Highlights
-
•
The largest study of its kind in the field to date, including high-risk kennelled dogs, and for the first time, pet dogs and dogs from other cohorts.
-
•
A clearly identifiable link between disease and the emerging pathogens: canine respiratory coronavirus and canine pneumovirus.
-
•
Provides, substantial evidence of CIRD and the circulation of the novel pathogens studied in pet dogs, and dogs from other cohorts.
-
•
Demonstrates the role and limitations of current vaccine strategies in managing CIRD outbreaks, and the need for including emerging pathogens.
Keywords: Canine respiratory coronavirus (CRCoV), Canine pneumovirus (CnPnV), Canine influenza (CIV), Mycoplasma cynos, Canine infectious respiratory disease (CIRD), Kennel cough
Abstract
Canine infectious respiratory disease (CIRD) is a major cause of morbidity in dogs worldwide, and is associated with a number of new and emerging pathogens. In a large multi-centre European study the prevalences of four key emerging CIRD pathogens; canine respiratory coronavirus (CRCoV), canine pneumovirus (CnPnV), influenza A, and Mycoplasma cynos (M. cynos); were estimated, and risk factors for exposure, infection and clinical disease were investigated.
CIRD affected 66% (381/572) of the dogs studied, including both pet and kennelled dogs. Disease occurrence and severity were significantly reduced in dogs vaccinated against classic CIRD agents, canine distemper virus (CDV), canine adenovirus 2 (CAV-2) and canine parainfluenza virus (CPIV), but substantial proportions (65.7%; 201/306) of vaccinated dogs remained affected.
CRCoV and CnPnV were highly prevalent across the different dog populations, with overall seropositivity and detection rates of 47% and 7.7% for CRCoV, and 41.7% and 23.4% for CnPnV, respectively, and their presence was associated with increased occurrence and severity of clinical disease. Antibodies to CRCoV had a protective effect against CRCoV infection and more severe clinical signs of CIRD but antibodies to CnPnV did not.
Involvement of M. cynos and influenza A in CIRD was less apparent. Despite 45% of dogs being seropositive for M. cynos, only 0.9% were PCR positive for M. cynos. Only 2.7% of dogs were seropositive for Influenza A, and none were positive by PCR.
1. Introduction
Canine infectious respiratory disease (CIRD) is a major cause of morbidity and an important welfare issue for kennelled dog populations worldwide. Characterised by clinical signs such as coughing, nasal discharge and dyspnoea, it can persist for several weeks, often resulting in severe disease, such as bronchopneumonia, and on occasions, lead to death or result in euthanasia (Appel and Binn, 1987).
CIRD is a complex disease of multifactorial aetiology, where environmental, host and pathogen interactions influence disease susceptibility, severity and persistence. The pathogens traditionally associated with CIRD include canine parainfluenza virus (CPIV)(Appel and Percy, 1970), canine adenovirus type 2 (CAV-2)(Ditchfield et al., 1962), and Bordetella bronchiseptica (Bb) (Bemis, 1992), which act sequentially or synergistically to cause disease. Several multivalent vaccines targeting these agents are available for routine vaccination (Day et al., 2016). However outbreaks of respiratory disease continue despite their use, resulting in expensive treatment costs and delays in rehoming or training (Erles et al., 2004).
In recent years a number of newly emerging pathogens, including canine respiratory coronavirus (CRCoV) (Erles et al., 2003), canine pneumovirus (CnPnV)(Renshaw et al., 2010), canine influenza H3N8 (CIV)(Crawford et al., 2005), and Mycoplasma cynos (M. cynos)(Chalker et al., 2004), have been implicated in the development and persistence of CIRD, and are the subject of a comprehensive review (Priestnall et al., 2014).
Whilst it is widely recognised that these agents are all likely to play an important role in the onset or persistence of CIRD, the exact nature of their contribution to the pathogenesis of disease is yet to be determined. Most, if not all, studies to date have either been limited to the investigation of individual pathogens, or have been geographically restricted to a small number of kennels or a single outbreak, and no comprehensive survey of pet dogs has been completed. Thus the true extent of the prevalence of these novel agents, and the risk factors associated with exposure, infection and disease amongst different cohorts, have not been fully investigated.
To help address this we present data from a large multi-centre European study in which the prevalences of four of the most important and recently identified emerging CIRD pathogens (CRCoV, CnPnV, CIV/Influenza A and M. cynos) were estimated in both client-owned and kenneld dogs, and risk factors for exposure, infection and clinical disease were investigated.
2. Methods
2.1. Study design
Samples and signalment data were collected from dogs in animal shelters, breeding kennels and academic or private veterinary clinics across Europe by collaborating Investigator Centres between October 2011 and August 2013. Dogs were actively recruited onto the study and sampled upon presentation to the veterinarian if they fell into one of three different clinical groups: (A) clinically unaffected but exposed to acute CIRD-affected dogs, (B) acute CIRD-affected dogs (0–3 days post onset of CIRD), or (C) convalescent CIRD-affected dogs (10–12 days post onset of CIRD). Details of the clinical grouping, source (i.e. client-owned, shelter/rehoming kennel, hospital, breeding kennel), recorded or estimated age, vaccination history for Bb, CPIV, CAV-2 and CDV, and clinical respiratory score (1–5) at the time of sample collection were recorded. Briefly the clinical scores described previously (Erles et al., 2003) were defined as: 1) no respiratory signs, 2) mild cough, 3) cough and nasal discharge, 4) cough and nasal discharge with depression and/or inappetence, 5) cough and nasal discharge with depression and/or inappetence and clinical signs of lower respiratory tract disease.
2.2. Sampling
Viral nasal swabs (VNS) (Sterilin, UK), oropharyngeal Amies swabs (OAS) (Sterilin, UK) and serum samples were collected by local veterinary practitioners. All samples were collected as part of the standard veterinary diagnostic work-up for respiratory disease, and with informed consent for clinical research purposes (obtained by the attending veterinarian or collaborating centre). Samples were sent to investigator centres across Europe, where they were appropriately stored, batched and shipped to the Royal Veterinary College for analysis. Duplicate samples were retained by collaborating investigator centres for their own research purposes and to date one study relating to a subset of these has been published (Decaro et al., 2016). The study was approved by the Royal Veterinary College’s Ethical Review Board.
