Abstract
Sera from 38 free-ranging spotted hyenas (Crocuta crocuta) in the Serengeti ecosystem, Tanzania, were screened for exposure to coronavirus of antigenic group 1. An immunofluorescence assay indicated high levels of exposure to coronavirus among Serengeti hyenas: 95% when considering sera with titer levels of ≥1:10 and 74% when considering sera with titer levels of ≥1:40. Cubs had generally lower mean titer levels than adults. Exposure among Serengeti hyenas to coronavirus was also confirmed by a serum neutralisation assay and an ELISA. Application of RT-PCR to 27 fecal samples revealed viral RNA in three samples (11%). All three positive fecal samples were from the 15 juvenile animals (<24 months of age) sampled, and none from the 12 adults sampled. No viral RNA was detected in tissue samples (lymph node, intestine, lung) from 11 individuals. Sequencing of two amplified products from the S protein gene of a positive sample revealed the presence of coronavirus specific RNA with a sequence homology to canine coronavirus of 76 and 78% and to feline coronavirus type II of 80 and 84%, respectively. Estimation of the phylogenetic relationship among coronavirus isolates indicated considerable divergence of the hyena variant from those in European, American and Japanese domestic cats and dogs. From long-term observations of several hundred known individuals, the only clinical sign in hyenas consistent with those described for coronavirus infections in dogs and cats was diarrhea. There was no evidence that coronavirus infection in hyenas caused clinical signs similar to feline infectious peritonitis in domestic cats or was a direct cause of mortality in hyenas. To our knowledge, this is the first report of coronavirus infection in Hyaenidae.
Keywords: Coronavirus, Phylogenetic relationship, Spotted hyena, Serengeti
1. Introduction
Coronaviruses are enveloped RNA viruses that differ according to their antigenic reactivity. The antigenic group 1 includes feline coronavirus (FCoV), canine coronavirus (CCV), transmissible gastroenteritis virus (TGEV) and the human coronavirus HCV 229E (Horzinek et al., 1982, Wege et al., 1982). FCoV and CCV are chiefly transmitted by the exposure of susceptible individuals to virus in the feces of infected hosts (Pedersen et al., 1981, Tennant et al., 1993, Foley et al., 1997) and rarely in saliva (Addie and Jarrett, 2001). CCV and FCoV frequently cause relatively benign enteric infections in domestic dogs and cats, respectively, that are normally confined to the digestive tract and regional lymph nodes (Pedersen et al., 1981, Evermann et al., 1989). However, in a small percentage of FCoV infected domestic cats (Addie and Jarrett, 1992) more virulent strains may develop by mutations, which are able to invade and replicate in macrophages causing a virulent fatal disease termed feline infectious peritonitis (FIP) (Pedersen et al., 1981, Evermann et al., 1991, Poland et al., 1996, Vennema et al., 1998). FIP is an immune-mediated disease that involves an antibody-dependent enhancement (ADE) of virus infection and an immune complex-induced pathology (Horzinek and Osterhaus, 1979). Possible synergistic infections of coronavirus and other pathogens may result in severe clinical signs (Appel, 1988).
Strains of FCoV are classified into two types (I and II) that differ in the genes encoding the S proteins. The S proteins of type II strains are more closely related to those of CCV (91%) and porcine TGEV (81%) than to the S protein of FCoV type I strains (46%) (Motokawa et al., 1995, Wesseling et al., 1994). Recent evidence suggests that type II FCoV has arisen from double homologous RNA recombination events between FCoV type I and CCV (Herrewegh et al., 1998).
FCoV and CCV infect domestic cats and dogs worldwide. Prevalence of exposure to FCoV in domestic cat populations is normally between 20 and 50%, but may be as high as 90%, depending on husbandry conditions (Rolle and Mayr, 2002). Exposure to CCV in domestic dog populations also varies widely (16–70%) in relation to husbandry (Herbst et al., 1988, Buxbaum, 1993, Naylor et al., 2001). High levels of exposure to coronaviruses have been reported in some wild species of carnivores, including wolves (Canis lupis) in Alaska, where prevalence among adults varied between 32% in autumn and 76% in spring (Zarnke et al., 2001), and lions (Panthera leo) in the Serengeti National Park, Tanzania, where exposure was approximately 60% (Hofmann-Lehmann et al., 1996). In contrast, comparatively low levels of exposure to coronaviruses (3, 4 and 6%, respectively) have been reported in lions in Etosha National Park, Namibia (Spencer and Morkel, 1993), and in wild cats (Felis silvestris) on the European mainland (Leutenegger et al., 1999) and Scotland (Daniels et al., 1999).
