Graphical abstract
We review emerging and re-emerging diseases such as schistosomiasis, dengue, avian influenza, angiostrongyliasis and soil-transmitted helminthiasis that occurre in China, with particular emphasis on environmental and agricultural change.
Keywords: Environment, Agriculture, Emerging diseases, Infectious diseases of poverty, Case study, People's Republic of China
Highlights
-
•
We discuss the emergence and re-emergence of infectious diseases in developing countries.
-
•
Changes in the natural environment and agricultural systems, govern infectious diseases.
-
•
Case studies are presented from China.
-
•
Research priorities and response strategies, are offered for consideration.
Abstract
Changes in the natural environment and agricultural systems induced by economic and industrial development, including population dynamics (growth, urbanization, migration), are major causes resulting in the persistence, emergence and re-emergence of infectious diseases in developing countries. In the face of rapid demographic, economic and social transformations, the People's Republic of China (P.R. China) is undergoing unprecedented environmental and agricultural change. We review emerging and re-emerging diseases such as schistosomiasis, dengue, avian influenza, angiostrongyliasis and soil-transmitted helminthiasis that have occurred in P.R. China due to environmental and agricultural change. This commentary highlights the research priorities and the response strategies, namely mitigation and adaptation, undertaken to eliminate the resurgence of those infectious diseases.
1. Introduction
Economic growth, population dynamics (growth, urbanization, migration) and industrial development over the past 50 years have resulted in changes in the natural environment and agricultural systems leading to persistence, emergence and re-emergence of infectious diseases in developing countries (Aguirre and Tabor, 2008, Patz et al., 2000, Patz et al., 2008, WHO, 2013). Climate change and climate variability, in part the consequence of atmospheric pollution, add new layers to this conglomerate of basic driving forces, as do the numerous factors which underlie human over- and under-nutrition (Gage et al., 2008, Patz et al., 2008). Infectious diseases are nonlinearly correlated with poverty as the burden falls most heavily on poor people in developing countries (Guerrant and Blackwood, 1999), particularly on infants and children, e.g. about three million children die each year from malaria and diarrhoeal diseases alone. The disability-adjusted life year (DALY), a measure of overall disease burden (WHO, 2008), of diarrhoea and malaria of all age groups in 2004 accounted for 4.8% (72.8 million) and 2.2% (34 million) of total DALYs, respectively (WHO, 2004)
All infections involving an agent (or pathogen) and/or host(s) are already present in the environment. Some agents (or pathogens) require vectors or intermediate hosts to complete their life cycles, a situation which can amplify impact (Butler, 2012, McMichael, 2004). Environmental conditions have a broad-based influence by either favouring or hindering pathogens, vectors, host defences directly or by changing the habitats exploited by the participating species (host or infectious agent). On rare occasions, changing environmental conditions can provide the opportunity for new pathogen variants to emerge, for instance, new influenza viruses (Aguirre and Tabor, 2008, Epstein, 2001, Zell, 2004). Landscapes altered by agricultural development can also become more sensitive to changes in other environmental variables, setting up a feedback loop that further degrades the ecosystem affecting human society adversely. Poor agricultural practises, for example, have the power to directly degrade living conditions via malnutrition due to food shortage (Sheeran, 2008), while water-borne diseases emerge following water resource development (Hunter et al., 1993, Keiser et al., 2005, Steinmann et al., 2006, Yang et al., 2005). Indeed, most influenza pandemics appear to have an agricultural origin (Ho and Su, 2004, Zhong, 2008). There are many examples of interaction between environmental or agricultural changes and infectious diseases, e.g. the emergence of the severe acute respiratory syndrome (SARS) in the People's Republic of China (P.R. China) in 2003 (Zhong, 2008) and Lyme disease in Europe in 2000 (Aguirre and Tabor, 2008, Ogden et al., 2008, Randolph, 2008). Changed interaction between humans and wild or domestic animal populations comes to mind as the most probable facilitator of the emergence of bovine spongiform encephalopathy (BSE), avian influenza, Nipah virus and the human immunodeficiency virus (HIV) (Chastel, 2004, Genne, 2007, Kong et al., 2008, Snowden, 2008).
In view of new evidence on the interplay between environmental changes and the emergence or re-emergence of infectious diseases (WHO, 2012, 2013), the following issues require attention. First, environmental changes cause significant hazards to human health, which might exacerbate due to increased air pollution, declining water quality, inadequate sanitation, and toxic industrial discharges (Harpham and Tanner, 1995). Second, agricultural practices, including intensive use of fertilizers, pesticides, and other industrial input, degrade natural resources and the environment leading to a slowdown or even decline in agricultural growth in addition to negative impact on human health, biodiversity and ecosystems (Trias, 1987). Third, a specific disease can have either positive or negative effects on an individual or entire communities (McMichael, 2004, WHO, 2003). For example, an infectious disease outbreak can alert more people to take attention to the health development of a community, while poverty cannot be eliminated. Environmental degradation exacerbates malnutrition, disease, and injury, whereas infectious diseases of poverty cannot be alleviated in circumstances of hazardous lifestyle, and poverty (Zhou, 2012). Hence, all components of the eight Millennium Development Goals (MDG) are closely interlinked (Wagstaff et al., 2006) and concerted efforts are reputed to break the poverty cycle related to human health (Tyer-Viola and Cesario, 2010).
In the face of rapid demographic, economic and social transformations, P.R. China is undergoing unprecedented environmental and agricultural change. Consequences of this change are manifested in environmentally induced challenges such as reduced water run-off in some rivers and flooding in others, extreme weather events (floods, blizzards and dust storms) (Yang et al., 2005). Most recently, the 2008 earthquake in Sichuan province took the life of 70,000 people, about 1300 schools had to be reconstructed and 25 townships relocated (Hu et al., 2012). There is also growing concern about the potential future effects of climate change on agricultural productivity. While P.R. China has made progress in controlling and eliminating infectious diseases, important public health challenges such as tuberculosis, HIV/AIDS, schistosomiasis and hepatitis B persist (Yang et al., 2008). In recent years, the country has witnessed outbreaks of newly emerging infectious diseases (Aguirre and Tabor, 2008, Yan et al., 2012), including the occurrence of SARS in 2003, avian influenza in 2004, Streptococcus suis infection in 2005, angiostrongyliasis in 2006, enterovirus 71 (EV71) in 2008, and chikungunya fever in 2010.
In response to these challenges, it is important to investigate the links discussed above to facilitate interdisciplinary research from a variety of natural, social, and health sciences, and train professionals to learn from case studies. We present in this article a framework which integrates three characteristics of the interrelationship between the environment and infectious diseases and poverty: (i) environmental hazards; (ii) agricultural development; and (iii) social factors. Applying this framework to a number of case studies, we put forward recommendations for research priorities.
2. Environmental hazards and infectious diseases of poverty
There is a growing consensus among ecologists that ecological dysfunction, habitat fragmentation, climate change, and the accumulation of toxins are largely attributed to human activities (Table 1 ) (Watson, 2000). All of these have worked synergistically to diminish biodiversity and ecosystem function (Brook et al., 2008), which has promulgated the spread of infectious disease in wildlife and humans (McMichael et al., 2004). People in developing countries are more vulnerable to environmental degradation than those in the developed countries (McMichael et al., 2004).
Table 1.
