Abstract
Feline calicivirus (FCV) is a highly infectious respiratory pathogen of domestic cats. The prevalence of FCV in the general cat population is high, particularly in multi-cat households, largely because many clinically recovered cats remain persistently infected carriers. In order to assess how FCV circulates in such groups and to assess the contribution that each individual animal makes to the epidemiology of the disease, we have carried out the first detailed analysis of long-term shedding patterns of FCV in individual cats within naturally infected colonies. The prevalence of FCV in each of the groups on individual sampling occasions ranged from 0% to 91%, with averages for the individual colonies ranging from 6% to 75%. Within each of the colonies, one to three distinct strains of FCV were identified. Individual cats showed a spectrum of FCV shedding patterns over the sampling period which broadly grouped into three categories: those that shed virus relatively consistently, those that shed virus intermittently, and those that appeared never to shed virus. This is the first report identifying non-shedder cats that appear resistant to FCV infection over long periods of time, despite being continually exposed to virus. Such resistance appeared to be age related, which may have been immune-mediated, although by analogy with other caliciviruses, factors such as host genetic resistance may play a role. Given that a proportion of the population appears to be resistant to infection, clearly the cohort of cats that consistently shed virus are likely to provide an important mechanism whereby infection can be maintained in small populations.
Keywords: Feline, Calicivirus, Carrier, Prevalence, Shedding, Resistance, Colony
1. Introduction
Feline calicivirus (FCV) is a ubiquitous respiratory and oral pathogen of domestic cats (Gaskell et al., 2004a). Other clinical signs reported include lameness (Bennett et al., 1989, Dawson et al., 1994, Pedersen et al., 1983), a recently reported virulent systemic disease (Coyne et al., 2006a, Hurley et al., 2004, Pedersen et al., 2000, Schorr-Evans et al., 2003) and a possible association with chronic stomatitis (Knowles et al., 1989, Thompson et al., 1984). Vaccines against FCV are widely used in the domestic cat population, and although reasonably effective in conferring clinical protection, they do not prevent infection (Dawson et al., 1991, Gaskell et al., 1982, Gaskell et al., 2004b, Pedersen and Hawkins, 1995).
Of critical importance in the epidemiology of FCV is the development of an asymptomatic carrier state following recovery from acute disease characterised by more-or-less continuous virus shedding from the oropharynx (Povey et al., 1973, Wardley, 1976). The duration of this carrier state is variable, ranging in individual animals from months to years (Gaskell et al., 1982, Povey et al., 1973, Wardley, 1976, Wardley and Povey, 1977a). Povey et al. (1973) arbitrarily defined the FCV carrier state as an animal excreting virus for a minimum of 30 days post infection. Some experimental studies have shown that most cats are shedding virus at 30 days post infection, but by 75 days only about 50% of these are still persistently infected (Gaskell et al., 1982, Wardley and Povey, 1977a). The decline in the proportion of cats shedding virus appears to be exponential, with only a minority of animals becoming long-term carriers. In other experimental studies however, the carrier state has been less easily established, suggesting various host or virus factors may also play a role (Knowles et al., 1991, Pedersen and Hawkins, 1995).
FCV carriers have been shown to shed a fairly constant amount of virus which fluctuates around a mean for an individual cat. Such carriers have been defined as high, medium or low-level shedders, according to the mean titre of virus recovered from the oropharynx (Wardley, 1976). High-level carriers are easily detectable and are thought to be of considerable risk to susceptible cats. In contrast, low-level carriers may be more difficult to detect and a series of swabs may be required to detect shedding over the minimum 30-day period defined for FCV carriers (Povey et al., 1973).
Previous studies on the epidemiology of FCV in the general healthy cat population have been largely cross-sectional. Such studies have demonstrated that overall FCV prevalence ranges from approximately 15% to 31%, depending on the population of cats sampled, with higher prevalences generally being associated with larger groups of cats (Bannasch and Foley, 2005, Binns et al., 2000, Coutts et al., 1994, Harbour et al., 1991, Helps et al., 2005, Knowles et al., 1989, Pedersen et al., 2004, Radford et al., 2001b). Such figures are broadly similar to those found in prevalence studies carried out in healthy cats prior to the introduction of vaccination (Wardley et al., 1974), and support the finding that vaccination does not prevent infection or the development of the carrier state (Dawson et al., 1991, Gaskell et al., 1982, Gaskell et al., 2004b, Pedersen and Hawkins, 1995). In general, most of the infected animals in such studies are thought to be persistently infected carriers, although it has been recognised that some may be cats undergoing re-infection, or subclinical infection with an avirulent strain (Wardley et al., 1974).
Clearly FCV carrier cats provide an important source of infection for susceptible animals, particularly in large groups of cats such as in breeding, pet and rescue colonies. However, how FCV circulates in such groups of cats, and the contribution that each individual animal makes in terms of virus carriage to the epidemiology of the disease is not clear. In order to address this, an in-depth longitudinal study of FCV prevalence and viral shedding patterns in five naturally infected cat colonies over a 15–46-month period was carried out. Demographic characteristics and other individual cat and colony factors were also obtained in order to determine possible risk factors for FCV carriage.
2. Materials and methods
2.1. Study sample
FCV isolates were obtained from five geographically distinct, naturally infected colonies of domestic cats. All cats within each of the colonies were annually vaccinated against FCV, using either a commercial live-attenuated vaccine or a commercial killed vaccine. Each of the five colonies was privately owned and had been in existence for approximately 5–15 years. The characteristics of each of the five colonies are summarised in Table 1 . Additional details are provided elsewhere (Coyne, 2005).
Table 1.