In this study, we investigated levels of exposure to coronavirus among spotted hyenas (Crocuta crocuta), in the Serengeti ecosystem, Tanzania, by testing hyena sera for antibodies against coronaviruses of the antigenic group 1. Additionally fecal and organ samples were tested for coronavirus-specific RNA by RT-PCRs targeting three different regions of the viral genome. Long-term monitoring of a large population of known individuals (East et al., 2001, Hofer and East, 1995) permitted an assessment of the effect of exposure to coronavirus on free-ranging spotted hyenas.
Spotted hyenas belong to the family Hyaenidae, and thus are phylogenetically more closely related to members of the family Felidae than those of the family Canidae (Wayne et al., 1989). For this reason, they may be more susceptible to pathogens that infect members of the Felidae than those that infect members of the Canidae (Haas et al., 1996). Furthermore, members of the Hyaenidae may maintain viral strains that are distinct ‘hyena’ variants. For example, Serengeti spotted hyenas maintain a non-symptomatic rabies strain that does not cause mortality and is genetically distinct from the virulent strain of rabies that circulates in bat-eared foxes (Otocyon megalotis) and white-tailed mongooses (Ichneumia albicauda) in the Serengeti ecosystem, and domestic dogs in Tanzania (East et al., 2001).
There are 26 carnivore species in the Serengeti ecosystem (Sinclair and Arcese, 1995), including large populations of several Felidae and Canidae species (Hofer and East, 1995) that may be reservoirs for coronavirus. The spotted hyena, which is the most numerous large carnivore species in the ecosystem, with a population estimated at approximately 9000 animals (Hofer and East, 1995), may also be a potential host for coronavirus. Currently, little is known about coronavirus infection in most Serengeti carnivore populations (Hofmann-Lehmann et al., 1996) and what strains of coronavirus infect these populations. It is also not known whether such infections are maintained in single or multi-species associations. A large (over 1 million) human population (Campbell and Hofer, 1995) and associated domestic cats and dogs live within a 45 km zone outside the western boundary of the Serengeti National Park. These domestic animals may be a large reservoir for coronavirus, although the contribution of domestic animals to the epidemiology of coronavirus in carnivore species in the Serengeti is currently unknown.
2. Materials and methods
2.1. Study population
Samples were obtained from spotted hyenas in the Serengeti ecosystem, mostly from a study population consisting of several hundred individually recognized spotted hyenas that were members of five study groups. Individuals in study groups were regularly monitored in terms of behavior and demography for between 7 and 13 years. Serengeti hyenas live in large social groups (clans) with approximately 45 adults and subadults (Hofer and East, 1993, Hofer and East, 1995). All female clan members breed, cubs are reared communally in a den for the first 12 months of life and are normally nursed only by their mother for a period of 12–18 months (Hofer and East, 1995). Serengeti hyenas have a large lifetime range as all clan members other than den-bound cubs travel (commute) on average 40 km from their territory to forage in areas containing large migratory herds during 46–62% of the year (Hofer and East, 1993). The age of individuals when blood and fecal samples were taken was determined from known life histories of individually recognized study animals. Cubs were individuals ≤12 months of age, yearlings were older than 12 months and younger than 24 months of age, and adults were two or more years of age. The term juvenile is used to refer to immature animals less than 2 years of age.
2.2. Specimens
We analysed sera from 38 individuals, including 28 adults and 10 juveniles. The mean age of adults and juveniles was 58.1 ± 22.3 months and 8.2 ± 7.0 months of age, respectively. We attempted to detect viral nucleic acids in tissue samples (four intestine, six lymph nodes, and a lung sample) from 10 juvenile hyenas (mean age 6.0 ± 5.6 months) and one adult. We also attempted to detect viral nucleic acids in fecal samples from 27 individuals, including 12 adult (mean age 76.8 ± 42.2 months) and 15 juvenile animals (mean age 9.6 ± 5.2). Samples were collected between June 1994 and July 2002. Following collection samples were stored and transported at ≤−70 °C.
