Abstract
Influenza virus-like sequences of H17N10 and H18N11 were identified in bats, despite there has been no live virus isolated. The genetic analysis indicated that they have distinct but relatively close evolutionary relationships to known influenza A viruses. However, the infectivity and adaptation of bat influenza viruses in avian species remain unclear. In this study, two modified bat influenza viruses cH9cN2/H17 and cH9cN2/H18 containing HA and NA coding regions replaced with those of H9N2 influenza A virus were generated in the background of the H17N10 or H18N11 viruses. These two modified viruses replicated less efficiently than wild type H9N2 virus in cultured chicken cells. The mini-genome assay showed that viral ribonucleoproteins (vRNPs) of H9N2 has significantly higher polymerase activity than that of bat influenza viruses in avian cells. In chicken study, compared with H9N2 virus, which replicated and transmitted efficiently in chickens, the cH9cN2/H17 and cH9cN2/H18 viruses only replicated in chicken tracheas with lower titers. Pathological examination showed that the H9N2 caused severer lesions in lung and trachea than the modified bat influenza viruses. Notably, the cH9cN2/H18 transmitted among chickens, but not cH9cN2/H17, and chicken IFN-β antagonism results showed that H18N11 NS1 protein inhibited chicken IFN-β response more efficiently than H17N10 NS1 protein in avian cells. Taken together, our data indicated that the internal genes of bat influenza viruses adapted poorly to chickens, while the internal genes of H18N11 seemed to adapt to chickens better than H17N10.
Keywords: Bat influenza virus, H17N10, H18N11, H9N2
Bat is the natural reservoir of most of deadly zoonotic viruses, including rabies virus, Ebola virus, and SARS coronavirus (Buceta and Johnson, 2017; Cyranoski, 2017; Streicker et al., 2016). Recently, two novel influenza A-like virus genomes designated as H17N10 and H18N11 were identified from bat specimens, indicating that bat is a reservoir of a new group of influenza viruses (bat influenza virus) which are phylogenetically related to influenza A viruses (IAVs) (Tong et al., 2012, 2013). Recently, two more H18N11 strains have been identified from fruit bats, Brazil. These two H18N11 strains shared 93.5%–96.9% nucleotide identity among all 8 genomic segments with the previous H18N11 strain found in Peru, 2010, suggesting that bat H18N11 subtype virus is diverse and wide spread in bat species (Campos et al., 2019). However, no infectious virus has been isolated from bats. Studies have shown that neither hemagglutinin (HA) nor neuraminidase (NA) of bat influenza A-like viruses has similar functions as those of IAVs (Garcia-Sastre, 2012; Li et al., 2012; Sun et al., 2013; Zhu et al., 2012, 2013). On the other hand, the internal genes of bat influenza A-like viruses have been demonstrated to be functional to support virus replication by generating chimeric bat influenza viruses, which contain six internal genes of bat influenza A-like virus and two chimeric surface genes (coding regions of HA and NA genes flanked by bat virus packaging signals) of IAVs (Juozapaitis et al., 2014; Zhou et al., 2014).
Previous study showed that bat influenza virus has limited genetic and protein compatibility with influenza A and influenza B viruses, and the internal genes of bat influenza virus are well adapted to mammalian, i.e. mice (Ciminski et al., 2017; Tefsen et al., 2014; Yang et al., 2017; Zhao et al., 2016; Zhou et al., 2014). However, whether the bat influenza viruses would adapt to avian species and pose potential threat to birds remains largely unclear. H9N2 is one of the major subtypes of IAVs circulating in poultry in China since 1993, which has a wide host range from birds to mammals (Guo et al., 2000; Sun et al., 2010). Although H9N2 is a low pathogenic virus in birds, co-infection with other pathogens often causes significant economic loss to poultry industry (Chu et al., 2016; Kishida et al., 2004; Pan et al., 2012). In addition, the cross species infection of avian origin influenza viruses often cause pandemic in humans. To explore the adaptation of bat influenza viruses to avian, two chimeric viruses cH9cN2/H17 and cH9cN2/H18 were generated and evaluated in vitro and in vivo, which the surface genes contain open reading frames (ORFs) from H9N2 and internal genes from H17N10 and H18N11.
