Abstract
Immunohistochemical, viral and bacterial isolation techniques were used to study the distribution and localization of porcine reproductive and respiratory syndrome virus (PRRSV) and Haemophilus (H.) parasuis in experimentally infected pigs. Thirty pigs seronegative to PRRSV and H. parasuis were divided into four groups. Group A pigs (10 animals) were inoculated with both virus and bacteria; group B pigs (10 animals) were inoculated with bacteria, group C pigs (five animals) were inoculated with virus and group D pigs (five animals) were kept as negative controls. All pigs of groups A and C became infected with PRRSV, according to virological techniques used (immunohistochemistry, virus isolation and virus serology). Lung, heart and tonsils were the most frequently immunolabeled tissues, and monocyte/macrophage lineage cells were the target for PRRSV in all tissues. All pigs in groups A and B also became infected with H. parasuis based on immunohistochemical and bacterial isolation results. Serosal surfaces, lung and tonsils were the most frequently immunolabeled tissues, and bacteria were found in monocyte/macrophage lineage cells as well as within neutrophil cytoplasm. No differences in terms of bacterial distribution or localization in tissues of pigs of groups A and B were detected. These results suggest that there is no influence of the previous infection with PRRSV in the occurrence of H. parasuis infection.
Keywords: Porcine reproductive and respiratory syndrome virus, Haemophilus parasuis, Pig-viruses, Pig-bacteria
1. Introduction
Clinical and diagnostic evidence have suggested that epizootic outbreaks caused by porcine reproductive and respiratory syndrome virus (PRRSV) in a naive herd mediate a sequence of clinical events that include a high prevalence of secondary viral and bacterial infections, mainly in nursery and fattening pigs (Pijoan et al., 1994). However, experimental demonstration of this hypothesis has not been fully accomplished. Furthermore, until now, trials to demonstrate direct association between PRRSV and Pasteurella multocida (Cooper et al., 1995; Carvalho et al., 1997), Haemophilus (H.) parasuis (Cooper et al., 1995; Solano et al., 1997), Salmonella cholerae-suis (Cooper et al., 1995), Actinobacillus pleuropneumoniae (Pol et al., 1997), swine influenza virus (Van Reeth, 1997), porcine respiratory coronavirus (Van Reeth et al., 1996), and Aujeszky's disease virus (Albina et al., 1995) have been unsuccesful. Attempts to demonstrate an interaction between PRRSV and Streptococcus suis (Galina et al., 1994; Cooper et al., 1995) and Mycoplasma hyopneumoniae (Albina et al., 1995; Van Alstine et al., 1996; Thacker et al., 1998), have shown contradictory results. On the other hand, Kubo et al. (1995)found more severe lesions in dually infected pigs with PRRSV and H. hyorrhinis than in singly infected pigs.
Although the hypothesis in which the infection by PRRSV potentiates secondary bacterial infections has not been demonstrated throughout the mentioned studies, just few of them have been focused on the association or relationship between PRRSV and bacteria in tissues or organs. The purpose of the present study is to determine the presence and relationship of PRRSV and H. parasuis antigens in tissues of dually infected pigs using specific immunohistochemical techniques on formalin-fixed, paraffin-embedded tissues.
2. Materials and methods
2.1. Experimental design
The design for this experiment has been previously described (Solano et al., 1997). Thirty 13–16-day old conventional pigs from a farm seronegative to PRRSV and Haemophilus parasuis were used. Animals were randomly divided into four groups (A, B, C and D). Pigs from group A (n = 10) and C (n = 5) were intranasally inoculated with the American reference strain of PRRSV (ATCC VR-2332) with a total dose of 105 TCID50 per animal on day 0 of experiment. Pigs from groups A and B (n = 10) were intratracheally inoculated with 29755 H. parasuis strain, serovar 5, at a total dose of 107 colony forming units (CFU) per animal on day 5 post-viral inoculation (PVI). Pigs from group D (n = 5) were kept as negative controls. Pigs were necropsied at the time of death, and the surviving pigs were euthanised at day 10 PVI. Pigs were bled at days PVI 0 and 5, and at the time of death. At necropsy, tissues including brain, lung, heart, liver, spleen, tonsil, thymus, intestine, lymph nodes (superficial inguinal, submandibular and mediastinal) and kidney were fixed in 10% neutral-buffered formalin, paraffin embedded, and sectioned at 4 μm. One section of each tissue was included in the study, except for lung and brain. Four sections of lung (including one from cranial lobe, one from medial lobe and two from caudal lobe) and three sections of brain (including one from cerebellum, another from pons and the other from cerebral cortex) were examined.
