Abstract
Monoclonal antibodies to porcine epidemic diarrhoea virus (PEDV) membrane protein M were prepared and used for the comparative assessment of three blocking ELISA variants to detect PEDV. The competitive blocking ELISA (CB-ELISA) format showed the highest sensitivity, allowing detection of 102.5 plaque-forming units of PEDV/ml in culture medium. Its specificity was verified by inclusion of control samples containing transmissible gastroenteritis virus (TGEV) and rotavirus A in each analysis. Eighty porcine field samples of faeces obtained from 38 herds affected with diarrhoea were examined, and PEDV was found in 15 (19%) samples from 6 (16%) herds. The suitability of the CB-ELISA for the screening herds in epizootiologic situations is discussed.
Keywords: Porcine epidemic diarrhoea virus (PEDV), Blocking ELISA, Viral gastroenteritis of swine, Monoclonal antibodies
1. Introduction
Along with transmissible gastroenteritis coronavirus (TGEV) and rotaviruses, the coronavirus causing porcine epidemic diarrhoea (PEDV) ranks among the economically most important viruses causing diarrhoea in pigs, particularly in piglets. In Europe, PEDV causes gastroenteritis in pigs of all ages with high morbidity but low mortality (Pensaert and DeBouck, 1978, Pensaert et al., 1981, Pritchard et al., 1999), whereas in Asia (Japan, Korea and China) mortality reaches high percentages (30–80%) in suckling piglets (Takahashi et al., 1983, Shibata et al., 2001). Three major structural proteins occur in coronaviruses: the glycoprotein S (Mr 150–220 K) found in the viral protrusions (corona), the nucleocapsid phosphoprotein N (Mr 45–57 K), and the membrane protein M (Mr 20–30 K) (Saif, 1993, Utiger et al., 1995).
Conventional diagnostic methods of porcine viral gastroenteritis are based on the detection of either antibodies or the virus. Before local immunity is actively established, the intestine of the piglet is protected against infection by maternal antibodies obtained through colostrum and, later on, mainly through milk. Serological examination allows assessing earlier contacts of sows with the virus, but even litters from seropositive sows are not protected against infection if the milk contains non-protective antibody levels. Therefore, information on a current epizootologic situation in a herd is best obtained by virus detection. Besides virus isolation, immunohistochemical and electron microscopic detection methods are often used (Guscetti et al., 1998, Kim et al., 1999, Rodák et al., 1999). As these techniques are labourious and time demanding, only few samples are usually examined. This holds also for highly sensitive and specific methods of molecular virology (PCR, RT-PCR) (Ishikawa et al., 1997, Kweon et al., 1997, Kim et al., 2000), although miniaturization and automation of e.g. real-time PCR methods are gradually reaching the veterinary diagnostic laboratory.
Of the ELISA variants the double antibody sandwich form of the assay (DAS), with specificity checked by blocking, has been described for PEDV detection (Callebaut et al., 1982; van Nieuwstadt and Zetstra, 1991, Carvajal et al., 1995). The objective of the present study was to verify modifications of the ELISA blocking method for the detection of PEDV in culture media and faecal samples, and to use the optimal one in routine examination of field samples.
2. Material and methods
2.1. Viral and control antigens
The reference strain of PEDV (CV-777) was propagated in Vero cells grown in minimum essential medium Eagle (MEM) in the presence of trypsin (Hofmann and Wyler, 1988). As soon as the cytopathic effect was fully developed, the virus was released from the cells by repeated freezing/thawing. After centrifugation (2000 × g for 15 min) of the culture medium, the pellet was resuspended in 1/100 of the original medium volume in phosphate buffer saline (PBS) as crude viral antigen (V-Ag). Purified V-Ag was prepared (Hofmann and Wyler, 1990) by ultracentrifugation in saccharose gradients (Beckman, Optima LE-80K Ultracentrifuge, USA) from the supernatant of the infectious culture medium (without cell debris). Similarly, crude control antigens (C-Ag) were prepared from non-infected cell lines. All antigens were kept at −80 °C before use. Culture media with defined virus concentrations (determined as plaque-forming units-PFU/ml medium) were used for determination of the sensitivity of the ELISA method by box titration.
V-Ag and C-Ag were prepared similarly by propagation of TGEV (collection of animal pathogenic microorganisms, Brno; strain CAPM V-344), and group A rotavirus (strain OSU and our own isolates) in the porcine kidney (PK-15) and monkey kidney (MA-104) cell lines, respectively.
