fitzgerald T.A., Snodgrass D.R. Evidence for two serotype G3 subtypes among equine rotaviruses. J. Clin. Microbiol. 1992;30:485–491. doi: 10.1128/jcm.30.2.485-491.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G.F., Begg A.P. Prevalence of G and P serotypes among equine rotaviruses in the faeces of diarrhoeic foals. Arch. Virol. 1996;141:1077–1089. doi: 10.1007/BF01718611. [DOI] [PubMed] [Google Scholar]
- Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009) Arch. Virol. 2010;155:133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlet M., Reggeti F., Pina C.I., Liprandi F. Equine rotaviruses with G14 serotype specificity circulate among venezuelan horses. J. Clin. Microbiol. 1994;32:2609–2612. doi: 10.1128/jcm.32.10.2609-2612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlet M., Isa P., Conner M.E., Liprandi F. Antigenic and molecular analyses reveal that the equine rotavirus strain H-1 is closely related to porcine, but not equine, rotaviruses: Interspecies transmission from pigs to horses? Virus Genes. 2001;22:5–20. doi: 10.1023/a:1008175716816. [DOI] [PubMed] [Google Scholar]
- Ciarlet M., Estes M.K. Encyclopedia of Environmental Microbiology. John Wiley & Sons, Inc.; New York: 2003. Rotaviruses; pp. 2753–2773. [Google Scholar]
- Collins P.J., Cullinane A., Martella V., O'Shea H. Molecular characterization of equine rotavirus in Ireland. J. Clin. Microbiol. 2008;46:3346–3354. doi: 10.1128/JCM.00995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner M.E., Darlington R.W. Rotavirus infection in foals. Am. J. Vet. Res. 1980;41:1699–1703. [PubMed] [Google Scholar]
- Dickson J., Smith V.W., Coackley W., McKean P., Adams P.S. Rotavirus infection of foals. Aust. Vet. J. 1979;55:207–208. doi: 10.1111/j.1751-0813.1979.tb15293.x. [DOI] [PubMed] [Google Scholar]
- Durham P.J.K., Stevenson B.J., Farquharson B.C. Rotavirus and coronavirus associated diarrhoea in domestic animals. N. Z. Vet. J. 1979;27:30–32. doi: 10.1080/00480169.1979.34595. [DOI] [PubMed] [Google Scholar]
- Dwyer R.M., Powell D.G., Osborne M., Roberts A.W. Evaluation of two commercially available kits for the detection of rotavirus in faeces of healthy and diarrhoeic foals. Equine Vet. J. 1988;20:60. [Google Scholar]
- Dwyer R.M., Powell D.G., Roberts W., Donahue M., Lyons E.T., Osborne M., Woode G. A study of the etiology and control of infectious diarrhea among foals in central Kentucky. Proceedings of the 36th Annual Convention of the American Association of Equine Practitioners; Lexington, Kentucky, 2–5 December 1990; 1990. pp. 337–355. [Google Scholar]
- Dwyer R.M. Equine rotavirus. In: Sellon D.C., Long M.T., editors. Equine Infectious Diseases. W.B. Saunders; Saint Louis: 2007. pp. 181–183. [Google Scholar]
- Dyall-Smith M.L., Lazdins I., Tregear G.W., Holmes I.H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc. Natl. Acad. Sci. U.S.A. 1986;83:3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn W., Huan-Chun C. Serological survey of the prevalence of rotavirus antibodies in horses. Tierarztl. Umsch. 1987;42:22–23. [Google Scholar]
- Elschner M., Schrader C., Hotzel H., Prudlo J., Sachse K., Eichhorn W., Herbst W., Otto P. Isolation and molecular characterisation of equine rotaviruses from Germany. Vet. Microbiol. 2005;105:123–129. doi: 10.1016/j.vetmic.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Eugster A.K., Whitford H.W., Mehr L.E. Concurrent rotavirus and salmonella infections in foals. J. Am. Vet. Med. Assoc. 1978;173:857–858. [PubMed] [Google Scholar]
