Abstract
Disease outbreaks characterized by reproductive failure and/or neurologic disorders, which are commonly referred as “Porcine Reproductive and Neurologic Syndrome (PRNS)”, were observed in many swine farms in Iowa and other states. Although an infectious cause was suspected to account for the disease, no conclusive diagnosis had been reached with respect to conventional infectious agents. Extensive laboratory diagnostic investigation on suspect cases repeatedly resulted in the isolation of a cytopathic enveloped virus of 50–60 nm in size from nervous and second lymphoid tissues and sera and, to reflect its unknown identity, named “Virus X”. The presence of virus particle with morphological characteristics similar to Virus X in tissues from affected animals was also observed on thin-section positive-staining electron microscopy. Isolates of Virus X were not readily recognized by antibodies raised against any known viruses pathogenic to swine but by antisera collected from animals surviving clinical episode, indicating that Virus X is likely a previously unrecognized agent. Pregnant sows experimentally inoculated with Virus X (ISUYP604671) or homogenate (filtrate) of tissues from a clinically affected animal developed clinical signs and pathological changes similar to field observations including the loss of pregnancy. Furthermore, caesarian-derived, colostrum-deprived young pigs developed mild encephalomyelitis lesions in brains after experimental inoculation with the virus or the tissue homogenate although clinical neurologic signs were not observed. More importantly, Virus X was re-isolated from all inoculated animals while control pigs remained negative for the virus during the study. Collectively, Virus X is a novel viral agent responsible for PRNS and remains to be further characterized for taxonomical identity.
Keywords: Pig, Neurologic disorder, Reproductive failure, Novel pestivirus
1. Introduction
Wastage of pigs due to reproductive failure results in significant economic losses to the swine industry. Death losses of sexually mature breeding animals and market hogs add more economic burden to producers. In many situations, both infectious and non-infectious causes can account for reproductive problems and mortality; infectious diseases are considerably more important because of the rapid transmission between animals and many times the lack of effective preventive measures.
Since at least 1995, pork producers and swine practitioners have reported disease outbreaks characterized primarily by early infertility, hence commonly referred to as “sow infertility syndrome” or “growth depression syndrome” (Yoon and Zimmerman, 1998). Typically, producers have observed an acute decline in farrowing rate due to increased early or delayed returns followed by a prolonged period (up to 2 years) of sub-optimal farrowing performance. Loss of pregnancy most commonly occurs in the early stages of gestation (i.e., 30–55 days), although abortion and reproductive failure were reported on sows at later gestations. Affected animals which were confirmed pregnant at 30 days either aborted or demonstrated irregular return to estrus. Besides reproductive disorder, many of the affected animals were presented with neurological signs, such as posterior weakness/paralysis, ataxia, bar chewing, and head pressing/banging (Yoon, 2003). The neurologic symptoms in affected individuals progressed, with death in 2 or 3 days as the usual outcome. Central nervous signs were also observed in younger pigs such as nursery and fattening pigs. To reflect various clinical manifestations, the newly identified disease has also been commonly referred to as “Porcine Reproductive and Neurologic Syndrome (PRNS)”. Reproductive and neurologic problems were not, however, always present simultaneously.
The incidence of the problem seems to continue growing. The pattern of spread within and between herds implies the involvement of an infectious agent. To date, affected herds have been observed in both PRRSV-infected and PRRSV-free swine herds in many states including Iowa (Yoon, 2003). Although many cases with similar clinical presentations have been presented to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL), a conclusive diagnosis could not be reached to date (Yoon and Zimmerman, 1998). Laboratory testing including serology, has not yet demonstrated the role of any recognized reproductive or neurologic pathogens of swine associated with the symptoms described above, including foreign or exotic viral agents. No bacterial or viral pathogens were consistently identified in index cases. Furthermore, there is no evidence that the condition is toxin-related, or associated with any particular management practice or pig genotype. Therefore, the disease was thought to be due to a previously unrecognized agent. With that hypothesis, the following extensive laboratory diagnostic investigations were conducted to identify the causative agent.
2. Materials and methods
2.1. Case definition
Index animals were selected for diagnostic investigations from herds showing the following clinical presentations: Sows exhibiting an unusually high early return or abortion after 30 but before 50 days of gestation; animals that were lethargic, restless, and/or dyspneic particularly after exercise prior to losing the pregnancy; some of the affected animals were also febrile and pale. Besides reproductive failure, some of the affected pregnant sows and weaned pigs also showed neurological signs such as posterior weakness, paresis, ataxia, lameness, head pressing or banging and aggressive behavior often leading to abrasions on the forehead, nose and front legs as well as abortion. Neonates showed “shaking” and many times appeared to be blind as those animals could not walk in one direction. Central nervous signs were also observed in grower-finishers. Some pigs recovered but the majority of affected animals died within several days of showing clinical signs. Sometimes affected animals died suddenly without any overt proceeding clinical signs. To avoid any potential confusion, animals from known PRRS-positive herds were not included in the study.
