Abstract
We report the genetic and biological characterisation of a novel pantropic canine coronavirus (CCoV), strain 450/07, which caused the death of a 60-day-old miniature pinscher. At the genetic level, this virus was strictly related to the prototype strain CB/05, but displayed some unique features. After experimental infection with the new pantropic isolate, most inoculated dogs showed diarrhoea and acute lymphopenia. Gross lesions and histological changes were mainly evident in the gut and lymphoid tissues, although some animals showed remarkable changed also in parenchymatous organs. The viral RNA was detected in the faeces and/or internal organs of most pups. These findings seem to indicate that strain 450/07 is able to spread to internal organs (mainly lymphoid tissues), causing lymphopenia but inducing a mild disease.
Keywords: Pantropic canine coronavirus, Sequence analysis, Biological characterisation
1. Introduction
Coronaviruses are large, enveloped, single-stranded RNA viruses that cause respiratory and/or enteric disease in mammals and birds. In dogs, three coronaviruses have been described so far. Canine coronavirus (CCoV) type I (CCoV-I) and type II (CCoV-II) are enteric viruses belonging to the old antigenic group 1 (Decaro and Buonavoglia, 2008, Decaro and Buonavoglia, 2011), recently classified as a new genus Alphacoronavirus (Carstens, 2009). According to the new taxonomy, CCoVs are included in the same species, Alphacoronavirus-1, as feline and some swine coronaviruses (Carstens, 2009). Canine respiratory coronavirus (CRCoV), first reported in UK by Erles et al. (2003), is a Betacoronavirus (Carstens, 2009) causing respiratory distress especially when associated to other pathogens (Buonavoglia and Martella, 2007, Decaro et al., 2007c). CCoV-I and CCoV-II are widely distributed in Europe (Decaro et al., 2010b, Decaro et al., 2011a) and are usually responsible for mild to moderate gastroenteritis in pups, although death can occur as a consequence of simultaneous infections by both genotypes (Pratelli et al., 2004, Decaro et al., 2005) or by other pathogens (Decaro et al., 2004a, Decaro et al., 2006, Decaro et al., 2007a, Decaro et al., 2007b). CCoV infection is usually restricted to the gastroenteric tract, and associated with anorexia, diarrhoea and vomiting (Tennant et al., 1991). However, in 2005 a fatal outbreak of CCoV-II infection was reported in pups housed in a pet shop of Apulia region, Italy (Buonavoglia et al., 2006). Coronavirus RNA (strain CB/05) was detected in the internal organs of the pups, presenting severe gross lesions, while other canine pathogens were not detected. Experimental infections of seronegative dogs reproduced the disease with the severity varying with the age of the infected animals (Buonavoglia et al., 2006, Decaro et al., 2008). In addition, strain CB/05 was able to infect dogs with immunity to enteric CCoVs, causing both enteric and systemic signs, chiefly lymphopenia (Decaro et al., 2010a). Subsequent experiments revealed that lymphopenia is the most prominent finding after infection by pantropic CCoV, with a marked long-lasting depletion of the circulating CD4+ T lymphocytes (Marinaro et al., 2010). Upon genomic analysis, strain CB/05 showed a high degree of homology to reference CCoV strains (Decaro et al., 2007d). Unique point mutations were present at the N-terminus of the spike (S) protein, including an Asp/His to Asn change at position 125. In addition, a unique 38-nucleotide deletion was mapped within ORF3b.
In the present paper, the isolation of a novel pantropic CCoV strain is reported. The strain was characterised molecularly by sequencing the 3′ end of the RNA genome. Also, the virus was characterised biologically by experimental infection of seronegative dogs.
2. Methods
2.1. Clinical case
In October 2007, a 60-day-old miniature pinscher died from systemic disease characterised by fever (39.5–40 °C), leucopenia (4.7 × 109 cells/L, reference range 6–17 × 109 cells/L), mainly lymphopenia (0.5 × 109 cells/L, reference range 1.0–4.8 × 109 cells/L), lethargy, anorexia, vomiting and haemorrhagic diarrhoea. One week before death, the pup had been bought in a pet shop in Apulia region, Italy, and ten days before had been administered a single dose of a vaccine containing canine parvovirus, canine distemper virus, canine adenovirus type 2, Leptospira canicola and Leptospira icterohaemorrhagie. Supportive therapy with antibiotics (amoxicillin/clavulanic acid) and fluids (lactated Ringer's solution) was not effective and the clinical conditions continued to deteriorate until the pup died.
