Abstract
Canine coronavirus (CCoV) is widespread in dogs in several countries and causes mild enteric illness evolving to severe enteritis in young pups.
In in vitro cultures canine coronaviruses generally induce extensive cell death, however nature of the events leading to cell death remains largely unknown.
We analysed the induction of cytopathic effect by CCoV in a canine fibrosarcoma cell line (A-72) in order to characterize the apoptotic effect in homologous cell system.
Following CCoV infection A-72 cell line, which is permissive to CCoV, showed reduced growth rate, as detected by MTT assay, a standard colorimetric assay for measuring cellular proliferation, and underwent to apoptotic death. Starting from 24 h after CCoV infection, cells morphology appeared dramatically changed, with cells rounding and detachment from culture surface. Morphologic and biochemical features of apoptosis, such as blebbing of the plasma membrane, translocation of phosphatidilserine to cell surface and annexin V positive staining, nuclear fragmentation, apoptotic bodies formation and DNA laddering, were detected in CCoV-infected cells. Propidium iodide staining of infected culture indicated the appearance of hypodiploid DNA peak corresponding to apoptotic cell population. Commonly to other animal coronavirus infection caspase-3 is likely to contribute to the execution phase of apoptosis induced by CCoV in A-72 cells since we found activation of enzymatic activity as well as procaspase-3 activating cleavage. Apoptotic death of infected cells is detrimental as it causes cell and tissue destruction as well as inflammatory responses. Therefore in the case of CCoV associated gastroenteritis, apoptosis of epithelial mucosa cells may be responsible for pathology induced by CCoV infection.
Keywords: Apoptosis, Canine coronavirus (CCoV), Canine fibrosarcoma cells (A-72), Caspases, FACS analysis
1. Introduction
The Coronaviridae family is a group of enveloped viruses with a large, positive-stranded RNA genome of about 30 kb which comprises two genera, coronavirus and torovirus. Coronaviruses are classified into three groups (I–III) (Gonzalez et al., 2003) based on serological cross-reactivity and phylogenetic analysis. Group I coronaviruses, to which canine coronavirus (CCoV) belongs, includes important animal pathogens such as porcine transmissible gastroenteritis virus (TGEV), porcine epidemic diarrhea virus (PEDV) and feline coronaviruses (FCoVs), as well as human coronavirus (HCoV) 229E and the recently identified HCoV-NL63.
Groups II and III coronavirus also include pathogens of veterinary relevance and a fourth group has been proposed for the human SARS-CoV, that is considered responsible of a viral zoonotic disease (Marra et al., 2003, Rota et al., 2003).
In general, coronaviruses cause a wide spectrum of diseases in humans and animals but primarily infect the respiratory and gastrointestinal mucosa (Siddell, 1995). These infections are generally acute, and destruction of the lining epithelia is considered to be a central event in their pathogenesis.
CCoV, firstly recognized by Binn et al. (1974) as a cause of mild enteric illness in military dogs, is associated to moderate or severe enteritis in young pups. Studies by several investigators found that CCoV is widespread in dogs in several countries and that in young or stressed animals, or in combination with other pathogens, such as canine parvovirus, bacteria or parasites the symptoms are more severe or fatal (Pratelli et al., 1999, Appel et al., 1979).
In in vitro cultures, CCoV generally induces extensive cell death, however nature of the events leading to cell death in coronavirus-infected cells remains largely unknown.
Virus-induced apoptosis is associated to several virus infections in vitro and in vivo, especially to RNA viruses, including members of Coronaviridae family (Hardwick, 1997, O’Brien, 1998, Liu et al., 2001, Liu et al., 2003) and recently the human SARS-CoV has also been reported to induce apoptosis in Vero cells (Yan et al., 2004). Apoptosis is a physiological process defined by typical changes in cellular morphology and biochemical features including DNA fragmentation, cytoplasm vacuolization, chromatin condensation and margination and cellular breakdown into apoptotic bodies (Kerr et al., 1972). Intracellular pathways leading to apoptotic death can be activated either by viruses or by triggering cellular pathways that activate apoptotic program of the cell. In any case, virus-induced apoptosis could be detrimental for the host and it is a common event in lytic viral infections. Several animal and human coronaviruses are reported as acute lytic infections and apoptotic cell death can be one possible mechanism of cell damage induced by these viruses.