2.2. PRRSV immunohistochemistry
The immunohistochemical technique used was an avidin–biotin–peroxidase method based on a previously published procedure (Halbur et al., 1994). Briefly, tissue sections were placed on silane-coated (3-(trietoxysilil)-propilamine) slides; then, inhibition of endogenous peroxidase activity was made by immersion of slides in a 3% hydrogen peroxide in methanol solution for 30 min. Antigen retrieval was performed with enzymatic treatment (Protease type XIV) in tris-buffered saline (TBS, pH = 7.4) for 10 min. Blocking was done for 1 h with a 10% normal goat serum in TBS. As a primary antiserum diluted 1 : 1000 in TBS, monoclonal antibody SDOW17 (Nelson et al., 1993) was incubated overnight at 4°C. Secondary antibody (biotinylated goat anti-mouse linking antibody) and peroxidase-conjugated avidin were used at 1 : 200 and 1 : 100 dilutions, respectively, for 1 h at room temperature both. Sections were finally incubated in diaminobenzidine (DAB)–hydrogen peroxide staining solution for 8 min and counterstained with Harris's hematoxylin. Negative controls consisted of lack of addition of the primary antisera and lung and tonsil tissues from a healthy pig seronegative against PRRSV and H. parasuis.
2.3. PRRSV isolation
Viremia was determined at day 0 and 5 PVI and at necropsy. An established cell line, CL2621, was used for virus isolation and propagation (Collins et al., 1992).
2.4. PRRSV serology
Serum samples obtained at day 0 of the experiment and at necropsy were tested for anti-PRRSV antibodies using an indirect fluorescent antibody (IFA) test, which used VR-2332 PRRSV-infected porcine alveolar macrophages as a primary cell culture (Yoon et al., 1992).
2.5. H. parasuis immunohistochemistry
The immunohistochemical technique used was also an avidin–biotin–peroxidase method based on a published procedure (Segalés et al., 1997). This procedure did not include antigen retrieval, and primary antiserum (kindly donated by Dr. A. Méndez-Trigo, Bayer Animal Health, Worthington, MN, USA), raised in rabbits against formalin-killed H. parasuis serovar 5 (Nagasaki strain), was previously adsorbed with dry porcine liver powder at a concentration of 100 mg ml−1 of diluted antiserum. Primary antiserum was used at 1 : 500 dilution in TBS, and incubated overnight at 4°C. Secondary antibody (biotinylated goat anti-rabbit linking antibody) and peroxidase-conjugated avidin were used at 1 : 400 and 1 : 100 dilutions, respectively, for 1 h at room temperature both. Sections were finally incubated in diaminobenzidine (DAB)–hydrogen peroxide staining solution for 2 min and counterstained with Harris's hematoxylin. Negative controls consisted of lack of addition of the primary antisera and lung and tonsil tissues from a healthy pig seronegative against PRRSV and H. parasuis.
2.6. Haemophilus parasuis isolation
Isolation of H. parasuis was attempted following a previously published procedure (Solano et al., 1997).
3. Results
3.1. Clinical evaluation
No clinical signs were observed after PRRSV inoculation, except for a very mild increase in rectal temperature. Pigs inoculated with H. parasuis developed hyperthermia (up to 41.5°C) and, due to the presence of central nervous system clinical signs such as opisthotonus, recumbency and tremors, or sudden death, some animals were euthanised or died on day 2 post-bacterial inoculation (PBI) (pigs No. 3, 14, 15, 18 and 19), day 3 PBI (pigs No. 5, 6, 9, 11, 17 and 20), and day 4 PBI (pig No. 7). The remaining pigs were euthanised on day 10 PVI (Table 1 ). No other clinical signs were recorded during the experimental period.