Necropsy revealed gross lesions in the alimentary tract (haemorrhagic enteritis), tonsils (necrotic areas), lungs (fibrinous pneumonia), liver (enlargement and areas of necrosis), spleen (enlargement) and kidneys (degeneration of the cortex). At post-mortem examination, samples were collected from brain, lungs, spleen, liver, mesenteric lymph nodes, kidneys, and intestinal content for molecular and virological investigations. Histopathology was not carried out as all tissue samples were frozen at −20 °C.
2.2. Molecular and virological investigations
After DNA and RNA purifications using commercial kits, intestinal contents and tissue samples collected from the dead dog were screened for major canine viral pathogens, as previously described (Decaro et al., 2007d). Characterisation of the CCoV genotypes was carried out by a type-specific real-time RT-PCR assays (Decaro et al., 2005).
The sequence of the 3′ end of the genome of the pantropic CCoV strain detected in the lungs was determined as previously described (Decaro et al., 2007d) and registered in GenBank (http://www.ncbi.nlm.nih.gov) under accession number GU146061. Phylogenetic and molecular evolutionary analyses were conducted using Mega4.0 (Tamura et al., 2007). Phylogenetic trees, based on the spike (S) and membrane (M)-nucleocapsid (N) proteins of strain 450/07 were elaborated using both parsimony and neighbour-joining methods, supplying a statistical support with bootstrapping over 1000 replicates.
The lung of the pup was homogenised (10%, w/v) in D-MEM with antibiotics (penicillin 5000 IU/mL, streptomycin 2500 μg/mL, amphotericin B 10 μg/mL), and inoculated into canine fibroma (A-72) cells. Infected cells were monitored daily for the occurrence of cytopathic effect (CPE) and, after 5 days of incubation, they Alphacoronaviurs-1 antigen was detected by an immunofluorescence (IF) assay using a monoclonal antibody targeting the N protein (courtesy of Dr. Gill Chappuis, Merial, France).
2.3. Experimental infection of dogs
Nine 10-to-11-week-old CCoV-seronegative beagles from the same litter were used for this challenge study after a 7-days acclimatisation period (authorisation no. 57/2006-C from Italian Ministry of Health). Each dog was individually housed for the administration of the challenge and to monitor clinical signs. All animals were weighed on study days −3, −1, 3, and 5. On study day 0, the six dogs in treatment group T02 were challenged with a total volume of 4 mL (3.0 mL orally and 1.0 mL intra-nasally; 0.5 mL per nostril) containing 105 TCID50/mL of strain 450/07 (3rd passage on A-72 cells). Three control dogs in group T01 received the same volume of a cryolysate of uninfected A-72 cells by the same route of administration. All dogs were monitored daily for clinical signs by the attending veterinarian starting on day −1 (before challenge) and for as long as the animals remained in the study. The general health of each animal was assessed using the scoring system described in Table 1 . EDTA-blood samples were collected from the jugular vein on study days −3, 0 (pre-challenge), 3, and 5 (day of necropsy) for complete and differential blood cell counts. Rectal temperatures (°C) were measured at arrival, on study day −3, and then daily until study day 5. Nasal and rectal swabs were collected on study days −3, −1, 3, and 5.
Table 1.
Scoring system for general health observations during the challenge trial.