Summary of management characteristics of the five colonies studied
| Colony ID | Characteristics | Breed type | Classificationa | Number of cats (minimum–maximum) |
|---|---|---|---|---|
| A | Breeding | Devon Rex | Semi-open | 21–30 |
| Showing | DSHb | |||
| Pet | ||||
| B | Breeding | Oriental | Semi-open | 11–13 |
| Showing | Siamese | |||
| C | Breeding | BSHc | Semi-closed | 6–19 |
| Showing | ||||
| D | Pet | DSH | Open | 29–34 |
| DLHd | ||||
| E | Breeding | BSH | Semi-open | 18–39 |
| Showing | DSH | |||
| Pet | ||||
The classification of each colony is based on cat movement and colony management procedures. Open: all cats having external access and unrestricted contact with all other cats within the colony; semi-open: some cats having external access and contact with some other cats within the colony; semi-closed: all cats within the colony having no external access and limited contact with some of the other cats within the colony.
DSH: domestic short haired.
BSH: British short haired.
DLH: domestic long haired.
2.2. Sampling procedure and virus isolation
A questionnaire relating to each sampled cat, including demographic characteristics, medical history and vaccination status as well as colony management procedures were recorded at the first visit. The presence or absence of clinical signs was also noted.
Oropharyngeal swabs were taken into 2 ml of virus transport medium and virus isolation carried out for FCV and feline herpesvirus-1 (FeHV-1) as described previously (Binns et al., 2000, Coutts et al., 1994, Knowles et al., 1990). Individual compliant cats from each colony were sequentially sampled over a 15–46-month period (Table 2 ). The prevalence of FCV in each colony was determined for each sampling visit as the number of positive cats divided by the number of cats sampled on that specific occasion. Serological studies could not be carried out in this study since serum samples were not available from these private colonies due to UK Home Office licensing regulations.
Table 2.
Summary of FCV prevalence observed in each of the five colonies
| Colony ID | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
E |
|||||
| Time (months) | FCV prevalence | Time (months) | FCV prevalence | Time (months) | FCV prevalence | Time (months) | FCV prevalence | Time (months) | FCV prevalence |
| 0 | 11/16 (69%) | 0 | 4/6 (67%) | 0 | 0/5 (0%) | 0 | 10/20 (50%) | 0 | 2/18 (11%) |
| 4 | 5/15 (33%) | 1a | 4/5 (80%) | 3 | 1/4 (25%) | 1 | 15/23 (65%) | 3 | 1/14 (7%) |
| 19 | 4/20 (20%) | 4 | 6/11 (55%) | 6 | 2/5 (40%) | 6 | 12/28 (43%) | 5 | 0/11 (0%) |
| 23 | 11/21 (52%) | 9 | 9/11 (82%) | 10 | 0/10 (0%) | 14 | 12/21 (57%) | 5 | 0/21 (0%) |
| 28 | 4/17 (24%) | 15 | 10/11 (91%) | 13 | 1/19 (5%) | 20 | 17/27 (63%) | 8 | 1/24 (4%) |
| 30b | 6/10 (60%) | 17 | 0/7 (0%) | 25 | 13/25 (52%) | 12 | 0/18 (0%) | ||
| 30 | 3/9 (33%) | 24 | 0/12 (0%) | 33 | 13/27 (48%) | 15 | 2/25 (8%) | ||
| 31 | 12/27 (44%) | 39 | 3/21 (14%) | 21 | 3/29 (10%) | ||||
| 37 | 9/22 (41%) | 46 | 3/25 (12%) | ||||||
| Overall FCV prevalence | 65/157 (41%) | 33/44 (75%) | 4/62 (6%) | 98/217 (45%) | 9/160 (6%) | ||||
FCV prevalence was calculated as the number of cats positive for FCV/the number of cats sampled on that specific occasion (%).
FeHV-1 also isolated at this time point at a prevalence of 1/5 (20%).
FeHV-1 also isolated at this time point at a prevalence of 5/10 (50%).
2.3. Viral titrations
Viral titres for each positive sample were determined as described previously and expressed as log10 50% tissue culture infective doses (TCID50) per 0.1 ml of sample (Knowles et al., 1990). In order to assess the error rate associated with titrations 25% of the samples were subjected to repeat analysis. Of these, 98% were repeatable within ±0.3 (log10 TCID50) of the original titre (data not presented).
2.4. Sequencing of viral strains
In order to identify the number of distinct strains of FCV circulating in each of the colonies, molecular analysis of FCV isolates obtained from individual cats was performed, essentially according to previously published protocols (Coyne et al., 2006b). Briefly, a 529-nucleotide region of the FCV capsid gene, spanning variable region C and hypervariable region E (Neill, 1992, Seal et al., 1993) were amplified using a single stage PCR, using either previously published primers (PercP1 and PercP2; Coyne et al., 2006b) or a primer set consisting of forward primer ArmsP1 (5′-CCCTTCGTCTTTCAGGCCAACCG-3′) and reverse primer ArmsP2 (5′-CCTCGCCAATCCCAGTGTAGCC-3′); all other conditions remained the same as previously described (Coyne et al., 2006b). All amplicons were purified (QIAquick PCR purification kit; QIAGEN Ltd.) and sequenced bi-directionally using corresponding PCR primers according to standard protocols (ABI Prism BigDye terminators Version 3.0 cycle sequencing kits; Applied Biosystems). Consensus sequences were assembled (ChromasPro v 1.32; Technelysium Pty) and analysed using programmes available in the GCG package (Deveraux et al., 1984). All sequences generated in this study have been submitted to Genbank with accession nos.: DQ397674–DQ397814.
2.5. Statistical analysis
Risk factors for FCV carriage were carried out on individual cats from endemically infected colonies who had a minimum of two swabs taken at least 30 days apart (Povey et al., 1973) and the colony to which they belonged was consistently infected with FCV (i.e. had at least one positive cat present on each sampling occasion).
Statistical analyses were carried out using Minitab for Windows 14 (Minitab Inc., USA). Comparisons between viral titres were analysed using the Mann–Whitney test. Univariate associations were examined using binary logistic regression for 2 × 2 tables and χ 2-test for 3 × 2 tables. The critical probability was taken as p ≤ 0.05 for a two-sided alternative hypothesis. Individual cat predictor variables screened for association with FCV carriage included the age of the cat categorised into two groups, i.e. 3 years and under and over 3 years; gender; breed; vaccination status and history of upper respiratory tract disease (URTD). Colony predictor variables included colony identification (e.g. A, B, C, D, or E) and classification (semi-open, semi-closed or open).