2.3. Serology
Serum samples were tested for antibodies against coronaviruses of the antigenic group 1 by indirect immunofluorescence assay (Moestl, 1983) using Crandell feline kidney cells (CRFK) grown in microtiter plates. All sera were tested in two-fold serial dilutions (1:10 to 1:320). The conjugate used was a Fluorescein-conjugated AffiniPure Goat Anti-Cat IgG (Jackson Immuno Research Lab. Inc., West Grove, PA, USA). Preliminary tests had shown that no fluorescence was achieved using an anti-dog IgG conjugate. The highest dilution showing a clear cytoplasmic fluorescence was recorded positive. For confirmation, two additional assays were used for 16 sera: the microserum neutralisation assay and the commercially available FIP Antibody Test Kit DiaSystems® CELISA FIP (IDEXX Lab. Inc., Westbrook, ME, USA), which is known to have a low sensitivity, but a high specificity (Moestl, unpublished data; Viefhues et al., 1990). The serum neutralisation assay was performed in CRFK cells inoculated with 100 TCID50 (tissue culture infectious dose 50%)/0.1 ml of TGEV (strain Purdue) in microtiter plates. The sera were used in two-fold serial dilutions (1:4 to 1:64) and the titer was recorded as the 50% inhibition dilution.
2.4. RT-PCR
RNA extraction from fecal and organ samples was performed using a commercially available kit (QIAamp Viral RNA Mini Kit, Qiagen, Valencia, CA, USA). Extracts were tested for coronaviral RNA by RT-PCR using primers published by Herrewegh et al. (1995), which detect the highly conserved 3′-untranslated region of the viral genome and is suitable for the detection of feline and canine coronaviruses. RT-PCR was performed as a single-tube assay using a commercially available kit (One Step RT-PCR Kit, Qiagen, Valencia, CA, USA). The amplification product had a size of 177 bp.
For the differentiation between FCoV type I and FCoV type II/CCV, we used RT-PCR assays to target two different regions of the S protein gene (Table 1 ) following Benetka et al. (2004) (position 4238–4512 for FCoV type I (GenBank Acc. No. D32044) and 469–700 for FCoV type II/CCV (GenBank Acc. No. AR017842)), and Posch et al. (2001) (position 1036–1375 for FCoV type I (GenBank Acc. No. D32044), and 65–725 for FCoV type II/CCV (GenBank Acc. No. X06170)). The first RT-PCR was done as a single-tube assay using a commercially available kit (One Step RT-PCR Kit, Qiagen, Valencia, CA, USA) with a reaction volume of 25 μl (Table 2 ). PCR products were analysed by electrophoresis (1% agarose gel, 1 h and 10 min, 90 V) and visualized by ethidium bromide staining. As a molecular weight marker, a 100 bp ladder (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) was used. Bands were visualized with UV illumination and photographed using the Eagle Eye TM II UV gel imaging system (Stratagene, La Jolla, CA, USA). The primers used and the sizes of the amplification products are shown in Table 1 and the cycler schemes in Table 2. The amplified DNA of specimens exhibiting a positive RT-PCR result was sequenced. The amplified product was extracted from the gel (QIA Quick Gel Extraction Kit, Qiagen, Valencia, CA, USA), sequencing PCR was done by ABI PRISM Big Dye™ Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Alameda, CA, USA). Sequences were determined using the sequence analyser ABI PRISM 310 Genetic Analyser PE Applied System.
Table 1.
RT-PCRs for the differentiation between feline coronavirus (FCoV) type I and FCoV type II/canine coronavirus (CCV)
| Primer name | Sequence | Orientation | Length of the amplified product (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| FCoV type I (RT-PCR, Benetka et al., 2004) | ||||
| Fecv1bf | 5′-ttgaccttgactggctcaac-3′ | Sense | 275 | 60 |
| Fecv1br | 5′-cgtccacagagatgccaata-3′ | Antisense | ||
| FCoV type II (RT-PCR, Benetka et al., 2004) | ||||
| Fecv2bf | 5′-aggttgttgtggatgcatag-3′ | Sense | 232 | 60 |
| Fecv2br | 5′-acggtcaagttcgtcaagta-3′ | Antisense | ||
| FCoV type I (RT-PCR, Posch et al., 2001) | ||||
| FCoV type I f | 5′-cctgtaccatcgtggtctaa-3′ | Sense | 340 | 48 |
| FCoV type I r | 5′-ctcgaacagttggtggaagt-3′ | Antisense | ||
| FCoV type II (RT-PCR, Posch et al., 2001) | ||||
| FCoV type II f | 5′-gtgccatgattgtgctcgta-3′ | Sense | 661 | 48 |
| FCoV type II r | 5′-gcagtgcttgagcgtgaata-3′ | Antisense | ||
Table 2.