| Parameter | Result | Score |
|---|---|---|
| General appearance | Normal | 0 |
| Depressed/lethargic | 2 | |
| Difficulties in breathing | 3 | |
| Death | 20 | |
| Dehydration | None (<4%): not detectable | 0 |
| Mild (4–5%): subtle loss of skin elasticity | 1 | |
| Moderate (6–8%): definite delay in return of skin to normal position; eyes possibly sunken in orbits; slightly prolonged capillary refill time; possibly dry mucous membranes | 2 | |
| Severe (10–15%): tented skin standing in place; prolonged capillary refill time; eyes sunken in orbits; dry mucous membranes possible signs of shock (increased heart rate, weak pulses); death imminent | 3 | |
| Diarrhoea | None | 0 |
| Soft | 1 | |
| Liquid | 2 | |
| Bloody | 3 | |
| Rectal temperature | 37.0–39.4 °C | 0 |
| ≥39.5 °C | 2 | |
| <37.0 °C | 3 | |
| Body weight | Weight loss | 2 |
| No weight loss | 1 | |
| Weight gain | 0 |
Five days post-challenge, all dogs were sedated by intravenous administration of 10 mg/kg of body weight of Zoletil® 100 (Virbac S.r.l., Italy) and euthanised by intravenous administration of 0.5 mL/kg of body weight of Tanax® (Intervet Italia, Italy). During necropsy, samples for histopathology and IHC were collected from small intestine, mesenteric, intermandibular and popliteal lymph nodes, lung, liver, spleen, and kidney, and stored in formalin. Fresh tissue samples were also collected in D-MEM and RNA Later Solution (QIAGEN S.p.A.) for viral isolation and real-time RT-PCR assays, respectively, and kept at −70 °C until used. Formalin-embedded samples were cut in 3-μm sections and stained with haematoxylin–eosin (H&E) for histological examination or treated with an anti-Alphacoronavirus-1 monoclonal antibody binding to the N protein for immunohistochemistry (IHC) (Buonavoglia et al., 2006). EDTA-blood samples and faecal swabs collected intra-vitam, as well as tissue samples collected at necropsy examination, were tested for CCoV by virus isolation on A-72 cells and/or by real-time RT-PCR (Decaro et al., 2004b).
3. Results
3.1. Identification of a CB/05-like strain in internal organs of the dead dog
The intestinal content and tissue samples of the dead dog (450/07) tested negative for all viral pathogens with the eccepition of CCoV. By real-time RT-PCR (Decaro et al., 2004b), the highest viral RNA load (5.81 × 106 RNA copies/μL of template) was found in the lung sample, but the virus was also detected in the intestinal content (8.57 × 105 RNA copies/μL of template), mesenteric lymph nodes (2.31 × 105 RNA copies/μL of template), spleen (1.78 × 104 RNA copies/μL), liver (3.43 × 103 RNA copies/μL), kidney (7.09 × 103 RNA copies/μL). Only traces of CCoV RNA (4.36 × 101 RNA copies/μL) were detected in the brain sample. The virus (strain 450/07) was characterised as CCoV-II by means of the genotype-specific TaqMan assays (Decaro et al., 2005) and as CCoV-IIa by means of subgenotype-specific RT-PCR assays (Decaro et al., 2010b).
The lung sample was used for virus isolation as it contained the highest viral load. Viral growth was observed by the first passage, as CPE appeared in the infected cells and CCoV antigen was detected by IF staining. Three serial passages were carried out in order to obtain a stock virus (105 TCID50/mL) of strain 450/07 for the challenge experiment. The stock virus was tested for the presence of other canine viral pathogens and for sterility from aerobe and anaerobe bacteria, mycoplasmas and mycetes and no contaminant agent was detected.
The 3′ end of the genome of strain 450/07 was amplified from the lung material through amplification of seven overlapping fragments. The obtained sequence was 8618 nt long and contained the full length of ORFs 2, 3a, 3b, 3c, 4, 5, 6, 7a and 7b. The S protein (ORF2 product) was 1454 aa in length and it was highly related (99.5% aa identity) to the prototype pantropic strain CB/05. A high degree of genetic relatedness was found also in the other structural proteins, including the envelope (E, 82 aa, 100% aa identity), membrane (M, 262 aa, 99.2% identity) and nucleocapsid (N, 382 aa, 99.7% identity) proteins, encoded by ORFs 4, 5, and 6, respectively. The products of ORF 3a (71 aa), 3b (22 aa), 7a (101 aa) and 7b (213 aa) were intact with respect to the prototype strain CB/05. ORF3b contained the same 38-nt deletion as strain CB/05, but an additional 164-nt deletion was present in ORF3c. This deletion altered the frame shift, introducing an early stop codon at nt 4824 and affecting the correct synthesis of the ORF3c product.
Upon phylogenetic analysis, strain 450/07 clustered with strain CB/05 in the S (Fig. 1 ) and M/N protein sequences. Genetic relatedness with the recombinant CCoV/transmissible gastroenteritis virus strains (CCoV-IIb) (Decaro et al., 2009, Decaro et al., 2011b) was also evident in the M/N sequences (data not shown).
Fig. 1.