To exclude potential nonspecificity due to cross-reactivity with other agents, crude V-Ag (PED, TGE, rota) and C-Ag were tested for specificity in all ELISA methods used. Purified PED V-Ag was used for immunisation of mice and for Western blot analysis.
2.2. Experimental infection
Two hysterectomy-derived, colostrum-deprived piglets kept in sterile box were fed sterile condensed milk containing 7% of fat. On the day 19 they were orally infected with 2.2 × 104 PFU PEDV strain CV-777. In one of the piglets, diarrhoea appeared 24 hpi and the animal died 48 hpi. The intestinal contents were collected for EM and ELISA virus detection. Small intestine samples were frozen in liquid nitrogen and kept at −80 °C before immunohistochemical examination. In the second piglet, diarrhoea occured 48 hpi, and serial faecal samples were collected during one week. Seven weeks post infection, the piglet was orally challenged with 1.1 × 105 PFU PEDV and sacrificed 12 days later by exsanguination under total anaesthesia. The titre of PEDV antibodies in the obtained serum (SwSpos.) was determined by indirect ELISA (51,200). Good results were obtained by comparative estimation of PEDV blocking activities of this serum and the swine PEDV sera kindly provided by Dr. Pensaert (Belgium) and Dr. Möstl (Austria). PEDV negative (SwSneg.) swine blood serum was obtained by exsanguination of the 21-day-old uninfected piglet. Both sera were diluted to various extents in the blocking ELISA methods. The immunoglobulin fraction was obtained from positive serum by ion exchange chromatography (SwAPEDV) and used as a binding antibody in the blocking ELISA methods.
2.3. Electron microscopic (EM) examination
Rota- and coronaviruses were detected by electron microscopic examination of samples of piglet faeces and culture media after negative staining with 2% ammonium molybdate solution in water, pH 7.0 (Šmíd et al., 1993).
2.4. Imunoperoxidase (IP) test
Slides with monolayers of infected (PEDV, TGEV, rotavirus A) cell lines were rinsed with PBS and fixed with acetone (15 min at 20 °C) before the cytopathic effect developed. Endogenous peroxidase was inhibited before examination (Li et al., 1987). Preparations with uninfected cells were prepared similarly. The presence of virus in infected cells was detected by monoclonal antibodies by direct and indirect IP tests. The reactions were read after 3–5 min incubation in a substrate solution containing chromogen AEC (3-amino 9-ethylcarbazole, Sigma).
2.5. SDS-PAGE and Western blot analysis
Purified PED V-Ag and a low molecular weight standard (LMW; Pharmacia, Sweden) were treated for 5 min at 100 °C in a reducing solution (sample buffer with 2-mercaptoethanol, Bio-Rad), and separated by discontinuous electrophoresis (Laemmli, 1970) in a 12% polyacrylamide gel (Bio-Rad Labs). After transfer to a nitrocellulose (NC) membrane (0.2 μ; Bio-Rad) and separation of the lanes, the strips were soaked in PBS containing 0.5% Tween 20 for 30 min. The NC membrane lane with LMW was stained with colloid gold (Moeremans et al., 1985), and lanes with V-Ag were used for Western blot (WB) analysis. The reactions were visualised by incubation with peroxidase conjugates to swine or mouse immunoglobulins and subsequently in a solution containing the chromogen DAB (3,3′diaminobenzidine, Fluka).
2.6. Monoclonal antibodies (mAb)
Inbred mice of the line BALB/c were repeatedly immunised by subcutaneous (s.c.) administration of purified V-Ag emulsified in complete or incomplete Freund's adjuvant. Three to five days before fusion, mice obtained intraperitoneal (i.p.) booster injections of PED V-Ag in PBS. Hybridomas were prepared according to Galfrè and Milstein (1981) by fusion of splenic lymphoid cells with cells of the myeloma line Sp2/0. Selected hybridomas with optimal results in indirect ELISA were cloned, checked by WB and IP tests and used for preparation of ascitic fluids. Monoclonal antibodies (mAb) purified from ascitic fluids by precipitation with ammonium sulphate and ion exchange chromatography (mAbPEDV) were stored at −18 °C in 50% glycerol or used for the preparation of peroxidase conjugates (HRPO-mAbPEDV).