Reaction schemes for differentiating RT-PCRs
| Reaction | Reaction volume (μl) | Reaction scheme | Number of cycles |
|---|---|---|---|
| Reverse transcription | 25 and 20, respectively | 50 °C, 30 min; 95 °C, 15 min | 1 |
| PCR, Benetka et al. (2004) | 25 | 94 °C, 30 s; 60 °C, 30 s; 72 °C, 30 s | 40 |
| PCR, Posch et al. (2001) | 20 | 94 °C, 30 s; 50 °C, 1 min; 72 °C, 30 s | 45 |
Nucleotide sequences of the hyena isolate were compared with known sequences from FCoV and CCV isolates from domestic cats and dogs from Europe, USA and Japan. Phylogenetic relationships among sequences were reconstructed by the maximum-likelihood method using PAUP∗ Version 4b4-10 (Swofford, 2002). Initially, a hierarchical series of likelihood ratio tests (hLRTs) in the Modeltest program (Posada and Crandall, 1998) was applied to find the most appropriate substitution model for the data set.
2.5. Statistical analysis
Statistical analyses were performed using SYSTAT 9.0 (SPSS Science Inc., Chicago, IL, USA). All statistical tests were two-tailed. Mean ages are quoted ± standard deviations.
3. Results
3.1. Serology
Using the immunofluorescence assay, sera from 36 of 38 individuals (95%) had antibody titer levels of ≥1:10 and 28 individuals (74%) had antibody titer levels of ≥1:40. Thus, irrespective of whether titers of ≥1:10 or titers of ≥1:40 were considered evidence of exposure to coronavirus, our analysis indicated a high level of exposure to the virus among Serengeti hyenas. Considering all sera, there was a nearly significant trend for adults to have higher titer levels than cubs (Fig. 1 , Mann–Whitney U-test, U = 139.5, P = 0.08, 28 adults, 7 cubs). High titer levels (≥1:320) were found in six spotted hyenas (4 adults, 2 yearlings, 0 cubs) representing 16% of the tested individuals.
Fig. 1.
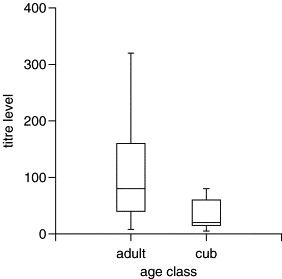
Comparison of coronavirus serum antibody titers determined by an indirect immunofluorescence assay for 28 adult hyenas (≥24 months of age) and 7 cubs (≤12 months of age) (Mann–Whitney U-test, U = 139.5, P = 0.08). The central line marks the median titer, each box shows the range of 50% of the values around the median, and the vertical lines indicate the total range of values.
The serum neutralisation assay indicated 13 positive individuals of 16 tested (results from three individuals could not be interpreted) with titers ranging from 1:8 to 1:32. Sera that produced titers of ≥1:10 with the immunofluorescence assay were also positive with the serum neutralisation assay. Four sera were positive with the ELISA, and three of these sera produced the highest (≥1:320) immunofluorescence titers.
3.2. RT-PCR
Using the RT-PCR described by Herrewegh et al. (1995) no positive result was achieved in any of the 11 tissue samples. In contrast, RT-PCR analysis of 27 fecal samples revealed bands of expected size in three samples. Only bands from a sample from one cub contained sufficient DNA for sequencing. This sample was submitted to differentiating RT-PCRs. Both differentiating RT-PCRs for FCoV type I were negative, but amplification products of expected sizes (232 and 661 bp, respectively) were obtained with the RT-PCRs for FCoV type II/CCV. The specificity of all three amplification products was shown by the results of sequencing. The sequence homologies between the three amplification products of the positive hyena sample and feline and canine coronavirus reference strains confirmed the specificity of the positive PCR results and revealed high percentages of homology to FCoV type II and CCV strains, but low homology to FCoV type I (Table 3 ).
Table 3.