To generate cH9cN2/H17 and cH9cN2/H18 virus, the chimeric HA vRNA (cH9) were generated as previously described (Zhou et al., 2014), which contained the coding region from A/mink/China/02/2014 (H9N2-wt) (Xue et al., 2018), flanked by putative cis-acting terminal packaging signals from either H17N10 or H18N11 (Gao and Palese, 2009; Watanabe et al., 2003), and all ATG in the packaging signal region were mutated to ATA. The cN2 was constructed with the same strategy (Fig. 1 A). The six internal gene segments of A/little yellow-shouldered bat/Guatemala/164/2009 (H17N10) were synthesized by GENEWIZ (SZ, CHINA) and sub-cloned into pHW2000 vector (Hoffmann et al., 2000). The resulting plasmids (pHWH17-PB2, pHW-H17-PB1, pHW-H17-PA, pHW-H17-NP, pHW-H17-M, and pHW-H17-NS) were confirmed by sequencing. The six internal gene plasmids of A/flat-faced bat/ Peru/033/2010 (H18N11) were obtained from Dr. Wenjun Ma (Kansas State University). Chimeric cH9cN2/H17 virus was generated by transfecting human embryonic kidney 293 T (HEK-293 T) with reverse genetic plasmids of the cH9, cN2, and the six internal genes of H17N10. The reassortant cH9cN2/H18 virus was constructed with the same strategy. The rescued viruses and H9N2-wt were propagated and titrated on MDCK cells.
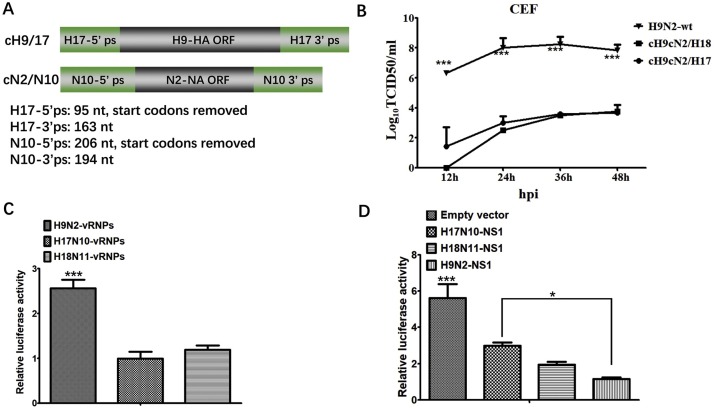
Fig. 1.
Schematic diagram of chimeric cH9/H17 and cN2/N10, growth kenetics, polymerase activity, IFN-β antagonism assay. A: green bars represent packaging signal sequence from HA and NA genes of the A/little yellow-shouldered bat/Guatemala/164/2009 (H17N10); gray bars represent HA or NA coding regions from HA and NA genes of A/mink/China/02/2014(H9N2); cH9/H18 and cN2/N11 genes were generated similarly; B: growth dynamics of cH9cN2/H17, cH9cN2/H18, and H9N2-wt viruses on CEF cells. Monolayer of CEF cells were infected with 0.01 MOI of each virus, and samples were collected at indicated time points. The virus titers were measured in MDCK cells. C: polymerase activity of vRNPs of H17N10, H18N11, and H9N2. D: IFN-β antagonism activity of NS1 proteins of H17N10, H18N11 and H9N2-wt. Each data point indicates the MEAN ± SEM of three independent experiments, ***P < 0.001, *P < 0.05 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
To evaluate growth kinetics of reassortant viruses in chicken cells, monolayers of chick embryo fibroblasts (CEF) cells were infected with each virus at the indicated multiplicity of infection (MOI = 0.01). The supernatants from infected cells were collected at different time points (12, 24, 36, and 48 hpi) and titrated on MDCK cells in 96-well plates. The virus titers were determined as TCID50 per mL by the Reed and Muench method. All three viruses were able to replicate on CEF cells. The H9N2-wt virus replicated more efficiently than cH9cN2/H17 and cH9cN2/H18 reassortant viruses on CEF cells, suggesting that the chimeric bat influenza viruses showed limited adaptation to avian cells (Fig. 1B).