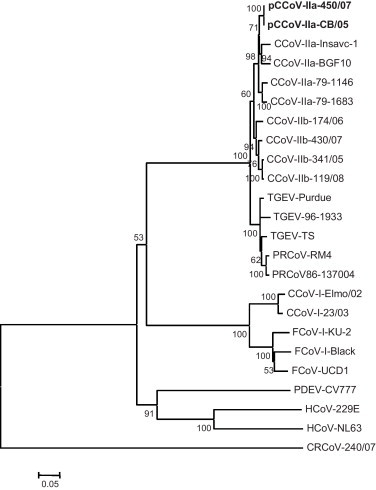
Neighbour-joining tree based on the spike protein of members of genus Alphacoronavirus. For phylogenetic tree construction, the following CoV strains were used (GenBank accession numbers are reported in parentheses): porcine transmissible gastroenteritis virus (TGEV) Purdue (NC_002306), TS (DQ201447), 96-1933 (AF104420); porcine respiratory coronavirus (PRCoV) RM4 (Z24675), 86-137004 (X60089); CCoV-IIa CB/05 (DQ112226), Insavc-1 (D13096), BGF10 (AY342160); CCoV-IIb 341/05 (EU856361), 174/06 (EU856362), 430/07 (EU924790), 118/08 (EU924791); CCoV-I Elmo/02 (AY307020), 23/03 (AY307021); feline coronavirus (FCoV) type I Black (EU186072), KU-1 (D32044), UCD-1 (AB088222); FCoV-II 79-1146 (NC_007025), 79-1183 (X80799); HCoV-229E (NC_002645), PEDV-CV777 (NC_003436), HCoV-NL63 (NC_005831). The tree is rooted on the Betacoronavirus canine respiratory coronavirus (CRCoV) 240/05 (EU999954). A statistical support was provided by bootstrapping over 1000 replicates. The scale bars indicate the estimated numbers of amino acid substitutions per site.
3.2. Clinical, post-mortem, histopathological and virological findings in experimentally infected dogs
All six dogs in T02 and one of the three control dogs in T01 lost weight on day 3 after challenge compared to their (baseline) weight on day −1. Only one dog from T02 group had fever on day 4, but three more dogs in T02 showed a numerical increase in their rectal temperature within the normal range after challenge. This change in range was not observed in T01 control dogs. Two dogs in group T02 were found depressed or lethargic on days 3 and 4 after challenge and two dogs of the same group were mildly to severely dehydrated on days 3 and 4 after challenge. Soft faeces or diarrhoea were observed in four T02 dogs on days 3 and 4 after challenge. All six dogs in T02 had lymphadenomegalia on days 3, 4 or 5 in one of the dogs, on days 4 and 5 in two dogs and on day 5 in the other three dogs. One T02 dog had also nasal secretion on days 0, 1 and 4 post challenge and another challenged dog had decreased capillar refill and pale mucosa on day 5. Based on the scoring system reported in Table 1, two of the three control pups had a clinical score of zero because they did not show any clinical signs. One of the T01 dogs lost weight on days 3 and 5 after challenge but did not show any other clinical sign. This pup had therefore a score of 4, with its group showing a mean score of 1.3. However, all six pups in T02 group showed a variety of clinical sings, which lasted between 1 and 3 days and therefore their scores ranged from 2 to 19 (mean 5.7).
Day −3 and day 0 lymphocyte and WBC counts per μL were averaged and were used as baseline levels to compare to the day 3 post-challenge levels. Lymphocyte counts decreased ≥30% in five dogs in T02, but less than 30% in the rest of dogs in T02 and T01. WBC counts decreased ≥30% in four of six dogs in T02, but less than ≥30% in the dogs left in T02 and in all the control dogs. The reduction in the lymphocyte counts was particularly dramatic for one T02 dog (75% with respect to the baseline values), that was the one that started showing clinical signs earlier and also showed the most severe clinical signs.
At necropsy, controls did not show any remarkable lesion, whereas four infected pups displayed mild to severe enteritis in one or more intestinal segments and five-presented enlargement of the mesenteric lymph nodes, that were congested and with scattered haemorrhages. Splenomegaly and involvement of popliteal and/or intermandibular lymph nodes were observed in one and two T02 pups, respectively. Lung congestion and haemorrhages on the thymus were detected only in one infected pup, whereas in four pups of group T02 abundant abdominal fluid was present.