Percentages of homology between three amplification products of a positive hyena fecal sample and feline and canine coronavirus reference strains
| Amplified region/reference strain | 3′ UTR (Herrewegh et al., 1995) | S protein gene (Benetka et al., 2004) | S protein gene (Posch et al., 2001) |
|---|---|---|---|
| CCV-INSAVC-1 | 97 | 76 | 78 |
| FCoV type I KU2 | Not done | 54 | <50 |
| FCoV type I UCD3 | 99 | Not done | Not done |
| FCoV type II 79-1146 | 98 | 80 | 84 |
Comparison of a 429 bp fragment in the 5′ region of the S protein gene (Posch et al., 2001) from the hyena isolate with that from FCoV type II and CCV isolates derived from European, American and Japanese domestic cats and dogs, revealed that the hyena isolate had 20 unique sites and 118 sites where it differed from at least one of the other strains analysed. There was no obvious pattern to the locations of these differences, with respect to other strains of CCV and FCoV type II and the absence of shared blocks of homology with either CCV or FCoV type II genes suggests that the hyena isolate is probably not the result of recombination events between CCV and FCoV type II genes. The level of divergence of the hyena isolate from strains of CCV and FCoV based on maximum-likelihood values (Fig. 2 ), suggests an early split from a common ancestor and sufficient isolated evolution in hyenas to form a new coronavirus subgroup that is distinct from the domestic feline- and canine-like subgroups.
Fig. 2.
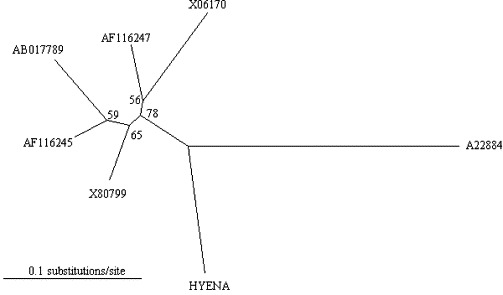
Phylogenetic relationship between coronavirus isolates from a spotted hyena (HYENA) in the Serengeti National Park, feline enteric coronavirus (X80799), feline infectious peritonitis virus (X06170), canine coronavirus (AF116247, AB017789, AF116245), and the canine coronavirus vaccine strain CCVInSAVC-1 (A22884). The unrooted maximum-likelihood tree (−ln L = 1581.61) based on the 429 bp segment of the 5′ region of the S gene (Posch et al., 2001) was recovered under the best-fit model (HKY + G) selected by hLRTs in Modeltest Version 3.06 (Posada and Crandall, 1998). Base frequencies: A = 0.3042, C = 0.1875, G = 0.1908, T = 0.3175; transition/transversion ratio: 1.6559; gamma distribution shape parameter: 0.2864. Numbers at nodes represent the percentage of 100 bootstrap replicates.
Comparison of a 96 nucleotide fragment in the 3′ region of the S protein gene (Benetka et al., 2004) from the hyena isolate with that from CCV and FCoV type II derived from European, American and Japanese domestic dogs and cats revealed that the hyena isolate had no unique sites and only differed at 27 sites from at least one of the other strains analysed.
Of the 27 fecal samples investigated (12 from adults, 15 from juveniles <24 months of age) the three positive samples with clear bands were all juvenile animals between 5.2 and 16.9 months of age. These results suggest that approximately 11% of the population (20% of juveniles) excreted coronavirus and that juveniles may excrete virus more frequently than adults.
4. Discussion
To our knowledge, we demonstrate for the first time high levels of exposure to coronavirus and excretion of coronavirus among free-ranging spotted hyenas. Sequence analyses of the coronavirus isolate in the feces of a spotted hyena in the Serengeti ecosystem demonstrated that this hyena isolate was more closely related to FCoV type II and CCV than FCoV type I (Table 3). Furthermore, a phylogenetic analysis indicated that the Serengeti hyena isolate should be considered as a new coronavirus subgroup that is distinct from the domestic feline and domestic canine subtypes (Fig. 2).
This study also demonstrates high levels of exposure of Serengeti spotted hyenas to coronavirus, regardless of whether titer levels of ≥1:10 or ≥1:40 were considered as evidence of exposure (95 and 74%, respectively). Comparison of results from the current analysis with those obtained following screening with a serum neutralization test of 37 sera collected from the same population between 1988 and 1991 (68% exposure, 25/37 positive sera, East and Hofer, unpublished data) indicate a high level of exposure to coronavirus among spotted hyenas in the Serengeti ecosystem during more than 10 years of monitoring. The high levels of exposure, we report in Serengeti spotted hyenas are similar to those reported for lions in the same ecosystem (Hofmann-Lehmann et al., 1996).