Table 1.
PRRSV and H. parasuis immunohistochemical results in groups A, B and C
| Group | Pig No. | Day | Tisues |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PVI | Brain/meningesa |
Lung/pleura |
Heart/pericardium |
Spleen/capsule |
Liver/capsule |
Tonsil |
Kidney |
|||||||||
| PRRSVb | HPc | PRRSV | HP | PRRSV | HP | PRRSV | HP | PRRSV | HP | PRRSV | HP | PRRSV | HP | |||
| A | 1 | 10 | − | − | − | − | + | − | − | − | − | − | − | + | − | − |
| 2 | 10 | − | − | + | + | + | − | − | − | − | − | − | − | − | − | |
| 3 | 7 | − | + | + | + | − | − | − | − | − | − | + | − | − | − | |
| 4 | 10 | − | − | − | + | − | + | − | + | − | + | − | + | − | + | |
| 5 | 8 | − | − | − | − | − | − | − | − | − | − | + | − | − | − | |
| 6 | 8 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | |
| 7 | 9 | + | + | + | + | + | + | − | + | − | + | + | + | + | + | |
| 8 | 10 | − | − | − | + | − | − | − | − | − | − | − | − | − | − | |
| 9 | 8 | − | + | − | + | − | − | − | − | − | − | − | − | − | − | |
| 10 | 10 | − | + | + | + | − | + | − | + | − | − | − | + | − | − | |
| Total group A | 1 / 10 | 5 / 10 | 4 / 10 | 7 / 10 | 3 / 10 | 3 / 10 | 0 / 10 | 3 / 10 | 0 / 10 | 2 / 10 | 3 / 10 | 4 / 10 | 1 / 10 | 2 / 10 | ||
| B | 11 | 8 | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| 12 | 10 | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| 13 | 10 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 14 | 7 | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| 15 | 7 | − | − | − | + | − | − | − | − | − | − | − | + | − | − | |
| 16 | 10 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 17 | 8 | − | + | − | + | − | + | − | + | − | + | − | − | − | + | |
| 18 | 7 | − | − | − | + | − | − | − | − | − | − | − | + | − | − | |
| 19 | 7 | − | + | − | + | − | − | − | − | − | − | − | + | − | − | |
| 20 | 8 | − | + | − | + | − | + | − | + | − | − | − | − | − | − | |
| Total group B | 0 / 10 | 5 / 10 | 0 / 10 | 8 / 10 | 0 / 10 | 4 / 10 | 0 / 10 | 4 / 10 | 0 / 10 | 3 / 10 | 0 / 10 | 5 / 10 | 0 / 10 | 3 / 10 | ||
| C | 21 | 10 | − | − | − | − | + | − | − | − | − | − | + | − | − | − |
| 22 | 10 | − | − | + | − | + | − | − | − | − | − | + | − | − | − | |
| 23 | 10 | − | − | − | − | + | − | − | − | − | − | − | − | − | − | |
| 24 | 10 | − | − | + | − | + | − | − | − | − | − | + | − | − | − | |
| 25 | 10 | − | − | − | − | + | − | − | − | − | − | + | − | − | − | |
| Total group C | 0 / 5 | 0 / 5 | 2 / 5 | 0 / 5 | 5 / 5 | 0 / 5 | 0 / 5 | 0 / 5 | 0 / 5 | 0 / 5 | 4 / 5 | 0 / 5 | 0 / 5 | 0 / 5 | ||
Tissue 1/Tissue 2 means result in Tissue 1 and/or Tissue 2.
bPRRSV immunohistochemical result.
cH. parasuis immunohistochemical result.
+ – positive immunohistochemical result; − – negative immunohistochemical result.