2.2. Biological samples
Serum, whole blood and tissues (brain, secondary lymphoid tissues, kidney, liver) were collected from each animal and used for laboratory testing. Secondary lymphoid tissues included tonsil, regional lymph nodes and spleen. When available, fetal thoracic fluid and tissues were collected from aborted or freshly dead fetuses. Placenta and uterus were also collected from sows.
Buffy coat cells were fractionated from EDTA blood by gradient centrifugation and used for testing. Sera and fetal thoracic fluids were tested as collected. Tissues were tested after 20% (w/v) homogenate was made in Earle's balanced salt solution (Sigma Chemical Co., St. Louis, MO, USA) and filter-sterilized through 0.22-μm membrane filters (Fisher Scientific Co, Houston, TX, USA).
2.3. Immunofluorescence microscopy on frozen tissue sections
The FA test was performed on cryosections of brain, tonsil, kidney and lymph nodes from clinically affected animals submitted to ISU-VDL to detect viral antigens. Sections were attached to prepared glass slides and fixed by immersing in cold 100% cold acetone. Fixed tissue sections were then stained with many FA conjugates against different viruses. Fixed tissues were also stained with commercially obtained polyclonal antiserum for ruminant/caprine pestivirus antibodies (440-BDV 9001) from the National Veterinary Services Laboratory (Ames, IA, USA). The bovine serum was diluted 1:80 in the 0.01 M phosphate-buffered saline (PBS) at pH 7.4 for tissue material from sows. Slides flooded with the antibodies were then incubated at 37 °C for 1 h in a humid condition and then rinsed with PBS three times. The antigen–antibody reaction in tissues was visualized by staining tissue sections with optimally diluted goat anti-bovine IgG (H+L) conjugated with FITC (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD, USA). Swine sera collected from naturally or experimentally infected pigs were used in the same manner but FITC-labeled anti-porcine IgG (H+L) was used instead of anti-bovine conjugate. Slides were then observed under a fluorescence microscope.
2.4. Polymerase chain reaction assays
All clinical specimens were tested by polymerase chain reaction (PCR) based tests for known swine viral pathogens, such as porcine reproductive and respiratory syndrome virus (PRRSV) of European and North American genotypes (type 1 and 2, respectively), porcine circovirus (PCV) type 1 and 2, group 1–3 porcine enterovirus (PEV) (currently group 1 and 2 PEV are classified into porcine teschovirus, whereas group 3 remains as enterovirus), influenza A virus, porcine parvovirus (PPV), porcine reovirus type 1, 2 and 3, porcine cytomegalovirus (PCMV), pseudorabies virus (PRV), transmissible gastroenteritis virus (TGEV), porcine respiratory coronavirus (PRCV), porcine epidemic diarrhea virus (PEDV), Japanese encephalitis B virus (JEV), porcine endogenous retrovirus (PERV) type 1, porcine lymphotropic herpesvirus type 1 (PLHV-1), swine hepatitis E virus (sHEV), bovine viral diarrhea virus (BVDV), West Nile virus (WNV), members of genus Pestivirus, and members of alpha Togavirus. A multiplex PCR was used for detecting PCV DNA as previously described (Pogranichnyy et al., 2000). A PCR assays previously described (Ellis et al., 1999) was employed for detecting PPV DNA. Reverse transcription (RT)-PCR assays were used for detecting RNA of PRRSV (Yoon et al., 1999), group 1–3 PEV (Zell et al., 2000), CSFV (Wirz et al., 1993, Hofmann et al., 1994), BVDV (Ridpath and Bolin, 1998, Vilcek et al., 1994), JEV and WNV (Scaramozzino et al., 2001), PERV (Akiyoshi et al., 1998), and alpha-togaviruses (Powers et al., 2001). Detection of SIV or TGEV/PRCV genome in samples was attempted using multiplex RT-PCR assays established in ISU-VDL (Harmon and Yoon, 1999).
2.5. Virus isolation attempts
Virus isolation was attempted on all samples using various cell lines and primary cells of porcine origin. All cell lines and primary cells were confirmed by virus isolation technique, polymerase chain reaction (PCR) based assay and antigen-capturing ELISA (Syracuse Bioanalytical, Inc., Ithaca, NY, USA) to be free of bovine viral diarrhea virus (BVDV) prior to and during use. All cell lines were also confirmed by a commercial PCR test (American Tissue Culture Collection, Manassas, VA, USA) to be free of Mycoplasma spp.