2.7. Peroxidase conjugates
Rabbit antibodies to swine and mouse immunoglobulins (RASwIg, RAMoIg) or swine antibodies to mouse immunoglobulins (SwAMoIg) were purified from hyperimmune sera by affinity chromatography. These antibodies and monoclonal antibodies were conjugated with horseradish peroxidase (HRPO, type VI-A, Sigma) using the periodate method (Boorsma and Streefkerk, 1979). For IP, WB and ELISA tests, the stock conjugate solutions adjusted to 1 mg of immunoglobulin/ml were diluted 100× to 10,000× in PBS containing 0.05% Tween 20 and 0.5% lactalbumin hydrolysate (PBST-LAH).
2.8. Indirect ELISA
PEDV antibodies in swine and mouse sera, in culture media of hybridomas and in purified mAbPEDV preparations were assayed using the indirect ELISA method. PED V-Ag and C-Ag diluted 500× to 2000× with carbonate–bicarbonate buffer pH 9.6 (50 μl/well) were alternatingly adsorbed in vertical rows to the wells of microtitre plates (Nunc-Immunoplate, PolySorp, Denmark) during overnight incubation in a humid chamber. Optimal antigen dilutions were determined by box titration. For examination, the pairs of wells with V-Ag and C-Ag were incubated with diluted serum samples for 1 h at 37 °C. After another incubation with the conjugates to swine or mouse immunoglobulins, the reactions were visualised by incubation with chromogen TMB (3,3′,5,5′ tetramethyl-benzidine, Sigma) solution. After 15 min, the reaction was stopped by addition of 1 M H2SO4, and absorbances were measured spectrophotometrically at 450 nm. Control wells filled with the diluent without serum (blank) at the first incubation, or with control negative and positive serum were included in each examination. Samples showing a difference in optical density of at least 0.1 (after subtraction of absorbances of blank wells) between the wells with bound V-Ag and C-Ag were classified as positive.
2.9. Blocking ELISA
Sensitivity and specificity of three variants of a blocking ELISA were determined by box titration using faeces of an experimentally PEDV infected piglet 48 hpi. The wells of microtitre plates (Nunc-Immuno Plate, MaxiSorp, Denmark) were precoated with PEDV antibodies in carbonate–bicarbonate buffer, pH 9.6 (50 μl/well; 5 μg Ig/ml). During the first incubation (2 h at 37 °C), pairs of precoated wells were filled with 50 μl mixture of faeces with SwSneg./pos. PBST-LAH containing 0.5 M NaCl and 1 mg EDTA.Na2 /ml was used for sample dilution at the first incubation. After rinsing (4 times with PBST after every incubation), the second incubation (1 h at 37 °C) with 50 μl of detection antibodies (HRPO-mAbPEDV or HRPO-SwAMoIg) followed. Pairs of wells filled with the diluent (blank) or the mixtures of control V-Ag (PEDV, TGEV, rotavirus A) and SwSneg./pos. during the first incubation were included in each analysis. The samples were regarded as positive if the net absorbance (NA), i.e. the difference of average absorbance in the wells incubated with SwSneg./pos. was >0.1, and the reactions were blocked by >50% in the wells with SwSpos. Blocking percentages were determined using the formula: %B = 100 − [(A SwSpos. × 100):(A SwSneg.)]. The following variants of the blocking ELISA method were investigated.
2.9.1. Variant 1
Double antibody sandwich ELISA (DAS-ELISA). Binding antibody mAbPEDV was used. The first incubation with antigen test samples was performed for 2 h, the second incubation with the conjugate HRPO-mAbPEDV for 1 h.
2.9.2. Variant 2
Competitive blocking ELISA (CB-ELISA). Binding antibody SwAPEDV was used. After 1 h of incubation with the antigen test samples, the wells were supplemented with 50 μl diluent containing 4 μg mAbPEDV/ml, and the incubation continued for another hour. The second, 1 h incubation was done using the conjugate HRPO-SwAMoIg.
2.9.3. Variant 3
Double antibody sandwich ELISA (DAS-ELISA). Binding antibody SwAPEDV was used. The first incubation with the antigen test samples was performed for 2 h and the second 1 h incubation was conducted with the conjugate HRPO-mAbPEDV.
2.10. Field samples
In total, 80 faecal samples from piglets with diarrhoea on 38 farms were examined. Most samples were from piglets younger than 21 days. After delivery to the laboratory, they were diluted in 2–3 vol of Earle's medium, centrifuged (15 min, 3000 × g), and the supernatants examined by electron microscopy and blocking ELISA methods. Samples were kept at −80 °C before analysis.