Infectious diseases with an important environmental contribution in developing countries.
| Infectious diseases of poverty | Burden of disease attributable to environmental causes | Environmental risks |
|---|---|---|
| Diarrhoeal diseases | 94% of the 1.8 million annual deaths | Unsafe drinking water and inadequate sanitation |
| Lower respiratory infections (LRI) | 1.5 million deaths annually (41% of the LRI disease burden) | Exposure to indoor smoke from solid fuels and outdoor (ambient) air pollution |
| Vector-borne disease | Over 500,000 deaths annually | Modifiable environmental factors (such as poorly designed irrigation and water systems; poor housing and settlement; deforestation and ecosystem change/degradation) |
P.R. China is experiencing extraordinary environmental changes, thus eliciting many human health challenges. Environmental change, such as climate changes and agricultural changes, will clearly alter the areas of transmission and the intensity of important vector-borne diseases still not eliminated in the country, such as dengue and schistosomiasis.
2.1. Schistosomiasis
Schistosomiasis is caused by infection by with the blood fluke Schistosoma japonicum through water contact, as the intermediate host is a snail. Historical records show a high correlation between schistosomiasis transmission intensity and environmental or ecological factors, such as temperature, vegetation and rainfall (Yang et al., 2005, Zhou et al., 2008). More recently, research focused on the impact of climate change on schistosomiasis transmission based on the fact that January mean temperature of ≤0 °C restricts the distribution of Oncomelania hupensis, the intermediate host of S. japonicum (Mao, 1990). The impact of global warming on the transmission of schistosoma japonica can be grouped into (i) direct and (ii) indirect effects (Fig. 1 ). It has been reported that the 0–1 °C isotherm in January, a crucial feature determining O. hupensis survival, has shifted latitude from 33°15′N to 33°41′N between the 1960s and the 1990s (Yang et al., 2005). This shift translates to a potential surface area expansion of 41,335 km2 (Yang et al., 2005). An estimated additional 20.7 million people are thus potentially at risk of S. japonicum infection in central P.R. China (Yang et al., 2005). In view of the current estimate that 779 million people are at risk of schistosomiasis worldwide (Steinmann et al., 2006), the 20.7 million additional at-risk Chinese translates to 2.7% on the global scale. Although moderate, this increase will have profound public health and economic implications. However, these figures are dwarfed by might be in store in the longer perspective. Using combined indexes, namely temperature thresholds of both the parasite and the intermediate host snail, together with predicted temperature increases in P.R. China of 0.9 °C by 2030 and 1.6 °C by 2050, indicates that the areas at risk of schistosomiasis may grow to as much as of 662,373 km2 and 783,883 km2 by 2030 and 2050, respectively. By 2050, this translates to 8.1% of the surface area of P.R. China. The same study also suggests that the transmission intensity would increase in the areas endemic for schistosomiasis today due to higher temperature (Zhou et al., 2008).
Fig. 1.
Direct and indirect effects of global warming on the transmission of S. japonicum.
By considering the aforementioned scenarios, supported by modelling studies, mitigation and adaptation strategies to minimize the impacts of climate changes have been initiated in the potential risk areas. For instance, a surveillance and response system has been set up in P.R. China, but in order to monitor subtle changes in intermediate host snail habitats due to global warming and other natural or human-made ecological transformations, the establishment of early warning systems (EWS) are necessary.
2.2. Dengue
Dengue is a re-emerging infectious disease transmitted by mosquitoes, mainly Aedes aegypti and Aedes albopictus. It comes in four slightly different serotypes and has been an emerging public-health problem in P.R. China, as well as in other parts of Southeast Asia for some time (Chastel, 2004). Early documented Chinese epidemics of dengue in 1902, 1915 and 1922 in Penghu Islet, Taiwan (King et al., 2000) estimated infection rates as high as 80%. Two subsequent outbreaks occurred in 1931 and 1942–1943 (Wu et al., 2003). In mainland P.R. China, dengue fever was probably imported at the beginning of the 20th century, and epidemics occurred in the cities of Shanghai, Hangzhou, Guangzhou and Haikou in the 1920s and the 1940s. A severe outbreak occurred in 1978 in Fushan, Guangdong province, with a total of 22,122 cases infected with the DEN-4 dengue virus variant (Fan et al., 2004, Qiu et al., 1993, Zhao, 1981). Since then, epidemics with high incidence rates were mainly reported from the south-eastern part of P.R. China with hotspots in Guangdong, Fujian, Guangxi, and Hainan provinces (Fan et al., 2004, Huang, 1982, Li et al., 1986, Qiu et al., 1991, Yang et al., 1989). Due to globalization and rapid urban development, a shift of occurrence of dengue cases from rural to urban and peri-urban areas has been observed in recent years (Wenming et al., 2005, Zhang et al., 2005).
Based on historic monthly mean temperature in January from 1950s to 2001 at eight meteorological stations, along with the threshold temperature for continued transmission of 21 °C, the transmission trend and intensity of dengue fever have been analyzed in Hainan province (Yu et al., 2005). Winter temperatures in this province are increasing at a pace projecting the 21 °C contour (and thus the potential risk for infection) to have moved 190 km northward by 2050. More than half of Hainan province would then be suitable for transmission of dengue fever virus during the entire year (Yu et al., 2005). Another study found that winter monthly average temperature increases by as little as 1–2 °C, would make the northern part of Hainan province a potential transmission area during the entire year (Chen et al., 2002). The transmission intensity in the southern part will also be enhanced, which could transit into a region of stable endemicity (Chen et al., 2002).
3. Agricultural practice and infectious diseases of poverty
The green revolution that began in P.R. China in the 1970s has led to rapid growth in agricultural production and to a large reduction of poverty (Huang et al., 2002). However, agriculture practises, such as intensive use of fertilizers, pesticides, and irrigation, have also caused biodiversity loss due to toxicity and underground water depletion (Tilman et al., 1997). Other forms of industrial impact, typical of the green revolution have also degraded natural resources and the environment, including increased soil salinity, and wind and water erosion (Folke et al., 2004). The 2008 Chinese milk scandal was a safety incidence involving an infant formula that contained involving additive food components that turned out to be contaminated with melamine (Chan et al., 2009). Environmental degradation, in turn, has led to a slow down or even decline in agricultural growth, in addition to its negative impact on human health, biodiversity and ecosystems (Trias, 1987), which reflected in outbreaks of avian influenza and angiostrongyliasis (Lv et al., 2008, Martin et al., 2011).
3.1. Avian influenza
The highly pathogenic avian influenza (HPAI) in poultry (H5 and H7 strains) has high mortality rates and often causes sudden death. Avian influenza leads to the destruction of the entire flocks, causing serious economic losses (Wang et al., 2008). The first strain of the HPAI virus (H5N1) was isolated from geese in P.R. China in 1996 (Chen et al., 2004). According to the Ministry of Agriculture, P.R. China experienced 50 epidemics by this strain in 16 provinces in 2004 (Zeng, 2006). There were 144,900 bird incident cases, 129,100 bird deaths, and a total of 9,045,000 birds were culled. About 32 H5N1 HPAI epidemics occurred in 13 provinces in 2005. Among them, one was a migratory bird epidemic in Qinghai province, and a total of 163,100 bird incident cases with 154,600 deaths, and 22,571,200 culled birds (Zeng, 2006). Another 10 poultry epidemic outbreaks occurred in seven provinces in 2006, including migratory birds in Qinghai and Tibet, as well as three HPAI outbreaks that took place in Hunan, Guangdong and Tibet in 2007 (Chen, 2009, Wu, 2009). The underscored demonstrate that both domestic and wild waterfowl can die from this infection.