Cells were prepared in 48-well plates and 25-cm2 tissue culture flasks and grown in Minimum Essential Medium (MEM, Mediatech, Inc., Herdon, VA, USA) supplemented with 10% (v/v) BVDV-free fetal bovine serum (Sigma Chemical Co., St. Louis, MO, USA) or 5% (v/v) horse serum (Sigma Chemical Co.), 20 mM l-glutamine (Gibco/BRL Life Science, Grand Island, NY, USA), and an antibiotic-antimycotic mixture (Sigma Chemical Co.). After the confluent monolayer was formed, samples (0.25 ml/well and 1ml/flask) were inoculated into each flask in duplicate or triplicate. After 1-h incubation at 37 °C, cells were rinsed with fresh growth medium and were incubated further in a humid 37 °C incubator with 5% CO2 supply. Alternatively, samples were inoculated in cell suspension and left for 24 h before material was removed. Inoculated cells were then observed daily for cytopathic effect (CPE) until 7 days post inoculation (PI). When CPE was evident in more than 70% of cell monolayer, cell culture medium was harvested and inoculated onto freshly prepared cells in duplicate. For cases in which no CPE was evident by 7 days PI, cells were subjected to 2 cycles of freeze thawing at −70 °C and 35 °C, respectively, and then cell lysate was inoculated into freshly prepared cells in the same manner as above.
At day 2 to 4 PI, regardless of the presence or absence of CPE, one set of the inoculated cells was fixed, after cell culture media were harvested, by immerging them in cold 80% aqueous acetone and subjected for an immunofluorescence microscopy using fluorescent isothiocynate (FITC)-labeled antibodies (USDA National Veterinary Services Laboratories, Ames, IA, USA; Rural Technologies, Inc., Brookings, SD, USA; DBA American Bioresearch, Inc., Seymour, TN, USA) raised against known swine viral pathogens, such as PRRSV type 1 and 2, PRV, PCMV, PCV (type 1 and 2), PPV, encephalomyocarditis virus (EMCV), PEV, TGEV, hemagglutinating encephalomyelitis virus (HEV), swine influenza virus (SIV) of H1N1 and H3N2 subtypes, influenza A virus, porcine reovirus, rabies virus and pestiviruses (BVDV/BDV). In addition, the cells were reacted with sera collected from animals affected but surviving during the course of natural infection. For pig and bovine sera, anti-porcine IgG (H+L) and anti-bovine IgG (H+L) conjugated with FITC (Kirkegaard and Perry Laboratories, Inc.) were used as secondary antibody to visualize specific antigen–antibody complex. At the same time another set of the inoculated cells was subjected to freeze thawing and then cell lysates were tested by PCR assays for known swine viral pathogens described above under PCR.
Each cell culture fluid and corresponding unused cell lysates that were negative for all viral agents described above were combined for each sample and inoculated again on each cell for further propagation for use in other laboratory testing such as electron microscopy (EM) or animal studies.
2.6. Electron microscopy
To assess morphological characteristic of the viral isolate, cells inoculated with potential virus material and cell culture fluid containing the virus as well as pig tissues were examined by electron microscopic techniques. Cells inoculated with virus material and tissues were examined by thin-section positive-staining EM. Inoculated cells were incubated at 37 °C in a humid 5% CO2 atmosphere for up to 120 h PI. At between 48 and 120 h PI, the cells were harvested using a cell scraper and pelleted by low-speed centrifugation. Each cell pellet was fixed in 2% (w/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.05M phosphate-buffered saline (PBS, pH 7.2) for 48 h at 4 °C. Samples were rinsed once in PBS followed by 2 washes in 0.1 M cacodylate buffer (pH 7.2) and then fixed in 1% osmium tetroxidate in 0.1 M cacodylate buffer for 1 h at ambient temperature. The samples then were dehydrated in a graded ethanol series, cleared with ultra pure acetone, infiltrated and embedded using a modified EPON epoxy resin (Embed 812, Electron Microscopy Science, Fort Washington, PA, USA). Resin blocks were polymerized for 48 h at 70 °C. Thick and ultra-thin sections were made using a Reichert Ultracut S ultra microtome (Leeds Precision Instruments, Minneapolis, MN, USA). Ultra-thin sections were collected onto copper grids and counterstained with 5% uranyl acetate in 100% methanol for 15 min followed by Sato's lead stain for 10 min. Images were captured using a JEOL 1200EX scanning and transmission electron microscope (Japan Electro Optic Laboratories, Akishima, Japan).
Viruses in cell culture fluid were examined by a negative-staining EM as previously described (Hsiung, 1982). Viral particles were pelleted by centrifugation using SW41 rotor in an ultracentrifuge (Optima LE 80K; Beckman, Fullerton, CA, USA) for 3 h at 160,000 × g and resuspended in a small quantity of PBS. Ten microliters of the virus suspension were applied to the carbon-coated grid. Excessive PBS was dried out with an absorbent paper and stained with 4% potassium phosphotungstate acid (PTA) for 5 min at ambient temperature. Grids were then examined under an electron microscope and digital images of virions were captured using a JEOL 1200EX scanning and transmission electron microscope (Japan Electro Optic Laboratories).