The pathogenetic mechanisms of cell injury by CCoV have not been disclosed so far, therefore in this study we examined the induction of cytopathic effect by CCoV in a canine cell line (A-72) in order to characterize the apoptotic effect in homologous cell system.
2. Materials and methods
2.1. Cells and reagents
CCoV 1–71 strain (ATCC VR 809) (courtesy by Dott. Cordioli, IZS Lombardia and Emilia Romagna, Italy) was grown on A-72 cell line (canine fibrosarcoma) on single well at a multiplicity of infection (moi) of 1 or 0.1 to obtain a viral cytopathic effect (CPE) within 2 days. The cells were cultivated in Eagle MEM with sodium pyruvate (1 μM/ml) and 10% fetal calf serum.
2.2. Cell proliferation assay
Cell proliferation of parental and CCoV infected A-72 cells was assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay that is a standard colorimetric assay for measuring cellular proliferation according to the manufacturer's instructions (Roche Diagnostics). Briefly, 104 cells were seeded per well in 96-wells plates and infected with 1 or 0.1 moi. Cell proliferation by MTT analysis was examined by measuring absorbance at 405 nm 48, 72 or 96 h after CCoV infection. The analysis was repeated twice with three replicates.
2.3. Microscopic examination and nuclear staining with Hoechst
A-72 cells in 35 mm culture plates were infected with CCoV as described above. At indicated times post infection (p.i.) cells were examined under the inverted phase-contrast microscope and images were taken with the attached camera.
For detection of alterations in nuclear chromatin, cells were fixed in 3.7% paraformaldehyde in PBS (pH 7.4) for 10 min, permeabilized with 0.2% Triton-X 100 in PBS for 5′, stained with Hoechst 33342 fluorochrome (SIGMA) and analysed under fluorescence microscope.
2.4. Annexin V staining and flow cytometer analysis
Translocation of phosphatidylserine to the external surface of apoptotic cell membrane was examined by staining of fixed samples for infected and control A-72 cells with anti-annexinV–biotin conjugated mAb, followed by incubation with streptavidin-PE-conjugated mAb before permeabilization and nuclear staining (see Section 2.3). Double stained (annexin V/Hoechst 33342) samples were then examined under fluorescence microscope.
Incubation of CCoV infected and control A-72 cells with anti-annexin V- FITC conjugated monoclonal antibody (mAb) was performed for quantitating annexin V positive cells by FACS analysis, using CellQuest software.
2.5. Purification of cellular genomic DNA and DNA fragmentation analysis
Cellular genomic DNA was extracted from subconfluent A-72 cells infected with CCoV (moi 1) by incubating cells in 10 mM Tris–HCl (pH 8.0) containing 10 mM EDTA and 0.5% Triton X-100, for 5 min on ice. After centrifugation (4000 × g for 10 min at 4 °C) the supernatants were incubated with RNAse A (5 μg/ml, SIGMA) overnight at 37 °C, followed by 2 h incubation at 50 °C with proteinase K (200 μg/ml, SIGMA) and 0.5% SDS. After extraction with phenol/chloroform (1:1, v/v) fragmented DNA was precipitated with ethanol and 5 M NaCl and analysed in 1.5% agarose gel with 50 bp DNA Step Ladder.
2.6. Propidium iodide (PI) staining and DNA content assay
For PI staining adherent and floating A-72 cells were collected 33 h p.i. with CCoV and washed in citrate buffer (250 mM sucrose, 40 mM trisodium citrate, 20% DMSO). Cell pellets were incubated for 10 min at room temperature (RT) with 0.03% trypsin solution containing 3.4 mM trisodium citrate, 1.5 mM spermine, 0.5 mM Tris and 0.01% NP-40 (pH 7.6). The samples were further incubated with addition of trypsin inhibitor and RNAse A. Cells were then stained with PI (0.4 mg/ml) for 10 min at RT. The samples were analysed by FACScalibur (Becton Dickinson) flow cytometer and the percentage of apoptotic cells was determined using CellQuest software.