3. Results
3.1. Monoclonal antibodies
After verification of the specificity of hybridomas producing mAbPEDV by WB analysis, ELISA and IP detection of PEDV antigen in infected and non-infected cells, three preparations (3C8/2H6; C2/D5; 2B7) were selected for further use. All were of the isotype IgG1 and reacted with the membrane protein M (Mr 20–30 K) in WB analysis (Fig. 1 ). ELISA titres of mAbPEDV solutions containing 4 mg Ig/ml ranged between 64,000 and 1,024,000. The mixtures of the same concentrations (w/v) of all three mAb (conjugated or unconjugated) were used in blocking ELISA examinations. Cross reactivity with other viral antigens could not be detected using any of the methods mentioned (Table 2 ; group of samples 1).
Fig. 1.
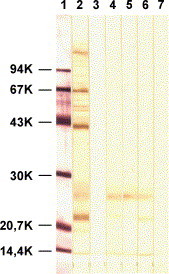
Western blot analysis of PEDV antibodies. After separation in 12% polyacrylamide gel, purified PEDV and a low molecular weight standard (LMW) were transferred to a nitrocellulose membrane. A part of the membrane with the LMW standard was stained with colloid gold (lane 1). The other lanes with PEDV were incubated with swine antiserum to PEDV (lane 2), mAbPEDV 3C8/2H6 (lane 4), C2/D5 (lane 5), 2B7 (lane 6), or with diluting solution alone (lanes 3 and 7). After incubation with peroxidase conjugates to swine (lanes 2 and 3) and mouse (lanes 4–7) immunoglobulins, the reaction was visualised by incubation in a solution containing the chromogen DAB.
Table 2.
Sensitivity and specificity of PEDV detection in control V-Ag samples and in field samples of faeces by the CB-ELISA method
| Group of samples | Sample tested | EM | Dilution of samples |
Absorbances in wells |
NA | %B | E | ||
|---|---|---|---|---|---|---|---|---|---|
| Ag/faeces | SwSneg./pos. | SwSneg. | SwSpos. | ||||||
| 1 | PED V-Ag | C+ | 40× | 40× | 1.822 | 0.070 | 1.752 | 96.2 | + |
| TGE V-Ag | C+ | 40× | 40× | 0.025 | 0.018 | 0.007 | 28.0 | − | |
| Rota V-Ag | R+ | 40× | 40× | 0.024 | 0.039 | −0.015 | −62.5 | − | |
| 2 | 676/2 | C+ | 2× | 40× | 0.503 | 0.050 | 0.453 | 90.1 | + |
| 716/2 | C− | 2× | 40× | 0.728 | 0.295 | 0.433 | 59.5 | + | |
| 726/3 | C− | 2× | 40× | 1.652 | 0.282 | 1.370 | 82.9 | + | |
| 3 | 708/3 | C− | 2× | 40× | 1.765 | 1.210 | 0.555 | 31.4 | − |
| 708/3 | C− | 2× | 20× | 1.671 | 0.506 | 1.165 | 69.7 | + | |
| 829/2 | C− | 2× | 40× | 2.104 | 1.147 | 0.957 | 45.5 | − | |
| 829/2 | C− | 2× | 20× | 1.792 | 0.267 | 1.525 | 85.1 | + | |
| 4 | 771/4 | C+ | 2× | 40× | 0.094 | 0.041 | 0.053 | 56.4 | − |
| 771/4 | C+ | 2× | 500× | 0.899 | 0.184 | 0.715 | 79.5 | + | |
| 837 | ND | 2× | 40× | 0.076 | 0.045 | 0.031 | 40.8 | − | |
| 837 | ND | 2× | 500× | 0.296 | 0.016 | 0.280 | 94.6 | + | |
| 5 | 833/2 | C− | 2× | 40× | 0.120 | 0.070 | 0.050 | 41.7 | − |
| 833/2 | C− | 2× | 500× | 0.038 | 0.047 | −0.009 | −23.7 | − | |
| 6 | 663/3 | C− | 2× | 40× | 0.090 | 0.085 | 0.005 | 5.6 | − |
| 668 | C− | 2× | 40× | 0.016 | 0.017 | −0.001 | −6.3 | − | |
Effect of SwSneg./pos. dilution on the values of net absorbance, % blocking and final evaluation. Dilutions of samples with positive values NA > 0.1 and %B > 50 are in bold; EM (+/−): positive and negative results of rotavirus (R) and coronavirus (C) detection obtained by electron microscopic examination; NA: net absorbance; %B: percentage of reaction blocking; E: evaluation of samples by CB-ELISA as PEDV positive (+) or negative (−); ND: not done; PED, TGE, Rota V-Ag: mean absorbance obtained by examination of crude viral antigens.