2.7. Effect of DNA inhibitors on virus growth
Mitomycin C and 5-bromo-2-deoxyuridine (BUDR) are known to inhibit the replication of DNA viruses in cells. To determine if Virus X contains a DNA genome, virus titration was performed using a microtitration infectivity assay in the presence and absence of these chemicals as previously described (Benfield et al., 1992). In brief, confluent monolayer of CL ISUVDL33 and CL ISUVDL99 cells were prepared in 96-well plates and inoculated with virus suspensions (100 μl/well) which were prepared by a serial 10-fold dilution technique. After 1-hr of absorption at 37 °C, the virus inoculum was removed and cells replenished with MEM supplemented with 40 or 160 μg/ml of BUDR (Sigma Chemical Co.) or 2 or 20 μg/ml of mitomycin C (Sigma Chemical Co.). Plates were incubated for an additional 48 h; virus titer was determined by the presence of visible CPE and then confirmed by IFA staining using rabbit antiserum raised against virus isolate designated ISUYP604671 from a PRNS case. As controls for RNA and DNA viruses, PRRSV and PRV, respectively were concurrently titrated under the same conditions described above.
2.8. Animal inoculation studies
2.8.1. Inoculation of pregnant sows at 30 days of gestation
Seven sows at 4th or 5th parity were purchased from a high-health-status commercial vendor and transferred to an animal holding facility with farrowing crates and bred through artificial insemination. The facility was operated and managed at BSL2 compliance. Once pregnancy was confirmed by an ultrasound technique, 5 sows were selected and used for the study. Four of the 5 sows at 30 days of gestation were inoculated intranasally, subcutaneously and intramuscularly with one of the following biological materials: (a) cell culture fluid containing virus isolate designated ISUYP604671; (b) homogenate of tissues collected from a clinically affected sow; (c) serum collected from the clinically affected sow. All inoculated animals were kept in the same room but individually in farrowing crates for 30 days. The one remaining sows served as sham-inoculated control and was kept in a separate room. After inoculation, all animals were monitored for changes in behavior and any reproductive problems. In addition, the sows were bled every seven days. At the termination of the study, all sows were euthanized, and various tissues (brain, tonsil, lung, spleen, lymph nodes, kidney, liver, placenta, spinal cord, uterus, uterine fluid) and, if present, fetuses were collected from each sow for histopathology, virus isolation and/or serology.
2.8.2. Inoculation of CDCD pigs
Seven 7-week-old caesarian-derived-colostrum-deprived (CDCD) pigs were used to evaluate the clinical effect of Virus X on young swine. Animals were divided to 3 groups. Group 1 (n = 3) was inoculated with cell culture material containing Virus X (ISUYP604671) via intranasal, intramuscular and intravenous routes. Group 2 (n = 3) was inoculated via the same routes with serum from an animal (Sow 800) which was experimentally infected with the virus in the previous animal study. Group 3 (n = 1) was inoculated with virus-free cell culture fluid and served as sham-inoculated negative control. All animals were monitored for the first 2 weeks after inoculation for changes in rectal temperature and behavior and for clinical signs. One pig per group except group 3 was euthanized and necropsied on days 7, 10 and 14 PI. Tissues (brain, tonsil, lung, spleen, kidney, lymph nodes) were collected from each pig for both virus assays and histopathology. Animals were bled at days 0, 7, 10 and 14 PI to collect whole blood and serum. At day 14 PI all remaining animals were euthanized and necropsied.
2.9. Histopathology
Tissues were fixed by immersing in 10% neutral buffered formalin immediately after collection. Fixed tissues were processed, embedded in paraffin, and sectioned according to the standard protocol established at ISU-VDL. Sections were then stained with hematoxylin and eosin for microscopic examination.
3. Results
3.1. Pathological evaluation of clinical cases
In many cases, external examination revealed trauma to the face and head of clinically affected pigs probably as a result of head-banging and head-pressing behaviors. Traumatic lesions were also seen in the extremities or shoulders possibly due to ataxia or recumbency.
While affected animals showed distinct clinical signs, gross lesions were not present except for the presence of petichial hemorrhage in lymph nodes and sometimes on the serosa of many organs. Microscopically, some lesions limited to the brain were detected as mild focal to locally extensive nonsuppurative perivascular cuffing, gliosis, and lymphoplasmacytic encephalitis. Hepatitis and interstitial pneumonia were other lesions frequently observed in the affected animals.
3.2. Isolation and preliminary characterization of virus isolates
A cytopathic agent that could pass through a 0.22 μm membrane filter was repeatedly and consistently isolated from serum, tonsil, lymph nodes and/or brain from clinically affected animals. Because of its unknown identity, the agent was tentatively named “Virus X”. Two initial isolates were designated ISU-KJY96 and ISUYP604671, respectively. Besides Virus X, several other viruses, such as PEV, porcine reovirus and PRRSV, were also inconsistently and infrequently isolated from suspect animals.
Morphologically, Virus X was an enveloped virus with the size of approximately 50–55 nm in diameter. As shown in Fig. 1 , the virion contains an icosahedral core and acquires its envelope by budding through the endoplasmic reticulum of infected cells. Overall, Virus X resembles members of the family Arteriviridae, Flaviviridae or Togaviridae.