2.7. Caspase activity assay
The biological activity of canine caspase-3 in cytosolic extract was measured by colorimetric CaspACE Assay System (Promega Corporation) according to the manufacturer's instruction. Pan-caspase inhibitor ZVAD-fmk (final concentration of 100 μM) was added directly to triplicate wells contemporary to CCoV infection, in order to specifically inhibit activation of caspases. CCoV infected A-72 cells were harvested 48 h p.i., washed with PBS and then resuspended in 100 μl of hypotonic cell lysis buffer. Cells were lysed by repeated freeze-thawing cycles (five rounds) followed by centrifugation to remove cell debris. The supernatants were collected and protein concentrations were determined by Lowry method. Fifty microliters of cell lysates contaning 100 μg of protein were mixed with caspase assay buffer, DMSO and DTT and 2 μl of 10 mM DEVD-pNA substrate. After incubation at 37 °C for 4 h, the release of chromophore was masured by determining the absorbance at 405 nm in a 96-wells plate. Background readings from cell lysates and buffers were subtracted from O.D. of the samples and resulting values were plotted.
2.8. Western blot analysis and caspase-3 cleavage
The cleavage of procaspase-3 to large subunits (32 kDa) of the active form was detected in cell extracts by Western Blot analysis. CCoV infected and uninfected A-72 cells were harvested and lysed with boiling 2× sample buffer. Lysates were centrifuged, and proteins were quantitated by Lowry method. Fifty micrograms of total proteins were run on 10% SDS-PAGE and separated proteins were transferred to PVDF membrane, which was stained with Ponceau S (SIGMA) to verify equal loading of lanes. Membranes were blocked overnight at 4 °C in TBS containing 5% non-fat dry milk/0.1%Tween 20. A rabbit polyclonal serum anti-caspase-3 (Stratagene) (1:2000), specifically reactive with caspase-3 of canine origin, was used as 1st Ab followed by incubation with HRP-conjugated anti-rabbit IgG as secondary serum (1:60,000, Pierce). As positive control for procaspase cleavage, Jurkart cell line treated with mAb CH-11Cl (MBL) against human anti-Fas was employed. Bands were visualized by chemiluminescence detection (ECL, Pierce) and autoradiography.
3. Results
3.1. Cell proliferation of CCoV infected A-72 cells
CCoV infection of A-72 cells resulted in a rapid CPE, starting 24 h p.i and characterized by cells detachment that was much more evident at 48 h p.i. In order to quantitate the cytotoxic effect of CCoV infection in A-72 cells, proliferation rate of infected cell line was analysed by MTT assay, which examines cell viability by measuring their metabolic activity. Results shown in Fig. 1 indicated that 48 h after CCoV infection proliferation rate was reduced by two/three times compared to the uninfected control cells at the two different moi choosen for infection. Proliferation rate progressively decreased five-six times over time up to 96 h p.i., suggesting a reduced A-72 cells proliferation due to cell death.
Fig. 1.
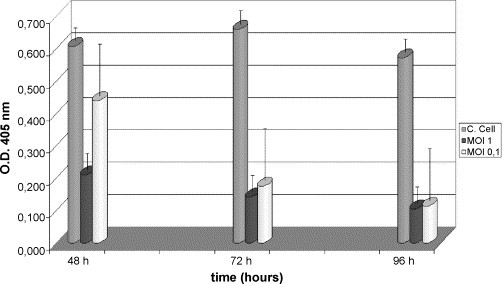
Cell proliferation of A-72 cells by MTT assay. Cell growth was analysed by MTT assay at 48, 72 and 96 h p.i. with CCoV at different moi. Grey column: A-72 control; black column: A-72 infected-cells (1 moi); white column: A-72 infected-cells (0.1 moi).