3.2. PRRSV immunohistochemistry
Immunohistochemical results of virus antigen detection are summarized in Table 1. PRRSV antigen was detected in several tissue sections from six out of 10 pigs of group A and all pigs of group C. No PRRSV antigen was detected in pigs of groups B and D. Immunolabeling was located within the cytoplasm of macrophage/monocyte lineage cells of lung, brain, heart, tonsils, thymus, Peyer's patches and kidney. Three tissues, lung (six out of 15 pigs), heart (8 / 15) and tonsils (7 / 15), were the most frequently immunolabeled. In all cases, a low number of positive cells were observed.
Lungs showed immunolabeling in the cytoplasm of interstitial and alveolar macrophages (Fig. 1 ); no other cell type had positive labeling. Heart showed presence of virus antigen in cytoplasm of histiocytes, mainly located near blood vessels, and tonsils had positive cells whose morphology resembled those of macrophage and dendritic cells. The rest of tissues had very few positive cells, and all of them were histiocytic cells. One pig from group A (pig No. 7) had immunolabeling in macrophages of the fibrinous inflammatory infiltrate of the brain.
Fig. 1.
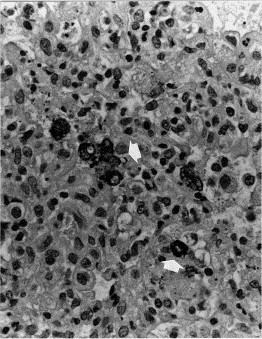
Lung of pig No. 7 (group A). Note presence of interstitial macrophages with presence of PRRSV antigen within the cytoplasm (arrows). Avidin–biotin–immunoperoxidase. Hematoxylin counterstain X350.
3.3. PRRSV isolation and serology
Results are summarized in Table 2 . All pigs from group A and three out of five from group C had virus in sera on day 5 PVI. At necropsy, PRRSV was isolated from sera of three pigs of group A and two of group C, and seven pigs of group A and four of group C had seroconverted against the virus with titers ranging from 1 : 64 to 1 : 1024. One pig from group C (No. 25) did not have positive viral isolation neither at day 5 PVI nor at day 10 PVI, and was seronegative at necropsy day. Negative results on virus isolation and PRRSV serology were obtained from samples of pigs of groups B and D.
Table 2.
PRRSV isolation and serology of pigs from groups A and C
| Group | Pig No. | Virus isolation from seruma |
Serologyb | |
|---|---|---|---|---|
| Day 5 PVI | Necropsy day | |||
| A | 1 | + | + | 1 : 64 |
| 2 | + | + | 1 : 1024 | |
| 3 | + | − | 1 : 64 | |
| 4 | + | − | 1 : 256 | |
| 5 | + | − | 1 : 256 | |
| 6 | + | − | 1 : 256 | |
| 7 | + | − | 1 : 256 | |
| 8 | + | + | Neg. | |
| 9 | + | − | Neg. | |
| 10 | + | − | Neg. | |
| Total group A | 10 / 10 | 3 / 10 | 7 / 10c | |
| C | 21 | + | + | 1 : 256 |
| 22 | − | − | 1.256 | |
| 23 | + | − | 1 : 1024 | |
| 24 | + | + | 1 : 1024 | |
| 25 | − | − | Neg. | |
| Total group C | 3 / 5 | 2 / 5 | 4 / 5c | |
Virus isolation.
bSerology:positive titres ranging from 1:16 to 1:1024; Neg. – seronegative pig.
cNumber of pigs that seroconverted at necropsy day.
+ – presence of cytopathic effect on CL2621 cells and positive staining against PRRSV.
− – absence of cytopathic effect on CL2621 cells and negative staining against PRRSV.
3.4. Haemophilus parasuis immunohistochemistry
Immunohistochemical results of bacterial antigen detection are summarized in Table 1. H. parasuis antigen was detected in several tissue sections from nine out of 10 pigs from group A and eight out of 10 pigs from group B. No bacterial antigen was detected in pigs from groups C and D. Immunolabeling was located within the cytoplasm macrophage/monocyte and polymorphonuclear lineage cells in the fibrino-purulent inflammatory reaction of meninges (Fig. 2 ), lung, pleura, pericardium, liver, spleeen, tonsils, Peyer's patches, lymph nodes and kidney. Serosal surfaces, including meninges (10 / 20) and pleura (6 / 20), lung (14 / 20) and tonsils (9 / 20) were the most frequently immunolabeled tissues. In most cases, a high number of positive cells were observed.