- Fahey K.J., Snodgrass D.R., Campbell I., Dawson A.M., Burrells C. IgG1 antibody in milk protects lambs against rotavirus diarrhea. Vet. Immunol. Immunopathol. 1981;2:27–33. doi: 10.1016/0165-2427(81)90036-2. [DOI] [PubMed] [Google Scholar]
- Flewett T.H., Bryden A.S., Davies H., Woode G.N., Bridger J.C., Derrick J.M. Relation between viruses from acute gastroenteritis of children and newborn calves. Lancet. 1974;2:61–63. doi: 10.1016/s0140-6736(74)91631-6. [DOI] [PubMed] [Google Scholar]
- Flewett T.H., Bryden A.S., Davies H. Virus diarrhoea in foals and other animals. Vet. Rec. 1975;96:477. [PubMed] [Google Scholar]
- Fukai K., Saito T., Fukuda O., Hagiwara A., Inoue K., Sato M. Molecular characterisation of equine group A rotavirus, Nasuno, isolated in Tochigi prefecture, Japan. Vet. J. 2006;172:369–373. doi: 10.1016/j.tvjl.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Garaicoechea L., Miño S., Ciarlet M., Fernández F., Barrandeguy M., Parreño V. Molecular characterization of equine rotaviruses circulating in Argentinean foals during a 17-year surveillance period (1992–2008) Vet. Microbiol. 2011;148:150–160. doi: 10.1016/j.vetmic.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Gentsch J.R., Glass R.I., Woods P., Gouvea V., Gorziglia M., Flores J., Das B.K., Bhan M.K. Identification of group-A rotavirus gene-4 types by polymerase chain-reaction. J. Clin. Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Varghese V., Sinha M., Kobayashi N., Naik T.N. Evidence for interstate transmission and increase in prevalence of bovine group B rotavirus strains with a novel VP7 genotype among diarrhoeic calves in Eastern and Northern states of India. Epidemiol. Infect. 2007;135:1324–1330. doi: 10.1017/S0950268806007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Shintani T., Kobayashi N. Evidence for the porcine origin of equine rotavirus strain H-1. Vet. Microbiol. 2012;158:410–414. doi: 10.1016/j.vetmic.2012.02.037. [DOI] [PubMed] [Google Scholar]
- Goto H., Tsunemitsu H., Horimoto M., Shimizu K., Urasawa T., Furuya K., Urasawa S., Ohishi H., Ikemoto Y. A seroepizootiological study on rotavirus infection in horses. Bull. Equine Res. Inst. 1981;1981(18):129–135. [Google Scholar]
- Gouvea V., Glass R.I., Woods P., Taniguchi K., Clark H.F., Forrester B., Fang Z.Y. Polymerase chain-reaction amplification and typing of rotavirus nucleic-acid from stool specimens. J. Clin. Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Santos N., Docarmotimenetsky M. Identification of bovine and porcine rotavirus-G types by PCR. J. Clin. Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.Y., Estes M.K. Proteolytic enhancement of rotavirus infectivity – biologic mechanisms. Virology. 1980;101:432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Gulati B.R., Yadav R.R., Singh B.K. Epidemiological studies on equine rotavirus infection in foals of organized farms in India. Indian J. Anim. Sci. 2009;79:3–5. [Google Scholar]
- Halaihel N., Liévin V., Alvarado F., Vasseur M. Rotavirus infection impairs intestinal brush-border membrane Na+-solute cotransport activities in young rabbits. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G587–G596. doi: 10.1152/ajpgi.2000.279.3.G587. [DOI] [PubMed] [Google Scholar]
- Hardy M.E., Woode G.N., Xu Z., Williams J.D., Conner M.E., Dwyer R.M., Powell D.G. Analysis of serotypes and electropherotypes of equine rotaviruses isolated in the United States. J. Clin. Microbiol. 1991;29:889–893. doi: 10.1128/jcm.29.5.889-893.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M.E., Gorziglia M., Woode G.N. The outer capsid protein VP4 of equine rotavirus strain H-2 represents a unique VP4 type by amino-acid-sequence analysis. Virology. 1993;193:492–497. doi: 10.1006/viro.1993.1152. [DOI] [PubMed] [Google Scholar]