In spite of avian influenza virus infection mainly affecting poultry production, there is growing evidence that it is increasing in virulence and pathogenicity, thus spreading the infection to many other species such as zoo-captive tigers and leopards, domestic pigs, cats and dogs (Crawford et al., 2005, Keawcharoen et al., 2004, Kida et al., 1994). Avian influenza also affects humans by increasing the opportunity of generating new influenza viruses by gene rearrangement involving the human influenza virus. The outbreak of avian flu in Hong Kong in 1997 showed, for the first time, that avian influenza can directly infect people and cause death (Kim et al., 2011). Therefore, the public health significance of avian flu, especially the HPAI type, needs to be re-evaluated. On 8 August 2006, the Chinese Ministry of Health announced the first laboratory-confirmed human pulmonary infection with HPAI in P.R. China. Seven, 12 and five human infections with HPAI resulting in five, eight and three deaths were reported in 2005, 2006 and 2007, respectively. In December 2008, eight cases of human infection of HPAI (H5N1) were reported, five of which was fatal (Chen, 2009, Wu, 2009).
Avian influenza caused by H5N1 virus in humans is a rare but severe disease that must be closely monitored and studied because of the potential of the virus to evolve in ways that might start a pandemic. A pandemic might occur if the following three conditions are met: (1) a new influenza virus emerges (such as avian influenza H5N1); (2) the virus infects humans (this has occurred with H5N1, although it is still relatively rare); and (3) the virus spreads efficiently and in a sustained manner from human to human (Department of Epidemic and Pandemic Alert and Response, 2008). Although the disease is not yet transmitted efficiently from person to person, transmission of influenza A/H5N1 virus through air has been proved ferrets (Herfst et al., 2012). Thus, an EWS supported by a surveillance system with rapid and sensitive diagnostics is required to prevent such a catastrophe (Cui et al., 2011, Martin et al., 2011).
3.2. Angiostrongyliasis
Angiostrongyliasis, caused by the nematode Angiostrongylus cantonensis, is an emerging food-borne disease that was first described in the brown rat (Rattus norvegicus) and the black rat (Rattus rattus) in Guangzhou (formerly Canton), P.R. China, in 1933 (Deng et al., 2011). Transmission to humans is primarily by consumption of raw snails. The first case of human angiostrongyliasis in mainland P.R. China was diagnosed in 1984 (Lv et al., 2009a). During the past decade, the number of cases has sharply increased (Lv et al., 2010).
The largest outbreak thus was occurred in Beijing in 2006, peaking in August and involving 160 reported cases, 100 of whom were hospitalized with four deaths. Outbreak investigations found that 75.1% of the patients had eaten raw apple snails (Pomacea canaliculata) or raw giant African land snails (Achatina fulica) (Lv et al., 2008, Lv et al., 2009b). The import and sale of infected P. canaliculata was the likely trigger for this angiostrongyliasis outbreak. Among the different factors facilitating the spread and transmission of A. cantonensis in P.R. China, the two invasive mollusk species P. canaliculata and A. fulica, play a central role (Lv et al., 2009b, Lv et al., 2010). Indeed, P. canaliculata and A. fulica have invaded the southern part of P.R. China after being imported from South America (through Taiwan) in the 1980s and East Africa in the 1930s. While both snail species were imported as food, they also became established in the wild fauna and are now common in the southern part of P.R. China (Deng et al., 2011, Lv et al., 2006). Vast habitats with suitable environmental conditions are found in many parts of the country. As a result, there is a need for strengthening food safety inspections and food-borne disease surveillance (Lv et al., 2009a).
4. Social status and infectious disease of poverty
Most non-members of the Organization for Economic Cooperation and Development (OECD) are low-income economies with a low human development index (HDI) and are regarded as developing countries. In non-OECD countries, the majority of the burden of disease can be attributed to communicable disorders, including infectious diseases. Particularly in the poor population of Asia, most of the disease burden is due to neglected tropical diseases, in which soil-transmitted helminthiasis contribute significantly (Hotez and Ehrenberg, 2010). Some scientists have even suggested that the level of soil-transmitted helminth infections in a given population could be used as an index for its economic status (Ziegelbauer et al., 2012). For example, the economic status that impacts on the poor significantly comes from the cumulative detrimental health effects of infectious and parasitic diseases (WHO, 2001). Some infectious pathogens have serious effects on human capital, manifested via substantial morbidity and disease-induced mortality (Bonds et al., 2010).
4.1. Soil-transmitted helminthiasis
In P.R. China, the first and second nationwide survey to determine the distribution of human intestinal parasites were conducted in 1988–1992 and 2003, respectively. The first survey revealed an overall infection rate of intestinal parasites of 62.6%, with an average infection rate of hookworm at 17.2%, Ascaris lumbricoides at 47% and Trichuris trichiura at 18.8%. The second national survey revealed that soil-transmitted helminth infections decline substantially by 407 million infections compared with that in the first national survey (CONSIHPD, 2005). The standardized rate of hookworm, A. lumbricoides and T. trichiura infections had decreased by 61%, 71%, and 74%, respectively (CONSIHPD, 2005, Xu et al., 1995, Yu et al., 1994). Although the infection rate tended to increase gradually from the north to the south of the country (CONSIHPD, 2005), the infection rates in some southern areas, i.e. Jiangsu, Guangdong, Shanghai and Zhejiang provinces/municipalities were lower than those of many other central and southern provinces/autonomous regions/municipalities due to large-scale control activities, rapid local economical development, and improved living standards of the farmers (CONSIHPD, 2005, Yu et al., 1994). In some less-developed counties and townships in central and western P.R. China, the number of soil-transmitted helminth infections remained high and difficult to control due to complicated natural, social, and behavioural factors. The inaccessibility of transportation as well as budgetary constraints were key impediments for parasitic disease control (CONSIHPD, 2005, Yu et al., 1994).
Different regions have identified specific diseases as priorities for control, for instance, trichinosis in western P.R. China and soil-transmitted helminthiasis in impoverished regions of central and eastern parts of the country. Correlation analysis of economic, cultural and sanitary parameters (e.g. age, sex, educational level, profession and ethnicity) and parasitic infections in three different strata, including 726 sampled counties, indicates that correlations exist between those social parameters and overall infection rate of STH, including A. lumbricoides, hookworm and T. trichiura. For instance, the 10–14 year age group had the highest soil-transmitted helminths infections at 23%, followed by the 5–9 year age group at 22%. Children, women, and ethnic minorities are the most vulnerable populations to be targeted within all disease-specific interventions (CONSIHPD, 2005, Schratz et al., 2010, Ziegelbauer et al., 2012). Besides the three factors, temperature and humidity was also found to influence the overall infection rates for A. lumbricoides, hookworm and Taenia solium.
5. Discussion
Evidence derived from the case studies presented here suggests that the vicious cycle of poverty and infectious diseases are affected by factors pertaining to environmental changes, agricultural practices as well as the social situation (Utzinger et al., 2010, Vandemark et al., 2010, Yang et al., 2010). This cycle cannot be interrupted without a clear understanding of the interplay between environment, agriculture, and infectious diseases of poverty (Butler, 2012, Zhou, 2012). There is a pressing need to examine quantitatively and qualitatively how these factors relate to one another (Fig. 2 ), with research priorities on the linkages and impacts radiating from the infectious diseases with the highest disease burden in poor populations attributed to various environmental causes.