3. Results
3.1. Characteristics of study sample
The age and sex distributions within each of the colonies are shown in Fig. 1, Fig. 2 . Considerable variation was seen between the colonies, depending on the nature of the colony and its purpose, e.g. whether it contained actively breeding animals or purely pet cats.
Fig. 1.
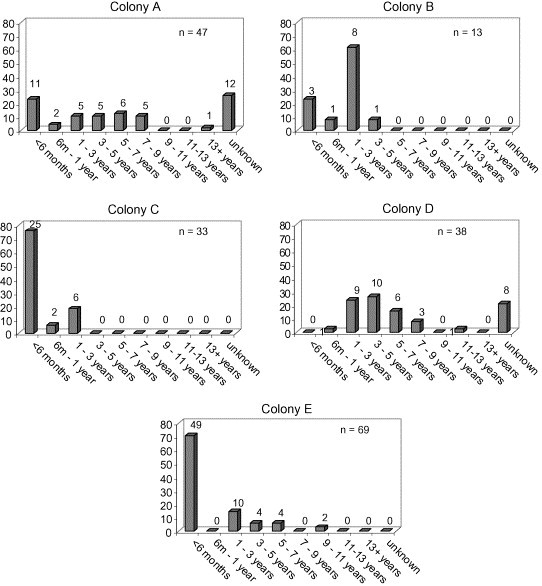
Histograms showing age distribution as a percentage of cats within each colony sampled. The number above each column represents the number of cats in each age category.
Fig. 2.
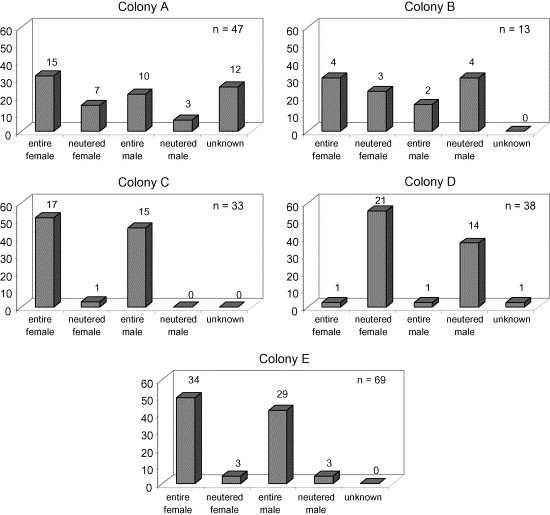
Histograms showing gender distribution of cats as a percentage of cats within each colony sampled. The number above each column represents the number of cats in each gender category.
3.2. Feline calicivirus prevalence
The prevalence of FCV infection over the sampling period in each of the colonies ranged from 20% to 69% for colony A, 55% to 91% for colony B, 0% to 40% for colony C, 12% to 65% for colony D, and 0% to 11% for colony E (Table 2). Although colonies A, B, and D had a markedly higher overall prevalence (41%, 75%, and 45%, respectively) than C and E (both 6%), there appeared to be no association for overall prevalence with classification of colony type.
3.3. Clinical signs
The majority of cats that were shedding FCV during this study were clinically healthy, except for a number of cats in colonies B and D. In colony B signs included chronic oculonasal discharge, sneezing and coughing, and in colony D, varying degrees of chronic gingivitis were observed.
On one occasion, in each of colonies A and B, feline herpesvirus-1 (FeHV-1) was also isolated from a small number of cats (5/10 colony A; 1/5 colony B) (Table 2, Table 3 ). Acute clinical signs of URTD were observed in colony A on this occasion.
Table 3.
(a–e) FCV viral shedding and strain patterns of individual cats within each of the five colonies
 |
 |
In each colony cats are broadly divided into three groups, consistent shedders (C) positive on ≥75% occasions; intermittent shedders (I) positive on <75% occasions; non-shedders (N) never positive for FCV. For all colonies coloured squares represent FCV isolated, viral strain identified and viral titre (expressed as log10 TCID50 per 0.1 ml sample); empty square (□) represents no FCV isolated; grey square ( ) represents FCV isolated but no strain information available; grey square (
) represents FCV isolated but no strain information available; grey square ( ) represents mixed FCV infection with two distinct viral strains; ns indicates that cat was not sampled; * Indicates cat was not part of the colony at the time of sampling; na represents cat was FCV positive but viral titre was not available; aFeHV-1 isolated. For individual colonies: (3a) Colony A, black square (
) represents mixed FCV infection with two distinct viral strains; ns indicates that cat was not sampled; * Indicates cat was not part of the colony at the time of sampling; na represents cat was FCV positive but viral titre was not available; aFeHV-1 isolated. For individual colonies: (3a) Colony A, black square ( ) is strain A1 and orange square (
) is strain A1 and orange square ( ) is strain A2; (3b) Colony B, brown square (
) is strain A2; (3b) Colony B, brown square ( ) is strain B1, green square (
) is strain B1, green square ( ) is strain B2, and blue square (
) is strain B2, and blue square ( ) is strain B3; (3c) Colony C, purple square (
) is strain B3; (3c) Colony C, purple square ( ) is strain C1; (3d) Colony D, red square (
) is strain C1; (3d) Colony D, red square ( ) is strain D1, and blue square (
) is strain D1, and blue square ( ) is strain D2, recombinant virus is cat 24; (3e) Colony E, pink square (
) is strain D2, recombinant virus is cat 24; (3e) Colony E, pink square ( ) is strain E1 and yellow square (
) is strain E1 and yellow square ( ) is strain E2.
) is strain E2.
3.4. Feline calicivirus shedding patterns
Individual cats within the five cat colonies appeared to show a spectrum of FCV shedding patterns that broadly grouped into three categories (Table 3a–e). At one end of the spectrum, cats were identified where the majority of the swabs (≥75%) were positive for FCV over the whole sampling period (“consistent shedders”). In contrast at the other end of the spectrum, some cats appeared to be consistently negative (“non-shedders”). In between, other cats were identified with a more intermittent shedding pattern, where the number of positive swabs ranged from 12% to 73% over the sampling period (“intermittent shedders”). These criteria are descriptive rather than absolute, as a number of factors will influence shedding patterns, including the precise sampling procedure.