- Higgins W.P., Gillespie J.H., Schiff E.I., Pennow N.N., Tanneberger M.J. Infectivity and immunity studies in foals with cell culture-propagated equine rotaviruses. Equine Infectious Diseases V: Proceedings of the Fifth International Conference; Lexington, Kentucky, 7–10 October 1987; 1987. pp. 241–247. [Google Scholar]
- Hoshino Y., Wyatt R.G., Greenberg H.B., Kalica A.R., Flores J., Kapikian A.Z. Isolation and characterization of an equine rotavirus. J. Clin. Microbiol. 1983;18:585–591. doi: 10.1128/jcm.18.3.585-591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R.G., Greenberg H.B., Kalica A.R., Flores J., Kapikian A.Z. Isolation, propagation, and characterization of a second equine rotavirus serotype. Infect. Immun. 1983;41:1031–1037. doi: 10.1128/iai.41.3.1031-1037.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R.G., Greenberg H.B., Flores J., Kapikian A.Z. Serotypic similarity and diversity of rotavirus of mammalian and avian origin as studied by plaque-reduction neutralization. J. Infect. Dis. 1984;149:694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Imagawa H., Ando Y., Sugiura T., Wada R., Hirasawa K., Akiyama Y. Isolation of foal rotavirus in MA-104 cells. Bull. Equine Res. Inst. 1981;1981(18):119–128. [Google Scholar]
- Imagawa H., Wada R., Hirasawa K., Akiyama Y., Oda T. Isolation of equine rotavirus in cell-cultures from foals with diarrhea. Jpn. J. Vet. Sci. 1984;46:1–9. doi: 10.1292/jvms1939.46.1. [DOI] [PubMed] [Google Scholar]
- Imagawa H., Wada R., Kamada M., Kumanomido T., Fukunaga Y., Hirasawa K. Experimental infection of equine rotavirus in foals. Bull. Equine Res. Inst. 1984;1984(21):65–71. [Google Scholar]
- Imagawa H., Fukunaga Y., Kanemaru T., Kamada M. Detection of equine rotavirus in feces by latex agglutination. Bull. Equine Res. Inst. 1989;1989(26):47–52. [Google Scholar]
- Imagawa H., Tanaka T., Sekiguchi K., Fukunaga Y., Anzai T., Minamoto N., Kamada M. Electropherotypes, serotypes, and subgroups of equine rotaviruses isolated in Japan. Arch. Virol. 1993;131:169–176. doi: 10.1007/BF01379088. [DOI] [PubMed] [Google Scholar]
- Imagawa H., Ishida S.I., Uesugi S., Masanobu K., Fukunaga Y., Nakagomi O. Genetic analysis of equine rotavirus by RNA–RNA hybridization. J. Clin. Microbiol. 1994;32:2009–2012. doi: 10.1128/jcm.32.8.2009-2012.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa H., Wada R., Sugita S., Fukunaga Y. Passive immunity in foals of mares immunised with inactivated equine rotavirus vaccine. Equine Infectious Disease VIII: Proceedings of the Eighth International Conference; Dubai, 23–26 March 1998; 1998. pp. 201–205. [Google Scholar]
- Imagawa H., Kato T., Tsunemitsu H., Tanaka H., Sato S., Higuchi T. Field study of inactivated equine rotavirus vaccine. J. Equine Sci. 2005;16:35–44. [Google Scholar]
- Isa P., Snodgrass D.R. Serological and genomic characterization of equine rotavirus VP4 proteins identifies 3 different P-serotypes. Virology. 1994;201:364–372. doi: 10.1006/viro.1994.1302. [DOI] [PubMed] [Google Scholar]
- Isa P., Wood A.R., Netherwood T., Ciarlet M., Imagawa H., Snodgrass D.R. Survey of equine rotaviruses shows conservation of one P genotype in background of two G genotypes. Arch. Virol. 1996;141:1601–1612. doi: 10.1007/BF01718285. [DOI] [PubMed] [Google Scholar]