Fig. 2.
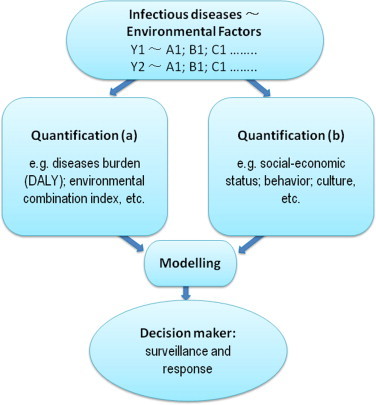
Research priority diagram between infectious diseases and environmental factors.
To begin to understand the correlations between environment, agriculture and infectious diseases, the following four research priorities are proposed:
-
1.
Identification of an appropriate set of metrics of infectious diseases and environmental/agricultural factors (Hunter, 2003, WHO, 2012, Yang et al., 2010). Previous estimates were either too broad to be useful or too narrow to cover the full spectrum of environmental challenges. In addition, metrics applied in different regions were not commensurable. A standardized set of metrics would help by spotting current problems and identifying priority environmental issues.
-
2.
Definition of risk factors, quantitatively and qualitatively, due to infectious diseases and the environment (Chen et al., 2002, Yu et al., 2005). Environmental and health challenges come in several forms which vary with the level of wealth and development. Without a standardized index, comparisons between various diseases are hazardous. The use of the DALY metric is a step in the right direction but it needs further development, e.g. by inclusion also of subtle pathology. For example, some of the neglected tropic diseases have considerable subtle morbidity which is inefficiently taken into consideration in the current version of DALY estimation (Jia et al., 2007, Jia et al., 2011, King et al., 2005).
-
3.
Establishment of models to evaluate the proportion of a particular disease that is influenced by a single or interacting risk factors (Yang et al., 2006, Zhou et al., 2008). To evaluate the relationships between these factors, robust and accurate statistics and mathematical models are required, which allow prompt regional and global responses to changes in the transmission intensity of the infectious disease in question.
-
4.
The design of disease-related policies and programmes for decision-makers (Lv et al., 2009a, Odermatt et al., 2010). Fuelled by advances in information technology, data-driven decision-making has and become a “game-changer” for surveillance and response activities (Yang et al., 2012). In the policy domain, quantitative and qualitative performance metrics should reshape activities as it is necessary to apply a similar, data-driven, fact-based empirical approach to provide concrete evidence for health policy makers (Malone et al., 2010).
To response to the challenges posed by global changes in environment, agriculture and health, two research priorities, as the principal strategies for managing or reducing the risks of environmental or agricultural change, need to be taken into account. One is mitigation, i.e. to limit the risk factors, and the other is adaptation, which means that although all efforts must be made in seeking to limit the negative impacts of industrial development, some degree of inevitable environmental or agriculture changes should be accepted. Mitigation and adaptation share the same ultimate purpose, which is to reduce undesirable impacts (Swart and Raes, 2007). The bottom line is that the more effective mitigations undertaken now, the less need will there be for adaptation in the future. The most successful example is the current responses to climate change, which is undertaken by a majority of all countries (Dowlatabadi, 2007, Willbanks and Sathaye, 2007). However, even the most rigorous mitigation efforts cannot avoid further impacts of climate change because of historically committed emissions, making action on adaptation essential. At the same time, adaptation cannot be allowed to rule indefinitely, so urgent mitigation is needed to avoid the worst effects of climate change. Investigations needed to answer how to best balance these demands are proposed to be implemented in community-based studies involved by local stakeholders to get more evidence-based results (Willbanks and Sathaye, 2007).
Before a course of action with regard to response is taken, an informative, timely, and useful assessment is required (Yan et al., 2012). Such an assessment includes the following five steps:
-
(a)
Determination of the scope of assessment which could either be the geographic range (e.g. country, province, county) or the heterogeneity at different scales.
-
(b)
Description of the existing distribution and intensity of infectious diseases, and the environmental or agricultural determinants to which these diseases are sensitive. For instance, to understand the current status of malaria transmission in P.R. China, the most important environmental conditions influencing its expression, such as temperature, rainfall and relative humidity, should be analyzed.
-
(c)
Identification of the current strategies, policies, and measures designed to reduce the burden of diseases due to the environmental changes at hand.
-
(d)
Estimation of the future potential health impacts using scenarios of future changes in the most important variables such as climate and socioeconomic development.
-
(e)
Identification of additional adaptation policies and measures needed to reduce potential negative health impacts which may call for interdisciplinary or intersectoral collaboration. Based on the assessment study, more suitable strategies to tackle the problems caused by infectious diseases of poverty could be adapted to effectively minimize the impacts by global changes of environment and agriculture.
In conclusion, the emergence and re-emergence of infectious diseases caused by changes of the environmental and agricultural systems should be monitored and modelled to better understand the interplay between the different variables and disease. The specific four-pronged approach (Yang et al., 2010), including identifying metrics (Lv et al., 2009b, Steinmann et al., 2006), defining risk factors quantitatively and qualitatively (Lv et al., 2006, Matola et al., 1987, McMichael et al., 2004, Xu et al., 2000), establishment of models to explore the interactions among risk factors (Keiser et al., 2005, Li et al., 1986, Lv et al., 2010, Yang et al., 2006, Zhou et al., 2008), and designing policies and programmes for specific disease for decision makers (Collins et al., 2012, Lv et al., 2008, Yang et al., 2005), are proposed to identify the multi-risk factors related to the global changes in environment, agriculture, and health. A response strategy based on mitigation and adaptation is important to interrupt the transmission of infectious diseases (McMichael, 2004). Finally, the assessment plays the key role in providing information to adaptation of intervention strategies that tailored to local settings in order to reduce the impact of global changes (Kong et al., 2008). Such an integrated and trans-disciplinary approach should provide tangible evidence for policy-makers regarding the best possible and the most cost-effective interventions (Huang et al., 2002, Hunter, 2003, Zhou, 2012) (see Box 1, Box 2 ).
Box 1. Key points of the review.
-
•
There is a vicious cycle between poverty and infectious diseases affected by factors of environmental changes, agricultural practices and social status.
-
•
Emerging and re-emerging diseases, e.g. schistosomiasis, dengue, avian influenza, and angiostrongyliasis, can be limited to environmental and agricultural change in P.R. China.
-
•
A four-pronged approach, including identifying metrics, defining risk factors quantitatively and qualitatively, establishment of risk-factor models, and designing policies and programmes for specific disease for decision makers, is used to identify the multi-risk factors with global changes.
-
•
The response strategy with mitigation and adaptation is of importance to interrupt the transmission of infectious diseases.
-
•
The assessment plays the key role in adaptation of intervention strategies readily tailored to the local settings to reduce the impact of global changes.
Box 2. Key papers in the field.
-
•
Aguirre, A.A., Tabor, G.M., 2008. Global factors driving emerging infectious diseases. Annals of the New York Academy of Sciences, 1119, 1–3.