3.2. Cellular and nuclear morphology of CCoV infected cells
To characterize the CPE of CCoV on A-72, we examined whether the cell death was due to apoptotic effect. Morphology of A-72 cells after CCoV infection was analysed by microscope examination at different times p.i. Results shown in Fig. 2 (upper panel) 48 h p.i., indicated a characteristic CPE with cells rounding up and shrinking, loss of cell contact with neighboring cells and detachment from the culture plates, that could be detected 30 h p.i. and rapidly extended to major part of the cell monolayer. The morphological changes observed in infected cells were those typical of apoptotic cells.
Fig. 2.
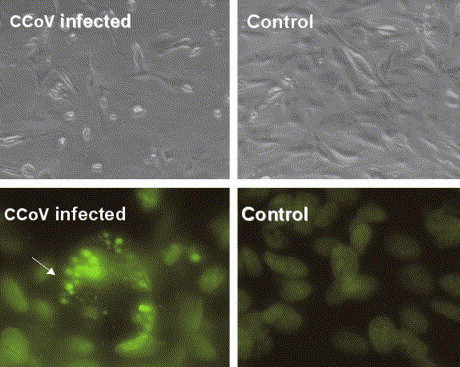
CCoV infection induces cell death in A-72 canine cell line. Upper panels: 48 h after infection (p.i.) with CCoV (moi 0.1), cells were examined for cytopathic effect under phase contrast microscope. Lower panels: nuclear staining with Hoechst 33324. A-72 cell line infected with CCoV (48 h p.i.) showing nuclear condensation and fragmentation with typical apoptotic bodies (indicated by arrow). Control label indicates parallel uninfected A-72 cell culture.
Nuclei of CCoV infected cells were stained with Hoechst 33342 which binds to and stains the DNA in condensed chromatin. Fluorescence microscope examination (Fig. 2 lower panel) indicated that 48 h after CCoV infection nuclei of infected cells appeared condensed, intensively stained and fragmented; typical apoptotic bodies, that are membrane bound portion of fragmented chromatin, could also be detected. In contrast, nuclei of control non-infected cells were uniformly stained without condensation. These results suggested that apoptotic changes of cell and nuclear morphology could be induced in A-72 cells by CCoV infection.
3.3. Annexin V staining of CCoV infected cells
Alterations of the plasma membrane, with translocation of phosphatidylserine from inner side of the plasma membrane to external surface is a hallmark of the early stages of apoptosis. To further characterize the CPE induced by CCoV as apoptotic cell death we performed double staining of control and infected cells with anti-annexin V–biotin/streptavidin-PE conjugated mAbs and Hoechst 33342, followed by fluorescence microscope observation. The results indicated that 33 h after CCoV infection about 20% of cells were double stained with annexin V/Hoechst 33342; this percentage increased up to 90% 48 h p.i.; in Fig. 3A red fluorescence of annexin V/PE is shown for a representative sample double stained with Hoechst 33342. Annexin V positive cells after CCoV infection were further examined by flow cytometry analysis (Fig. 3B); consistently to the microscopic examination, virus infected cells resulted to be positively stained with annexin V, suggesting that CCoV infection induced membrane alterations characteristic of apoptotic cells.
Fig. 3.
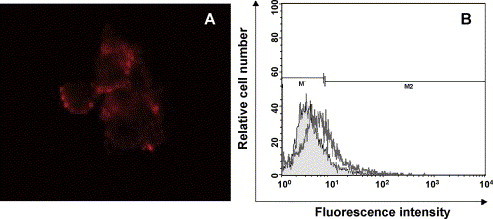
Annexin V analysis. (A) CCoV infected A-72 cells were stained with anti-annexin V–biotin/streptavidin-PE mAbs and analysed under fuorescence microscope. (B) CCoV infected A-72 cells were stained with anti-annexin V- FITC mAb and analysed by FACS. Filled histogram represents mock infected A-72 cells, empty histogram is relative to CCoV infected cells.
3.4. Detection of sub-G0/G1 cell population by PI staining and FACS analysis in CCoV infected cells
Rates of apoptosis after CCoV infection was determined by the appearance of hypodiploid DNA peaks on propidium iodide staining and flow cytometric analysis (Fig. 4 ). Apoptotic cells show a decreased DNA content that is detected by the appearance of a sub-G0/G1 cell population by flow cytometer analysis. The sub-G0/G1 cell population in control cells was at the background level of 4.06%. In contrast, 24 h p.i. higher percentage (15.34%) of CCoV infected cells shifted to sub-G0/G1 fraction; this percentage increased to 29.94% 36 h after CCoV infection, indicating that infected cells were killed by apoptosis.