- Jourdan N., Brunet J.P., Sapin C., Blais A., Cotte-Laffitte J., Forestier F., Quero A.-M., Trugnan G., Servin A.L. Rotavirus infection reduces sucrase-isomaltase expression in human intestinal epithelial cells by perturbing protein targeting and organization of microvillar cytoskeleton. J. Virol. 1998;72:7228–7236. doi: 10.1128/jvi.72.9.7228-7236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A.R., Greenberg H.B., Wyatt R.G., Flores J., Sereno M.M., Kapikian A.Z., Chanock R.M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981;112:385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kanitz C.L. Identification of an equine rotavirus as a cause of neonatal foal diarrhoea. Proceedings of the 22nd annual convention of the American Association of Equine Practitioners; Dallas, TX, 28 November–1 December 1976; 1976. pp. 155–165. [Google Scholar]
- Kurtz J.B., Lee T.W., Parsons A.J. The action of alcohols on rotavirus, astrovirus and enterovirus. J. Hosp. Infect. 1980;1:321–325. doi: 10.1016/0195-6701(80)90008-0. [DOI] [PubMed] [Google Scholar]
- Labbé M., Charpilienne A., Crawford S.E., Estes M.K., Cohen J. Expression of rotavirus VP2 produces empty corelike particles. J. Virol. 1991;65:2946–2952. doi: 10.1128/jvi.65.6.2946-2952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans N., Springthorpe V.S., Sattar S.A. Chemical disinfection of human rotavirus-contaminated inanimate surfaces. J. Hyg. (Lond.) 1986;97:163–173. doi: 10.1017/s0022172400064445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren O., Peregrin A.T., Persson K., Kordasti S., Uhnoo I., Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- Malherbe H.H., Strickla M. Simian virus SA11 and related O agent. Arch. Gesamte Virusforsch. 1967;22:235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Ciarlet M., Heiman E., Arijs I., Delbeke T., McDonald S.M., Palombo E.A., Iturriza-Gómara M., Maes P., Patton J.T., Rahman M., Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J., Ciarlet M., McDonald S.M., Attoui H., Banyai K., Brister J.R., Buesa J., Esona M.D., Estes M.K., Gentsch J.R., Iturriza-Gomara M., Johne R., Kirkwood C.D., Martella V., Mertens P.P.C., Nakagomi O., Parreno V., Rahman M., Ruggeri F.M., Saif L.J., Santos N., Steyer A., Taniguchi K., Patton J.T., Desselberger U., Van Ranst M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG) Arch. Virol. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J., Mino S., Papp H., Potgieter C., Novo L., Heylen E., Zeller M., Garaicoechea L., Badaracco A., Lengyel G., Kisfali P., Cullinane A., Collins P.J., Ciarlet M., O'Shea H., Parreno V., Banyai K., Barrandeguy M., Van Ranst M. Complete molecular genome analyses of equine rotavirus A strains from different continents reveal several novel genotypes and a largely conserved genotype constellation. J. Gen. Virol. 2012;93:866–875. doi: 10.1099/vir.0.039255-0. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Otto P., Ciarlet M., Desselberger U., Van Ranst M., Johne R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012;157:1177–1182. doi: 10.1007/s00705-012-1273-3. [DOI] [PubMed] [Google Scholar]
- McIntosh K. Diagnostic virology. In: Fields B.N., Knipe D.M., Howley P.M., Chanock R.M., Melnick J.K., Monath T.P., editors. Fields Virology. Lippincott-Raven Publishers; Philadelphia: 1996. p. 407. [Google Scholar]
- Mebus C.A., Underdah N.R., Rhodes M.B. vol. 233. Research bulletin/Agricultural experiment station; University of Nebraska: 1969. pp. 1–16. (Calf Diarrhea (Scours): Reproduced With a Virus From a Field Outbreak). [Google Scholar]