2.3. Detection of viral pathogens
The tip of each VNS was immersed in 1 ml of DMEM medium (Sigma, Dorset, UK) and vigorously mixed. RNA was extracted from 200 μl of the resulting swab fluid using the RNeasy Mini Kit (Qiagen, Crawley, UK) as recommended by the manufacturer, and eluted in 30 μl of RNAse free water. Five microliters of RNA was reverse transcribed into cDNA using Random Hexameres (Qiagen, Crawley, UK) and Improm II reverse transcriptase (Promega, Southampton, UK) according to the manufacturer’s protocol. CRCoV and CnPnV were detected as described previously by PCR (Erles et al., 2003) and real-time PCR (Mitchell et al., 2013b) respectively. Influenza A was detected using primers targeting a conserved region of the matrix gene (Ellis and Zambon, 2001), and the GoTaq polymerase system (Promega, Southampton, UK).
2.4. Detection of mycoplasma spp
The tip of each OAS was placed in 1 ml of Mycoplasma Experience (ME) liquid media (Mycoplasma Experience Ltd, Bletchingley UK), vigorously mixed and incubated at 37 °C with 5% CO2. Cultures were monitored daily (for up to 3 days) for signs of mycoplasma growth, as indicated by the presence of turbidity and or colour change. Once growth was observed, or at 3 days post inoculation with no apparent growth, the cells from 0.7 ml of the liquid culture were pelleted and the DNA extracted using the DNeasy Mini Kit (Qiagen, Crawley, UK) as recommended by the manufacturer. DNA was eluted in 100 μl of elution buffer and analysed using a pan-Mycoplasma Spp. PCR (Kobayashi et al., 1995), followed by a specific M. cynos PCR (Chalker et al., 2004) as previously described.
2.5. Detection of antibodies
The antibody status of each dog for CRCoV, CnPnV and M. cynos was determined by ELISA using the following antigens:
2.5.1. CRCoV ELISA antigen
Recombinant CRCoV hemagglutinin esterase (HE) protein. The CRCoV HE protein (strain 4182) was expressed using the BacMagic™ recombinant baculovirus expression system (Merck, UK) at 5MOI in Spodoptera frugiperda 9 (SF9) cells. The antigen was prepared by lysing infected cells with lysis buffer (1% Igepal, 50 mM NaH2PO4, 300 mM NaCl, pH8) at 3 days post infection. The CRCoV HE antigen was standardised to a mock infected SF9 lysate which served as the control antigen, and 0.8 micrograms of total protein per well was used to coat the plate.
2.5.2. CnPnV ELISA antigen
Freeze-dried murine pneumovirus and control antigen (Churchill Applied Biotechnology Ltd, Huntingdon) was prepared and used as described previously (Mitchell et al., 2013b).
2.5.3. M. cynos ELISA antigen
M. cynos (UK strain 491) cell pellets were washed three times in PBS, then resuspended in 5 ml PBS, and freeze thawed. Six nano-grams per well of total M. cynos protein in 50 μl of PBS was used to coat the plate. Wells coated with PBS only were used as a background control.
2.5.4. Briefly for each ELISA
Antigen was adsorbed overnight at 4 °C onto 96 well Nunc Maxisorb™ ELISA plates, and blocked with 5% milk in 0.05% PBS Tween. Serum samples were diluted 1:100 in blocking buffer and 50 μl dispensed in duplicate onto positive and control antigens and incubated at 37 °C for 1 h. Following three washes with 0.05% PBST, the anti-dog IgG peroxidase conjugate (Sigma-Aldrich, Poole) secondary antibody was diluted 1:2500 (CRCoV, CnPnV) or 1:5000 (M. cynos) in blocking buffer, and 50 μl dispensed into each well and incubated. The plates were washed as before and bound antibody was detected using Sigma-Fast™ OPD peroxidase substrate (Sigma Aldrich, UK) according to the manufacturer’s instructions, and stopped with 2 M H2SO4. Optical densities (OD) were measured at 490 nm (Optimax automatic plate reader Molecular Devices, Wokingham). Positive and negative control sera were included in duplicate on each plate. For each serum sample, the average OD value for the negative antigen was subtracted from that of the positive antigen to give the average corrected OD value. The positive cut-off was defined as the average corrected OD value for a panel of reference serum (IgG negative for the antigen used) plus three times the standard deviation. Samples were considered positive if the ‘average corrected OD value’ was greater than or equal to the positive cut-off value for the ELISA.
2.5.5. Influenza A ELISA
The influenza antibody status was determined via two independent methods:
-
1)
Freeze-dried equine influenza A (H3N8 Miami) and control antigen (Churchill Applied B Biotechnology Ltd, Huntingdon) were used according the manufacturers recommendation and validated for use with canine samples using a panel of CIV (H3N8) positive and negative control serum (Zoetis Animal Health, Kalamazoo). The ELISA was performed as described above using a 1:2500 dilution of secondary antibody.
-
2)
The commercially available ID Screen Influenza A Antibody competition assay. The assay was performed according to the manufacturer’s protocol using a 1:10 dilution of canine serum as previously described (Pratelli and Colao, 2014). The assay was validated using the controls supplied with the kit and a panel of CIV (H3N8) positive and negative control serum (Zoetis Animal Health, Kalamazoo).
2.6. Statistical analysis
Data were recorded in Excel spreadsheets (Microsoft) and imported into Stata/SE 13 software (Statacorp) for analysis. Continuous variables were summarised using mean (standard deviation) if normally distributed and median (minimum, maximum) if non-normally distributed. Categorical variables were summarised using numbers and percent in each category and 95% confidence intervals (CI) around prevalence estimates calculated using the Clopper-Pearson method. For the purposes of these analyses the clinical score variable was collapsed from the original categorisation of 1–5 (as detailed above) to a three-point ‘severity score’ of ‘no disease’ (clinical score 1), ‘mild to moderate disease’ (clinical score 2 and 3) and ‘severe disease’ (clinical score 4 and 5). The presence or absence of disease was also assessed, where the original clinical score of 2–5 indicated the presence of disease and 1 indicated no disease.
Variations in disease presence and severity by different factors (source, age, country and vaccination status), and variations in individual pathogen prevalence and seroprevalence in association with source, clinical group, disease presence and severity were examined using Chi-square/Fisher’s exact tests and univariable logistic regression. Multivariable logistic regression was used to examine relationships where more than one factor was significantly associated with the outcome at the 25% level (univariable screening p-value <0.25).
3. Results
3.1. Descriptive statistics
Descriptive statistics relating to dogs and samples included in the study are summarised in Table 1 . Samples and signalment data were obtained from 572 dogs (Table 1). Of these, 45.6% (n = 261) were client-owned dogs, 46.2% (n = 264) were from shelters and 8.2% (n = 47) were from other sources (predominantly a single veterinary hospital). Overall, 24.1% (n = 138) were clinically unaffected dogs that had been exposed to acute CIRD-affected dogs, 49.3% (n = 282) were acute CIRD-affected dogs and 26.6% (n = 152) were convalescent (10 days post onset of acute disease and may or may not have still been showing clinical signs of CIRD). Approximately equal proportions (∼20%) of samples came from Italy, Greece, Hungary and France respectively with smaller proportions (∼10%) from Spain and Netherlands. Estimated age was recorded for 542 dogs and ranged from 5 weeks to 15 years with a median of 3 years.