As cubs tended to have lower titer levels than adults (Fig. 1), they may be more prone to active coronavirus infection following exposure than adults. This may explain the more frequent detection of viral nucleic acids in their feces than in the feces of adults. Spotted hyenas often defecate in communal latrines (Kruuk, 1972), and young spotted hyenas stationed at clan communal dens use communal latrines within the vicinity of the den (East and Hofer, unpublished). In addition, during ritualized greeting ceremonies, hyenas sniff the anal area of greeting partners (East et al., 1993). Thus, infection of susceptible hyenas via contact with virus-laden feces is likely to play an important role in the transmission of the coronavirus within the spotted hyena population. This is in accordance with current knowledge of transmission of coronavirus in domestic dogs and cats, where the fecal-oral route plays the most important role and first infection usually occurs within a few weeks of birth (Addie and Jarrett, 1990, Foley et al., 1997). Either current infection or repeated exposure to coronavirus may have increased antibody production and induced the high titer levels (≥1:320) found in some hyenas.
Exposure among Serengeti hyenas was confirmed by three different methods for antibody detection, and results from the immunofluorescence assay correlated well with those produced by the serum neutralisation assay. The ELISA that was applied is known to show a high specificity of 95–100% (Moestl, unpublished data; Viefhues et al., 1990), and confirmed positive results in sera that produced high titers with the immunofluorescence assay.
Long-term monitoring of a large population of individually known spotted hyenas revealed no evidence that coronavirus infection resulted in mortality or clinical signs compatible with FIP other than diarrhea. This observation is supported by the fact that no coronaviral nucleic acids could be detected in organ samples. However, as young spotted hyenas in the Serengeti suffer high levels of mortality during periodic outbreaks of canine distemper (Haas et al., 1996), it is possible that coronavirus may act as a cofactor in exacerbating the clinical signs of other pathogens such as canine distemper virus.
By sequencing specific regions of the 3′ region of the S protein gene, we found that the coronavirus isolate from a Serengeti spotted hyena was more homologous with FCoV type II and CCV than FCoV type I (Table 3). As it is known that FCoV type II arose from recombination events between FCoV type I and CCV, the high level of homology between FCoV type II, CCV and the isolate from the Serengeti spotted hyena is expected. Our phylogenetic analysis suggests that the spotted hyena isolate has split from a common ancestor and has undergone sufficient isolated evolution to result in a new coronavirus subgroup that is distinct from the domestic feline- and canine-subtypes (Fig. 2). This would suggest that high levels of exposure among Serengeti hyenas are mainly due to intra-specific infection. However, as spotted hyenas occasionally eat the feces of carnivores such as lions, contact with coronavirus contained in feces of other wild carnivores is possible, and may contribute to levels of exposure and infection in spotted hyenas. It is currently unknown whether the isolate we have described in spotted hyenas also infects other Serengeti carnivores. Our genetic results also suggest that infection among Serengeti hyenas is more likely due to viral transmission within the hyena population than due to transmission from infected domestic cats or dogs in areas surrounding the National Park. To obtain a better understanding of the genetic relationship between coronavirus isolates present in the Serengeti ecosystem, and the involvement of different carnivore species in the transmission and maintenance of the virus in carnivore populations, further genetic characterization of isolates from different wild and domestic carnivore species is required. The clinical consequences of coronavirus infection in spotted hyenas should also be clarified.
Acknowledgements
We thank the Tanzanian Wildlife Research Institute and Tanzania National Parks for permission to conduct the study, the Fritz-Thyssen-Stiftung, the Max-Planck-Gesellschaft, the Stifterverband der Deutschen Wissenschaft, the Institute for Zoo and Wildlife Research, Berlin, and the Messerli Foundation for financial support, N. Burgener, W. Golla, C. Pallan, T. Shabani, D. Thierer, K. Walk and K. Wilhelm for assistance. The activities of E. Moestl for initiating this project are kindly acknowledged.
References
- Addie D.D, Jarrett O. Control of feline coronavirus infection in kittens. Vet. Rec. 1990;126:164. [PubMed] [Google Scholar]
- Addie D.D, Jarrett O. A study of naturally occurring feline coronavirus infections in kittens. Vet. Rec. 1992;130:133–137. doi: 10.1136/vr.130.7.133. [DOI] [PubMed] [Google Scholar]
- Addie D.D, Jarrett O. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Vet. Rec. 2001;148:649–653. doi: 10.1136/vr.148.21.649. [DOI] [PubMed] [Google Scholar]
- Appel M. Does canine coronavirus augment the effect of subsequent parvovirus infection? Vet. Med. 1988;83:360–366. [Google Scholar]
- Benetka V, Kuebber-Heiss A, Kolodziejek J, Nowotny N, Moestl K. Prevalence of feline coronavirus types I and II in cats with histopathologically verified feline infectious peritonitis. Vet. Microbiol. 2004;99:31–42. doi: 10.1016/j.vetmic.2003.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum, A., 1993. Seroprävalenz von Corona- und Rotaviren in offenen Hundepopulationen. Thesis. University of Veterinary Medicine, Vienna.