Fig. 2.
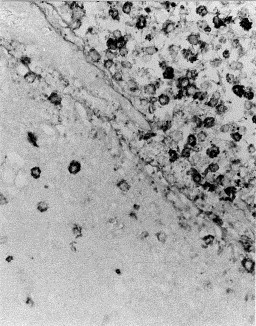
Meninges of pig No. 17 (group B). Presence of many mononuclear inflammatory cells with presence of H. parasuis antigen within the cytoplasm. Avidin–biotin–immunoperoxidase. No counterstain X200.
Lungs showed immunolabeling in the cytoplasm of alveolar macrophages as well as the cytoplasm of neutrophils in bronchopneumonic areas. Antigen distribution was multifocal, following a lobular pattern. Bacterial antigen was also present in the cytoplasm of monocytes within blood vessels of heart (Fig. 3 ) and liver. Tonsils had positive cells whose morphology resembled those of macrophages, and spleen had immunoreaction in foci of monocyte/macrophage lineage cells in the red pulp. Serosal surfaces showed labeling within cytoplasm of neutrophils and in a diffuse distribution among fibrin fibers. The rest of tissues showed very few positive cells, and the positive reaction was observed within cytoplasm of macrophages. No immunolabeling was observed in cases in which no polyserositis or bronchopneumonia occurred.
Fig. 3.
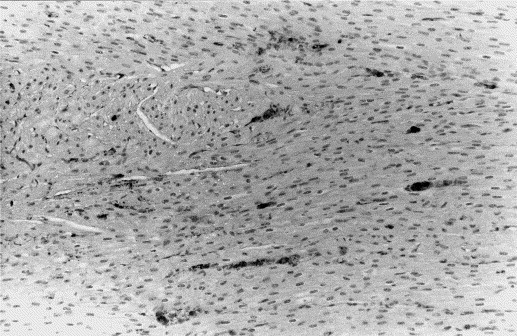
Heart of pig No. 14 (group B). Presence of H. parasuis antigen in the cytoplasm of monocytes within blood vessels of the myocardium. Avidin–biotin–immunoperoxidase. Hematoxylin counterstain X100.
Five pigs of group A (pigs No. 1, 2, 3, 7 and 10) showed both PRRSV and H. parasuis antigens in several tissues, but, except for the lung, minimal or no correlation between the presence of both antigens in the same tissue and location was found. Only pig No. 7 had a high correlation of presence of both antigens in the same location in most of examined tissues. Four pigs of group A (No. 4, 6, 8 and 9) had only H. parasuis antigen, and one pig of the same group (No. 5) had only PRRSV antigen.
3.5. Haemophilus parasuis isolation
These results are summarized in Table 3 . H. parasuis was isolated from at least one tested sample of all pigs from both groups, except for one pig in each group (pig No. 1 of group A, and pig No. 19 of group B). Best locations in order to recover bacteria were trachea, and peritoneal and articular fluid. Similar isolation results were obtained in viral and non-viral-infected groups (groups A and B, respectively). No H. parasuis was isolated from samples of pigs from groups C and D.
Table 3.
Isolation of H. parasuis from different locations in pigs of groups A and B
| Group | Pig No. | Samples |
|||||
|---|---|---|---|---|---|---|---|
| Lung | Tracheal swab | Pericardial swab | Peritoneal swab | CSFa | Joint swabb | ||
| A | 1 | − | − | − | − | − | − |
| 2 | + | + | − | − | − | − | |
| 3 | − | + | + | − | − | + | |
| 4 | − | + | − | − | − | + | |
| 5 | − | + | − | − | − | + | |
| 6 | − | + | − | + | − | + | |
| 7 | − | + | + | − | + | + | |
| 8 | − | − | − | + | − | − | |
| 9 | − | + | + | + | + | − | |
| 10 | − | − | − | + | + | − | |
| Total group A | 1 / 10 | 7 / 10 | 3 / 10 | 4 / 10 | 3 / 10 | 5 / 10 | |
| B | 11 | − | − | − | + | − | − |
| 12 | + | − | + | + | + | + | |
| 13 | − | + | − | − | − | − | |
| 14 | + | + | + | + | + | + | |
| 15 | − | + | + | + | − | − | |
| 16 | − | + | − | − | − | − | |
| 17 | − | − | + | + | − | − | |
| 18 | − | − | − | + | − | + | |
| 19 | − | − | − | − | − | − | |
| 20 | + | + | + | + | − | + | |
| Total group B | 3 / 10 | 5 / 10 | 5 / 10 | 7 / 10 | 2 / 10 | 4 / 10 | |
Cerebrospinal fluid.