-
•
Brook, B.W., Sodhi, N.S., Bradshaw, C.J.A., 2008. Synergies among extinction drivers under global change. Trends in Ecology and Evolution 25, 453–460.
-
•
Huang, J., Pray, C., Rozelle, S., 2002. Enhancing the crops to feed the poor. Nature 418, 678–684.
-
•
Keiser, J., Castro, M.C., Maltese, M.F., Bos, R., Tanner, M., et al., 2005. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. American Journal of Tropical Medicine and Hygiene 72, 392–406.
-
•
Kong, Q., Zheng, M., Casalone, C., Qing, L., Huang, S., et al., 2008. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. Journal of Virology 82, 3697–3701.
-
•
Lv, S., Zhang, Y., Liu, H.X., Hu, L., Yang, K., et al., 2009. Invasive snails and an emerging infectious disease: results from the first national survey on Angiostrongylus cantonensis in China. PLoS Neglected Tropical Diseases 3, e368.
-
•
Matola, Y.G., White, G.B., Magayuka, S.A., 1987. The changed pattern of malaria endemicity and transmission at Amani in the eastern Usambara mountains, north-eastern Tanzania. Journal of Tropical Medicine and Hygiene 90, 127–134.
-
•
McMichael, A.J., 2004. Environmental and social influences on emerging infectious diseases: past, present and future. Philosophical Transactions of the Royal Society B: Biological Sciences 359, 1049–1058.
-
•
McMichael, A.J., Campbell-Lendrum, D.H., Kovats, S., Edwards, S., Wilkinson, P.W., et al., 2004. Comparative quantification of health risks: global and regional burden of disease due to selected major risk factors. In: Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J.L., (Eds.), Global Climate Change, World Health Organization, Geneva, pp. 1543–1649.
-
•
Odermatt, P., Lv, S., Sayasone, S., 2010. Less common parasitic infections in Southeast Asia that can produce outbreaks. Advances in Parasitology 72, 409–435.
-
•
Steinmann, P., Keiser, J., Bos, R., Tanner, M., Utzinger, J., 2006. Schistosomiasis and water resources development: systematic review, meta-analysis and estimates of people at risk. Lancet Infectious Diseases 6, 411–425.
-
•
WHO, 2012. The Global Report for Research on Infectious Diseases of Poverty. World Health Organization, Geneva.
-
•
WHO, 2013. Research priorities for the environment, agriculture and infectious diseases of poverty. WHO Tech. Rep. Ser. 976, 1–125.
-
•
Yang, G.J., Utzinger, J., Lv, S., Qian, Y.J., Li, S.Z., et al., 2010. The Regional Network for Asian Schistosomiasis and Other Helminth Zoonoses (RNAS+): target diseases in face of climate change. Advances in Parasitology 73, 101–135.
-
•
Zeng, G., 2006. Strategic analysis on responding human avian flu and flu pandemic in China. Biomedical and Environmental Sciences 19, 158–161.
-
•
Zhou, X.N., Yang, G.J., Yang, K., Wang, X.H., Hong, Q.B., et al., 2008. Potential impact of climate change on schistosomiasis transmission in China. American Journal of Tropical Medicine and Hygiene 78, 188–194.
Funding
GJY is grateful to the National Nature Science Foundation (grant nos. 81102173 and 81273192), UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (no. A70530), and the Health Promotion Project, Outstanding Person Fund, Jiangsu Provincial Department of Health (2011). This work was partially supported by the Program for National S & T Major Program (grant nos. 2008ZX10004-011 and 2012ZX10004-220), and partially funded through a capacity building initiative for Ecohealth Research on Emerging Infectious Disease in Southeast Asia (grant no: 105509-029) supported by the International Development Research Centre (IDRC), the Canadian International Development Agency (CIDA) and the Australian Agency for International Development (AusAID) in partnership with the Global Health Research Initiative.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
We would like to thank Professor Anthony J. McMichael, Professor Corey Bradshaw and Dr. Johannes Sommerfeld, for their valuable comments on the draft manuscript. Many thanks to Dr. Robert Bergquist for critical reviewing the manuscript. Thanks also to the support from the World Health Organization (WHO) Country Office in China for providing a platform for activities of the WHO Reference Group on Environment, Agriculture and Infectious Diseases.
References
- Aguirre A.A., Tabor G.M. Global factors driving emerging infectious diseases. Ann. N. Y. Acad. Sci. 2008;1149:1–3. doi: 10.1196/annals.1428.052. [DOI] [PubMed] [Google Scholar]
- Bonds M.H., Keenan D.C., Rohani P., Sachs J.D. Poverty trap formed by the ecology of infectious diseases. Proc. R. Soc. B: Biol. Sci. 2010;277:1185–1192. doi: 10.1098/rspb.2009.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook B.W., Sodhi N.S., Bradshaw C.J.A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008;25:453–460. doi: 10.1016/j.tree.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Butler C.D. Infectious disease emergence and global change: thinking systemically in a shrinking world. Infect. Dis. Poverty. 2012;1:5. doi: 10.1186/2049-9957-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M., Lo C.K., Cheng L.S., Cheung T.C., Wong Y.C. Evaluation of testing capabilities for the determination of melamine in milk through an interlaboratory proficiency test programme during the melamine crisis. Food Addit. Contam. A: Chem. Anal. Control Expos. Risk Assess. 2009;26:1450–1458. doi: 10.1080/02652030903173627. [DOI] [PubMed] [Google Scholar]
- Chastel C. Emergence of new viruses in Asia: is climate change involved? Méd. Malad. Infect. 2004;34:499–505. doi: 10.1016/j.medmal.2004.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H5N1 avian influenza in China. Sci. China Life Sci. 2009;52:419–427. doi: 10.1007/s11427-009-0068-6. [DOI] [PubMed] [Google Scholar]
- Chen H., Deng G., Li Z., Tian G., Li Y., Jiao P., Zhang L., Liu Z., Webster R.G., Yu K. The evolution of H5N1 influenza viruses in ducks in southern China. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10452–10457. doi: 10.1073/pnas.0403212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.J., Li C.X., Lin M.H., Wu K.C., Wu K.L., Zhao Z.G. Study on the suitable duration for dengue fever (DF) transmission in a whole year and potential impact on DF by global warming in Hainan province. Chin. Trop. Med. 2002;2:31–34. [Google Scholar]
- Collins C., Xu J., Tang S. Schistosomiasis control and the health system in PR China. Infect. Dis. Poverty. 2012;1:8. doi: 10.1186/2049-9957-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSIHPD A national survey on current status of the important parasitic diseases in human population (Coordinating Office of the National Survey on the Important Human Parasitic Diseases) Chin. J. Parasitol. Parasit. Dis. 2005;23:332–340. [PubMed] [Google Scholar]