- Campbell, K., Hofer, H., 1995. People and wildlife: spatial dynamics and zones of interactions. In: Sinclair, A.R.E., Arcese, P. (Eds.), Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press, Chicago, pp. 534–570.
- Daniels M.J, Golder M.C, Jarrett O, Macdonald D.W. Feline viruses in wildcats from Scotland. J. Wildl. Dis. 1999;35:121–124. doi: 10.7589/0090-3558-35.1.121. [DOI] [PubMed] [Google Scholar]
- East M.L, Hofer H, Wickler W. The erect penis is a flag of submission in a female-dominated society: greeting ceremonies in Serengeti spotted hyenas. Behav. Ecol. Sociobiol. 1993;33:355–370. [Google Scholar]
- East M.L, Hofer H, Cox J.H, Wulle U, Wiik H, Pitra C. Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proc. Natl. Acad. Sci. U.S.A. 2001;98:15026–15031. doi: 10.1073/pnas.261411898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evermann J.F, McKeirnan A.J, Eugster A.K, Solozano R.F, Collins J.K, Black J.W, Kim J.S. Update on canine coronavirus infections and interactions with other enteric pathogens of the dog. Comp. Anim. Pract. 1989;19:6–12. [Google Scholar]
- Evermann J.F, McKeirnan A.J, Ott R.L. Perspectives on the epizootiology of feline enteric coronavirus and the pathogenesis of feline infectious peritonitis. Vet. Microbiol. 1991;28:243–255. doi: 10.1016/0378-1135(91)90079-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J.E, Poland A, Carlson J, Pedersen N.C. Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments. JAVMA. 1997;210:1307–1312. [PubMed] [Google Scholar]
- Haas L, Hofer H, East M.L, Wohlsein P, Liess B, Barrett T. Canine distemper virus infection in Serengeti spotted hyenas. Vet. Microbiol. 1996;49:147–152. doi: 10.1016/0378-1135(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Herbst W, Zhang X.M, Schliesser Th. Zur Seroprävalenz von Coronavirus-Infektionen beim Hund in der Bundesrepublik Deutschland. Berl. Münch. Tierärztl. Wschr. 1988;101:381–383. [PubMed] [Google Scholar]
- Herrewegh A.A.P.M, DeGroot R.J, Cepica A, Egberink H.F, Horzinek M.C, Rottier P.J.M. Detection of feline coronavirus RNA in feces. J. Clin. Microbiol. 1995;33:684–689. doi: 10.1128/jcm.33.3.684-689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A.P.M, Smeenk I, Horzinek M.C, Rottier P.J.M, DeGroot R.J. Feline coronavirus type II strains 79–1683 and 79–1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, East M.L. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. II. Intrusion pressure and commuters’ space use. Anim. Behav. 1993;46:559–574. [Google Scholar]
- Hofer, H., East, M.L., 1995. Population dynamics, population size, and the commuting system of Serengeti spotted hyaenas. In: Sinclair, A.R.E., Arcese, P. (Eds.), Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press, Chicago, pp. 332–363.
- Hofmann-Lehmann R, Fehr D, Grob M, Elgizoli M, Packer C, Martenson J.S, O’Brien S.J, Lutz H. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in East Africa. Clin. Diagn. Lab. Immunol. 1996;3:554–562. doi: 10.1128/cdli.3.5.554-562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzinek M.C, Osterhaus A.D.M.E. The virology and pathogenesis of feline infectious peritonitis. Arch. Virol. 1979;59:1–15. doi: 10.1007/BF01317889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzinek M.C, Lutz H, Pedersen N.C. Antigenic relationships among homologous structural polypeptides of porcine. Inf. Immun. 1982;37:1148–1155. doi: 10.1128/iai.37.3.1148-1155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk, H., 1972. The Spotted Hyena. University of Chicago Press, Chicago, USA.