To assess the infection and replication of cH9cN2/H17 and cH9cN2/H18 in chickens, a total of 48 6-week-old Specific Pathogen Free (SPF) chickens (Boehringer Ingelheim Vital Biotechology Co. Ltd. Beijing) were randomly allocated to four groups (12 per group), including three infected groups and one control group. Nine chickens in each infected group were intranasally inoculated with each virus at a dose of 105.5 TCID50 in 100 μL PBS, whereas each chicken in the mock group was inoculated with 100 μL PBS. To assess the transmissibility of each virus in chickens, three naïve chickens were comingled with infected chickens at 1 day post infection (dpi). All chickens were observed daily for clinical signs until the end of the experiment (14 days). Oropharyngeal and cloacal swabs were collected at 2, 4, and 6 days post infection/contact (dpi/dpc) to detect the virus shedding. Three chickens from each inoculated group were euthanized at both 3 and 5 dpi, and tissue samples (heart, liver, spleen, trachea, lung, kidney, pancreas, intestine, and bursa of fabricius) were collected for viral titration. For the histopathologic examination, lung and trachea tissues were fixed and processed routinely, and then stained with haematoxylin and eosin (H&E). For detection of viral NP antigen in lung and trachea sections, a rabbit anti-H1N1 NP polyclonal antibody (GenScript, USA) was used in IHC staining. The animal study was conducted in accordance to the guidelines of the Animal Care and Use Committee of Shanghai Veterinary Research Institute, and the protocols were approved by Chinese Academy of Agricultural Science. All data was analyzed by using analysis of variance (ANOVA) in GraphPad Prism version 6.0 (GraphPad software Inc, CA), a p-value <0.05 was considered statistically significant; p-value <0.001 was considered statistically highly significant.
All three viruses replicated in trachea of infected chickens (Table 1 ). H9N2-wt replicated more efficiently than cH9cN2/H17 and cH9cN2/H18 in trachea at 3 and 5 dpi, while only one to two cH9cN2/H17 or cH9cN2/H18 infected chickens showed virus positive at 3 and 5 dpi. Histopathological lesions were characterized by loss of ciliation, disruption of the epithelial architecture, and mild to severe inflammation in the tracheas of all infected chickens (Fig. 3). H9N2-wt infection caused severer lesions in trachea than the other two viruses (Fig. 3B). Abundant NP antigens were observed in tracheas of H9N2-wt infected chickens (Fig. 3F), while mild to moderate NP signals were detected in cH9cN2/H17 and cH9cN2/H18 infected chickens (Fig. 3G, H). H9N2 viruses attach and replicate efficiently in upper respiratory tract, since α-2,3-linked SAs are expressed on trachea of domestic birds. Whereas cH9cN2/H17 and cH9cN2/H18 carrying the H9 and N2 proteins replicated poorly in chicken trachea, suggesting the internal genes of bat influenza virus were limitedly adapted to avian species.
Table 1.
Virus titers in tissues of infected chickens. Nine chickens (6 weeks-old) were inoculated intranasally with the viruses at a dose of 105.5 TCID50. Three chickens from each group were euthanized at 3 dpi and5 dpi, and tissue samples (trachea, lung, pancreas, spleen, kidney and bursa of fabricus) were obtained for virus titration. a:2/3 infected chickens were positive for virus detection, and the titer values were shown as (mean ± SD); -: virus was not detected in any chicken.