Fig. 1.
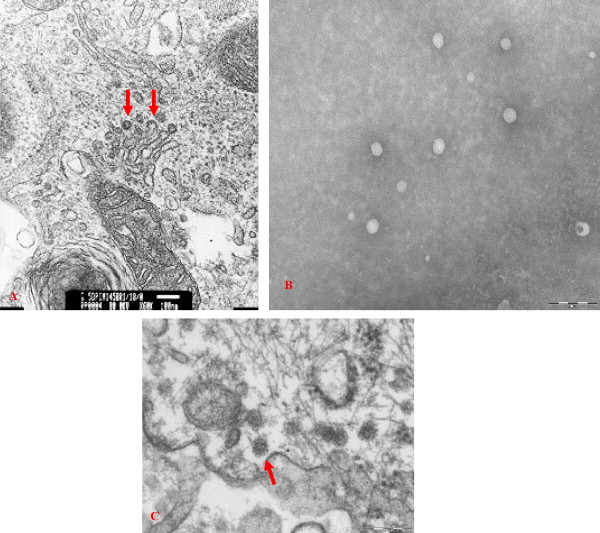
Electron photomicrographs of Virus X in CL ISUVDL13b cells (A) infected with isolate ISUYP604671, cell culture fluid (B) harvested from the infected cells and nerve cell (C) of the brain from an experimentally infected pig.
As summarized in Table 1 , infection and replication of the virus ISUYP604671, like PRRSV, was not negatively affected by the presence of BUDR and mitomycin C in cell culture media, whereas PRV replication was substantially inhibited by the treatment.
Table 1.
Effect of the presence of mitomycin C and 5-bromo-2 deoxyuridine (BUDR) in cell culture media on the replication of virus isolate (‘Virus X’) from PRNS case, pseudorabies virus (PRV) and type 2 porcine reproductive and respiratory syndrome virus (PRRSV) in vitro as determined by microtitration infectivity assay
| Virus | Type of nucleic acid | Media only | Media with BURD |
Media with mitomycin C |
||
|---|---|---|---|---|---|---|
| 40b | 160 | 2 | 20 | |||
| PRRSV | RNA | 106a | 106 | 106 | 106 | 106 |
| Virus X | Unknown | 104 | 104 | 104 | 104 | 104 |
| PRV | DNA | 108 | 106 | 103 | 106 | 103 |
Resulting virus titer (TCID50/ml) of the stock virus in the presence and absence of each chemical in the media.
Concentration (μg/ml) of each chemical in cell culture media.
Various cell lines and primary cells of porcine origin were permissive to Virus X and supported productive infection of the virus (Fig. 2 ). On immunofluorescence tests, Virus X did not cross-react with antibodies raised against PRRSV (type 1 and 2), PRV, SIV (both H1 and H3), TGEV, PPV, PCV (type 1 and 2), porcine reovirus, EMCV, PEV, HEV, PCMV and rabies virus. Interestingly, the virus appeared to be recognized to a degree by the polyclonal antiserum specific for BVDV as weak positive fluorescence was observed when Virus X infected cells were stained with the serum (Fig. 3 ). However, PCR results for 5′UTR did not support that Virus X was BVDV (both type 1 and 2) or CSFV. PCR assays also demonstrated that Virus X was not PRRSV (neither North American nor European genotypes), PCV (neither type 1 nor 2), PEV (groups 1, 2 and 3), influenza A virus, TGEV, PRCV, PEDV, PPV, sHEV, WNV, JEV, PERV, PLHV-1 or alpha-togaviruses.
Fig. 2.
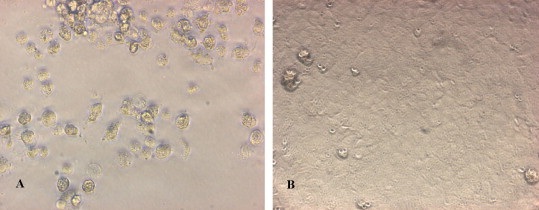
Cytopathology in cell line CL ISUVDL44 infected with Virus X (A) in comparison to uninfected cells (B) at 200× magnification.
Fig. 3.
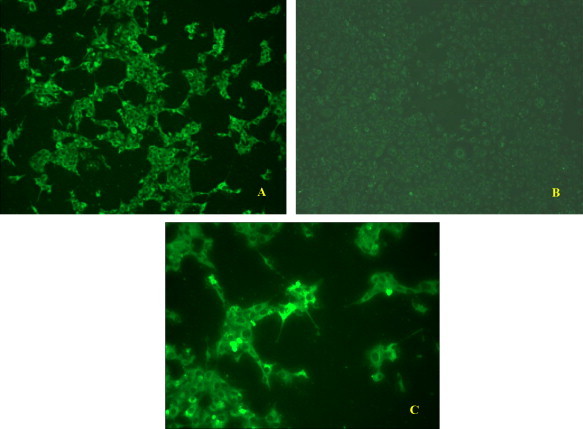
Immunofluorescence photomicroscopy of CL ISUVDL13b cells infected (A, C) and not infected (B) with Virus X. The cells were fixed at approximately 48 h post inoculation and stained with an anti-BVDV bovine polyclonal serum. Panel A and C are presented at 100× magnification, whereas panel C is presented at 200× magnification.