3.5. Viral strains
One hundred and forty-seven of 195 (75%) FCV isolates collected during the course of this study were sequenced successfully (Table 3a–e); in-depth sequence analysis of these isolates is presented elsewhere (Coyne, 2005). Each colony appeared to be infected with between one and three distinct strains, on the basis of previous reports where distinct strains of FCV appear to be separated by uncorrected nucleotide distances of >20% in hypervariable region E of the FCV capsid gene (Radford et al., 1997, Radford et al., 2000, Radford et al., 2003); a range of variability was also seen within a strain (Coyne, 2005). Thus colony A contained two strains, A1 and A2; colony B three strains, B1, B2 and B3; colony C one strain, C1; colony D two strains, D1 and D2; colony E two strains, E1 and E2. The shedding patterns of each of these strains by individual cats are shown in Table 3a–e. Interestingly, in colony E, one cat appeared to be infected with a virus similar to that present in the live-attenuated vaccine used in the colony (cat 2, colony E, strain E1). Not all isolates were sequenced successfully, and it is possible that other strains were present in some cats/colonies which may not have been detected by the primers used. Such PCR failure has been reported in other studies with FCV as well as for other caliciviruses (Green et al., 1995, Radford et al., 2001b).
Of the cats that were classified as consistent shedders, 59% (13/22) appeared to be infected with only one strain of virus during the course of the study (e.g. Table 3d, cat 2), whilst 23% (5/22) shed two different strains (e.g. Table 3d, cat 3). A similar pattern was also observed with the group of cats that were classified as intermittent shedders; 65% (30/46) shed the same strain of FCV during the study period (e.g. Table 3d, cat 10) and 15% (7/46) shed more than one strain (e.g. Table 3d, cat 13). Thus both groups (consistent and intermittent shedders) appear to contain a mixture of animals, with some consistently shedding the same virus strain, and others undergoing re-infection with a different strain. Co-infection of more than one distinct FCV strain was detected in two individual cats (cat 5, colony B; cat 1, colony D). A recombinant strain was also detected in colony D (Table 3a, cat 24) and has been reported elsewhere (Coyne et al., 2006b).
3.6. Viral titres
Viral titres in individual cats on each sampling occasion ranged from 0.3 to 3.3, for colony A; 0.3–2.7 for colony B; 0.3–1.0 for colony C; 0.3–4.0 for colony D; and 1.3–3.5, for colony E (Table 3a–e) (titres expressed as log10 TCID50 per 0.1 ml of sample). Interestingly, cats which were involved in an acute outbreak of disease at 30 months (colony A) shed higher levels of virus (2.0–3.3) at that time.
In some cats, individual titres were relatively consistent throughout the sampling period (e.g. cat 3 colony A, cat 4 colony B, and cat 7 colony D), whereas in others titres tended to fluctuate (e.g. cat 4 colony A, and cats 14 and 15 colony D). Four cats (1–4; colony D) which had relatively high titres initially, tended to show a decline in viral titre prior to cessation of virus shedding at 39 months (Table 3d, Fig. 3 ).
Fig. 3.
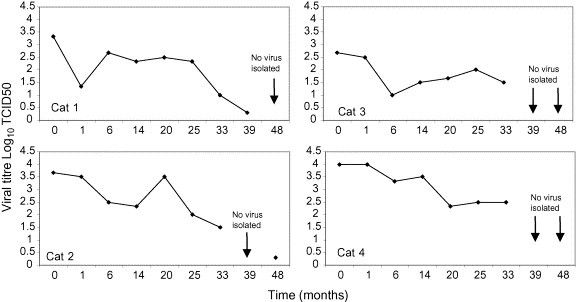
Plot of viral titres for four cats in colony D, showing a decline in the amount of FCV shed prior to cessation of viral shedding.
The overall mean titres for the sampling period for each cat ranged from 0.3 to 2.2, for colony A; 0.3–1.8 for colony B; 0.3–1.0 for colony C; 1.2–3.3, for colony D; and 2.4–2.7 for colony E (Fig. 4 ). Cats in colonies D and E shed significantly more virus than those in colonies A, B and C (p < 0.01), and cats in colony E shed significantly more virus than those in colony D (p < 0.01) (Fig. 4).
Fig. 4.
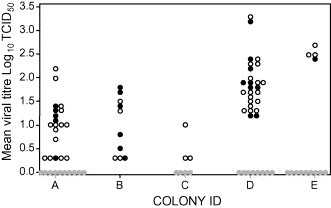
Comparison of mean viral titres of FCV shed by individual cats within each colony. Solid circle (●) represents consistent shedders (≥75% samples positive over the whole sampling period); open circle (○) represents intermittent shedding (<75% samples positive over the whole sampling period); grey circle ( ) represents non-shedders (never positive for FCV over the whole sampling period).
) represents non-shedders (never positive for FCV over the whole sampling period).
Previous studies have classified individual cats into high, medium and low-level shedders depending on the mean monthly amounts of virus shed from the oropharynx (Wardley, 1976). In this study only two cats (4 and 24) in colony D shed mean amounts of virus equivalent to Wardley's high-level shedders (≥3.0), whereas the remaining cats were equivalent to medium (1.2–2.9) or low-level shedders (≤1.2). It should be noted that Wardley's figures appeared to be higher as they included a correction factor of log 1.3 to account for dilution of the saliva sample in the virus transport media.
Wardley (1976) also suggested that cats shedding virus intermittently tended to shed lower levels of virus. However, in our study, the intermittent shedders did not shed significantly less virus than consistent shedders (p > 0.05), either overall, or within a colony (Fig. 4).