- Crawford P.C., Dubovi E.J., Castleman W.L. Transmission of equine influenza virus to dogs. Science. 2005;310:482–485. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- Cui P., Hou Y., Xing Z., He Y., Li T., Guo S., Luo Z., Yan B., Yin Z., Lei F. Bird migration and risk for H5N1 transmission into Qinghai Lake, China. Vector-Borne Zoonot. Dis. 2011;11:567–576. doi: 10.1089/vbz.2009.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.H., Lv S., Lin J.Y., Lin R.X., Pei F.Q. An outbreak of angiostrongyliasis in Guanging, People's Republic of China: migrants vulnerable to an emerging disease. Southeast Asian J. Trop. Med. Pub. Health. 2011;42:1047–1053. [PubMed] [Google Scholar]
- Department of Epidemic and Pandemic Alert and Response . World Health Organization; Geneva: 2008. Pandemic Influenza Preparedness and Mitigation in Refugee and Displaced Populations:WHO Guidelines for Humanitarian Agencies. [Google Scholar]
- Dowlatabadi H. On integration of policies for climate and global change. Mitig. Adapt. Strat. Glob. Change. 2007;12:651–663. [Google Scholar]
- Epstein P.R. Climate change and emerging infectious diseases. Microbes Infect. 2001;3:747–754. doi: 10.1016/s1286-4579(01)01429-0. [DOI] [PubMed] [Google Scholar]
- Fan J., Pan Z., Zhao J., Zheng D., Tuo D., Zhao P. Ecological management model of agriculture-pasture ecotone based on the theory of energy and material flow: a case study in Houshan dryland area of Inner Mongolia. Ying Yong Sheng Tai Xue Bao. 2004;15:579–583. [PubMed] [Google Scholar]
- Folke C., Carpenter S., Walker B., Scheffer M., Elmqvist T., Gunderson L., Holling C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 2004;35:557–581. [Google Scholar]
- Gage K.L., Burkot T.R., Eisen R.J., Hayes E.B. Climate and vectorborne diseases. Am. J. Prevent. Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Genne D. Bad bats? Rev. Méd. Suisse. 2007;3 2273–2274, 2276–2277. [PubMed] [Google Scholar]
- Guerrant R.L., Blackwood B.L. Threats to global health and survival: the growing crises of tropical infectious diseases—our “unfinished agenda”. Clin. Infect. Dis. 1999;28:966–986. doi: 10.1086/514765. [DOI] [PubMed] [Google Scholar]
- Harpham T., Tanner M. Earthscan; London: 1995. Urban Health in Developing Countries: Progress and Prospects. [Google Scholar]
- Herfst S., Schrauwen E.J., Linster M., Chutinimitkul S., de Wit E., Munster V.J., Sorrell E.M., Bestebroer T.M., Burke D.F., Smith D.J., Rimmelzwaan G.F., Osterhaus A.D.M.E., Fouchier R.A.M. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M.S., Su I.J. Preparing to prevent severe acute respiratory syndrome and other respiratory infections. Lancet Infect. Dis. 2004;4:684–689. doi: 10.1016/S1473-3099(04)01174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Ehrenberg J.P. Escalating the global fight against neglected tropical diseases through interventions in the Asia Pacific region. Adv. Parasitol. 2010;72:31–53. doi: 10.1016/S0065-308X(10)72002-9. [DOI] [PubMed] [Google Scholar]
- Hu Y., Wang J.F., Li X.H., Ren D., Mu D.H., Wang Y.P., Wen C.M., Zhu J., Liang J. Application of Bayesian geostatistical modeling for the assessment of risk for child mortality during the 2008 earthquake in Wenchuan, People's Republic of China. Geospatial Health. 2012;6:247–255. doi: 10.4081/gh.2012.142. [DOI] [PubMed] [Google Scholar]
- Huang J., Pray C., Rozelle S. Enhancing the crops to feed the poor. Nature. 2002;418:678–684. doi: 10.1038/nature01015. [DOI] [PubMed] [Google Scholar]
- Huang Z.X. Epidemiologic investigation on dengue fever in northern Zhanxian County of Hainan Island. Zhonghua Yi Xue Za Zhi. 1982;62:605–609. [PubMed] [Google Scholar]
- Hunter J.M., Rey L., Chu K.Y., Adekolu-John E.O., Mott K.E. World Health Organization; Geneva: 1993. Parasitic Diseases in Water Resources Development. [Google Scholar]
- Hunter P.R. Climate change and waterborne and vector-borne disease. J. Appl. Microbiol. 2003;94(Suppl.):37S–46S. doi: 10.1046/j.1365-2672.94.s1.5.x. [DOI] [PubMed] [Google Scholar]
- Jia T.W., Utzinger J., Deng Y., Yang K., Li Y.Y., Zhu J.H., King C.H., Zhou X.N. Quantifying quality of life and disability of patients with advanced schistosomiasis japonica. PLoS Neglect. Trop. Dis. 2011;5:e966. doi: 10.1371/journal.pntd.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T.W., Zhou X.N., Wang X.H., Utzinger J., Steinmann P., Wu X.H. Assessment of the age-specific disability weight of chronic schistosomiasis japonica. Bull. World Health Org. 2007;85:458–465. doi: 10.2471/BLT.06.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J., Oraveerakul K., Kuiken T.A. Avian influenza H5N1 in tigers and leopards. Emerg. Infect. Dis. 2004;10:2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Castro M.C., Maltese M.F., Bos R., Tanner M., Singer B.H., Utzinger J. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am. J. Trop. Med. Hyg. 2005;72:392–406. [PubMed] [Google Scholar]
- Kida H., Ito T., Yasuda J., Shimizu Y., Itakura C., Shortridge K.F., Kawaoka Y., Webster R.G. Potential for transmission of avian influenza viruses to pigs. J. Gen. Virol. 1994;75:2183–2188. doi: 10.1099/0022-1317-75-9-2183. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lo F.K., Cheuk K.K., Kwong M.S., Goggins W.B., Cai Y.S., Lee S.S., Griffiths S. Knowledge of avian influenza (H5N1) among poultry workers, Hong Kong, China. Emerg. Infect. Dis. 2011;17:2319–2321. doi: 10.3201/eid1712.110321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.C., Wu Y.C., Chao D.Y., Lin T.H., Chow L., Wang H.T., Ku C.C., Kao C.L., Hwang K.P., Lam S.K., Gubler D.J. Major epidemics of dengue in Taiwan in 1991–2000: related to intensive virus activities in Asia. Dengue Bull. (Geneva: World Health Org.) 2000;24:1–10. [Google Scholar]
- King C.H., Dickman K., Tisch D.J. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- Kong Q., Zheng M., Casalone C., Qing L., Huang S., Chakraborty B., Wang P., Chen F., Cali I., Corona C., Martucci F., Iulini B., Acutis P., Wang L., Liang J., Wang M., Li X., Monaco S., Zanusso G., Zou W.Q., Caramelli M., Gambetti P. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J. Virol. 2008;82:3697–3701. doi: 10.1128/JVI.02561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.S., Yang F.R., Song J.C., Gao H., Tang J.Q., Zou C.H., Hu B.N., Wen S.R., Qiu F.X. Etiologic and serologic investigations of the 1980 epidemic of dengue fever on Hainan Island, China. Am. J. Trop. Med. Hyg. 1986;35:1051–1054. doi: 10.4269/ajtmh.1986.35.1051. [DOI] [PubMed] [Google Scholar]