- Medici K.C., Barry A.F., Alfieri A.F., Alfieri A.A. Porcine rotavirus groups A B, and C identified by polymerase chain reaction in a fecal sample collection with inconclusive results by polyacrylamide gel electrophoresis. J. Swine Health Prod. 2011;19:146–150. [Google Scholar]
- Miño S., Matthijnssens J., Badaracco A., Garaicoechea L., Zeller M., Heylen E., Van Ranst M., Barrandeguy M., Parreño V. Equine G3P[3] rotavirus strain E3198 related to simian RRV and feline/canine-like rotaviruses based on complete genome analyses. Vet. Microbiol. 2013;161:239–246. doi: 10.1016/j.vetmic.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Monini M., Biasin A., Valentini S., Cattoli G., Ruggeri F.M. Recurrent rotavirus diarrhoea outbreaks in a stud farm, in Italy. Vet. Microbiol. 2011;149:248–253. doi: 10.1016/j.vetmic.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Morris A.P., Scott J.K., Ball J.M., Zeng C.Q.-Y., O’Neal W.K., Estes M.K. NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- Nemoto M., Hata H., Higuchi T., Imagawa H., Yamanaka T., Niwa H., Bannai H., Tsujimura K., Kondo T., Matsumura T. Evaluation of rapid antigen detection kits for diagnosis of equine rotavirus infection. J. Vet. Med. Sci. 2010;72:1247–1250. doi: 10.1292/jvms.10-0064. [DOI] [PubMed] [Google Scholar]
- Nemoto M., Imagawa H., Tsujimura K., Yamanaka T., Kondo T., Matsumura T. Detection of equine rotavirus by reverse transcription loop-mediated isothermal amplification (RT-LAMP) J. Vet. Med. Sci. 2010;72:823–826. doi: 10.1292/jvms.09-0446. [DOI] [PubMed] [Google Scholar]
- Nemoto M., Tsunemitsu H., Imagawa H., Hata H., Higuchi T., Sato S., Orita Y., Sugita S., Bannai H., Tsujimura K., Yamanaka T., Kondo T., Matsumura T. Molecular characterization and analysis of equine rotavirus circulating in Japan from 2003 to 2008. Vet. Microbiol. 2011;152:67–73. doi: 10.1016/j.vetmic.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Nemoto M., Tsunemitsu H., Murase H., Nambo Y., Sato S., Orita Y., Imagawa H., Bannai H., Tsujimura K., Yamanaka T., Matsumura T., Kondo T. Antibody response in vaccinated pregnant mares to recent G3BP 12 and G14P 12 equine rotaviruses. Acta Vet. Scand. 2012;54(1):63–67. doi: 10.1186/1751-0147-54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherwood T., Wood J.L.N., Townsend H.G.G., Mumford J.A., Chanter N. Foal diarrhoea between 1991 and 1994 in the United Kingdom associated with Clostridium perfringens, rotavirus, Strongyloides westeri and Cryptosporidium spp. Epidemiol. Infect. 1996;117:375–383. doi: 10.1017/s0950268800001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.F.E., Brown F., Bridger J.C., Woode G.N. Characterization of a rotavirus. Nature. 1975;258:631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Ntafis V., Fragkiadaki E., Xylouri E., Omirou A., Lavazza A., Martella V. Rotavirus-associated diarrhoea in foals in Greece. Vet. Microbiol. 2010;144:461–465. doi: 10.1016/j.vetmic.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Offit P.A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J. Virol. 1986;57:376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta C., Hoshi A., Goto H., Tsunoda N., Tagami M., Akita H. Epizootiological and virological studies of foal diarrhea associated with serotype 3 rotavirus. Jpn. J. Vet. Sci. 1990;52:1049–1056. doi: 10.1292/jvms1939.52.1049. [DOI] [PubMed] [Google Scholar]
- Ojeh C.K., Cusack T.M., Yolken R.H. Evaluation of the effects of disinfectants on rotavirus RNA and infectivity by the polymerase chain reaction and cell-culture methods. Mol. Cell. Probes. 1995;9:341–346. doi: 10.1016/s0890-8508(95)91652-0. [DOI] [PubMed] [Google Scholar]