3.3. Outcome of experimental inoculation
3.3.1. Sow study
Clinical, pathological and virological observations on pregnant sows after experimental inoculation are summarized in Table 2 . During a 30-day observation period, inoculated sows became viremic. Yet, all animals remained normal in their behavior during the study period. No abortion was observed in any of the inoculated sows.
Table 2.
Summary of reproductive record and pathological lesions of sows experimentally infected with Virus X isolate ISUYP604671 at early gestation
| Sows (inoculum) | Average # of litter in previous gestations | No. of fetuses recovered at necropsy | Significant gross lesionsa | Significant microscopic lesionsa | Recovery of Virus X from sow/fetus |
|---|---|---|---|---|---|
| 800 (serum) | 11.4 | 3 | Yes | Yes | Yes/Yes |
| 21622 (virus isolate-P2) | 13.3 | 0 | No | No | Yes/NAb |
| 30021 (virus isolate-P3) | 11.2 | 13 | No | No | Yes/Yes |
| 30172 (virus isolate-P1) | 8.4 | 10 | Yes | Yes | Yes/Yes |
| 30120 (sham inoculum) | 10.2 | 6 | No | No | No/No |
The virus inocula were prepared at various cell passages (P).
Significant gross lesions were hemorrhages in inguinal lymph node, decolorization of uterus (green and brown) and possible embryonic death of fetuses. Microscopically, necrotic edema in the lymph node, mineralization plaques in uterus and necrotic debris in the lumen were observed.
NA: not applicable.
At the termination of the study, no lesions were observed in the control sow. In contrast, gross lesions, such as hemorrhages in inguinal lymph node and decolorization of the uterus (green and brown), were observed in the sows inoculated with materials containing Virus X (ISUYP604671). Embryonic or fetal death was also apparent in some of the inoculated sows. One of the 4 inoculated sows did not have any fetus at necropsy although no significant lesions were observed. Microscopically, necrotic edema in the lymph node, mineralization plaques in uterus and necrotic debris in the lumen of the uterus were observed, supporting the possibility of embryonic death and/or fetal reabsorption.
Virus X was recovered from tissues (spleen, liver, jejunum, uterus, serum, tonsil) of the inoculated sows, confirming the replication of Virus X in the animals, particularly in secondary lymphoid organs. In addition, the virus was also isolated from fetal tissues such as lung, spleen, heart and kidney, suggesting transplacental transmission of the virus.
3.3.2. CDCD pig study
All inoculated pigs became viremic during a 14-day observation period but did not show any overt clinical signs by the end of the study. The sham-inoculated negative control pigs remained negative for Virus X in the blood (Table 3 ).
Table 3.
Summary of clinical, pathological and virological observations on 7-week-old caesarian-derived-colostrum-deprived pigs infected with Virus X isolate (ISUYP604671), clinical specimen (serum) containing the virus or cell culture medium
| Pig ID | Inoculums | DPI at necropsya | Clinical signs | Lesionsb | Detection of Virus X in serum/tissue |
|---|---|---|---|---|---|
| 274 | Serum 604671 | 7 | No | Yes | Positive/positive |
| 275 | Isolate ISUYP604671 | 7 | No | Yes | Negative/positive |
| 302 | Isolate ISUYP604671 | 10 | No | No | Positive/positive |
| 303 | Serum 604671 | 14 | No | Yes | Positive/positive |
| 304 | Serum 604671 | 10 | No | Yes | Positive/positive |
| 675 | Isolate ISUYP604671 | 14 | No | Yes | Positive/positive |
| 273 | Medium | 14 | No | No | Negative/negative |
Day post inoculation.
Gross lesion: hemorrhagic, enlarged and swollen lymph node. Microscopic lesions: multifocal perivascular accumulations of mononuclear cells in meninges, mild edema with occasional degenerating neurons, interstitial pneumonia.
At each necropsy, significant gross lesions were not apparent although spleen and lymph nodes collected from pigs at days 7 and 10 PI looked edematous. Microscopically, nonsuppurative meningoencephalitis was observed in 5 of the 6 inoculated pigs (Fig. 4 ). Nonsuppurative interstitial pneumonia (Fig. 5 ) and nonsuppurative periportal hepatitis were other lesions commonly observed in the inoculated pigs. Neither gross nor microscopic lesions were present in tissues/organs of the control pig.
Fig. 4.