Fig. 4.
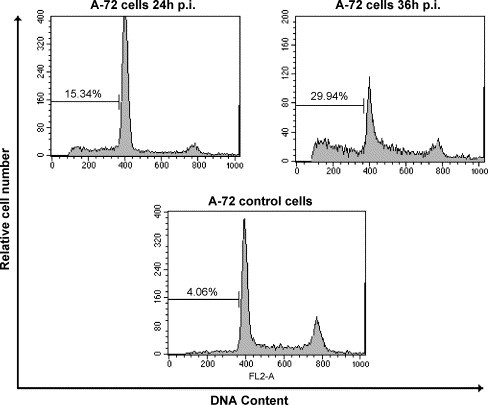
Flow cytometry analysis of CCoV infected A-72 cells. The samples were analysed by FACScalibur flow cytometer at indicated times p.i., after propidium iodide staining. The percentage of apoptotic cells in the sub-G0/G1 region is shown above marker.
3.5. Nucleic acid fragmentation
Internucleosomal degradation of DNA, with appearance of DNA ladders due to activation of nuclear endonucleases is considered an hallmark in most cells undergoing apoptosis. To further ascertain that the sub-G0/G1 cell population was the result of apoptotic death, DNA in CCoV infected A-72 cells was extracted for DNA fragmentation analysis. As expected, DNA laddering was not detected in control cells, whereas in CCoV infected cells degradation of the DNA resulted in production of oligonucleosomal DNA ladders (Fig. 5 ). This result clearly indicates that CCoV infection induces DNA ladder fragmentation, that is typical of apoptotic cells.
Fig. 5.
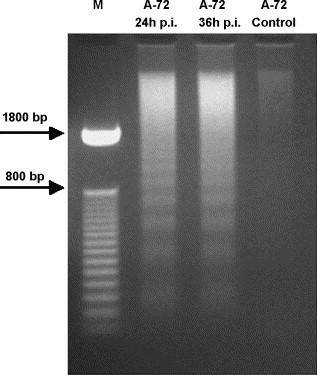
DNA fragmentation analysis in A-72 cell line infected with CCoV at indicated times p.i. and in control cells. M: 50 bp DNA Step Ladder.
3.6. Caspase-3 activation by CCoV infection
Apoptosis is mediated by sequential activation of caspases, which are constitutively present in most cells as inactive proenzymes. Caspases operate in an intracellular cascade leading to the activation of downstream effector caspases-3, 6 and 7. We analysed whether CCoV infection induced caspase-3 in infected A-72 cells. The results (Fig. 6 A) indicated a 10-fold increase of caspase-3 activity 33 h after CCoV infection compared to the control A-72 cells. Pre-treatment of cells with the caspase inhibitor ZVAD-fmk prevented caspase-3 activation by CCoV infection since the O.D. values obtained in these latter cells were similar to those of controls. These results suggest that caspase-3 pathway may be activated by CCoV induced apoptosis.
Fig. 6.
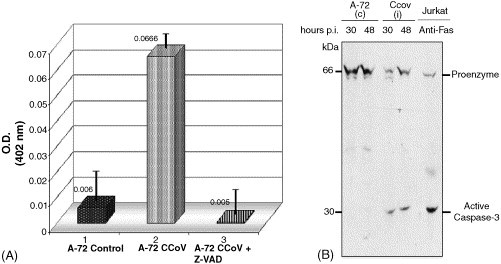
Caspase-3 activation by CCoV infection. (A) Caspase activity was measured by colorimetric assay in control (A-72 control) and CCoV infected cells untreated (A-72 CCoV) or treated with caspase-3 inhibitor ZVAD (A-72 CCoV + ZVAD). The results are expressed as the means of two independent experiments. (B) Proteolytic cleavage of procaspase-3 was detected by immunoblotting of 50 μg of cell lysates from uninfected A-72 cell line (c) or CCoV infected cells (i) obtained at indicated times p.i. (30 and 48 h). Apoptotic Jurkat cells treated with anti-Fas mAb was included in this analysis as positive control for procaspase-3 cleavage.