Table 1.
Study population and sample distribution.
| Variable |
Seropositive |
Pathogen detected |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRCoV |
CnPnV |
M. cynos |
CRCoV |
CnPnV |
||||||||||
| Dogs |
(n = 525) |
(n = 559) |
(n = 555) |
|||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| Total* | 572 | 100 | 247 | 47 | 219 | 41.7 | 236 | 45 | 43 | 7.7 | 130 | 23.4 | ||
| Source | Client | 261 | 45.6 | 86 | 36.7 | 51 | 21.8 | 92 | 39.3 | 20 | 7.9 | 46 | 18.3 | |
| Shelter | 264 | 46.2 | 139 | 55.6 | 137 | 54.8 | 137 | 54.8 | 21 | 8.1 | 73 | 28.2 | ||
| Other | 47 | 8.2 | 22 | 53.7 | 31 | 75.6 | 7 | 17 | 2 | 4.4 | 11 | 24.4 | ||
| Clinical Group | Unaffected | 138 | 24.1 | 73 | 55.3 | 67 | 50.8 | 55 | 41.7 | 8 | 5.9 | 28 | 20.6 | |
| Acute | 282 | 49.3 | 109 | 42.9 | 90 | 35.4 | 97 | 38.2 | 27 | 9.8 | 60 | 22.2 | ||
| Convalescent | 152 | 26.6 | 65 | 46.8 | 62 | 44.6 | 84 | 60.4 | 8 | 5.4 | 42 | 28.2 | ||
| Country | Italy | 130 | 22.7 | 37 | 31.9 | 25 | 21.5 | 38 | 32.8 | 4 | 3.1 | 22 | 16.9 | |
| Greece | 112 | 19.6 | 37 | 38.5 | 26 | 27.1 | 50 | 52.1 | 9 | 8.6 | 23 | 21.9 | ||
| Hungary | 109 | 19 | 38 | 39.2 | 42 | 43.3 | 50 | 51.5 | 8 | 7.4 | 34 | 32.4 | ||
| France | 101 | 17.7 | 70 | 72.2 | 68 | 70.1 | 60 | 61.9 | 11 | 11.5 | 24 | 25.3 | ||
| Spain | 61 | 10.7 | 39 | 63.9 | 23 | 37.7 | 26 | 42.6 | 3 | 4.9 | 9 | 14.8 | ||
| Netherlands | 59 | 10.3 | 26 | 44.8 | 35 | 60.3 | 12 | 20.7 | 8 | 13.6 | 18 | 30.5 | ||
| Severity Score | 1 (healthy) | 191 | 33.4 | 108 | 59.3 | 100 | 55 | 87 | 47.8 | 11 | 5.9 | 42 | 22.5 | |
| 2 (mild-moderate) | 317 | 55.4 | 119 | 41.3 | 102 | 35.4 | 121 | 42 | 22 | 7 | 67 | 21.5 | ||
| 3 (severe) | 64 | 11.2 | 20 | 36.4 | 17 | 30.9 | 28 | 51 | 10 | 17 | 21 | 37.5 | ||
| Disease | N | 191 | 33.4 | 108 | 59.3 | 100 | 54.9 | 87 | 47.8 | 11 | 5.9 | 42 | 22.5 | |
| Y | 381 | 66.6 | 139 | 40.5 | 119 | 34.7 | 149 | 43.4 | 32 | 8.6 | 88 | 23.9 | ||
| Vaccination | CDV (n = 403) | N | 72 | 17.9 | – | – | – | – | – | – | – | – | – | – |
| Y | 331 | 82.1 | – | – | – | – | – | – | – | – | – | – | ||
| CAdV (n = 405) | N | 87 | 21.5 | – | – | – | – | – | – | – | – | – | – | |
| Y | 318 | 78.5 | – | – | – | – | – | – | – | – | – | – | ||
| CPIV (n = 381) | N | 149 | 39.1 | – | – | – | – | – | – | – | – | – | – | |
| Y | 232 | 60.9 | – | – | – | – | – | – | – | – | – | – | ||
| Bb (n = 307) | N | 241 | 78.5 | – | – | – | – | – | – | – | – | – | – | |
| Y | 66 | 21.5 | – | – | – | – | – | – | – | – | – | – | ||
Clinical signs of respiratory disease were observed in 66.6% (n = 381) of the 572 dogs. Just over half of the dogs (55.4%; n = 317) showed signs of mild to moderate disease and 11.2% (n = 64) showed signs of more severe disease.
In total, 525 serum samples were examined for the presence of CRCoV, CnPnV, Influenza A or M.cynos antibodies and 559 viral nasal swabs (VNS) and 566 oropharyngeal Amies swabs (OAS) were analysed for the presence of the pathogens.
The overall estimated seroprevalence in this study population was 47% (247/525; 95% CI: 42.7–51.4%) for CRCoV, 41.7% (219/525; 95% CI: 37.4–46.1%) for CnPnV, 45% (236/525; 95% CI: 40.6–49.3%) for M. cynos, and 2.7% (6/220; 95% CI: 1.0–5.8%) for Influenza A.
The overall estimated prevalence of each pathogen detected by PCR was 7.7% (43/559; 95% CI: 5.6–10.2%) for CRCoV, 23.4% (130/555; 95% CI: 20–27.2%) for CnPnV, 0.9% (n = 5/566, CI) for M. cynos, and 0% (0/511) for Influenza A.
3.2. Disease presence and severity was associated with source and a history of vaccination
Significant univariable variations in the presence and severity of disease were associated with source, country, vaccination and age (data not shown). Dogs with clinical signs were significantly younger (median 3 years; range 5 weeks to 15 years) than those without (median 4 years; range 5 weeks to 15 years) (p = 0.003), with an inverse relationship between age and disease severity. Whilst clinical disease was clearly prevalent amongst dogs both with and without a history of vaccination (Bb, CPIV, CAV-2 and CDV) the odds of disease were significantly reduced in dogs vaccinated against CPIV, CAV-2, and CDV, but not Bb.