3.2. Imunoperoxidase (IP) test
Uninfected and infected cell lines (PEDV, TGEV, rotavirus A) and cryostat sections of the small intestine of the experimentally PEDV infected piglet were used for verification of mAbPEDV specificity by direct and indirect IP tests. Positive reactions in the form of red granules were visible in the cytoplasm of PEDV infected cells only. Uninfected control cells and TGEV- and rotavirus A-infected cells were always negative.
3.3. Comparison of blocking ELISA methods
The results obtained with the three variants of the blocking ELISA method were compared by means of box titrations in microtitre plate wells precoated with monoclonal or polyclonal PEDV antibodies. Mixtures containing faeces of an experimentally infected piglet collected 48 hpi and SwSneg. or SwSpos. at working dilutions of 1:2 to 1:128 or 1:10 to 1:640, respectively, were examined. The average absorbance, the NA and %B values of the reactions obtained with SwS dilutions 1:10 and 1:160 are shown in Table 1 . In variant 1 with binding mAbPEDV and detection HRPO-mAbPEDV antibodies, the faecal sample was positive only at the dilutions 1:2 and 1:8. In variant 3 with binding SwAPEDV and detection HRPO-mAbPEDV antibodies, a slightly higher sensitivity was detected. Optimal results were obtained using variant 2, with binding SwAPEDV and detection HRPO-SwAMoIg antibodies. The sample was tested positive (NA > 0.1; %B > 50) even at an antigen dilution of 1:128. Table 1 shows that at high PEDV concentrations and low SwS concentrations, the absorbance values were consistently high, although the %B qualified the result as negative. By contrast, low absorbance values were obtained at a low PEDV concentration, while the %B was positive. The CB-ELISA method using dilutions of faeces and SwSneg./pos. 1:2 and 1:40, respectively, was selected for routine examination of field samples. Under the selected conditions (NA > 0.1; %B > 50), sample evaluation was highly specific as established by examination of selected positive and negative samples in eight wells (n = 8). The calculated coefficients of variation CV% ranged from 3.2 to 5.7%. Only with negative samples giving very low absorbance values, CV% occasionally exceeded 10%.
Table 1.
Detection of PED virus in a faecal sample from an experimentally infected SPF piglet using three variants of monoclonal blocking ELISA methods
| ELISA variant | Dilution of faces | Results obtained with different dilutions of faeces and swine sera |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SwS dilution 10× |
SwS dilution 160× |
||||||||
| Neg. | Pos. | NA | %B | Neg. | Pos. | NA | %B | ||
| (1) DAS-ELISA mAbPEDVa HRPO-mAbPEDVb | 2× | 1.012 | 0.161 | 0.851 | 84.1 | 1.120 | 1.001 | 0.119 | 10.6 |
| 8× | 0.420 | 0.123 | 0.297 | 70.7 | 0.657 | 0.470 | 0.187 | 28.5 | |
| 32× | 0.190 | 0.119 | 0.071 | 37.4 | 0.203 | 0.183 | 0.020 | 9.9 | |
| 128× | 0.155 | 0.174 | −0.019 | −12.2 | 0.153 | 0.193 | −0.040 | −26.1 | |
| (2) CB-ELISA SwAPEDVa HRPO-SwAMoIgb | 2× | 1.531 | 0.094 | 1.437 | 93.3 | 1.382 | 1.012 | 0.370 | 26.8 |
| 8× | 0.762 | 0.066 | 0.696 | 91.3 | 1.382 | 0.582 | 0.800 | 57.9 | |
| 32× | 0.246 | 0.063 | 0.183 | 74.4 | 0.992 | 0.143 | 0.849 | 85.6 | |
| 128× | 0.126 | 0.068 | 0.058 | 46.0 | 0.250 | 0.112 | 0.138 | 55.2 | |
| (3) DAS-ELISA SwAPEDVa HRPO-mAbPEDVb | 2× | 1.131 | 0.171 | 0.960 | 84.9 | 1.068 | 0.868 | 0.200 | 18.7 |
| 8× | 0.340 | 0.120 | 0.220 | 64.7 | 0.890 | 0.484 | 0.405 | 45.5 | |
| 32× | 0.142 | 0.123 | 0.019 | 13.4 | 0.468 | 0.241 | 0.227 | 48.5 | |
| 128× | 0.157 | 0.121 | 0.037 | 22.9 | 0.247 | 0.171 | 0.076 | 30.8 | |
Pairs of microtitre plate wells precoated with PEDV binding antibodies were incubated with mixtures containing a faecal sample from a piglet 48 hpi diluted 1:2 to 1:128 and swine PEDV negative or positive serum diluted 1:10 and 1:160; NA: Net absorbance. Differences of mean absorbance in wells incubated with SwSneg.–SwSpos.; %B: reduction of absorbance in the wells incubated with SwSpos. in comparison with the wells incubated with SwSneg.; Bold letters indicate dilutions at which positive results were obtained (NA > 0.1 and %B > 50).