H&E staining of intestinal sections from all infected pups showed diffuse lymphocytic and plasmacellular infiltration of the lamina propria with fusion of the duodenal villi and focal ectasia of the cryptae containing desquamated epithelial cells (Fig. 2a). A diffuse lymphocytic depletion was evident in the mesenteric lymph nodes and/or spleen of these animals, with two pups displaying the same histologic changes also in the popliteal lymph nodes. Lung sections from one pup displayed marked leucocyte infiltration of alveolar walls, capillary congestion, multifocal hyperplasia of myocytes, and focal hyperplasia of the bronchial and bronchiolar epithelia; rare macrophages, erythrocytes and desquamated epithelial cells were present in the alveolar lumen (Fig. 2b). Liver and kidney displayed some histopathological changes only in three and two pups, respectively, that consisted of fatty degeneration of hepatocytes and multifocal hyperaemia and ectasia of the renal cortex. By IHC, Alphacoronavirus-1 N antigen was found to be widespread in the apical cells of the intestinal villi (Fig. 2c) and mesenteric lymph nodes of five T02 pups, with positive cells being also detected in the lungs, spleen and popliteal lymph nodes (Fig. 2d) of some pups. The liver and lung tissues of all pups tested IHC negative for coronaviral antigen. No remarkable histopathological changes neither IHC detection of coronaviral antigen were evident in T01 animals.
Fig. 2.
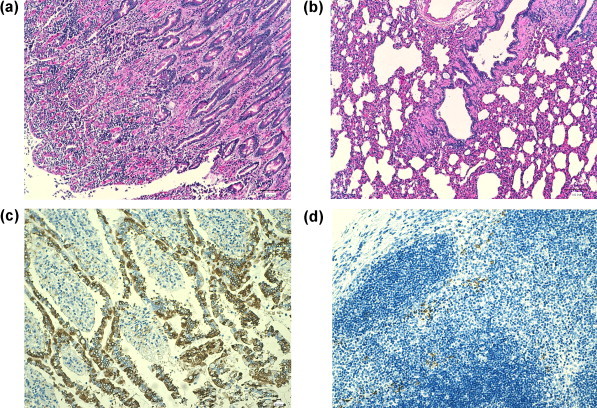
Histopathological (a, b) and immunohistochemical (c, d) findings in pups experimentally infected with strain 450/07. (a) Jejunum of dog 9319: fusion and desquamation of intestinal villi and inflammatory cell infiltration in the lamina propria (H&E, 10×). (b) Lung of dog 9314: diffuse leucocyte infiltration of alveolar walls and epithelial desquamation (H&E, 10×). (c) Ileum of dog 9319: Alphacoronavirus-1 antigen (brown stained) in epithelial cells (IHC with monoclonal antibody, 10×). (d) Popliteal lymph node of dog 9322: Alphacoronavirus-1 antigen (brown stained) in lymphocytic cells (IHC with monoclonal antibody, 20×).
By real-time RT-PCR (Decaro et al., 2004b), three T02 pups were excreting virus on days 3 and/or 5 in their faeces, with titres ranging from 2.75 × 103 to 1.07 × 107 CCoV RNA copies per μL of template, and the viral RNA was detected in only one day 5 blood sample of a pup in T02 (titre of 5.73 × 101 RNA copies per μL). As expected on the basis of the viral loads, CCoV was only isolated from a day 3 rectal swab from a pup in T02 group. Several tissues collected from five infected pups tested positive by real-time RT-PCR, with the highest titres being detected in the lymphoid tissues. CCoV was isolated from mesenteric lymph nodes in three dogs, from duodenum and jejunum in one dog and from ileum in two dogs from T02. Neither nasal swabs from T02 dogs nor any samples from T01 dogs tested positive to CCoV by real-time RT-PCR or virus isolation.