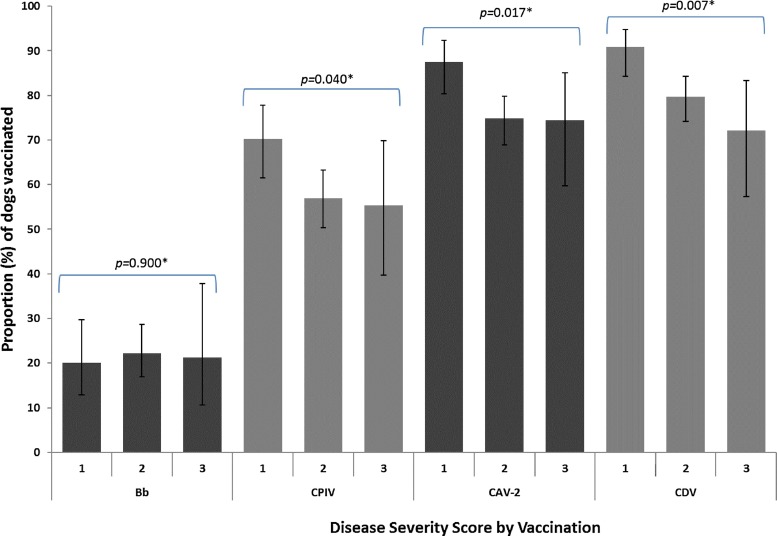
Variations in disease presence in association with country and age were no longer significant in multivariable analysis. However, reduced odds of disease remained significantly associated with a history of vaccination for CPIV, CAV-2 or CDV with an independent effect of reduced odds of disease in shelter dogs and dogs from other sources compared with client-owned dogs (Table 2 ). Further examination of the vaccination effect indicated that the proportion of dogs with a history of vaccination against CPIV, CAV-2 and CDV reduced significantly as the severity of disease increased (Fig. 1 ).
Table 2.
Final multivariable model for presence of clinical disease (N = 406).
| Variable | OR | p | 95% CI | ||
|---|---|---|---|---|---|
| Source | Client | Ref | – | – | – |
| Shelter | 0.1 | <0.001 | 0.06 | 0.19 | |
| Other | 0.22 | <0.001 | 0.1 | 0.45 | |
| Vaccination (CPIV, CAV-2, CDV) | N | Ref | – | – | – |
| Y | 0.3 | 0.002 | 0.15 | 0.66 | |
Fig. 1.
Proportion of vaccinated dogs with different clinical disease severity scores.
Vaccinations: (Bb) Boredetella bronchiseptica, (CPIV) canine parainfluenza, (CAV-2) canine adenovirus-2, (CDV) canine distemper virus. The disease severity score is indicted on the x-axis by 1) Healthy, 2) mild to moderate, and 3) Severe respiratory disease. Error bars show 95% confidence interval. *Chi-squared/Fisher’s exact test p-value.
3.3. Significant variations in pathogen seroprevalence were associated with country, source, age, antibody status and clinical group, but varied by the pathogen studied
Significant univariable variations in CRCoV seroprevalence were detected by country, age, source, disease status, clinical score, and CnPnV seropositivity (data not shown). Dogs with antibodies to CRCoV were significantly less likely to develop respiratory disease (OR: 0.5, p < 0.001), with odd ratios of 0.5 (p < 0.001) and 0.4 (p = 0.003) for dogs with clinical scores of 2 and 3 respectively compared to dogs with no clinical signs. Following multivariable analysis, independent effects of country, age and CnPnV antibody status remained significant (Table 3 ). Dogs from France and Spain had significantly increased odds of being seropositive (OR 4.8, p < 0.001 and OR 4.1, p < 0.001 respectively) compared with dogs from Italy. Dogs were also significantly more likely to be CRCoV seropositive with increasing age (OR 1.1; p = 0.001), or if they were also seropositive for CnPnV (OR 2.7, p < 0.001).
Table 3.
Final multivariable models for pathogen seropositivity.
| Pathogen | Variable | OR | p | 95% CI | ||
|---|---|---|---|---|---|---|
| CRCoV (N = 496) | Country | Italy | Ref | |||
| Greece | 1.6 | 0.126 | 0.87 | 2.97 | ||
| Hungary | 1.4 | 0.331 | 0.73 | 2.51 | ||
| France | 4.8 | <0.001 | 2.34 | 9.69 | ||
| Spain | 4.1 | <0.001 | 2.06 | 8.34 | ||
| Netherlands | 1.5 | 0.289 | 0.72 | 3.00 | ||
| CnPnV IgG | Negative | Ref | ||||
| Positive | 2.7 | <0.001 | 1.77 | 4.00 | ||
| Age | 1.1 | 0.001 | 1.04 | 1.17 | ||
| CnPnV (N = 525) | Country | Italy | Ref | |||
| Greece | 1.4 | 0.331 | 0.71 | 2.81 | ||
| Hungary | 1.6 | 0.151 | 0.83 | 3.17 | ||
| France | 3.4 | 0.001 | 1.67 | 6.93 | ||
| Spain | 1.7 | 0.192 | 0.75 | 3.92 | ||
| Netherlands | 3.7 | 0.002 | 1.64 | 8.22 | ||
| Source | Client | Ref | ||||
| Shelter | 2.8 | <0.001 | 1.61 | 4.87 | ||
| Other | 8.0 | <0.001 | 3.33 | 19.26 | ||
| CRCoV IgG | Negative | Ref | ||||
| Positive | 2.7 | <0.001 | 1.79 | 4.08 | ||
| M. cynos IgG | Negative | Ref | ||||
| Positive | 1.8 | 0.005 | 1.20 | 2.76 | ||
| M. cynos (N = 496) | Country | Italy | Ref | |||
| Greece | 2.3 | 0.008 | 1.23 | 4.16 | ||
| Hungary | 1.6 | 0.171 | 0.82 | 3.03 | ||
| France | 2.5 | 0.012 | 1.22 | 5.27 | ||
| Spain | 2.2 | 0.028 | 1.08 | 4.46 | ||
| Netherlands | 0.7 | 0.334 | 0.29 | 1.51 | ||
| Source | Client | Ref | ||||
| Shelter | 1.8 | 0.024 | 1.08 | 3.15 | ||
| Other | 0.5 | 0.126 | 0.18 | 1.23 | ||
| Clinical Group | Clinically unaffected | Ref | ||||
| Acute | 1.3 | 0.287 | 0.79 | 2.17 | ||
| Convalescent | 2.8 | <0.001 | 1.60 | 4.87 | ||
| Age | 1.07 | 0.024 | 1.00 | 1.13 | ||
Significant univariable variations in CnPnV seroprevalence were detected by country, source, clinical group, disease status and clinical score (data not shown). Dogs with antibodies to CnPnV were significantly less likely to develop respiratory disease than those without (OR: 0.4, p < 0.001), with odds ratios of 0.5 (p < 0.001) and 0.4 (p = 0.002) for dogs with clinical scores of 2 and 3 respectively compared to those with no clinical signs of disease. In multivariable analysis, independent effects of country, source and CRCoV and M. cynos antibody status remained significant (Table 3). Dogs from France and the Netherlands had 3.4 (p = 0.001) and 3.7 (p = 0.002) times the odds of being seropositive compared with dogs from Italy. Dogs from shelters were almost 3 times (OR 2.8, p < 0.001) and dogs from other sources were 8 times (OR 8.0, p < 0.001) as likely to be CnPnV seropositive than client-owned dogs. The odds of being CnPnV seropositive were also significantly increased in association with CRCoV (OR 2.7, p = <0.001) and M. cynos (OR 1.8, p = 0.005) seropositivity.