- Leutenegger C.M, Hofmann-Lehmann R, Riols C, Liberek M, Worel G, Lups P, Fehr D, Hartmann M, Weilenmann P, Lutz H. Viral infections in free-living populations of the European wildcat. J. Wildl. Dis. 1999;35:678–686. doi: 10.7589/0090-3558-35.4.678. [DOI] [PubMed] [Google Scholar]
- Moestl K. Nachweis von Antikörpern gegen das Virus der Felinen Infektiösen Peritonitis in Katzenseren und Peritonealexsudaten. Wien. Tierärztl. Mschr. 1983;70:318–323. [Google Scholar]
- Motokawa K, Hohdatsu T, Aizawa C, Koyama H, Hashimoto H. Molecular cloning and sequence determination of the peplomer protein gene of feline infectious peritonitis virus type I. Arch. Virol. 1995;140:469–480. doi: 10.1007/BF01718424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor M.J, Monckton R.P, Lehrbach P.R, Deane E.M. Canine coronavirus in Australian dogs. Aust. Vet. J. 2001;79:116–119. doi: 10.1111/j.1751-0813.2001.tb10718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C, Boyle J.F, Floyd K, Fudge A, Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981;42:368–377. [PubMed] [Google Scholar]
- Poland A.M, Vennema H, Foley J.E, Pedersen N.C. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J. Clin. Microbiol. 1996;34:3180–3184. doi: 10.1128/jcm.34.12.3180-3184.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Posch A, Posch U, Kuebber-Heiss A, Stur I, Seiser M, Moestl K. Feline Coronaviren: Differenzierung der Typen I und II mittels RT-PCR und deren Vorkommen in österreichischen Katzenpopulationen. Wien. Tierärztl. Mschr. 2001;88:235–243. [Google Scholar]
- Rolle, M., Mayr, A., 2002. Infektiöse Peritonitis der Katze. In: Mayr, A. (Ed.), Medizinische Mikrobiologie, Infektions- und Seuchenlehre, 7th ed. Enke, Stuttgart, pp. 273–275.
- Sinclair, A.R.E., Arcese, P., 1995. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press, Chicago, USA.
- Spencer J.A, Morkel P. Serological survey of sera from lions in Etosha National Park. S. Afr. J. Wildl. Res. 1993;23:60–61. [Google Scholar]
- Swofford, D.L., 2002. PAUP* beta Version. Phylogenetic Analysis using Parsimony (* and Other Methods). Sinauer Associated, Sunderland, USA.
- Tennant B.J, Gaskell R.M, Jone R.C, Gaskell C.J. Studies on the epizootiology of canine coronavirus. Vet. Rec. 1993;132:7–11. doi: 10.1136/vr.132.1.7. [DOI] [PubMed] [Google Scholar]
- Vennema H, Poland A, Foley J, Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150–157. doi: 10.1006/viro.1998.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viefhues G, Uysal O, Heusinger A. Der Nachweis von Antikörpern gegen das Virus der felinen infektiösen Peritonitis (FIPV) mit dem Diasystems™ FIP Test Kit im Vergleich zur indirekten Immunofluoreszenz. Tierärztl. Umschau. 1990;45:490–492. [Google Scholar]
- Wayne, R.K., Benveniste, R.E., Janczewski, D.N., O’Brien, S.J., 1989. Molecular and biochemical evolution of the carnivora. In: Gittleman, J.L. (Ed.), Carnivore Behavior, Ecology, and Evolution. Chapman & Hall, London, pp. 465–593.
- Wege H, Siddell St, TerMeulen V. The biology and pathogenesis of coronaviruses. Curr. Top. Microbiol. Immun. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Wesseling J.G, Vennema H, Godeke G, Horzinek M.C, Rottier P.J.M. Nucleotide sequence and expression of the spike gene of canine coronavirus and comparison with the S proteins of feline and porcine coronaviruses. J. Gen. Virol. 1994;75:1789–1794. doi: 10.1099/0022-1317-75-7-1789. [DOI] [PubMed] [Google Scholar]
- Zarnke R.L, Evermann J, Ver Hoef J.M, McNay M.E, Boertje R.D, Gardner C.L, Adams L.G, Dale B.W, Burch J. Serological survey for canine coronavirus in wolves from Alaska. J. Wildl. Dis. 2001;37:740–745. doi: 10.7589/0090-3558-37.4.740. [DOI] [PubMed] [Google Scholar]