| Lung | Trachea | Heart | Liver | Kidney | Spleen | Pancreas | Intestine | Bursa of fabricius | ||
|---|---|---|---|---|---|---|---|---|---|---|
| cH9cN2/H17 | 3 dpi | 0/3(−) | 2/3a(2.24 ± 0.37) | 0/3(−) | 0/3(−) | 0/3(−) | 0/3(−) | 3/3(2.08 ± 0.52) | 0/3(−) | 0/3(−) |
| 5 dpi | 0/3(−) | 1/3(1.5) | 0/3(−) | 0/3(−) | 0/3(−) | 1/3(1.98) | 2/3(1.63 ± 0.18) | 0/3(−) | 1/3(1.98) | |
| cH9cN2/H18 | 3 dpi | 0/3(−) | 1/3(1.98) | 0/3(−) | 0/3(−) | 0/3(−) | 0/3(−) | 3/3(1.75 ± 0.43) | 0/3(−) | 0/3(−) |
| 5 dpi | 0/3(−) | 1/3(1.25) | 0/3(−) | 0/3(−) | 0/3(−) | 0/3(−) | 2/3(2.38 ± 0.18) | 0/3(−) | 0/3(−) | |
| H9N2-wt | 3 dpi | 3/3(4.33 ± 1.81) | 3/3(7.083 ± 0.52) | 1/3(2.5) | 0/3(−) | 1/3(5.75) | 1/3(3.5) | 1/3(1.5) | 1/3(1.5) | 1/3(3.75) |
| 5 dpi | 3/3(5.33 ± 0.52) | 3/3(7 ± 0.43) | 1/3(3.5) | 0/3(−) | 3/3(6.83 ± 0.63) | 3/3(5.67 ± 0.52) | 3/3(5.08 ± 0.29) | 3/3(4.42 ± 0.14) | 3/3(4 ± 1.49) |
Fig. 3.
Haematoxylin and eosin (H&E) and IHC staining for microscopic trachea sections. Chickens were inoculated intranasally with the virus at 105.5 TCID50/100 u L/chicken. The trachea was collected at 5 dpi. A, E: mock group. B, F: H9N2-wt infected group, C, G: cH9cN2/H17 infected group. D, H: cH9cN2/H18 infected group.
In lower respiratory tract, H9N2-wt replicated in lungs with higher titers, while the virus was undetectable in lung tissue of chickens infected with either cH9cN2/H17 or cH9cN2/H18 (Table 1). Histopathological tests showed that severer lung lesion was observed in the lung section of H9N2-wt infected chickens, such as alveolar interstitium areas infiltrated with lymphocytes and degenerate neutrophils (Fig. 4B). In the cH9cN2/H18 and cH9cN2/H17 infected chickens, only medium numbers of lymphocytes were observed in the interstitium areas (Fig. 4C, D). Strong NP antigen signal was detected in H9N2-wt infected chicken lung (Fig. 4F). In contrast, only limited NP signal was observed in the lungs of cH9cN2/H18 and cH9cN2/H17 infected chickens (Fig. 4G, H).
Fig. 4.
Haematoxylin and eosin (H&E) and IHC staining for microscopic lung sections. Chickens were inoculated intranasally with the virus at 105.5TCID50/100 u L/chicken. The lungs were collected at 5 dpi. A, E: mock group, B, F: H9N2-wt infected group, C, G: cH9cN2/H17 infected group, D, H: cH9cN2/H18 infected group.
H9N2-wt was detected in all organs except liver of infected chickens, while cH9cN2/H17 was only detected in trachea, spleen, and pancreas, similarly cH9cN2/H18 was only detected in trachea and pancreas, suggesting that chimeric bat influenza viruses showed limited replication in chickens compared with the systemic infection in chickens caused by H9N2-wt (Table 1). Viral shedding results showed that all three chickens inoculated by H9N2-wt shed virus through oropharyngeal route at 2 and 4 dpi, and only two infected chickens shed virus through cloaca at 2 dpi (Fig. 2 ). In addition, two out of three contact chickens in H9N2-wt group shed virus through both oropharynx and cloaca at 2 dpc, which suggested that H9N2-wt is transmissible among chickens. Nevertheless, virus was undetectable in oropharyngeal and cloacal swabs from cH9cN2/H17 inoculated and contact chickens (Fig. 2). Notably, lower levels of cH9cN2/H18 virus were detected in oropharyngeal and cloacal swabs in one infected chicken at 2 dpi, and one to two contact chickens shed virus from oropharynx and cloaca at 2 dpc with lower titers (Fig. 2), suggesting that cH9cN2/H18 showed better transmission efficiency than cH9cN2/H17 in chickens.