4. Discussion
In the present paper, we report the isolation of a novel pantropic CCoV strain from the lungs of a pup that died after systemic disease. The virus was characterised at the genetic level, displaying a close relatedness (more than 99% aa identity) to the prototype strain CB/05 in all the analysed proteins. Like strain CB/05, strain 450/07 displayed the unique mutation Asn-125 in the S protein, and the 38-nt deletion in ORF3b. These genetic signatures are not shared by all pantropic CCoV strains (Decaro et al., manuscript in preparation). An additional 164-deletion, affecting the protein frame shift, was mapped in ORF3c. Coronavirus accessory protein genes are believed to be dispensable for replication in vitro, but they are strictly maintained during infection of the natural hosts (Lorusso et al., 2008). In both the pantropic CCoV strains CB/05 and 450/07, the accessory protein 3b was 49 aa shorter than expected. In strain 450/07 the ORF3c product was likely not expressed due to the presence of an early stop codon in the 5′ end of the gene. Similar deletions in the accessory protein genes of feline coronavirus have been suggested to play a role in the enhanced virulence showed by the hypervirulent biotype feline infectious peritonitis virus (Haijema et al., 2007). Enterotropic CCoV-II strains genetically related to CB/05 have been detected in recent years, but none of them displayed deletions in the accessory protein genes (Decaro et al., 2007d). Thus, strain 450/07 may have originated from the prototype virus CB/05 through an additional 164-nt deletion in ORF3c.
At the biological level, the isolates CB/05 and 450/07 were both able to infect pups and to spread to their internal organs, causing systemic disease and lymphopenia, although the severity of clinical signs and involvement of lymphoid tissues seemed to be more pronounced in CB/05 infected dogs. These apparent variations could be accounted for by differences in the breed and age of the infected dogs, in the dose and cell passage of the viruses used for the challenge and in the clinical score adopted to evaluate the induced disease. Variations in virulence have been observed in different challenge experiments using the same batch of CB/05 isolate (Decaro et al., 2008, Marinaro et al., 2010). A comprehensive study using simultaneously both viruses would help evaluate more precisely if the two strains differ in their pathogenic potential.
The isolation of a new pantropic CCoV strain from a dead pup suggests that this variant is circulating in dogs, as described in recent investigations (Ntafis et al., 2012, Zicola et al., 2012), stressing the need to develop homologous vaccines on the basis of the poor cross-protection induced by enteric CCoV (Decaro et al., 2010a).
Acknowledgements
This work was supported by a Pfizer Animal Health grant. We thank Vincenzo Ferrara and Carlo Armenise for the supervision and management of the dogs used in this study. We also acknowledge Donato Narcisi and Arturo Gentile for their excellent technical assistance.
Footnotes
The DDBJ/EMBL/GenBank accession number of the sequence reported in this paper is GU146061.
References
- Buonavoglia C., Martella V. Canine respiratory viruses. Vet. Res. 2007;38:355–373. doi: 10.1051/vetres:2006058. [DOI] [PubMed] [Google Scholar]
- Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E.B. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses. Arch. Virol. 2009;155:133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia C. An update on canine coronaviruses: viral evolution and pathobiology. Vet. Microbiol. 2008;132:221–234. doi: 10.1016/j.vetmic.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia C. Canine coronavirus: not only an enteric pathogen. Vet. Clin. North Am. Small Anim. Pract. 2011;38:799–814. doi: 10.1016/j.cvsm.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Camero M., Greco G., Zizzo N., Tinelli A., Campolo M., Fratelli A., Buonavoglia C. Canine distemper and related diseases: report of a severe outbreak in a kennel. New. Microbiol. 2004;27:177–182. [PubMed] [Google Scholar]
- Decaro N., Pratelli A., Campolo M., Elia G., Martella V., Tempesta M., Buonavoglia C. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J. Virol. Methods. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Ricci D., Elia G., Desario C., Campolo M., Cavaliere N., Di Trani L., Tempesta M., Buonavoglia C. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J. Virol. Methods. 2005;130:72–78. doi: 10.1016/j.jviromet.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Desario C., Bellacicco A.L., Camero M., Manna L., D’aloja D., Buonavoglia C. First detection of canine parvovirus type 2c in pups with haemorrhagic enteritis in Spain. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2006;53:468–472. doi: 10.1111/j.1439-0450.2006.