We further analysed correlation between induction of caspase-3 activity upon CCoV infection and the processing of the proenzyme to the active proteolytic fragment, by immunoblotting analysis in parallel samples from uninfected A-72 cells and CCoV infected, 30 and 48 h p.i. Fig. 6B shows that CCoV infection induces processing of caspase-3 from its pro-form of about 60 kDa to the large active proteolityc subunit of 32 kDa. No processing of caspase-3 could be found in uninfected control cells. These results suggest that CCoV induced apoptosis is caspase 3-dependant.
4. Discussion
The sudden emergence of SARS and the more recent discovery of another human CoV (HCOV-NL63) has sparked wide interest in coronavirus biology and pathogenesis. Moreover the indication of an animal reservoir or a range of animal species susceptible to SARS-CoV infection (Martina et al., 2003) addressed to more detailed studies on the animal coronavirus pathogenesis.
Virus-induced apoptosis is a common mechanism of cell damage associated to virus infection also described for some coronavirus, e.g. TGEV, avian infectious bronchitis virus (IBV) and feline infectious peritonitis virus (FIPV), mouse hepatitis virus (MHV) and recently reported also in human SARS-CoV. (Haagmans et al., 1996, Liu et al., 2001, Yan et al., 2004, Liu et al., 2003).
Scant informations have been available so far for CCoV, whose associated disease, although mild, turn into severe illness mainly in presence of coinfections with other viral or bacterial pathogens, that are commonly hosted by young dogs. Recently an outbreak of a severe lethal disease in pups caused by a highly pathogenic CCoV strain was reported (Buonavoglia et al., 2006). Moreover, in contrast to human diseases, for which a considerable number of studies have been conducted on the relationship between apoptosis and viral diseases, the relationship between apoptosis and dog infections remains largely unknown.
By using A-72 cell line, an homologous cell system, which is permissive to CCoV, we characterized apoptotic death of infected cells. Starting from 24 h after CCoV infection, cells morphology appeared dramatically changed, with cells rounding and detachment from culture surface. Morphologic and biochemical features of apoptosis, such as blebbing of the plasma membrane, translocation of phosphatidilserine to cell surface and annexin V staining, nuclear fragmentation, apoptotic bodies formation and DNA laddering, were detected in CCoV-infected cells.
Caspases are cysteine proteases which play fundamental roles in apoptotic responses of cells to different stimuli. In particular, caspase-3 can be considered a key enzyme involved in apoptosis since it has been reported to be indispensable for chromatin condensation and DNA fragmentation in all cell types. However only recently (Sano et al., 2004) canine caspase-3 cDNA sequence has been available and as consequence reagents for detection of caspase-3 cleavage have not been in usage until now.
We found that caspase-3 activity is induced by CCoV infection in A-72 cell line, suggesting that caspase-3 is likely to contribute to the execution phase of apoptosis and that apoptotic pathways, common to other animal coronavirus infections (e.g. IBV, TGEV), are also operating in canine cells.
Virus-induced apoptosis is a complex and important aspect of the pathogenesis of viral infections. In fact, in the case of virus-infected cells, the induction of cell death can either reduce viral spread in the host by early killing of infected cells, or facilitate viral progeny dissemination, through apoptotic bodies, which hide virus antigens and limit induction of inflammatory and immune responses (Koyama et al., 2000).
Although it limits spreading of virus, apoptotic death of infected cells is still detrimental as it causes cell and tissue destruction as well as inflammatory responses. Therefore in the case of CCoV associated gastroenteritis, apoptosis of epithelial mucosa cells may be responsible for pathology induced by CCoV infection. Although mechanisms other than apoptosis could be operating in vivo to favour viral yields in acute infection, as suggested for TGEV (Kim et al., 2000), we believe that studies on apoptosis mechanism could lead to a better understanding of CCoV pathology, particularly in the case of highly pathogenic CCoV strain.