Significant univariable variations in M.cynos seroprevalence were detected in association with country, source, clinical group, and CRCoV and CnPnV antibody status. No significant association between M. cynos antibody status and the presence or severity of clinical disease was observed (data not shown). Independent effects of country, source, clinical group and age remained significant in multivariable analysis (Table 3). Dogs from Greece, France and Spain were 2.3 (p = 0.008), 2.5 (p = 0.012) and 2.2 (p = 0.028) times as likely to be seropositive for M. cynos as those from Italy, respectively. Shelter dogs were almost twice as likely as pet dogs to be seropositive for M. cynos (OR 1.8; p = 0.024), and convalescent dogs almost three times as likely to be seropositive than clinically unaffected but exposed dogs (OR 2.8; p = < 0.001). The odds of M. cynos seropositivity also increased with age (OR 1.06; p = 0.024).
The overall seroprevalence of Influenza A was 2.7% (6/220; 95% CI: 1.0–5.8%) with good correlation between the two ELISA protocols used. The small number of seropositive dogs precluded further statistical analysis. However, of the six positive dogs five were from a shelter (5/114; 4.4%) and one was client-owned (1/91; 1.1%). Three were acute with mild signs of clinical disease, two convalescent with no clinical signs of disease, and one healthy but exposed.
3.4. Significant variations in pathogen presence were associated with country, source, clinical score and antibody status, but varied by the pathogen studied
Significant univariable variations in CRCoV presence were detected with country, age, clinical score, CRCoV and CnPnV seropositivity (data not shown). Independent associations with country, clinical score and the presence of CRCoV antibody remained significant in multivariable analysis (Table 4 ). Dogs from Greece (OR 12.2; p = 0.02), Hungary (OR 8.9; p = 0.045), France (OR 28; p = 0.002) and Spain (OR 22.5; p = 0.004), at significantly increased odds of CRCoV positivity compared with dogs from Italy. Dogs with severe clinical signs were 3.5 times as likely to be positive for CRCoV as those with no signs of clinical disease (p = 0.029), but were almost half as likely to be positive for CRCoV if they had antibodies to the virus (OR 0.4; p = 0.015).
Table 4.
Final multivariable models for pathogen detection.
| Pathogen | Variable | OR | p value | 95% CI | ||
|---|---|---|---|---|---|---|
| CRCoV (N = 517) | Country | Italy | Ref | |||
| Greece | 12.2 | 0.02 | 1.48 | 100.27 | ||
| Hungary | 8.9 | 0.045 | 1.05 | 76.62 | ||
| France | 28 | 0.002 | 3.32 | 236.08 | ||
| Spain | 8.7 | 0.066 | 0.86 | 87.34 | ||
| Netherlands | 22.5 | 0.004 | 2.71 | 187.35 | ||
| Severity Score | 1 | Ref | ||||
| 2 | 1.9 | 0.155 | 0.78 | 4.51 | ||
| 3 | 3.5 | 0.029 | 1.13 | 10.59 | ||
| CRCoV IgG | Negative | Ref | ||||
| Positive | 0.4 | 0.015 | 0.17 | 0.82 | ||
| CnPnV (N = 555) | Country | Italy | Ref | |||
| Greece | 1.3 | 0.409 | 0.68 | 2.55 | ||
| Hungary | 2.3 | 0.007 | 1.26 | 4.40 | ||
| France | 1.7 | 0.127 | 0.85 | 3.40 | ||
| Spain | 0.9 | 0.762 | 0.37 | 2.05 | ||
| Netherlands | 2.0 | 0.061 | 0.97 | 4.21 | ||
| Severity Score | 1 | Ref | ||||
| 2 | 1.1 | 0.803 | 0.66 | 1.69 | ||
| 3 | 2.1 | 0.029 | 1.07 | 4.20 | ||
| CRCoV PCR | Negative | Ref | ||||
| Positive | 2.03 | 0.040 | 1.03 | 3.98 | ||
Significant univariable variations in CnPnV presence were detected with country, source and clinical score, but not with the presence or absence of CnPnV antibody (data not shown). Independent effects of country, clinical score and CRCoV status remained significant in multivariable analysis (Table 4). Dogs from Hungary (OR 2.3; p = 0.007) had twice the odds of CnPnV positivity as dogs from Italy. The presence of CRCoV was associated with a two-fold increase in the odds of CnPnV presence (OR 2.0, p = 0.040) and dogs with severe clinical disease had more than twice the odds of CnPnV positivity as those with no clinical disease (p = 0.029).
Overall 47.9% (n = 271/566, CI) of dogs were positive for Mycoplasma Spp. and 0.9% (n = 5/566, CI) were positive for M. cynos (data not shown). The small number of M. cynos positive dogs (n = 5) precluded further analysis. However, four were shelter dogs and one was client-owned. Three had severe respiratory signs (one requiring euthanasia) whilst two dogs were non-clinical. The trachea, lung, bronchial lymph node, palatine tonsil and thymus from the euthanized dog were analysed and all, with the exception of the lung, were positive for M. cynos.
None of the 511 dogs screened for the presence of Influenza A by PCR were positive (data not shown).
4. Discussion
This study examined the occurrence of CIRD, and four of the most important emerging pathogens associated with the disease in different dog populations across Europe. Key variables (e.g. clinical presentation, housing, vaccination etc.) and their relationship with disease occurrence, severity, pathogen exposure and presence were also analysed.