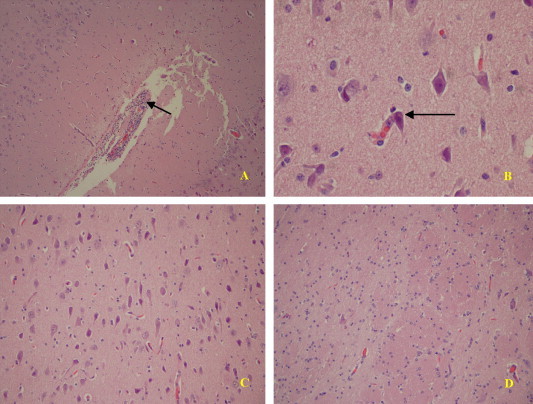
Photomicrographs of representative lesions in brains from CDCD pigs inoculated with Virus X. (A) Moderate multifocal perivascular accumulations of mononuclear cells in meninges. (B) Occasional neurons appear in early stages of necrosis. (C) Mild edema with occasional degenerating neurons. (D) Normal brain tissue.
Fig. 5.
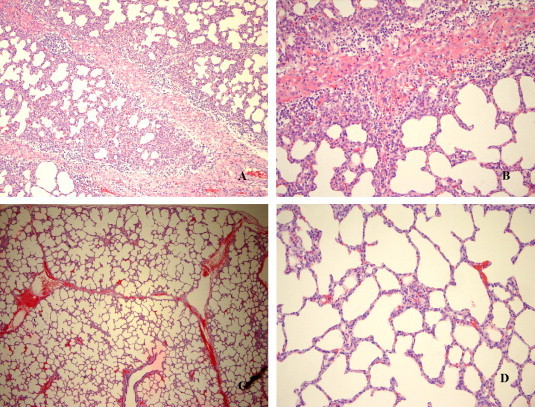
Photomicrographs of representative lesions in lung tissues from CDCD pigs experimentally infected animals with Virus X. (A) Interstitial pneumonia with infiltration of mixed but primarily mononuclear cells in interlobular septa as well as interstitial space. (B) Extensive nonsuppurative interstitial pneumonia with congestion. (C and D) Section of normal lung at different magnifications (C) at 100× magnification and (D) at 200× magnification.
Virus X was recovered from tissues of all inoculated pigs regardless of the presence or absence of detectable lesions. Brain tissues from 4 of the 5 pigs with meningitis or encephalitis were positive for Virus X. The presence of virus particles with morphological characteristics similar to Virus X in brain tissues was confirmed by EM (Fig. 1). The virus was most frequently recovered from tonsils. Spleens and lymph nodes also harbored the virus.
4. Discussion
Porcine reproductive and neurologic syndrome brought a challenge to the diagnostic community since no definitive diagnosis as to the cause could be made. Although death loss and reproductive failure in breeding females is not an unusual event in many farms for various reasons, clinical presentations of reproductive problems along with neurologic disorders were a unique feature of PRNS. Unfortunately, diagnostic investigations focusing on all conventional infectious agents that had been implicated in reproductive and/or neurologic problems of pigs at various ages has been unfruitful or yielded inconsistent results. With the hypothesis that a previously unknown infectious agent could be responsible for the disease, extensive laboratory testing resulted in frequent isolation of a relatively small enveloped cytopathic virus (‘Virus X’) from clinically affected animals. Although one might say that Virus X is not necessarily a causative pathogen, isolation of the virus from nervous tissues collected from clinically ill animals with CNS signs is a convincing evidence suggesting that Virus X was likely responsible for the disease. Observations from the animal inoculation study with pregnant sows clearly indicate that Virus X is capable of crossing the placental barrier and causing the loss of pregnancy most likely due to embryonic or fetal death as evident by recovery of the virus from fetal tissues. Furthermore, development of meningitis or encephalitis lesions in the brains of young CDCD pigs after inoculation with Virus X also indicate that the virus may be able to cause neurologic disorder in infected animals, although the inoculated CDCD pigs did not show clinical central nervous signs under experimental conditions. Those lesions were due to the attack by Virus X since the virus was recovered from brain tissues. Since all these experimental findings match well with field observations of PRNS, Virus X is postulated to be the causative agent for PRNS as Koch's postulates were partially fulfilled. It is proposed then that Virus X shall be named “PRNS virus (PRNSV)”.