Binding antibodies.
Detection antibodies (conjugate).
3.4. CB-ELISA sensitivity determination
The culture medium from Vero cells infected with PEDV and containing 2.2 × 104 PFU/ml was analysed by box titration using the CB-ELISA. Mixtures of medium diluted 1:4 to 1:64 and porcine sera diluted 1:20 to 1:500 were examined. Positive NA and %B values were obtained at all dilutions of both medium and SwS (results not shown). The sensitivity of the CB-ELISA method was therefore estimated to exceed 102.5 PFU PEDV/ml.
3.5. Experimental infection
Serial faecal samples from a piglet experimentally infected with PEDV were examined using the CB-ELISA method. PEDV was detected on days 2–6 post infection, during 5 days after first signs of diarrhoea had appeared. In cryostat sections of the ileum of another piglet, which had died 48 hpi, PEDV antigen was detected in the cytoplasm of enterocytes by IP tests (not shown).
3.6. Field samples of faeces
Eighty faecal samples from 38 herds in which gastroenteritis had occurred were examined by the CB-ELISA method using routine dilutions of faeces (1:2) and SwS (1:40). The specificity of the CB-ELISA method was verified in each plate by examination of the crude V-Ag of PED, TGE and rotavirus A (Table 2; group of samples 1). The typical ELISA results obtained with some selected field samples are given in Table 2 (groups of samples 2–6). Positive values (NA > 0.1; %B > 50%) were found in seven samples (group 2). Other four samples with high absorbance were, because of the %B < 50, assessed as negative. At a lower SwS dilutions (1:20), these samples (group 3) were also positive. Interesting results were obtained with 12 samples showing low absorbance and %B values >40%. Considering the results shown in Table 1, we suspected that some of them could contain low concentrations of virus. When SwS were diluted 1:500, indeed 4 of these 12 tested samples were positive (group 4). Samples negative even at higher SwS dilutions are shown in group 5 and samples, which were evidently negative in basic dilutions in group 6. Altogether PEDV was detected in 15 samples (19%) from six herds (16%).
3.7. Comparison of electron microscopy and CB-ELISA examinations
The results obtained by EM and CB-ELISA was poorly correlated. By the EM examination, coronavirus was proved only in three faecal samples out of 15 that tested positive in the CB-ELISA. Conversely, in 65 samples, negative by the CB-ELISA method, coronavirus-like particles were detected in 9 (14%) cases.
4. Discussion
The most accurate information on the cause of gastroenteritis occurring in herds is obtained by detection of the causative agent. Therefore, detection methods are the necessary prerequisite for assessing the current epidemic situation in herds and for subsequent immunoprophylactic measures. Conventional methods (virus isolation, immunohistochemical detection of PEDV) are laborious and time consuming. Therefore, the objective of our study was to assess methods for the detection of viral agents. Blocking ELISA methods of PEDV detection in samples of piglet faeces in the form of double antibody sandwich ELISA) have been used previously. Polyclonal (Callebaut et al., 1982) or monoclonal (Carvajal et al., 1995) binding and detection antibodies, and standard dilution of SwSneg./pos. were employed. The sensitivity and specificity of these methods depended particularly on the quality of the antibodies. High specificity of PEDV detection is obtained particularly by the ELISA method using monoclonal antibodies. However, lower sensitivity of ELISA variants with conjugated mAb was detected in our experiments (Table 1). Therefore after the assessment of three variants, we selected the CB-ELISA based on the use of non-conjugated mAb as optimal. PEDV detection specificity reached by the CB-ELISA method was also proven by the negative results obtained by examinations of crude V-Ag samples of TGEV and rotavirus A (Table 2).