Acknowledgements
The authors grateful acknowledge Dr. Cordioli for supplying CCoV 1–71 strain; R. Di Pasquale and M. Nenci for technical assistance. This study was supported by grant from the Istituto Superiore di Sanità (C3CH “Canine Coronavirus: studies on SARS-CoV animal models).
References
- Appel M.J., Cooper B.J., Greisen H., Scott F., Carmichael L.E. Canine viral enteritis. I. Status report on corona- and parvo-like viral enteritides. Cornell. Vet. 1979;69(3):123–133. [PubMed] [Google Scholar]
- Binn L.N., Lazar E.C., Keenan K.P., Huxsoll D.L., Marchwicki R.H., Strano A.J. Recovery and characterization of a coronavirus from military dogs with diarrhea. Proc. Ann. Meet. U.S. Anim. Health Assoc. 1974;78:359–366. [PubMed] [Google Scholar]
- Buonavoglia C., Decaro N., Martella V., Elia G., Campolo M., Desario C., Castagnaro M., Tempesta M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006;12:492–494. doi: 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J.M., Gomez-Puertas P., Cavanagh D., Gorbalenya A.E., Enjuanes L. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 2003;148:2207–2235. doi: 10.1007/s00705-003-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., Egberink H.F., Horzinek M.C. Apoptosis and T-cell depletion during feline infectious peritonitis. J. Virol. 1996;70(12):8977–8983. doi: 10.1128/jvi.70.12.8977-8983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J.M. Virus-induced apoptosis. Adv. Pharmacol. 1997;41:295–336. doi: 10.1016/s1054-3589(08)61063-7. [DOI] [PubMed] [Google Scholar]
- Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br. J. Caner. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Kim O., Tai J.H., Chae C. Transmissible gastroenteritis virus induces apoptosis in swine testicular cell lines but not in intestinal enterocytes. J. Comp. Pathol. 2000;123:64–66. doi: 10.1053/jcpa.2000.0386. [DOI] [PubMed] [Google Scholar]
- Koyama A.H., Fukumori T., Fujita M., Irie H., Adachi A. Physiological significance of apoptosis in animal virus infection. Microbes Infect. 2000;2:1111–1117. doi: 10.1016/s1286-4579(00)01265-x. [DOI] [PubMed] [Google Scholar]
- Liu C., Xu H.Y., Liu D.X. Induction of caspase-dependent apoptosis in cultured cells by the avian coronavirus infectious bronchitis virus. J. Virol. 2001;75(14):6402–6409. doi: 10.1128/JVI.75.14.6402-6409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Cai Y., Zhang X. Induction of caspase-dependent apoptosis in cultured rat oligodendrocytes by murine coronavirus is mediated during cell entry and does not require virus replication. J. Virol. 2003;22:11952–11963. doi: 10.1128/JVI.77.22.11952-11963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Martina B.E., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G., Peiris J.S., Lim W., Osterhaus A.D. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien V. Viruses and apoptosis. J. Gen. Virol. 1998;79:1833–1845. doi: 10.1099/0022-1317-79-8-1833. [DOI] [PubMed] [Google Scholar]
- Pratelli A., Tempesta M., Roperto F.P., Sagazio P., Carmichael L., Buonavoglia C. Fatal coronavirus infection in puppies following canine parvovirus 2b infection. J. Vet. Diagn. Invest. 1999;11:550–553. doi: 10.1177/104063879901100615. [DOI] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Sano J., Oguma K., Kano R., Hasegawa A. Characterization of canine caspase-3. J. Vet. Med. Sci. 2004;66:563–567. doi: 10.1292/jvms.66.563. [DOI] [PubMed] [Google Scholar]
- Siddell S.G. Plenum Press; New York: 1995. The Coronaviridae. [Google Scholar]
- Yan H., Xiao G., Zhang J., Hu Y., Yuan F., Cole D.K., Zheng C., Gao G.F. SARS coronavirus induces apoptosis in Vero E6 cells. J. Med. Virol. 2004;73:323–331. doi: 10.1002/jmv.20094. [DOI] [PMC free article] [PubMed] [Google Scholar]