Fig. 2.
Viral titers in oropharyngeal and cloacal swabs of infected and contact chickens. Nine chickens (6-week-old) were inoculated intranasally with the viruses at 105.5TCID50/100 u L/chicken. Three naïve chickens were introduced into the isolators at 1 day post infection (dpi). Virus shedding in oropharyngeal (OP) and cloacal (CL) swabs of infected and contact groups was monitored at 2, 4, and 6 dpi/dpc.
To further explore the potential mechanism of the poor adaptation of chimeric bat influenza virus in chickens, polymerase activity and IFN inhibition activity of bat influenza viruses were evaluated in avian cells. The mini-genome assay of H17N10, H18N11, and H9N2-wt vRNPs was conducted in chicken DF-1 cells as described previously (Zhou et al., 2014). The results showed that polymerase activity of H9N2-wt vRNPs was significantly higher than those of H17N10 and H18N11 in avian cells (Fig. 1C). NS1 protein is a multifunctional protein that is responsible to antagonize host antiviral response during viral infection (Krug, 2015). Bat influenza virus NS1 protein has been reported to bind double-stranded RNA and antagonize IFN-β response in mammalian cells (Turkington et al., 2015). To compare the chicken IFN-β antagonism ability of NS1 proteins, DF-1 cells were transfected with the indicated pCDNA-H9N2-NS1, pCDNA-H17N10-NS1 or pCDNA-H18N11-NS1 expression plasmids (0.2 μg/well), together with plasmids expressing firefly luciferase under the control of the chicken IFN-β promoter (pGL-chIFNβ-LUC, 0.2 μg/well), Renilla luciferase expressing plasmid pRL-TK (0.07 μg/well) and 0.2 μg poly(I:C). 24 h post transfection, the cells were lysed and subjected to dual-luciferase reporter assay (Promega, USA). The results showed that all the three NS1 proteins showed strong chicken IFN-β antagonism ability, whilst H9N2 NS1 inhibited IFN-β response more efficiently than bat NS1 in chicken cells (Fig. 1D). Notably, H17N10 NS1 inhibited chicken IFN-β response relatively less efficiently than H18N11 NS1 in chicken cells, suggesting that the stronger IFN antagonist of H18N11 NS1 might help the virus to adapt to avian species better than H17N10.
In addition, studies showed that the compatibility between surface proteins and the internal proteins is important for IAVs replication (Lakdawala et al., 2011; Ma et al., 2012; Rossman and Lamb, 2011). Interaction between M1 with cytoplasmic tails of HA and NA is critical for viral genome packaging and assembly in infected cells (Enami and Enami, 1996; Zhang et al., 2000). While the M1 protein of bat influenza viruses only share 78% identity with that of H9N2-wt, more than 50 amino acid substitutions in the M1 proteins between the H9N2 and H17N10/H18N11 were observed. Nevertheless, how these mutations affect incompatibility between H9N2 surface proteins and the M1 proteins of bat viruses needs further study.