- Park S.I., Jeong Y.J., Kim H.J., Park J.G., Kang S.Y., Woo S.K., Kim C.H., Jung C.H., Kang M.I., Cho K.O. Genetically diverse group C rotaviruses cause sporadic infection in Korean calves. J. Vet. Med. Sci. 2011;73:479–482. doi: 10.1292/jvms.10-0280. [DOI] [PubMed] [Google Scholar]
- Parreno V., Marcoppido G., Vega C., Garaicoechea L., Rodriguez D., Saif L., Fernandez F. Milk supplemented with immune colostrum: protection against rotavirus diarrhea and modulatory effect on the systemic and mucosal antibody responses in calves experimentally challenged with bovine rotavirus. Vet. Immunol. Immunopathol. 2010;136:12–27. doi: 10.1016/j.vetimm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Payment P., Morin E. Minimal infective dose of the OSU strain of porcine rotavirus. Arch. Virol. 1990;112:277–282. doi: 10.1007/BF01323172. [DOI] [PubMed] [Google Scholar]
- Pearson N.J., Fulton R.W., Issel C.J., Springer W.T. Prevalence of rotavirus antibody in chickens and horses in Louisiana, USA. Vet. Rec. 1982;110:58–59. doi: 10.1136/vr.110.3.58. [DOI] [PubMed] [Google Scholar]
- Powell D.G., Dwyer R.M., Traub-Dargatz J.L., Fulker R.H., Whalen J.W., Srinivasappa J., Acree W.M., Chu H.J. Field study of the safety, immunogenicity, and efficacy of an inactivated equine rotavirus vaccine. J. Am. Vet. Med. Assoc. 1997;211:193–198. [PubMed] [Google Scholar]
- Puyalto-Moussu C., Taouji S. 28eme journee de la recherche equine 27 fevrier 2002. Les Haras Nationaux Direction du Developpement; Paris: 2002. Epidemiology and prophylaxis of neonatal diarrhoea in the foal - preliminary results of a study carried out in Normandy; pp. 25–33. [Google Scholar]
- Rodger S.M., Schnagl R.D., Holmes I.H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J. Virol. 1975;16:1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S.M., Holmes I.H., Studdert M.J. Characteristics of the genomes of equine rotaviruses. Vet. Microbiol. 1980;5:243–248. [Google Scholar]
- Rothnagel R., Zeng Q.Y., Estes M.K., Chiu W., Prasad B.V.V. 3-D structural studies on baculovirus-expressed rotavirus sub-assemblies. Biophys. J. 1994;66 pp A134–A134. [Google Scholar]
- Saif L.J., Redman D.R., Smith K.L., Theil K.W. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or non-immunized cows. Infect. Immun. 1983;41:1118–1131. doi: 10.1128/iai.41.3.1118-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S.A., Raphael R.A., Lochnan H., Springthorpe V.S. Rotavirus inactivation by chemical disinfectants and antiseptics used in hospitals. Can. J. Microbiol. 1983;29:1464–1469. doi: 10.1139/m83-225. [DOI] [PubMed] [Google Scholar]
- Schroeder B.A., Kalmakoff J., Holdaway D., Todd B.A. The isolation of rotavirus from calves, foals, dogs and cats in New Zealand. N. Z. Vet. J. 1983;31:114–116. doi: 10.1080/00480169.1983.34988. [DOI] [PubMed] [Google Scholar]
- Slovis N.M., Elam J., Estrada M., Thao M.F., Leutenegger C.M. Comprehensive analysis of infectious agents associated with diarrhea in foals in Central Kentucky. Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners; Baltimore, MD, 4–8 December 2010; 2010. pp. 262–266. [Google Scholar]
- Snodgrass D.R., Herring J.A. Action of disinfectants on lamb rotavirus. Vet. Rec. 1977;101 doi: 10.1136/vr.101.4.81-a. pp. 81–81. [DOI] [PubMed] [Google Scholar]