- Lv S., Zhang Y., Chen S.R., Wang L.B., Fang W., Chen F., Jiang J.Y., Li Y.L., Du Z.W., Zhou X.N. Human angiostrongyliasis outbreak in Dali, China. PLoS Neglect. Trop. Dis. 2009;3:e520. doi: 10.1371/journal.pntd.0000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S., Zhang Y., Liu H.X., Hu L., Yang K., Steinmann P., Chen Z., Wang L.Y., Utzinger J., Zhou X.N. Invasive snails and an emerging infectious disease: results from the first national survey on Angiostrongylus cantonensis in China. PLoS Negl. Trop. Dis. 2009;3:e368. doi: 10.1371/journal.pntd.0000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S., Zhang Y., Steinmann P., Zhou X.N. Emerging angiostrongyliasis in Mainland China. Emerg. Infect. Dis. 2008;14:161–164. doi: 10.3201/eid1401.061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S., Zhang Y., Steinmann P., Zhou X.N., Utzinger J. Helminth infections of the central nervous system occurring in Southeast Asia and the Far East. Adv. Parasitol. 2010;72:351–408. doi: 10.1016/S0065-308X(10)72012-1. [DOI] [PubMed] [Google Scholar]
- Lv S., Zhou X.N., Zhang Y., Liu H.X., Zhu D., Yin W.G., Steinmann P., Wang X.H., Jia T.W. The effect of temperature on the development of Angiostrongylus cantonensis (Chen, 1935) in Pomacea canaliculata (Lamarck, 1822) Parasitol. Res. 2006;99:583–587. doi: 10.1007/s00436-006-0198-8. [DOI] [PubMed] [Google Scholar]
- Malone J.B., Yang G.J., Leonardo L., Zhou X.N. Implementing a geospatial health data infrastructure for control of Asian schistosomiasis in the People's Republic of China and the Philippines. Adv. Parasitol. 2010;73:71–100. doi: 10.1016/S0065-308X(10)73004-9. [DOI] [PubMed] [Google Scholar]
- Mao C.P. People's Health Press; Beijing: 1990. Biology of Schistosome and Control of Schistosomiasis. [Google Scholar]
- Martin V., Zhou X., Marshall E., Jia B., Fusheng G., FrancoDixon M.A., DeHaan N., Pfeiffer D.U., Soares Magalhães R.J., Gilbert M. Risk-based surveillance for avian influenza control along poultry market chains in South China: the value of social network analysis. Prevent. Vet. Med. 2011;102:196–205. doi: 10.1016/j.prevetmed.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matola Y.G., White G.B., Magayuka S.A. The changed pattern of malaria endemicity and transmission at Amani in the eastern Usambara mountains, north-eastern Tanzania. J. Trop. Med. Hyg. 1987;90:127–134. [PubMed] [Google Scholar]
- McMichael A.J. Environmental and social influences on emerging infectious diseases: past, present and future. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2004;359:1049–1058. doi: 10.1098/rstb.2004.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J., Campbell-Lendrum D.H., Kovats S., Edwards S., Wilkinson P.W., Wilson T., Nicholls R., Hales S., Tanser F.C., Le Sueur D., Schlesinger M., Andronova N. Comparative quantification of health risks: global and regional burden of disease due to selected major risk factors. In: Ezzati M., Lopez A.D., Rodgers A., Murray C.J.L., editors. Global Climate Change. World Health Organization; Geneva: 2004. pp. 1543–1649. [Google Scholar]
- Odermatt P., Lv S., Sayasone S. Less common parasitic infections in Southeast Asia that can produce outbreaks. Adv. Parasitol. 2010;72:409–435. doi: 10.1016/S0065-308X(10)72013-3. [DOI] [PubMed] [Google Scholar]
- Ogden N.H., St-Onge L., Barker I.K., Brazeau S., Bigras-Poulin M., Charron D.F., Francis C.M., Heagy A., Lindsay L.R., Maarouf A., Michel P., Milord F., O’Callaghan C.J., Trudel L., Thompson R.A. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int. J. Health Geogr. 2008;7:24. doi: 10.1186/1476-072X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz J.A., Graczyk T.K., Geller N., Vittor A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Patz J.A., Olson S.H., Uejio C.K., Gibbs H.K. Disease emergence from global climate and land use change. Med. Clin. N. Am. 2008;92:1473–1491. doi: 10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Qiu F.X., Chen Q.Q., Ho Q.Y., Chen W.Z., Zhao Z.G., Zhao B.W. The first epidemic of dengue hemorrhagic fever in the People's Republic of China. Am. J. Trop. Med. Hyg. 1991;44:364–370. doi: 10.4269/ajtmh.1991.44.364. [DOI] [PubMed] [Google Scholar]
- Qiu F.X., Gubler D.J., Liu J.C., Chen Q.Q. Dengue in China: a clinical review. Bull. World Health Org. 1993;71:349–359. [PMC free article] [PubMed] [Google Scholar]
- Randolph S.E. Tick-borne encephalitis virus, ticks and humans: short-term and long-term dynamics. Curr. Opin. Infect. Dis. 2008;21:462–467. doi: 10.1097/QCO.0b013e32830ce74b. [DOI] [PubMed] [Google Scholar]
- Schratz A., Pineda M.F., Reforma L.G., Fox N.M., Le Anh T., Tommaso Cavalli-Sforza L., Henderson M.K., Mendoza R., Utzinger J., Ehrenberg J.P., Tee A.S. Neglected diseases and ethnic minorities in the Western Pacific Region: exploring the links. Adv. Parasitol. 2010;72:79–107. doi: 10.1016/S0065-308X(10)72004-2. [DOI] [PubMed] [Google Scholar]
- Sheeran J. The challenge of hunger. Lancet. 2008;371:180–181. doi: 10.1016/S0140-6736(07)61870-4. [DOI] [PubMed] [Google Scholar]
- Snowden F.M. Emerging and reemerging diseases: a historical perspective. Immunol. Rev. 2008;225:9–26. doi: 10.1111/j.1600-065X.2008.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis and estimates of people at risk. Lancet Infect. Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Swart B., Raes F. Making integration of adaptation and mitigation work: mainstreaming into sustainable development policies? Clim. Policy. 2007;7:288–303. [Google Scholar]
- Tilman D., Knops J., Wedin D., Reich P., Ritchie M., Siemann E. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277:1300–1302. [Google Scholar]
- Trias M. Population and development. Bol. Asoc. Chilena Protec. Familia. 1987;23:3–7. [PubMed] [Google Scholar]
- Tyer-Viola L.A., Cesario S.K. Addressing poverty, education, and gender equality to improve the health of women worldwide. J. Obstet. Gynecol. Neonat. Nurs. 2010;39:580–589. doi: 10.1111/j.1552-6909.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- Utzinger J., Bergquist R., Olveda R., Zhou X.N. Important helminth infections in Southeast Asia: diversity, potential for control and prospects for elimination. Adv. Parasitol. 2010;72:1–30. doi: 10.1016/S0065-308X(10)72001-7. [DOI] [PubMed] [Google Scholar]
- Vandemark L.M., Jia T.W., Zhou X.N. Social science implications for control of helminth infections in Southeast Asia. Adv. Parasitol. 2010;73:137–170. doi: 10.1016/S0065-308X(10)73006-2. [DOI] [PubMed] [Google Scholar]