The identification of bat influenza virus expanded the host reservoir of IAVs, although the origin and the evolution of bat influenza virus still remain unclear. Serological surveys indicated that bat influenza viruses are widespread in various bat species in Central and South America (Tong et al., 2013). Bats are distributed worldwide and migrate over long distances, which enhance the opportunity of interaction and interspecies transmission of viruses. Waterfowl are considered as reservoir of IAVs, including 16 HA and nine NA subtypes (Yoon et al., 2014). However, the potential of bat influenza virus infects birds needs to be explored. Zhou’s studies have shown that the packaging signals of most gene segments of the bat viruses are not compatible with those of canonical IAVs (Zhou et al., 2014). In this study, the H9N2 is unable to reassort with H17N10 or H18N11 using reverse genetic system which confirmed the previous finding (Data not shown). Nevertheless, the chimeric cH9 and cN2 genes containing packaging signals of H17N10 or H18N11 were compatible with the six internal genes of bat influenza viruses. Both of cH9cN2/H17 and cH9cN2/H18 replicated to significantly lower titer than H9N2-wt virus on CEF cells, suggesting the internal genes of bat influenza virus showed limited adaptation to avian species.
In chickens, H9N2-wt virus replicated efficiently in respiratory tract and other tissues, however, only lower level of virus was detected in trachea and pancreas of chimeric cH9cN2/H18 and cH9cN2/H17 viruses infected chickens. The H9N2-wt was shed and transmitted efficiently among chickens, and cH9cN2/H18 also showed transmissibility though limited, while cH9cN2/H17 was not transmissible in chickens. In addition, NS1 protein of H18N11 showed stronger IFN-β antagonism activity than H17N10 in avian cells. Taken together, all the present data indicated that the chimeric cH9cN2/H17 and cH9cN2/H18 showed limited adaptation to chickens, while the internal genes of H18N11 seemed to adapt to chickens better than H17N10.
Declaration of interest
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by the National Key Research and Development Plan (2016YFD0500204) and National Natural Science Foundation of China (no.31572543, no.31772753, no.31472206 and no.31402150), and Chinese Academy of Agricultural Sciences Young Talent Scientist Program (No. CAASQNYCKYYJ58).
References
- Buceta J., Johnson K. Modeling the Ebola zoonotic dynamics: Interplay between enviroclimatic factors and bat ecology. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos A.C.A., Goes L.G.B., Moreira-Soto A., de Carvalho C., Ambar G., Sander A.L., Fischer C., Ruckert da Rosa A., Cardoso de Oliveira D., Kataoka A.P.G., Pedro W.A., Martorelli L.F.A., Queiroz L.H., Cruz-Neto A.P., Durigon E.L., Drexler J.F. Bat Influenza A(HL18NL11) Virus in Fruit Bats, Brazil. Emerging Infect. Dis. 2019;25:333–337. doi: 10.3201/eid2502.181246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J., Zhang Q., Zhang T., Han E., Zhao P., Khan A., He C., Wu Y. Chlamydia psittaci infection increases mortality of avian influenza virus H9N2 by suppressing host immune response. Sci. Rep. 2016;6:29421. doi: 10.1038/srep29421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciminski K., Thamamongood T., Zimmer G., Schwemmle M. Novel insights into bat influenza a viruses. J. Gen. Virol. 2017;98:2393–2400. doi: 10.1099/jgv.0.000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. Bat cave solves mystery of deadly SARS virus - and suggests new outbreak could occur. Nature. 2017;552:15–16. doi: 10.1038/d41586-017-07766-9. [DOI] [PubMed] [Google Scholar]