3.7. Risk factors for FCV carriage
Seventy-nine cats fulfilled the criteria for analysis of risk factors associated with FCV carriage. These cats belonged to colonies A, B and D; colonies C and E were excluded from the analysis, as FCV was not consistently isolated from them. Of these 79 cats, 21 (27%) were classified as consistent shedders, 40 (51%) cats were classified as intermittent shedders, and 18 (22%) were classified as non-shedders. Age was found to be significantly associated with FCV carriage, with cats older than 3 years less likely to be shedding virus than those that were 3 years and under (p = 0.03; OR = 0.29). In addition, colony ID was found to be significantly associated with consistent FCV shedding (i.e. shedding virus on ≥75% occasions over the sampling period), with cats from colony B being more likely to be consistent shedders than those cats in colonies A and D (p = 0.016; OR = 6.25). There was no relationship between vaccination status (past or current) and FCV carriage (p = 0.35; OR = 0.36, p = 0.12; OR 2.27, respectively).
4. Discussion
Previous studies on the prevalence of FCV in the general cat population have essentially been cross-sectional in nature. Prevalence has ranged from 8% to 47%, largely depending on the population of cats sampled, with higher levels found in larger groups of animals (Binns et al., 2000, Coutts et al., 1994, Harbour et al., 1991, Helps et al., 2005, Wardley et al., 1974). In order to increase our understanding of how FCV circulates in large groups of cats and the contribution that individual cats make in terms of viral carriage to the epidemiology of the disease, we have carried out an in-depth analysis on FCV prevalence and viral shedding patterns over time, in five different cat colonies over a 15–46-month period.
The prevalence of FCV in this study varied considerably, both over time, and between and within colonies. Thus in some colonies, prevalence was very low over the whole sampling period (e.g. colony E, 0–11%) whereas in others, prevalence was much higher (e.g. colony B, 55–91%): such differences did not appear to be related to the colony classification, which included cat movement and colony management procedures, although a recently published study reported that less than excellent hygiene within cat colonies increased the prevalence of FCV (Helps et al., 2005). Virus was also present in each of the colonies despite active vaccination. Such variation in prevalence may be influenced by a number of host, population, management and viral factors, including, for example, the level of pre-existing immunity within the cats. It is also possible that colony prevalence was influenced by factors such as the sampling regimen, and the sensitivity of the cell culture detection system, which could have led to under estimation of the true prevalence.
Individual cats within the five colonies appeared to show a spectrum of FCV shedding patterns over the sampling period. These broadly grouped into three categories: those that shed FCV relatively consistently over long periods of time (consistent shedders), those that shed virus intermittently (intermittent shedders), and those that appeared not to shed virus throughout the study period (non-shedders). These distinctions are descriptive rather than absolute as a number of factors including sampling protocol may influence the results for an individual cat. Whether or not the cat that appears negative either on some or all occasions are truly negative, or whether it is a sampling artefact, is not clear. Nevertheless it does seem likely that animals vary in their sensitivity to FCV infection despite being in the same colony with similar exposure factors. This is clearly demonstrated by three adult cats in colony D that were siblings (cats 2 and 4, both consistent shedders, and cat 27, an intermittent shedder). Interestingly, similar spectrums of viral shedding patterns have been reported previously for feline coronavirus infections in cats (Addie and Jarrett, 2001, Foley et al., 1997).
It is likely that the consistent shedders, in particular, will play an important role in the epidemiology of FCV, acting as a reservoir of infection within the colony and a mechanism for maintaining infection in small populations. Identifying such cats would be crucial to eliminating virus from infected colonies. Why some animals appear to shed virus more consistently than others is not clear. There are a number of possible reasons, apart from the sampling procedure, that may account for this. For example, Wardley (1976) suggested that intermittent shedders may shed lower levels of virus, which sometimes fall below the sensitivity of the cell culture test, rather than truly fluctuating between a positive and negative state. However, in our study there was no significant difference in mean viral titres between the consistent and intermittent shedder groups. In addition, viral shedding patterns did not appear to be influenced by whether individual cats were infected with the same strain of virus over time, or were undergoing re-infection with a different strain.
Previous studies have shown that only a limited number of strains tend to predominate within such endemically infected colonies, in contrast to rescue shelters where many strains are present (Coyne, 2005, Radford et al., 2001b, Radford et al., 2003). This present study confirmed such findings in that each colony appeared to be infected with between one and three distinct strains of virus. It is possible that other strains were also present in some colonies, as not all isolates were amplifiable on PCR, possibly because of sequence variability particularly in the hypervariable region E of the FCV capsid region of the genome used for PCR (Neill, 1992, Radford et al., 2001b, Seal et al., 1993). Interestingly, one of the strains identified in colony E appeared to be the vaccine strain F9. This colony was known to use live F9-based vaccine, resulting in the possibility that F9 virus may have become established in the colony from the vaccine. Virus closely related to vaccine strain F9 has also been reported in other groups of cats (Horimoto et al., 2001, Radford et al., 2001a).
The sites and mechanism of persistence of FCV infection are unclear. FCV may be detected in epithelial cells in oropharyngeal and other tissues in persistently infected cats (Dick et al., 1989, Wardley and Povey, 1977b). In has been suggested that such sites of viral persistence may partially be protected from immune surveillance allowing a low level of virus replication to occur (Povey et al., 1973). As a consequence the emergence of distinct viral populations may occur, contributing to viral persistence. Previous studies have shown that alterations in the antigenic profile of FCVs isolated from carrier cats are highly suggestive of positive selection that is likely to be immune driven, and which may ultimately lead to the elimination of virus (Kreutz et al., 1998, Radford et al., 1998, Radford et al., 2003). In this context, we observed a decline in viral titre shed by four cats in colony D prior to the apparent elimination of virus, as has been seen in similar circumstances in foot and mouth disease virus (Hughes et al., 2002).
The majority of cats in this study shed medium or low levels of virus as defined by Wardley (1976). Only two cats could be classified as high-level shedders and as such would be likely to be of greater epidemiological importance. However, within endemically infected colonies, where there is generally close contact between cats, it is likely that all carriers have the potential to transmit infection. There was also evidence to suggest that the overall titre of virus shed in certain colonies was higher than others. For example, cats in colonies D and E shed significantly more virus than cats in A and C. This suggests that differences between the colonies such as host, viral or environmental factors may affect the amounts of virus shed and hence transmissibility.