bFrom the tibial-femoral joint.
+ – isolation of H. parasuis; − – no isolation of H. parasuis.
4. Discussion
The present study shows the antigen distribution and relationship of PRRSV and H. parasuis in dually infected pigs. All pigs inoculated with PRRSV became infected based on immunohistochemical, serological and viral isolation results. Viral serology at the end of the experiment showed a great variation in titres among animals of groups A and C; this fact suggests that pigs of this experiment were in different stages of PRRSV infection at the necropsy day or that individual non-determined factors might be involved in serologic response in those pigs. On the other hand, all pigs from groups A and B, also became infected with bacteria based on immunohistochemical and bacterial isolation results. As a whole, these data suggest that there is no influence of the previous infection with PRRSV on the occurrence of H. parasuis infection.
Lung and tonsils were the tissues in which both virus and bacteria were found most frequently. In these tissues, PRRSV can replicate in monocyte/macrophage lineage cells and other antigen presenting cells such as dendritic cells (Halbur et al., 1996; Rossow et al., 1996). H. parasuis was also found within the cytoplasm of mononuclear cells of the mentioned tissues, but the number of pigs with bacterial antigen in the lung and tonsils did not differ if PRRSV had been previously inoculated or not.
Mechanisms of pathogenesis of dual PRRSV infections remain to be elucidated. However, it has been demonstrated that PRRSV infection can induce high levels of interleukin-1β and interferon-α in lung lavage cells, which can play a role in antiviral defense and stimulate the non-specific pulmonary inflammatory response (Van Reeth, 1997). This fact may explain why PRRSV does not potentiate H. parasuis replication.
In conclusion, this study shows that there is no relationship between the presence of PRRSV and H. parasuis antigens in dually infected pigs, and confirms no interaction between aetiological agents in terms of antigen distribution. This data agreed with clinical and pathological findings of experimental studies in which no association between PRRSV and H. parasuis was demonstrated (Cooper et al., 1995; Solano et al., 1997).
Acknowledgements
The authors wish to thank Ruth Cory for correction of the manuscript.
References
- Albina E., Kobish M., Cariolet R., Morvan P., Kéranflec'h, Beaurepaire B., Hutet E., Labbé A. Le syndrome dysgénésique et respiratoire du porc (SDRP): étude expérimentale des effects de l'infection sur la résponse immunitaire et la résistance aux infections Aujeszky et Mycoplasma hyopneumoniae chez le porc en croissance. J. Rech. Porcine France. 1995;27:107–116. [Google Scholar]
- Carvalho L.F.O.S., Segalés J., Pijoan J. Effect of porcine reproductive and respiratory syndrome virus on subsequent Pasteurella multocida challenge in pigs. Vet. Microbiol. 1997;55:241–246. doi: 10.1016/S0378-1135(96)01324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Christianson W.T., Harris L., Hennings J.C., Shaw D.P., Goyal S.M., McCullough S., Morrison R.B., Joo H.J., Gorcyca D., Chladek D. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Cooper V.L., Doster A.R., Hesse R.A., Harris N.B. Porcine reproductive and respiratory syndrome: NEB-1 PRRSV infection did not potentiate bacterial pathogens. J. Vet. Diagn. Invest. 1995;7:313–320. doi: 10.1177/104063879500700303. [DOI] [PubMed] [Google Scholar]