In faecal samples of germ-free piglet experimentally infected with CV-777 strain of PEDV, shedding of the virus was demonstrated by CB-ELISA from 2 to 6 dpi. This is in correlation with other findings (Callebaut et al., 1982, van Nieuwstadt and Zetstra, 1991). However, long lasting shedding of PEDV until 11 dpi was detected after experimental infection of conventional piglets (Carvajal et al., 1995).
We have found that some samples might be incorrectly evaluated by both the DAS and CB-ELISA analysis of mixtures with standard dilutions of faeces and SwSneg./pos. All samples with high absorbance values but low blocking percentages were taken as positive in repeated analyses under different conditions of sample or SwSneg./pos. dilution. Also some samples with originally low absorbance but high percentages of blocking were similarly evaluated as positive (Table 2). Determination of viral agents in faecal field samples by EM and blocking ELISA method examinations may be affected by several factors. The time of sample collection after the first symptoms of diarrhoea had emerged and the conditions of shipment are important. High fragility of coronaviruses in the contents of intestines was proven (Aynaud and Bottreau, 1984). Therefore, the finding of the characteristic peplomer structures of coronaviruses by EM is less frequent than the finding of “corona-like particles”, which are in fact cellular substructures. The low correlation between the results obtained by EM and CB-ELISA may be explained by that fact.
Because of its sensitivity and specificity, the CB-ELISA method is seen as most suitable for routine detection of PEDV gastroenteritis. Similar results were obtained in our studies with TGEV (unpublished data) and group A rotavirus detection (Rodák et al., 2004). The objective of our ongoing studies is the implementation of these methods to obtain information on the incidence of viral gastroenteritis in pig herds. The methods will allow subsequent confirmation of the effect of targeted immunoprophylactic measures, including immunisation of sows or prophylactic administration of antiviral antibodies to piglets (Menšík et al., 1978, Shibata et al., 2001), according to current epidemiological situation.
Acknowledgements
This work was supported by projects QF 4051 and MZE 0002716201 of the Ministry of Agriculture of the Czech Republic. The authors wish to thank Dr. Hofmann (Institute of Virology, University of Zurich, Switzerland) for kindly providing us with the reference strain of PEDV (CV-777) and the Vero cell line. Mrs. Farníková, Mrs. Mikulská, Mrs. Křipačová, Miss Hoydenová and Miss Bačínská are greatly acknowledged for their skilful technical assistance.
References
- Aynaud J.M., Bottreau E. Transmissible gastroenteritis of swine: stability of coronavirus in gastric and intestinal contents. Annu. Rech. Vet. 1984;15:359–364. [PubMed] [Google Scholar]
- Boorsma D.M., Streefkerk J.G. Periodate or glutaraldehyde for preparing peroxidase conjugates? J. Immunol. Methods. 1979;30:245–255. doi: 10.1016/0022-1759(79)90098-x. [DOI] [PubMed] [Google Scholar]
- Callebaut P., deBouck P., Pensaert M. Enzyme-linked immunosorbent assay for the detection of the coronavirus-like agent and its antibodies in pigs with porcine epidemic diarrhoea. Vet. Microbiol. 1982;7:295–306. doi: 10.1016/0378-1135(82)90009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal A., Lanza I., Diego R., Rubio P., Cármenes P. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhoea virus and its antibodies. J. Vet. Diagn. Invest. 1995;7:60–64. doi: 10.1177/104063879500700109. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73:3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Guscetti F., Bernasconi C., Tobler K., van Reeth K., Pospischil A., Ackermann M. Immunohistochemical detection of porcine epidemic diarrhoea virus compared to other methods. Clin. Diagn. Lab. Immunol. 1998;5:412–414. doi: 10.1128/cdli.5.3.412-414.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhoea in cell culture. J. Clin. Microbiol. 1988;26:2235–2239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Enzyme-linked immunosorbent assay for the detection of porcine epidemic diarrhoea coronavirus antibodies in swine sera. Vet. Microbiol. 1990;21:263–273. doi: 10.1016/0378-1135(90)90037-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Sekiguchi H., Ogino T., Suzuki S. Direct and rapid detection of porcine epidemic diarrhoea virus by RT-PCR. J. Virol. Methods. 1997;69:191–195. doi: 10.1016/s0166-0934(97)00157-2. [DOI] [PubMed] [Google Scholar]