This study also identified for the first time a “non-shedder” group of cats, i.e. those that appear resistant to FCV infection over long periods of time, despite being continually exposed to virus. In this context, it is interesting that the two mostly negative colonies are British Shorthairs (colonies C and E) whilst the colonies with relatively high prevalence are either domestic shorthairs or of oriental type (colonies A, B, and D). Such resistance to infection may be as a result of genetic or acquired immunity. For human caliciviruses (noroviruses), resistance/susceptibility of an individual to infection with a given strain is associated with variability in the expression of surface carbohydrates belonging to the ABH histo-blood group antigens, which are used by the virus for cell attachment (Harrington et al., 2004, Hutson et al., 2003, Lindesmith et al., 2003). Recently the junctional adhesion molecule 1 (JAM-1) has been suggested to be a receptor for FCV (Makino et al., 2006) and it would be interesting to investigate JAM-1 polymorphisms in susceptible and resistant cats.
There is also evidence from our study that there may be an immune-mediated mechanism for the acquisition of resistance to infection in that older cats (more than 3 years) were significantly less likely to be shedding FCV than younger cats (less than 3 years). However, immunity following experimental FCV infection is not normally sterile (Dawson et al., 1991, Gaskell et al., 1982, Knowles et al., 1991). This study therefore suggests that other factors, such as repeated challenge or genetic differences (as above) may also play a role in generating resistance to infection. It is possible that serological studies might have clarified the immune status of the cats and whether there were differences between the shedding and non-shedding groups. However, since all of the cats in this study were regularly vaccinated, interpretation of antibody titres would have been problematic, since it would have been difficult to distinguish between neutralising antibodies present due to vaccination and those due to field infection. Based on risk factors for FCV carriage, there was no relationship between vaccination status (past or present vaccine) and FCV shedding.
Although most of the cats in this study were clinically normal there was some evidence of both acute and chronic disease in some of the colonies. Chronic disease was apparent in two of the colonies (B and D). In colony B clinical signs included chronic oculonasal discharges, sneezing and coughing. FeHV-1 was also detected in this colony on one occasion from one cat and is associated with acute and chronic URTD (Gaskell et al., 2004a). In colony D there was evidence of chronic stomatitis in some of the cats. This clinical syndrome has previously been associated with FCV infection (Knowles et al., 1991, Thompson et al., 1984). However, the significance of this association remains unclear. Although FCV has been shown to be associated with acute faucitis (Reubel et al., 1992) attempts to recreate this chronic syndrome experimentally have been unsuccessful (Dawson et al., 1991, Knowles et al., 1991, Poulet et al., 2000). Additional host factors, such as concurrent feline immunodeficiency virus (FIV) infection may also play a role in the aetiology of this disease (Dawson et al., 1991, Knowles et al., 1989). However, the FIV status of the majority of cats in colony D was recorded as negative at the beginning of the study (data not presented).
Acute disease was also seen in one of the colonies (A), coinciding with a relatively high prevalence of FCV in the colony and generally higher viral titres. However, in addition to FCV, FeHV-1 was also isolated and it was not clear which of the two viruses accounted for the acute clinical signs. FeHV-1 is generally associated with more severe disease than FCV and in this outbreak the clinical signs were more compatible with FeHV-1 (Gaskell et al., 2004a).
In summary, this work describes the first detailed analysis of long-term shedding patterns of individual cats in naturally infected populations. The study shows that FCV can remain at a relatively high prevalence in colonies of cats over long periods of time, despite widespread use of vaccination. The observed high prevalence is maintained by large numbers of generally asymptomatically infected cats in colonies where a small number of distinct FCV strains are circulating. A spectrum of FCV shedding patterns were identified with cats broadly grouping into three categories; consistent shedders, intermittent shedders and non-shedders. The basis of the apparent resistance of the non-shedders remains to be determined, but may either be immune-mediated, or possibly host receptor driven.
Acknowledgments
This work was kindly supported by the Petplan Charitable trust as part of KC's PhD thesis. SD is funded by Intervet. CP was funded by the University of Liverpool. The authors wish to thank the cat owners for allowing us to sample their cats.
References
- Addie D.D., Jarrett O. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Vet. Rec. 2001;148:649–653. doi: 10.1136/vr.148.21.649. [DOI] [PubMed] [Google Scholar]
- Bannasch M.J., Foley J.E. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J. Feline Med. Surg. 2005;7:109–119. doi: 10.1016/j.jfms.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D., Gaskell R.M., Mills A., Knowles J., Carter S., McArdle F. Detection of feline calicivirus antigens in the joints of infected cats. Vet. Rec. 1989;124:329–332. doi: 10.1136/vr.124.13.329. [DOI] [PubMed] [Google Scholar]
- Binns S.H., Dawson S., Speakman A.J., Cuevas L.E., Hart C.A., Gaskell C.J., Morgan K.L., Gaskell R.M. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. J. Feline Med. Surg. 2000;2:123–133. doi: 10.1053/jfms.2000.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts A.J., Dawson S., Willoughby K., Gaskell R.M. Isolation of feline respiratory viruses from clinically healthy cats at UK cat shows. Vet. Rec. 1994;135:555–556. [PubMed] [Google Scholar]
- Coyne, K.P., 2005. Epidemiology and evolution of feline calicivirus in naturally infected groups of domestic cats. PhD Thesis, University of Liverpool.