- Galina L., Pijoan C., Sitjar M., Christianson W.T., Rossow K.D., Collins J.E. Interaction between Streptococcus suis serotype 2 and porcine reproductive and respiratory syndrome in specific pathogen-free piglets. Vet. Rec. 1994;134:60–64. doi: 10.1136/vr.134.3.60. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Andrews J.J., Huffman E.L., Paul P.S., Meng X.J., Niyo Y. Development of a streptavidin-biotin immunoperoxidase procedure for the detection of porcine reproductive and respiratory syndrome virus antigen in porcine lung. J. Vet. Diagn. Invest. 1994;6:254–257. doi: 10.1177/104063879400600219. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Paul P.S., Frey M.L., Landgraf J., Eernise K., Meng X.J., Lum M.A., Andrews J.J., Rathje J.A. Comparison of the antigen distribution of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1996;33:159–170. doi: 10.1177/030098589603300205. [DOI] [PubMed] [Google Scholar]
- Kubo M., Kimura K., Kobayashi M., Shimizu M., Yamada S., Morozumi T., Kobayashi H., Mitani K., Ito N., Yamamoto K., Miura Y., Yamamoto T., Watanabe K. J. A. R. Q. 1995;29:201–205. [Google Scholar]
- Nelson E.A., Christopher-Hennings J., Drew T., Wensvoort G., Collins J.E., Benfield D.A. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J. Clin. Microbiol. 1993;31:3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijoan C., Solano G.I., Segalés J. PRRS virus and secondary disease. Proc. Allen D. Leman Conf. 1994;21:225–226. [Google Scholar]
- Pol J.M.A., Van Leengoed L.A.M.G., Stockhofe N., Kok G., Wensvoort G. Dual infections of PRRSV/Influenza or PRRSV/Actinobacillus pleuropneumoniae in the respiratory tract. Vet. Microbiol. 1997;55:259–264. doi: 10.1016/s0378-1135(96)01323-5. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Benfield D.A., Goyal S.M., Nelson E.A., Christopher-Hennings J., Collins J.E. Chronological immunohistochemical detection and localization of porcine reproductive and respiratory syndrome virus in gnotobiotic pigs. Vet. Pathol. 1996;33:551–556. doi: 10.1177/030098589603300510. [DOI] [PubMed] [Google Scholar]
- Segalés J., Domingo M., Solano G.I., Pijoan C. Immunohistochemical detection of Haemophilus parasuis serovar 5 in formalin-fixed, paraffin-embedded tissues of experimentally infected swine. J. Vet. Diagn. Invest. 1997;9:237–243. doi: 10.1177/104063879700900303. [DOI] [PubMed] [Google Scholar]
- Solano G.I., Segalés J., Collins J.E., Molitor T.W., Pijoan C. Porcine reproductive and respiratory syndrome virus (PRRSv) interaction with Haemophilus parasuis. Vet. Microbiol. 1997;55:247–257. doi: 10.1016/S0378-1135(96)01325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker E., Halbur P.G., Thacker B. Mycoplasma and PRRSV interactions – their possible role in PRDC. Proc. Am. Assoc. Swine Pract. 1998;29:351–356. [Google Scholar]
- Van Alstine W.G., Stevenson G.W., Kanitz C.L. Porcine reproductive and respiratory syndrome virus does not exacerbate Mycoplasma hyopneumoniae infection in young pigs. Vet. Microbiol. 1996;49:297–303. doi: 10.1016/0378-1135(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Van Reeth K., Nauwynck H., Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet. Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K. Pathogenesis and clinical aspects of a respiratory porcine reproductive and respiratory syndrome virus infection. Vet. Microbiol. 1997;55:223–230. doi: 10.1016/S0378-1135(96)01331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon I.J., Joo H.S., Christianson W.T., Kim H.S., Collins J.E., Morrison R.B., Dial G.D. An indirect fluorescent antibody test for the detection of antibody to swine infertility and respiratory syndrome virus in swine sera. J. Vet. Diagn. Invest. 1992;4:144–147. doi: 10.1177/104063879200400205. [DOI] [PubMed] [Google Scholar]