- Enami M., Enami K. Influenza virus hemagglutinin and neuraminidase glycoproteins stimulate the membrane association of the matrix protein. J. Virol. 1996;70:6653–6657. doi: 10.1128/jvi.70.10.6653-6657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Palese P. Rewiring the RNAs of influenza virus to prevent reassortment. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15891–15896. doi: 10.1073/pnas.0908897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A. The neuraminidase of bat influenza viruses is not a neuraminidase. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18635–18636. doi: 10.1073/pnas.1215857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.J., Krauss S., Senne D.A., Mo I.P., Lo K.S., Xiong X.P., Norwood M., Shortridge K.F., Webster R.G., Guan Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- Hoffmann E., Neumann G., Hobom G., Webster R.G., Kawaoka Y. "Ambisense" approach for the generation of influenza a virus: vRNA and mRNA synthesis from one template. Virology. 2000;267:310–317. doi: 10.1006/viro.1999.0140. [DOI] [PubMed] [Google Scholar]
- Juozapaitis M., Aguiar Moreira E., Mena I., Giese S., Riegger D., Pohlmann A., Hoper D., Zimmer G., Beer M., Garcia-Sastre A., Schwemmle M. An infectious bat-derived chimeric influenza virus harbouring the entry machinery of an influenza a virus. Nat. Commun. 2014;5:4448. doi: 10.1038/ncomms5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida N., Sakoda Y., Eto M., Sunaga Y., Kida H. Co-infection of Staphylococcus aureus or Haemophilus paragallinarum exacerbates H9N2 influenza a virus infection in chickens. Arch. Virol. 2004;149:2095–2104. doi: 10.1007/s00705-004-0372-1. [DOI] [PubMed] [Google Scholar]
- Krug R.M. Functions of the influenza a virus NS1 protein in antiviral defense. Curr. Opin. Virol. 2015;12:1–6. doi: 10.1016/j.coviro.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakdawala S.S., Lamirande E.W., Suguitan A.L., Jr, Wang W., Santos C.P., Vogel L., Matsuoka Y., Lindsley W.G., Jin H., Subbarao K. Eurasian-origin gene segments contribute to the transmissibility, aerosol release, and morphology of the 2009 pandemic H1N1 influenza virus. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Sun X., Li Z., Liu Y., Vavricka C.J., Qi J., Gao G.F. Structural and functional characterization of neuraminidase-like molecule N10 derived from bat influenza a virus. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18897–18902. doi: 10.1073/pnas.1211037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Liu Q., Bawa B., Qiao C., Qi W., Shen H., Chen Y., Ma J., Li X., Webby R.J., Garcia-Sastre A., Richt J.A. The neuraminidase and matrix genes of the 2009 pandemic influenza H1N1 virus cooperate functionally to facilitate efficient replication and transmissibility in pigs. J. Gen. Virol. 2012;93:1261–1268. doi: 10.1099/vir.0.040535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q., Liu A., Zhang F., Ling Y., Ou C., Hou N., He C. Co-infection of broilers with Ornithobacterium rhinotracheale and H9N2 avian influenza virus. BMC Vet. Res. 2012;8:104. doi: 10.1186/1746-6148-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman J.S., Lamb R.A. Influenza virus assembly and budding. Virology. 2011;411:229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicker D.G., Winternitz J.C., Satterfield D.A., Condori-Condori R.E., Broos A., Tello C., Recuenco S., Velasco-Villa A., Altizer S., Valderrama W. Host-pathogen evolutionary signatures reveal dynamics and future invasions of vampire bat rabies. Proc. Natl. Acad. Sci. U. S. A. 2016;113:10926–10931. doi: 10.1073/pnas.1606587113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Pu J., Jiang Z., Guan T., Xia Y., Xu Q., Liu L., Ma B., Tian F., Brown E.G., Liu J. Genotypic evolution and antigenic drift of H9N2 influenza viruses in China from 1994 to 2008. Vet. Microbiol. 2010;146:215–225. doi: 10.1016/j.vetmic.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Sun X., Shi Y., Lu X., He J., Gao F., Yan J., Qi J., Gao G.F. Bat-derived influenza hemagglutinin H17 does not bind canonical avian or human receptors and most likely uses a unique entry mechanism. Cell Rep. 2013;3:769–778. doi: 10.1016/j.celrep.