- Coyne K.P., Jones B.R.D., Kipar A., Chantrey J., Porter C.J., Barber P., Dawson S., Gaskell R.M., Radford A.D. A lethal outbreak associated with feline calicivirus (FCV) infection in cats: a possible case of virulent systemic FCV-associated disease. Vet. Rec. 2006;156(16):544–550. doi: 10.1136/vr.158.16.544. [DOI] [PubMed] [Google Scholar]
- Coyne K.P., Reed F.C., Porter C.J., Dawson S., Gaskell R.M., Radford A.D. Recombination of feline calicivirus within an endemically infected cat colony. J. Gen. Virol. 2006;87:921–926. doi: 10.1099/vir.0.81537-0. [DOI] [PubMed] [Google Scholar]
- Dawson S., Bennett D., Carter S.D., Bennett M., Meanger J., Turner P.C., Carter M.J., Milton I., Gaskell R.M. Acute arthritis of cats associated with feline calicivirus infection. Res. Vet. Sci. 1994;56:133–143. doi: 10.1016/0034-5288(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Dawson S., Smyth N.R., Bennett M., Gaskell R.M., McCracken C.M., Brown A., Gaskell C.J. Effect of primary-stage feline immunodeficiency virus infection on subsequent feline calicivirus vaccination and challenge in cats. Aids. 1991;5:747–750. doi: 10.1097/00002030-199106000-00016. [DOI] [PubMed] [Google Scholar]
- Deveraux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucl. Acid Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick C.P., Johnson R.P., Yamashiro S. Sites of persistence of feline calicivirus. Res. Vet. Sci. 1989;47:367–373. [PubMed] [Google Scholar]
- Foley J.E., Poland A., Carlson J., Pedersen N.C. Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments. J. Am. Vet. Med. Assoc. 1997;210:1307–1312. [PubMed] [Google Scholar]
- Gaskell C.J., Gaskell R.M., Dennis P.E., Woolridge M.J.A. Efficacy of an inactivated feline calicivirus (FCV) vaccine against challenge with United Kingdom field strains and its interaction with the FCV carrier state. Res. Vet. Sci. 1982;32:23–26. [PubMed] [Google Scholar]
- Gaskell, R.M., Radford, A.D., Dawson, S., 2004a. Feline infectious respiratory disease. In: E.A. Chandler, G., C.J. & Gaskell, R.M. (Eds.), Feline Medicine and Therapeutics, 3rd ed. Blackwell Publishing, pp. 577–595.
- Gaskell, R.M., Radford, A.D., Dawson, S., 2004b. Vaccination, In: Chandler, E.A., G., C.J. & Gaskell, R.M. (Eds.), Feline Medicine and Therapeutics, 3rd ed. Blackwell Publishing, pp. 13–18.
- Green J., Gallimore C.I., Norcott J.P., Lewis D., Brown D.W. Broadly reactive reverse transcriptase polymerase chain reaction for the diagnosis of SRSV-associated gastroenteritis. J. Med. Virol. 1995;47:392–398. doi: 10.1002/jmv.1890470416. [DOI] [PubMed] [Google Scholar]
- Harbour D.A., Howard P.E., Gaskell R.M. Isolation of feline calicivirus and feline herpesvirus from domestic cats 1980 to 1989. Vet. Rec. 1991;128:77–80. doi: 10.1136/vr.128.4.77. [DOI] [PubMed] [Google Scholar]
- Harrington P.R., Vinje J., Moe C.L., Baric R.S. Norovirus capture with histo-blood group antigens reveals novel virus–ligand interactions. J. Virol. 2004;78:3035–3045. doi: 10.1128/JVI.78.6.3035-3045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps C.R., Lait P., Damhuis A., Bjornehammar U., Bolta D., Brovida C., Chabanne L., Egberink H., Ferrand G., Fontbonne A., Pennisi M.G., Gruffydd-Jones T., Gunn-Moore D., Hartmann K., Lutz H., Malandain E., Mostl K., Stengel C., Harbour D.A., Graat E.A. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: experience from 218 European catteries. Vet. Rec. 2005;156:669–673. doi: 10.1136/vr.156.21.669. [DOI] [PubMed] [Google Scholar]
- Horimoto T., Takeda Y., Iwatsuki-Horimoto K., Sugii S., Tajima T. Capsid protein gene variation among feline calicivirus isolates. Virus Genes. 2001;23:171–174. doi: 10.1023/a:1011892120875. [DOI] [PubMed] [Google Scholar]
- Hughes G.J., Mioulet V., Haydon D.T., Kitching R.P., Donaldson A.I., Woolhouse M.E. Serial passage of foot-and-mouth disease virus in sheep reveals declining levels of viraemia over time. J. Gen. Virol. 2002;83:1907–1914. doi: 10.1099/0022-1317-83-8-1907. [DOI] [PubMed] [Google Scholar]
- Hurley K.E., Pesavento P.A., Pedersen N.C., Poland A.M., Wilson E., Foley J.E. An outbreak of virulent systemic feline calicivirus disease. J. Am. Vet. Med. Assoc. 2004;224:241–249. doi: 10.2460/javma.2004.224.241. [DOI] [PubMed] [Google Scholar]
- Hutson A.M., Atmar R.L., Marcus D.M., Estes M.K. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J. Virol. 2003;77:405–415. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles J.O., Dawson S., Gaskell R.M., Gaskell C.J., Harvey C.E. Neutralisation patterns among recent British and North American feline calicivirus isolates from different clinical origins. Vet. Rec. 1990;127:125–127. [PubMed] [Google Scholar]
- Knowles J.O., Gaskell R.M., Gaskell C.J., Harvey C.E., Lutz H. Prevalence of feline calicivirus, feline leukaemia virus and antibodies to FIV in cats with chronic stomatitis. Vet. Rec. 1989;124:336–338. doi: 10.1136/vr.124.13.336. [DOI] [PubMed] [Google Scholar]
- Knowles J.O., McArdle F., Dawson S., Carter S.D., Gaskell C.J., Gaskell R.M. Studies on the role of feline calicivirus in chronic stomatitis in cats. Vet. Microbiol. 1991;27:205–219. doi: 10.1016/0378-1135(91)90148-9. [DOI] [PubMed] [Google Scholar]
- Kreutz L.C., Johnson R.P., Seal B.S. Phenotypic and genotypic variation of feline calicivirus during persistent infection of cats. Vet. Microbiol. 1998;59:229–236. doi: 10.1016/s0378-1135(97)00158-2. [DOI] [PubMed] [Google Scholar]