Overall clinical disease occurred in approximately two-thirds of the dogs, with variations in the rate of occurrence and severity predominantly associated with housing and vaccination history. Whilst CIRD is generally regarded as a disease of kennelled dogs as shown here, we have also provided substantial evidence of CIRD in pet (client-owned) dogs, many of which had moderate to severe signs of disease, highlighting the need for further consideration of pet dogs in the occurrence and spread of CIRD, and the potential risk factors associated with this group (e.g. multi-dog households, attendance at dog training, doggy day care, recent boarding or kennelling). However, the high rate of CIRD observed in client-owned dogs, compared to kennelled dogs, is most likely explained by an inherent sampling bias. Healthy client-owned dogs are unlikely to have been presented or sampled as part of a normal veterinary investigation, except in some cases of multi-dog households, doggy day care etc. where CIRD has been problematic. In contrast, sampling of apparently healthy dogs in kennelled environments often forms part of a standard outbreak investigation.
Vaccination therefore is likely to have had the biggest influence on the occurrence and severity of disease in this study. Within the EU it is recommended that dogs are routinely vaccinated against CAV-2 and CDV as part of the core vaccinations they receive from puppyhood. The modified-live virus vaccines are administered parenterally, and are highly efficacious (Day et al., 2016). Some core vaccine formulations include CPIV but not Bb, and whilst separate modified-live intranasal vaccines for Bb and CPIV are availaible they tend to be administered only to high risk dogs (i.e. during periods of kennelling). Studies of CPIV and Bb vaccine efficacy are regulated by European monographs, requiring specific efficacy and safety requirmemnts including the onset and duration of immunity. However, published studies are limited and there is debate on the differing efficacies demonstrated (Day et al., 2016, Ellis, 2015, Ellis and Krakowka, 2012, Mitchell and Brownlie, 2015).
Here, a history of vaccination against the classic CIRD-associated pathogens CDV, CAV-2 and CPIV, significantly reduced the occurrence and severity of disease. However, vaccination against Bb did not, although this could be due to the small number of Bb vaccinated dogs (n = 66) in this study. Despite evidence of a protective effect substantial proportions of CDV, CAV-2 and CPIV vaccinated dogs remained affected by CIRD, many with severe clinical signs, supporting the widely accepted view that vaccination against these key agents may reduce disease occurrence and severity in some individuals or outbreaks, but is often poorly effective in others. Differences may be a result of vaccine formulation or protocol used, the biological properties and characteristics of the agent and host, or the involvement of newly identified or emerging pathogens (Erles et al., 2004, Priestnall et al., 2014)
Whilst these first two points fall beyond the scope of this study, in examining four of the most important emerging CIRD pathogens it is clear their role in CIRD may be significant for both kennelled and pet dogs. In particular CRCoV and CnPnV were highly prevalent across the different dog populations studied and their presence was positively associated with an increased occurrence and severity of clinical disease. The presence and involvement of M. cynos and influenza A in CIRD was however less apparent.
Almost half the dogs in this study were seropositive for CRCoV and 7.7% were positive for the virus, consistent with published data (Erles et al., 2003, Priestnall et al., 2006). Multivariable analysis showed that the likelihood of dogs having antibodies to CRCoV increased with age, and those with antibodies were significantly less likely to be positive for the virus. Dogs with increasing disease severity were significantly more likely to be positive for CRCoV. Dogs with antibodies to CRCoV that developed CIRD were less likely to develop the more severe clinical signs of disease. Younger dogs (less likely to have antibodies to CRCoV) were more likely to be infected with CRCoV, and had a greater rate of occurrence of CIRD, and developed more severe clinical signs. These findings are consistent with previously published data (Erles et al., 2004, Erles et al., 2003, Mitchell et al., 2013a), and strengthen the evidence for a causal relationship between CRCoV infection and CIRD, as well as for the protective effect of CRCoV antibodies against both CRCoV infection and clinical signs of CIRD in general. Whilst evidence of CRCoV in pet dogs has been reported previously (Mochizuki et al., 2008, Priestnall et al., 2006) this is the first study to link infection to clinical disease in this group.
Compared to CRCoV similar overall levels of CnPnV seropositivity (41.7%), but higher detection rates (23.4%) of CnPnV were observed and are consistent with published data (Mitchell et al., 2013b). Antibodies to CnPnV were detected in dogs from all three sources, demonstrating the susceptibility of both pet and kennelled dogs to this virus. Although shelter dogs and dogs from ‘other sources’ had significantly increased odds of being positive compared to pet dogs, the higher seroprevelce observed in dogs from ‘other sources’ is most likely due to the samples being derived predominantly from an outbreak of CIRD in a single veterinary hospital, where, 96% of dogs were seropositive and 33% were positive for the virus.
Dogs were significantly more likely to be CnPnV seropositive if they were also seropositive for CRCoV (and vice versa) and M. cynos, suggesting frequent co-infection, or co-circulation of one or more of these pathogens. In further support of this, dogs were significantly more likely to be positive for CnPnV (by PCR) if they were also positive for CRCoV (by PCR). Although only 17 such cases were observed in this study, precluding further analysis, CIRD is increasingly being considered as a complex infection where multiple agents act sequentially or synergistically to cause disease, and similar disease complexes in humans are the subject of several reviews Eg: (Bosch et al., 2013). It is likely therefore that co-infections with both the classic and novel agents of CIRD will contribute the increased likelihood of disease onset, severity and duration of CIRD. Given the limited amount work in the field that currently addresses this point however, this should be an important focus of future.
Dogs with increasing disease severity were significantly more likely to be positive for CnPnV, supporting a causal relationship with CIRD (Glineur et al., 2013, Mitchell et al., 2013b). Following multivariable analysis no significant relationship between the presence of CnPnV antibodies and infection or disease occurrence was observed. This relationship was however significant at the univariable level consistant with other published data (Mitchell et al., 2013b), suggesting that whilst CnPnV specific antibodies may confer some degree of protection, this was confounded in this instance by the effects of other factors. That said, it is well documented that antibody mediated immunity for related human and bovine respiratory syncytial viruses is poor, and individuals may be repeatedly re-infected throughout their lifetime with clinical signs of respiratory disease (Falsey, 2007, Hall et al., 1976, Van der Poel et al., 1993). A number of key pneumovirus features enable them to evade and modify host immune responses as reviwed by (Collins and Melero, 2011), and as such it would be vital to assess the genetic and antigenic diversity of circulating CnPnV strains to better understand CnPnV ecology and inform vaccine design and development.
Although studies are limited, M. cynos also represents a potentially important agent in the development and persistence of CIRD (Chalker et al., 2004, Rosendal, 1972). This is the first study to examine the seroprevalence of M. cynos, and overall 45% of dogs were seropositive, comparable to that of CRCoV and CnPnV. In multivariable analysis increased odds of M. cynos seropositivity were seen in shelter dogs (compared to pet dogs), and convalescent (compared to clinically unaffected) dogs and with age.