- Wagstaff A., Claeson M., Hecht R.M., Gottret P., Fang Q. Millennium development goals for health: what will it take to accelerate progress? In: Jamison D.T., Breman J.G., Measham A.R., Alleyne G., Claeson M., Evans D.B., Jha P., Mills A., Musgrove P., editors. Disease Control Priorities in Developing Countries. World Bank; Washington, DC: 2006. [PubMed] [Google Scholar]
- Wang H., Feng Z., Shu Y., Yu H., Zhou L., Zu R., Huai Y., Dong J., Bao C., Wen L., Wang H., Yang P., Zhao W., Dong L., Zhou M., Liao Q., Yang H., Wang M., Lu X., Shi Z., Wang W., Gu L., Zhu F., Li Q., Yin W., Yang W., Li D., Uyeki T.M., Wang Y. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008;371:1427–1434. doi: 10.1016/S0140-6736(08)60493-6. [DOI] [PubMed] [Google Scholar]
- Watson R.T. Cambridge University Press; 2000. Land Use, Land Use Change and Forestry. Intergovernmental Panel on Climate Change. [Google Scholar]
- Wenming P., Man Y., Baochang F., Yongqiang D., Tao J., Hongyuan D., Ede Q. Simultaneous infection with dengue 2 and 3 viruses in a Chinese patient return from Sri Lanka. J. Clin. Virol. 2005;32:194–198. doi: 10.1016/j.jcv.2004.04.010. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2001. Macroeconomics and Health: Investing in Health for Development—Report of the Commission on Macroeconomics and Health. [Google Scholar]
- WHO . World Health Organization; Geneva: 2003. Climate Change and Human Health: Risk and Responses. [Google Scholar]
- WHO . World Health Organization; Geneva: 2004. The World Health Report 2004. [Google Scholar]
- WHO . World Health Organization; Geneva: 2008. The Global Burden of Disease: 2004 Update. [Google Scholar]
- WHO . World Health Organization; Geneva: 2012. The Global Report for Research on Infectious Diseases of Poverty. [Google Scholar]
- WHO Research priorities for the environment, agriculture and infectious diseases of poverty. WHO Tech. Rep. Ser. 2013;976:1–125. [PubMed] [Google Scholar]
- Willbanks T.J., Sathaye J. Integrating mitigation and adaptation as a response to climate change: a synthesis. Mitig. Adapt. Strat. Glob. Change. 2007;12:957–962. [Google Scholar]
- Wu K.L., Changchien C.S., Kuo C.M., Chuah S.K., Lu S.N., Eng H.L., Kuo C.H. Dengue fever with acute acalculous cholecystitis. Am. J. Trop. Med. Hyg. 2003;68:657–660. [PubMed] [Google Scholar]
- Wu W. Control of avian influenza A H5N1 in China. Lancet Infect. Dis. 2009;9:460–461. doi: 10.1016/S1473-3099(09)70184-7. [DOI] [PubMed] [Google Scholar]
- Xu L.Q., Yu S.H., Jiang Z.X., Yang J.L., Lai L.Q., Zhang X.J., Zheng C.Q. Soil-transmitted helminthiases: nationwide survey in China. Bull. World Health Org. 1995;73:507–513. [PMC free article] [PubMed] [Google Scholar]
- Xu X.J., Wei F.H., Yang X.X., Dai Y.H., Yu G.Y., Chen L.Y., Su Z.M. Possible effects of the Three Gorges dam on the transmission of Schistosoma japonicum on the Jiang Han plain, China. Ann. Trop. Med. Parasitol. 2000;94:333–341. doi: 10.1080/00034983.2000.11813548. [DOI] [PubMed] [Google Scholar]
- Yan W.R., Nie S.F., Xu B., Dong H.J., Palm L., Diwan V.K. Establishing a web-based integrated surveillance system for early detection of infectious disease epidemic in rural China: a field experimental study. BMC Med. Inf. Decis. Making. 2012;12:4. doi: 10.1186/1472-6947-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Kong L., Zhao W., Wan X., Zhai Y., Chen L.C., Koplan J.P. Emergence of chronic non-communicable diseases in China. Lancet. 2008;372:1697–1705. doi: 10.1016/S0140-6736(08)61366-5. [DOI] [PubMed] [Google Scholar]
- Yang G.J., Utzinger J., Lv S., Qian Y.J., Li S.Z., Wang Q., Bergquist R., Vounatsou P., Li W., Yang K., Zhou X.N. The Regional Network for Asian Schistosomiasis and Other Helminth Zoonoses (RNAS+): target diseases in face of climate change. Adv. Parasitol. 2010;73:101–135. doi: 10.1016/S0065-308X(10)73005-0. [DOI] [PubMed] [Google Scholar]
- Yang G.J., Vounatsou P., Tanner M., Zhou X.N., Utzinger J. Remote sensing for predicting potential habitats of Oncomelania hupensis in Hongze, Baima and Gaoyou lakes in Jiangsu province, China. Geospatial Health. 2006;1:85–92. doi: 10.4081/gh.2006.283. [DOI] [PubMed] [Google Scholar]
- Yang G.J., Vounatsou P., Zhou X.N., Tanner M., Utzinger J. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia. 2005;47:127–134. [PubMed] [Google Scholar]
- Yang K., Zhou X.N., Wang X.H., Yang G.J., Jia T.W., He W.L. Establishment and application of spatio-temporal model of schistosomiasis japonica in a county in marshland region. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2012;24:14–20. [PubMed] [Google Scholar]
- Yang P.Y., Si B.Y., Yan G.Z., Xu P.F., Li R.X. Antigen signature analysis of dengue-2 viruses strains in Hainan China. Wei Sheng Wu Xue Bao. 1989;29:299–302. [PubMed] [Google Scholar]
- Yu S.H., Li Z.Q., Teng W.P., Cai J. Impact on the potential epidemic of dengue fever under warming winter in Hainan province. Chin. J. Epidemiol. 2005;26:25–28. [PubMed] [Google Scholar]
- Yu S.H., Xu L.Q., Jiang Z.X., Xu S.H., Han J.J., Zhu Y.G., Chang J., Lin J.X., Xu F.N. Nationwide survey of human parasite in China. Southeast Asian J. Trop. Med. Pub. Health. 1994;25:4–10. [PubMed] [Google Scholar]
- Zell R. Global climate change and the emergence/re-emergence of infectious diseases. Int. J. Med. Microbiol. 2004;293(Suppl. 37):16–26. doi: 10.1016/s1433-1128(04)80005-6. [DOI] [PubMed] [Google Scholar]
- Zeng G. Strategic analysis on responding human avian flu and flu pandemic in China. Biomed. Environ. Sci. 2006;19:158–161. [PubMed] [Google Scholar]
- Zhang F.C., Chen Y.Q., Lu Y.C., Wang J., Chen W.S., Hong W.X. Analysis on clinical and epidemiological characteristics of 1032 patients with Dengue fever in Guangzhou. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:421–423. [PubMed] [Google Scholar]
- Zhao H.L. The epidemic of dengue fever at Shiwanzhen of Forshan City in 1978 (author's transl) Zhonghua Yi Xue Za Zhi. 1981;61:466–469. [PubMed] [Google Scholar]
- Zhong N. Preparing for the next flu pandemic: from SARS to avian flu. Singap. Med. J. 2008;49:595–598. [PubMed] [Google Scholar]
- Zhou X.N. Prioritizing research for “One health-One world”. Infect. Dis. Poverty. 2012;1:1. doi: 10.1186/2049-9957-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.N., Yang G.J., Yang K., Wang X.H., Hong Q.B., Sun L.P., Malone J.B., Kristensen T.K., Bergquist N.R., Utzinger J. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008;78:188–194. [PubMed] [Google Scholar]
- Ziegelbauer K., Speich B., Mäusezahl D., Bos R., Keiser J., Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9:e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]