- Snodgrass D.R., Fahey K.J., Wells P.W., Campbell I., Whitelaw A. Passive immunity in calf rotavirus infections: maternal vaccination increases and prolongs immunoglobulin G1 antibody secretion in milk. Infect. Immun. 1980;28:344–349. doi: 10.1128/iai.28.2.344-349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D.R., Browning G.F. Characterisation of equine rotavirus. Equine Infectious Diseases VI: Proceedings of the Sixth International Conference; Cambridge, England, 7–11 July 1991.; 1991. pp. 219–223. [Google Scholar]
- Snodgrass D.R., Fitzgerald T.A., Campbell I., Browning G.F., Scott F.M.M., Hoshino Y., Davies R.C. Homotypic and heterotypic serological responses to rotavirus neutralization epitopes in immunologically naive and experienced animals. J. Clin. Microbiol. 1991;29:2668–2672. doi: 10.1128/jcm.29.11.2668-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springthorpe V.S., Grenier J.L., Lloydevans N., Sattar S.A. Chemical disinfection of human rotaviruses – efficacy of commercially-available products in suspension tests. J. Hyg. (Lond.) 1986;97:139–161. doi: 10.1017/s0022172400064433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland K.L., Lenihan P., Oconnor M.G., Condon J.C. Diarrhea in foals associated with rotavirus. Vet. Rec. 1982;111 doi: 10.1136/vr.111.18.421. pp. 421–421. [DOI] [PubMed] [Google Scholar]
- Studdert M.J., Mason R.W., Patten B.E. Rotavirus diarrhea of foals. Aust. Vet. J. 1978;54:363–364. doi: 10.1111/j.1751-0813.1978.tb02499.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S. Species-specificity and interspecies relatedness in VP4 genotypes demonstrated by VP4 sequence-analysis of equine, feline, and canine rotavirus strains. Virology. 1994;200:390–400. doi: 10.1006/viro.1994.1203. [DOI] [PubMed] [Google Scholar]
- Theil K.W., Grooms D.L., McCloskey C.M., Redman D.R. Group-B rotavirus associated with an outbreak of neonatal lamb diarrhea. J. Vet. Diagn. Invest. 1995;7:148–150. doi: 10.1177/104063879500700124. [DOI] [PubMed] [Google Scholar]
- Tsunemitsu H., Imagawa H., Togo M., Shouji T., Kawashima K., Horino R., Imai K., Nishimori T., Takagi M., Higuchi T. Predominance of G3B and G14 equine group A rotaviruses of a single VP4 serotype in Japan. Arch. Virol. 2001;146:1949–1962. doi: 10.1007/s007050170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Walker M. Isolation of rotavirus from foals with diarrhoea. Aust. J. Exp. Biol. Med. Sci. 1978;56:453–457. doi: 10.1038/icb.1978.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Makin T., Smith M., Krautil F. Enteritis in foals induced by rotavirus and enterotoxigenic Escherichia coli. Aust. Vet. J. 1982;58:20–23. doi: 10.1111/j.1751-0813.1982.tb00572.x. [DOI] [PubMed] [Google Scholar]
- van der Heide R., Koopmans M.P.G., Shekary N., Houwers D.J., van Duynhoven Y., van der Poel W.H.M. Molecular characterizations of human and animal group A rotaviruses in the Netherlands. J. Clin. Microbiol. 2005;43:669–675. doi: 10.1128/JCM.43.2.669-675.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada R., Imagawa H., Kamada M., Fukunaga Y., Kumanomido T. Pathological studies on foals experimentally infected with equine rotavirus. Bull. Equine Res. Inst. 1984;1984(21):72–80. [Google Scholar]
- Welch A.B., Thompson T.L. Physicochemical characterization of a neonatal calf diarrhea virus. Can. J. Comp. Med. 1973;37:295–301. [PMC free article] [PubMed] [Google Scholar]
- Woode G.N. Epizootiology of bovine rotavirus infection. Vet. Rec. 1978;103:44–46. doi: 10.1136/vr.103.3.44. [DOI] [PubMed] [Google Scholar]
- Woode G.N., Crouch C.F. Naturally occurring and experimentally induced rotaviral infections of domestic and laboratory animals. J. Am. Vet. Med. Assoc. 1978;173:522–526. [PubMed] [Google Scholar]