Under a laboratory setting, PRNSV could not be readily recognized by antibodies raised against various viral agents that have been implicated in diseases of swine except anti-BVDV polyclonal antibody. The positive cross-reactivity of PRNSV with anti-BVDV antibody is of particular interest with respect to taxonomical identification of the virus. The possibility that PRNSV could be CSFV was ruled out at the beginning of the investigation since US swine populations are known to be free of CSFV. Furthermore, diagnostic testing on some early PRNS suspect cases at the Foreign Animal Disease Diagnostic Laboratory of the National Veterinary Laboratory Service ruled out the presence of CSFV or its infection in those animals as well as the involvement of other foreign animal disease agents (Data not shown). Pigs are known to be susceptible to ruminant pestiviruses such as BVDV and BDV besides porcine pestivirus (i.e., CSFV). Although BVDV infection of pigs has been demonstrated both in the field and under experimental conditions, BVDV has not been considered to be a significant pathogen for pigs. Considering that PRNSV is readily spread among pigs and there is very minimum contact between pigs and ruminants in the US, it would be hard to believe that PRNSV is BVDV or BDV. This argument is supported by negative PCR results (5′ UTR) on isolates of PRNSV for BVDV and CSFV. However, based on its morphological similarity with pestiviruses and cross-reactivity with anti-BVDV antibody, the possibility that PRNSV is a pestivirus cannot be completely ruled out until proven otherwise. At the time of writing this manuscript, PRNSV is proposed to be a novel swine pestivirus which remains to be further characterized for its conclusive taxonomical identification. As a pestivirus is suspected to be responsible for PRNS, development of a specific diagnostic reagent for PRNSV is prudent.
References
- Akiyoshi D.E., Denaro M., Zhu H., Greenstein J.L., Banerjee P., Fishman J.A. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J. Virol. 1998;72:4503–4507. doi: 10.1128/jvi.72.5.4503-4507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Robison D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Ellis J., Krakowka S., Lairmore M., Haines D., Bratanich A., Clark E., Allan G., Konoby C., Hassard L., Meehan B., Martin K., Harding J., Kennedy S., McNeilly F. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J. Vet. Diagn. Invest. 1999;11:3–14. doi: 10.1177/104063879901100101. [DOI] [PubMed] [Google Scholar]
- Harmon K.M., Yoon K.-J. Application of PCR assay to differentiate two subtypes of swine influenza viruses. Swine Res. Rep. 1999:180–182. [Google Scholar]
- Hofmann M.A., Brechtbuhl K., Stauber N. Rapid characterization of new pestivirus strains by direct sequencing of PCR-amplified cDNA from the 5′ noncoding region. Arch. Virol. 1994;139:217–229. doi: 10.1007/BF01309467. [DOI] [PubMed] [Google Scholar]
- Hsiung G.D. third ed. Yale University Press; New Haven and London: 1982. Diagnostic Virology. pp. 68–86. [Google Scholar]
- Pogranichnyy R.M., Yoon K.-J., Harms P.A., Swenson S.L., Zimmerman J.J., Sorden S.D. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral. Immunol. 2000;13:143–153. doi: 10.1089/vim.2000.13.143. [DOI] [PubMed] [Google Scholar]
- Powers A.M., Brault A.C., Shirako Y., Strauss E.G., Kang W., Strauss J.H., Weaver S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001;75:10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridpath J.F., Bolin S.R. Differentiation of types 1a, 1b and 2 bovine viral diarrhoea virus (BVDV) by PCR. Mol. Cell. Probes. 1998;12:101–106. doi: 10.1006/mcpr.1998.0158. [DOI] [PubMed] [Google Scholar]
- Scaramozzino N., Crance J.M., Jouan A., DeBriel D.A., Stoll F., Garin D. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J. Clin. Microbiol. 2001;39:1922–1927. doi: 10.1128/JCM.39.5.1922-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek S., Herring A.J., Herring J.A., Nettleton P.F., Lowings J.P., Paton D.J. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 1994;136:309–323. doi: 10.1007/BF01321060. [DOI] [PubMed] [Google Scholar]
- Wirz B., Tratschin J.D., Muller H.K., Mitchell D.B. Detection of hog cholera virus and differentiation from other pestiviruses by polymerase chain reaction. J. Clin. Microbiol. 1993;31:1148–1154. doi: 10.1128/jcm.31.5.1148-1154.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K.-J. An unconventional reproductive disease in sows: clinical and diagnostic perspectives. Proceedings of the 11th Swine Disease Conference; Iowa State University, Ames, Iowa; 2003. p. 145. [Google Scholar]
- Yoon K.-J., Zimmerman J.J. Possible role of infectious agents in early infertility. Proceedings of the 6th Swine Disease Conference; Iowa State University, Ames, Iowa; 1998. p. 23. [Google Scholar]
- Yoon K.-J., Zimmerman J.J., Chang C.C., Cancel-Tirado S., Harmon K.M., McGinley M.J. Effect of challenge dose and route on porcine reproductive and respiratory syndrome virus (PRRSV) infection in young swine. Vet. Res. 1999;30:629–638. [PubMed] [Google Scholar]
- Zell R., Krumbholz A., Henke A., Birch-Hirschfeld E., Stelzner A., Doherty M., Hoey E., Dauber M., Prager D., Wurm R. Detection of porcine enteroviruses by nRT-PCR: differentiation of CPE groups I-III with specific primer sets. J. Virol. Methods. 2000;88:205–218. doi: 10.1016/s0166-0934(00)00189-0. [DOI] [PubMed] [Google Scholar]