- Kim O., Chae C., Kweon C. Monoclonal antibody-based immunohistochemical detection of porcine epidemic diarrhoea virus antigen in formalin-fixed, paraffin-embedded intestinal tissues. J. Vet. Diagn. Invest. 1999;11:458–462. doi: 10.1177/104063879901100512. [DOI] [PubMed] [Google Scholar]
- Kim O., Choi C., Kim B., Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT-PCR. Vet. Rec. 2000;146:637–640. doi: 10.1136/vr.146.22.637. [DOI] [PubMed] [Google Scholar]
- Kweon C.H., Lee J.G., Han M.G., Kang Y.B. Rapid diagnosis of porcine epidemic diarrhoea virus infection by polymerase chain reaction. J. Vet. Med. Sci. 1997;59:231–232. doi: 10.1292/jvms.59.231. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li C.Y., Ziesmer S.C., Lazcano-Villareal O. Use of azide and hydrogen peroxide as an inhibitor for endogeneous peroxidase in the immunoperoxidase method. J. Histochem. Cytochem. 1987;35:1457–1460. doi: 10.1177/35.12.2824601. [DOI] [PubMed] [Google Scholar]
- Menšík J., Salajka J., Štěpánek J., Ulmann L., Procházka Z., Dressler J. Use of polyvalent cow colostrum in prevention of enteric infections in calves and piglets. Annu. Rech. Vét. 1978;9:255–258. [PubMed] [Google Scholar]
- Moeremans M., Daniels G., de Mey J. Sensitive colloidal (gold or silver) staining of protein blots on nitrocellulose membranes. Analyt. Biochem. 1985;145:315–321. doi: 10.1016/0003-2697(85)90368-9. [DOI] [PubMed] [Google Scholar]
- Pensaert M.B., DeBouck P. A new coronavirus-like particle associated with diarrhoea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., DeBouck P., Reynolds D.J. An immunoelectron microscopic and immunofluorescent study on the antigenic relationship between the coronavirus-like agent CV-777 and several coronaviruses. Arch. Virol. 1981;68:45–52. doi: 10.1007/BF01315166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard G.C., Paton D.J., Wibberley G., Ibata G. Transmissible gastroenteritis and porcine epidemic diarrhoea in Britain. Vet. Rec. 1999;144:616–618. doi: 10.1136/vr.144.22.616. [DOI] [PubMed] [Google Scholar]
- Rodák L., Valíček L., Šmíd B., Nevoránková Z. Detection of porcine epidemic diarrhoea virus by a monoclonal antibody immunoperoxidase test. Vet. Med. -Czech. 1999;44:165–170. [Google Scholar]
- Rodák L., Šmíd B., Nevoránková Z., Smítalová R., Valíček L. Verification of sensitivity and specificity of group A rotavirus detection in piglets faeces with monoclonal blocking ELISA methods. J. Vet. Med. B. 2004;51:160–165. doi: 10.1111/j.1439-0450.2004.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J. Coronavirus immunogens. Vet. Microbiol. 1993;37:285–297. doi: 10.1016/0378-1135(93)90030-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata I., Ono M., Mori M. Passive protection against porcine epidemic diarrhoea (PED) virus in piglets by colostrum from immunized cows. J. Vet. Med. Sci. 2001;63:655–658. doi: 10.1292/jvms.63.655. [DOI] [PubMed] [Google Scholar]
- Šmíd B., Valíček L., Rodák L., Kudrna J. Electron microscopic demonstration of porcine epidemic diarrhoea virus in the Czech Republic. Vet. Med. -Czech. 1993;38:333–341. in Czech. [PubMed] [Google Scholar]
- Takahashi K., Okada K., Oshima K. An outbreak of swine diarrhoea of a new type associated with coronavirus-like particles in Japan. Jpn. J. Vet. Sci. 1983;45:829–832. doi: 10.1292/jvms1939.45.829. [DOI] [PubMed] [Google Scholar]
- Utiger A., Tobler K., Bridgen A., Suter M., Singh M., Ackermann M. Identification of proteins specified by porcine epidemic diarrhoea virus. In: Talbot P.J., Levy G.A., editors. Corona- and related viruses. Plenum Press; New York: 1995. pp. 287–290. [DOI] [PubMed] [Google Scholar]
- van Nieuwstadt A.P., Zetstra T. Use of two enzyme-linked immunosorbent assays to monitor antibody responses in swine with experimentally induced infection with porcine epidemic diarrhoea virus. Am. J. Vet. Res. 1991;52(7):1044–1050. [PubMed] [Google Scholar]