Whilst the seropositivity suggests exposure, very few dogs with M. cynos itself (n = 5) were detected. Of those that were, three had severe respiratory signs but two were clinically unaffected. Given the limited number of positive cases in this study it is not possible to comment on the prevalence of M. cynos or its relationship with clinical disease. However it is worth considering the limitations of the study which may have influenced this finding. Firstly, several mycoplasma species are carried by dogs, often as part of their normal respiratory flora (Chalker, 2005), however very limited data is available regarding their serological cross-reactivity (Rosendal, 1975). The possibility exists therefore that the use of whole cell ELISA antigen in this study may have resulted in an overestimate in M. cynos seropositivity. Secondly, oropharyngeal swabs collected as part of a standard veterinary investigation of CIRD were analysed. Previously respiratory tissues were shown to yield a much higher recovery rate of M. cynos (99.7–23.9%) compared to oropharyngeal swabs (0–0.9%) (Chalker et al., 2004), and indeed studies that have implicated M. cynos in CIRD were based on tissue sampling, particularly of the lower respiratory tract (Hong and Kim, 2012, Zeugswetter et al., 2007), the level of M. cynos detected in this study may therefore have been vastly underestimated and highlights an important consideration for the study of M. cynos in living dogs.
Influenza A (H3N8) caused significant respiratory disease in dogs in north America (Anderson et al., 2013, Crawford et al., 2005) with an estimated seroprevalence of 49% in high risk populations (Anderson et al., 2013). Subsequent studies from North and Central America and Asia have also identified a number of other influenza A subtypes which infect and cause disease in dogs, some of which have undergone cross-species transmission, including possible transmission between dogs and humans (Lin et al., 2012, Song et al., 2008, Song et al., 2012, Songserm et al., 2006). Assays were therefore selected on the basis that they would allow for the broad detection of Influenza A rather than specific subtypes (Damiani et al., 2012, Ellis and Zambon, 2001).In agreement with other recently published European studies (Damiani et al., 2012, Dundon et al., 2010) however very little evidence of influenza A was found in this study. All of the dogs were negative for Influenza A by PCR, and only 2.7% had evidence of exposure via antibody detection. Whilst influenza A appears relatively insignificant in the European dog population at present, given its rapid spread across north America and Asia, the increasing number of influenza A subtypes detected in dogs, and the increased movement of dogs into and across Europe, it may be only be a matter of time before it makes its appearance. Moreover, the co-existence of multiple types of influenza in dogs, with the potential to cross-species, should underline the importance of continued vigilance by both the veterinary and medical communities.
Our current understanding of the true prevalence and complexity of CIRD is limited by a lack of comprehensive investigations into causative agents and associated risk factors. This study, which has begun to unpick some of these key areas, is one of the largest studies undertaken in the field to date. Findings highlight the need for a far greater consideration of pet dogs and other dog populations, the role of current vaccine formulations and strategies in preventing and managing disease outbreaks, and the impact of classic and newly identified pathogens on disease onset, development and persistence. For pathogens such as CRCoV and CnPnV, the emerging data provide a clearly identifiable link with CIRD. However continued effort is required to characterise the epidemiology and pathogenesis of more elusive pathogens such as M. cynos, to ensure that they are not underestimated or overlooked.
Acknowledgements
Sample collection and analysis for CRCoV, M. cynos and influenza A virus was funded by Zoetis Animal Health. Screening for canine pneumovirus was supported by a grant awarded to Judy A. Mitchell and Joe Brownlie by the Petplan Charitable Trust. The authors would also like to thank C. Murrell and Y. Alver for technical assistance, and L. Harbour for administrative support.
References
- Anderson T.C., Crawford P.C., Dubovi E.J., Gibbs E.P., Hernandez J.A. Prevalence of and exposure factors for seropositivity to H3N8 canine influenza virus in dogs with influenza-like illness in the United States. J. Am. Vet. Med. Assoc. 2013;242:209–216. doi: 10.2460/javma.242.2.209. [DOI] [PubMed] [Google Scholar]
- Appel M.J., Binn L.N. Virus Infections of Carnivors. Elseiver Science Publishing Co; New York: 1987. Canine infectious tracheobronchitis short review: kennel cough; pp. 201–211. [Google Scholar]
- Appel M.J., Percy D.H. SV-5-like parainfluenza virus in dogs. J. Am. Vet. Med. Assoc. 1970;156:1778–1781. [PubMed] [Google Scholar]
- Bemis D.A. Bordetella and Mycoplasma respiratory infections in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 1992;22:1173–1186. doi: 10.1016/s0195-5616(92)50308-4. [DOI] [PubMed] [Google Scholar]
- Bosch A.A., Biesbroek G., Trzcinski K., Sanders E.A., Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9:e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker V.J., Owen W.M., Paterson C., Barker E., Brooks H., Rycroft A.N., Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- Chalker V.J. Canine mycoplasmas. Res. Vet. Sci. 2005;79:1–8. doi: 10.1016/j.rvsc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Collins P.L., Melero J.A. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford P.C., Dubovi E.J., Castleman W.L., Stephenson I., Gibbs E.P., Chen L., Smith C., Hill R.C., Ferro P., Pompey J., Bright R.A., Medina M.J., Johnson C.M., Olsen C.W., Cox N.J., Klimov A.I., Katz J.M., Donis R.O. Transmission of equine influenza virus to dogs. Science. 2005;310:482–485. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- Damiani A.M., Kalthoff D., Beer M., Muller E., Osterrieder N. Serological survey in dogs and cats for influenza A(H1N1)pdm09 in Germany. Zoonoses Public Health. 2012;59:549–552. doi: 10.1111/j.1863-2378.2012.01541.x. [DOI] [PubMed] [Google Scholar]