- Lindesmith L., Moe C., Marionneau S., Ruvoen N., Jiang X., Lindblad L., Stewart P., LePendu J., Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- Makino A., Shimojima M., Miyazawa T., Kato K., Tohya Y., Akashi H. Junctional adhesion molecule 1 is a functional receptor for feline calcivirus. J Virol. 2006;80(9):4482–4490. doi: 10.1128/JVI.80.9.4482-4490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J.D. Nucleotide sequence of the capsid protein gene of two serotypes of San Miguel sea lion virus: identification of conserved and non-conserved amino acid sequences among calicivirus capsid proteins. Virus Res. 1992;24:211–222. doi: 10.1016/0168-1702(92)90008-w. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Elliott J.B., Glasgow A., Poland A., Keel K. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 2000;73:281–300. doi: 10.1016/S0378-1135(00)00183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Hawkins K.F. Mechanisms for persistence of acute and chronic feline calicivirus infections in the face of vaccination. Vet. Microbiol. 1995;47:141–156. doi: 10.1016/0378-1135(95)00101-f. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Laliberte L., Ekman S. A transient febrile “limping” syndrome of kittens caused by two different strains of feline calicivirus. Feline Pract. 1983;13:26–35. [Google Scholar]
- Pedersen N.C., Sato R., Foley J.E., Poland A.M. Common virus infections in cats, before and after being placed in shelters, with emphasis on feline enteric coronavirus. J. Feline Med. Surg. 2004;6:83–88. doi: 10.1016/j.jfms.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet H., Brunet S., Soulier V., Leroy V., Goutebrouze S., Chappuis G. Comparison between acute oral/respiratory and chronic stomatitis/gingivitis isolates of feline calicivirus: pathogenicity, antigenicity profile and cross-neutralisation studies. Arch. Virol. 2000;145:243–261. doi: 10.1007/s007050050021. [DOI] [PubMed] [Google Scholar]
- Povey R.C., Wardley R.C., Jessen H. Feline picornavirus infection: the in vivo carrier state. Vet. Rec. 1973;92:224–229. doi: 10.1136/vr.92.9.224. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Bennett M., McArdle F., Dawson S., Turner P.C., Glenn M.A., Gaskell R.M. The use of sequence analysis of a feline calicivirus (FCV) hypervariable region in the epidemiological investigation of FCV related disease and vaccine failures. Vaccine. 1997;15:1451–1458. doi: 10.1016/s0264-410x(97)00059-5. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Dawson S., Ryvar R., Coyne K., Johnson D.R., Cox M.B., Acke E.F., Addie D.D., Gaskell R.M. High genetic diversity of the immunodominant region of the feline calicivirus capsid gene in endemically infected cat colonies. Virus Genes. 2003;27:145–155. doi: 10.1023/a:1025772409539. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Dawson S., Wharmby C., Ryvar R., Gaskell R.M. Comparison of serological and sequence-based methods for typing feline calcivirus isolates from vaccine failures. Vet. Rec. 2000;146:117–123. doi: 10.1136/vr.146.5.117. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Sommerville L., Ryvar R., Cox M.B., Johnson D.R., Dawson S., Gaskell R.M. Endemic infection of a cat colony with a feline calicivirus closely related to an isolate used in live attenuated vaccines. Vaccine. 2001;19:4358–4362. doi: 10.1016/s0264-410x(01)00191-8. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Sommerville L.M., Dawson S., Kerins A.M., Ryvar R., Gaskell R.M. Molecular analysis of isolates of feline calicivirus from a population of cats in a rescue shelter. Vet. Rec. 2001;149:477–481. doi: 10.1136/vr.149.16.477. [DOI] [PubMed] [Google Scholar]
- Radford A.D., Turner P.C., Bennett M., McArdle F., Dawson S., Glenn M.A., Williams R.A., Gaskell R.M. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats. J. Gen. Virol. 1998;79(Pt. 1):1–10. doi: 10.1099/0022-1317-79-1-1. [DOI] [PubMed] [Google Scholar]
- Reubel G.H., Hoffmann D.E., Pedersen N.C. Acute and chronic faucitis of domestic cats. A feline calicivirus-induced disease. Vet. Clin. N. Am. Small Anim. Pract. 1992;22:1347–1360. doi: 10.1016/s0195-5616(92)50131-0. [DOI] [PubMed] [Google Scholar]
- Schorr-Evans E.M., Poland A., Johnson W.E., Pedersen N.C. An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England. J. Feline Med. Surg. 2003;5:217–226. doi: 10.1016/S1098-612X(03)00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal B.S., Ridpath J.F., Mengeling W.L. Analysis of feline calicivirus capsid protein genes: identification of variable antigenic determinant regions of the protein. J. Gen. Virol. 1993;74:2519–2524. doi: 10.1099/0022-1317-74-11-2519. [DOI] [PubMed] [Google Scholar]
- Thompson R.R., Wilcox G.E., Clark W.T., Jansen K.L. Association of calicivirus infection with chronic gingivitis and pharyngitis in cats. J. Small Anim. Pract. 1984;25:207–210. [Google Scholar]
- Wardley R.C. Feline calicivirus carrier state. A study of the host/virus relationship. Arch. Virol. 1976;52:243–249. doi: 10.1007/BF01348021. [DOI] [PubMed] [Google Scholar]
- Wardley R.C., Gaskell R.M., Povey R.C. Feline respiratory viruses—their prevalence in clinically healthy cats. J. Small Anim. Pract. 1974;15:579–586. doi: 10.1111/j.1748-5827.1974.tb06538.x. [DOI] [PubMed] [Google Scholar]
- Wardley R.C., Povey R.C. The clinical disease and patterns of excretion associated with three different strains of feline caliciviruses. Res. Vet. Sci. 1977;23:7–14. [PubMed] [Google Scholar]
- Wardley R.C., Povey R.C. The pathology and sites of persistence associated with three different strains of feline calicivirus. Res. Vet. Sci. 1977;23:15–19. [PubMed] [Google Scholar]