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Elia G., Buonavoglia D., Colaianni M.L., Lorusso A., Mari V., Buonavoglia C. Infectious canine hepatitis: an “old” disease reemerging in Italy. Res. Vet. Sci. 2007;83:269–273. doi: 10.1016/j.rvsc.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Campolo M., Lo russo A., Mari V., Martella V., Buonavoglia C. Occurrence of severe gastroenteritis in pups after canine parvovirus vaccine administration: a clinical and laboratory diagnostic dilemma. Vaccine. 2007;25:1161–1166. doi: 10.1016/j.vaccine.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Mari V., Lucente M.S., Cordioli P., Colaianni M.L., Martella V., Buonavoglia C. Serological and molecular evidence that canine respiratory coronavirus is circulating in Italy. Vet. Microbiol. 2007;121:225–230. doi: 10.1016/j.vetmic.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Martella V., Elia G., Campolo M., Desario C., Cirone F., Tempesta M., Buonavoglia C. Molecular characterisation of the virulent canine coronavirus CB/05 strain. Virus Res. 2007;125:54–60. doi: 10.1016/j.virusres.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Lorusso A., Desario C., Mari V., Colaianni M.L., Elia G., Martella V., Buonavoglia C. Experimental infection of dogs with a novel strain of canine coronavirus causing systemic disease and lymphopenia. Vet. Microbiol. 2008;128:253–260. doi: 10.1016/j.vetmic.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Mari V., Campolo M., Lorusso A., Camero M., Elia G., Martella V., Cordioli P., Enjuanes L., Buonavoglia C. Recombinant canine coronaviruses related to transmissible gastroenteritis virus of swine are circulating in dogs. J. Virol. 2009;83:1532–1537. doi: 10.1128/JVI.01937-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Campolo M., Mari V., Desario C., Lucente M.S., Lorusso E., Kanellos T., Gibbons R.H., Buonavoglia C. Immunity after natural exposure to enteric canine coronavirus does not provide complete protection against infection with the new pantropic CB/05 strain. Vaccine. 2010;28:724–729. doi: 10.1016/j.vaccine.2009.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Mari V., Elia G., Addie D.D., Camero M., Lucente M.S., Martella V., Buonavoglia C. Recombinant canine coronaviruses in dogs, Europe. Emerg. Infect. Dis. 2010;16:41–47. doi: 10.3201/eid1601.090726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Billi M., Mari V., Elia G., Cavalli A., Martella V., Buonavoglia C. Western European epidemiological survey for parvovirus and coronavirus infections in dogs. Vet. J. 2011;187:195–199. doi: 10.1016/j.tvjl.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Mari V., Sciarretta R., Colao V., Losurdo M., Catella C., Elia G., Martella V., Del Giudice G., Buonavoglia C. Immunogenicity and protective efficacy in dogs of an MF59™-adjuvanted vaccine against recombinant canine/porcine coronavirus. Vaccine. 2011;29:2018–2023. doi: 10.1016/j.vaccine.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijema B.J., Rottier P.J.M., de Groot R.J. Feline coronaviruses: a tale of two-faced types. In: Thiel V., editor. Coronaviruses. Molecular and Cellular Biology. Caister Academic Press; Norfolk, UK: 2007. pp. 183–203. [Google Scholar]
- Lorusso A., Decaro N., Schellen P., Rottier P.J., Buonavoglia C., Haijema B.J., de Groot R.J. Gain, preservation and loss of a group 1a coronavirus accessory glycoprotein. J. Virol. 2008;82:10312–10317. doi: 10.1128/JVI.01031-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaro M., Mari V., Bellacicco A.L., Tarsitano E., Elia G., Losurdo M., Rezza G., Buonavoglia C., Decaro N. Prolonged depletion of circulating CD4+ T lymphocytes and acute monocytosis after pantropic canine coronavirus infection in dogs. Virus Res. 2010;152:73–78. doi: 10.1016/j.virusres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntafis V., Xylouri E., Mari V., Papanastassopoulou M., Papaioannou N., Thomas A., Buonavoglia C., Decaro N. Molecular characterization of a canine coronavirus NA/09 strain detected in a dog's organs. Arch. Virol. 2012;157:171–175. doi: 10.1007/s00705-011-1141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Decaro N., Tinelli A., Martella V., Elia G., Tempesta M., Cirone F., Buonavoglia C. Two genotypes of canine coronavirus simultaneously detected in fecal samples of dogs with diarrhea. J. Clin. Microbiol. 2004;42:1797–1799. doi: 10.1128/JCM.42.4.1797-1799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tennant B.J., Gaskell R.M., Kelly D.F., Carter S.D., Gaskell C.J. Canine coronavirus infection in the dog following oronasal inoculation. Res. Vet. Sci. 1991;51:11–18. doi: 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicola A., Jolly S., Mathijs E., Ziant D., Decaro N., Mari V., Thiry E. Fatal outbreaks in dogs associated with pantropic canine coronavirus in France and Belgium. J. Small Anim. Pract. 2012 doi: 10.1111/j.1748-5827.2011.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