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Tefsen B., Lu G., Zhu Y., Haywood J., Zhao L., Deng T., Qi J., Gao G.F. The N-terminal domain of PA from bat-derived influenza-like virus H17N10 has endonuclease activity. J. Virol. 2014;88:1935–1941. doi: 10.1128/JVI.03270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Li Y., Rivailler P., Conrardy C., Castillo D.A., Chen L.M., Recuenco S., Ellison J.A., Davis C.T., York I.A., Turmelle A.S., Moran D., Rogers S., Shi M., Tao Y., Weil M.R., Tang K., Rowe L.A., Sammons S., Xu X., Frace M., Lindblade K.A., Cox N.J., Anderson L.J., Rupprecht C.E., Donis R.O. A distinct lineage of influenza a virus from bats. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Zhu X., Li Y., Shi M., Zhang J., Bourgeois M., Yang H., Chen X., Recuenco S., Gomez J., Chen L.M., Johnson A., Tao Y., Dreyfus C., Yu W., McBride R., Carney P.J., Gilbert A.T., Chang J., Guo Z., Davis C.T., Paulson J.C., Stevens J., Rupprecht C.E., Holmes E.C., Wilson I.A., Donis R.O. New world bats harbor diverse influenza a viruses. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkington H.L., Juozapaitis M., Kerry P.S., Aydillo T., Ayllon J., Garcia-Sastre A., Schwemmle M., Hale B.G. Novel bat influenza virus NS1 proteins bind double-stranded RNA and antagonize host innate immunity. J. Virol. 2015;89:10696–10701. doi: 10.1128/JVI.01430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Watanabe S., Noda T., Fujii Y., Kawaoka Y. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J. Virol. 2003;77:10575–10583. doi: 10.1128/JVI.77.19.10575-10583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R., Tian Y., Hou T., Bao D., Chen H., Teng Q., Yang J., Li X., Wang G., Li Z., Liu Q. H9N2 influenza virus isolated from minks has enhanced virulence in mice. Transbound. Emerg. Dis. 2018;65:904–910. doi: 10.1111/tbed.12805. [DOI] [PubMed] [Google Scholar]
- Yang J., Lee J., Ma J., Lang Y., Nietfeld J., Li Y., Duff M., Li Y., Yang Y., Liu H., Zhou B., Wentworth D.E., Richt J.A., Li Z., Ma W. Pathogenicity of modified bat influenza virus with different M genes and its reassortment potential with swine influenza a virus. J. Gen. Virol. 2017;98:577–584. doi: 10.1099/jgv.0.000715. [DOI] [PubMed] [Google Scholar]
- Yoon S.W., Webby R.J., Webster R.G. Evolution and ecology of influenza a viruses. Curr. Top. Microbiol. Immunol. 2014;385:359–375. doi: 10.1007/82_2014_396. [DOI] [PubMed] [Google Scholar]
- Zhang J., Pekosz A., Lamb R.A. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Tefsen B., Li Y., Qi J., Lu G., Shi Y., Yan J., Xiao H., Gao G.F. The NS1 gene from bat-derived influenza-like virus H17N10 can be rescued in influenza A PR8 backbone. J. Gen. Virol. 2016;97:1797–1806. doi: 10.1099/jgv.0.000509. [DOI] [PubMed] [Google Scholar]
- Zhou B., Ma J., Liu Q., Bawa B., Wang W., Shabman R.S., Duff M., Lee J., Lang Y., Cao N., Nagy A., Lin X., Stockwell T.B., Richt J.A., Wentworth D.E., Ma W. Characterization of uncultivable bat influenza virus using a replicative synthetic virus. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Yang H., Guo Z., Yu W., Carney P.J., Li Y., Chen L.M., Paulson J.C., Donis R.O., Tong S., Stevens J., Wilson I.A. Crystal structures of two subtype N10 neuraminidase-like proteins from bat influenza a viruses reveal a diverged putative active site. Proc. Natl. Acad. Sci. U.S.A. 2012;109:18903–18908. doi: 10.1073/pnas.1212579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Yu W., McBride R., Li Y., Chen L.M., Donis R.O., Tong S., Paulson J.C., Wilson I.A. Hemagglutinin homologue from H17N10 bat influenza virus exhibits divergent receptor-binding and pH-dependent fusion activities. Proc. Natl. Acad. Sci. U.S.A. 2013;110:1458–1463. doi: 10.1073/pnas.1218509110. [DOI] [PMC free article] [PubMed] [Google Scholar]