- Day M.J., Horzinek M.C., Schultz R.D., Squires R.A., Vaccination Guidelines Group of the World Small Animal Veterinary A A. WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016;57:E1–E45. doi: 10.1111/jsap.2_12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Mari V., Larocca V., Losurdo M., Lanave G., Lucente M.S., Corrente M., Catella C., Bo S., Elia G., Torre G., Grandolfo E., Martella V., Buonavoglia C. Molecular surveillance of traditional and emerging pathogens associated with canine infectious respiratory disease. Vet. Microbiol. 2016;192:21–25. doi: 10.1016/j.vetmic.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield J., Macpherson L.W., Zbitnew A. Association of canine adenovirus (Toronto A 26/61) with an outbreak of laryngotracheitis (Kennel cough): a preliminary report. Can. Vet. J. 1962;3:238–247. [PMC free article] [PubMed] [Google Scholar]
- Dundon W.G., De Benedictis P., Viale E., Capua I. Serologic evidence of pandemic (H1N1) 2009 infection in dogs, Italy. Emerg. Infect. Dis. 2010;16:2019–2021. doi: 10.3201/eid1612.100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J.A., Krakowka G.S. A review of canine parainfluenza virus infection in dogs. J. Am. Vet. Med. Assoc. 2012;240:273–284. doi: 10.2460/javma.240.3.273. [DOI] [PubMed] [Google Scholar]
- Ellis J.S., Zambon M.C. Combined PCR-heteroduplex mobility assay for detection and differentiation of influenza A viruses from different animal species. J. Clin. Microbiol. 2001;39:4097–4102. doi: 10.1128/JCM.39.11.4097-4102.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J.A. How well do vaccines for Bordetella bronchiseptica work in dogs? A critical review of the literature 1977–2014. Vet. J. 2015;204:5–16. doi: 10.1016/j.tvjl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Dubovi E.J., Brooks H.W., Brownlie J. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 2004;42:4524–4529. doi: 10.1128/JCM.42.10.4524-4529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R. Respiratory syncytial virus infection in adults. Semin. Respir. Crit. Care Med. 2007;28:171–181. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- Glineur S.F., Renshaw R.W., Percopo C.M., Dyer K.D., Dubovi E.J., Domachowske J.B., Rosenberg H.F. Novel pneumoviruses (PnVs): evolution and inflammatory pathology. Virology. 2013;443:257–264. doi: 10.1016/j.virol.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.B., Geiman J.M., Biggar R., Kotok D.I., Hogan P.M., Douglas G.R., Jr. Respiratory syncytial virus infections within families. N. Engl. J. Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- Hong S., Kim O. Molecular identification of Mycoplasma cynos from laboratory beagle dogs with respiratory disease. Lab. Anim. Res. 2012;28:61–66. doi: 10.5625/lar.2012.28.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Yamamoto K., Eguchi M., Kubo M., Nakagami S., Wakisaka S., Kaizuka M., Ishii H. Rapid detection of mycoplasma contamination in cell cultures by enzymatic detection of polymerase chain reaction (PCR) products. J. Vet. Med. Sci. 1995;57:769–771. doi: 10.1292/jvms.57.769. [DOI] [PubMed] [Google Scholar]
- Lin D., Sun S., Du L., Ma J., Fan L., Pu J., Sun Y., Zhao J., Sun H., Liu J. Natural and experimental infection of dogs with pandemic H1N1/2009 influenza virus. J. Gen. Virol. 2012;93:119–123. doi: 10.1099/vir.0.037358-0. [DOI] [PubMed] [Google Scholar]
- Mitchell J.A., Brownlie J. The challenges in developing effective canine infectious respiratory disease vaccines. J. Pharm. Pharmacol. 2015;67:372–381. doi: 10.1111/jphp.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.A., Brooks H.W., Szladovits B., Erles K., Gibbons R., Shields S., Brownlie J. Tropism and pathological findings associated with canine respiratory coronavirus (CRCoV) Vet. Microbiol. 2013;162:582–594. doi: 10.1016/j.vetmic.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.A., Cardwell J.M., Renshaw R.W., Dubovi E.J., Brownlie J. Detection of canine pneumovirus in dogs with canine infectious respiratory disease. J. Clin. Microbiol. 2013;51:4112–4119. doi: 10.1128/JCM.02312-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Yachi A., Ohshima T., Ohuchi A., Ishida T. Etiologic study of upper respiratory infections of household dogs. J. Vet. Med. Sci. 2008;70:563–569. doi: 10.1292/jvms.70.563. [DOI] [PubMed] [Google Scholar]
- Pratelli A., Colao V. A population prevalence study on influenza infection in dogs in Southern Italy. New Microbiol. 2014;37:277–283. [PubMed] [Google Scholar]
- Priestnall S.L., Brownlie J., Dubovi E.J., Erles K. Serological prevalence of canine respiratory coronavirus. Vet. Microbiol. 2006;115:43–53. doi: 10.1016/j.vetmic.2006.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestnall S.L., Mitchell J.A., Walker C.A., Erles K., Brownlie J. New and emerging pathogens in canine infectious respiratory disease. Vet. Pathol. 2014;51:492–504. doi: 10.1177/0300985813511130. [DOI] [PubMed] [Google Scholar]
- Renshaw R.W., Zylich N.C., Laverack M.A., Glaser A.L., Dubovi E.J. Pneumovirus in dogs with acute respiratory disease. Emerg. Infect. Dis. 2010;16:993–995. doi: 10.3201/eid1606.091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S. Mycoplasmas as a possible cause of enzootic pneumonia in dogs. Acta Vet. Scand. 1972;13:137–139. [PubMed] [Google Scholar]
- Rosendal S. Canine mycoplasmas: serological studies of type and reference strains, with a proposal for the new species, Mycoplasma opalescens. Acta Pathol. Microbiol. Scand. B. 1975;83:463–470. doi: 10.1111/j.1699-0463.1975.tb00126.x. [DOI] [PubMed] [Google Scholar]
- Song D., Kang B., Lee C., Jung K., Ha G., Kang D., Park S., Park B., Oh J. Transmission of avian influenza virus (H3N2) to dogs. Emerg. Infect. Dis. 2008;14:741–746. doi: 10.3201/eid1405.071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Moon H.J., An D.J., Jeoung H.Y., Kim H., Yeom M.J., Hong M., Nam J.H., Park S.J., Park B.K., Oh J.S., Song M., Webster R.G., Kim J.K., Kang B.K. A novel reassortant canine H3N1 influenza virus between pandemic H1N1 and canine H3N2 influenza viruses in Korea. J. Gen. Virol. 2012;93:551–554. doi: 10.1099/vir.0.037739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T., Amonsin A., Jam-on R., Sae-Heng N., Pariyothorn N., Payungporn S., Theamboonlers A., Chutinimitkul S., Thanawongnuwech R., Poovorawan Y. Fatal avian influenza A H5N1 in a dog. Emerg. Infect. Dis. 2006;12:1744–1747. doi: 10.3201/eid1211.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Poel W.H., Kramps J.A., Middel W.G., Van Oirschot J.T., Brand A. Dynamics of bovine respiratory syncytial virus infections: a longitudinal epidemiological study in dairy herds. Arch. Virol. 1993;133:309–321. doi: 10.1007/BF01313771. [DOI] [PubMed] [Google Scholar]
- Zeugswetter F., Weissenbock H., Shibly S., Hassan J., Spergser J. Lethal bronchopneumonia caused by Mycoplasma cynos in a litter of golden retriever puppies. Vet. Rec. 2007;161:626–627. doi: 10.1136/vr.161.18.626. [DOI] [PubMed] [Google Scholar]