Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) continues to be a major problem to the pork industry worldwide. Increasing data indicate that PRRSV strains differ in virulence in infected pigs and are biologically, antigenically, and genetically heterogeneous. It is evident that the current vaccines, based on a single PRRSV strain, are not effective in protecting against infections with the genetically diverse field strains of PRRSV. The recent outbreaks of atypical or acute PRRS in vaccinated pigs have raised a serious concern about the efficacy of the current vaccines and provided the impetus for developing more effective vaccines. Special attention in this review is given to published work on antigenic, pathogenic and genetic variations of PRRSV and its potential implications for vaccine efficacy and development. Although there are ample data documenting the heterogeneous nature of PRRSV strains, information regarding how the heterogeneity is generated and what clinical impact it may have is very scarce. The observed heterogeneity will likely pose a major obstacle for effective prevention and control of PRRS. There remains an urgent need for fundamental research on this virus to understand the basic biology and the mechanism of heterogeneity and pathogenesis of PRRSV.
Keywords: Porcine reproductive and respiratory syndrome virus (PRRSV), Pig, Virus, Heterogeneity, Vaccine
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS), characterized by severe reproductive failure in sows and respiratory diseases in young pigs, was first recognized in the United States (U.S.) in 1987 (Keffaber, 1989, Hill, 1990). Since its appearance, PRRS has devastated the swine industry with tremendous economic losses (Polson et al., 1992). The causative agent of PRRS, porcine reproductive and respiratory syndrome virus (PRRSV), was first isolated by Wensvoort et al. (1991) in the Netherlands using porcine alveolar macrophages (PAM) and was designated as Lelystad virus (LV). In the U.S., PRRSV was first isolated and characterized in a continuous cell line ATCC CL2621 (Benfield et al., 1992, Collins et al., 1992). PRRSV is a small, enveloped, single positive-stranded RNA virus (Wensvoort et al., 1991, Benfield et al., 1992, Meulenberg et al., 1993a, Meulenberg et al., 1993b).
PRRS has now been recognized worldwide (Plana et al., 1992, Saito et al., 1996, Valicek et al., 1997, Madsen et al., 1998, Chueh et al., 1998) and is considered to be an economically important global disease. Although PRRSV strains identified from around the world cause similar diseases in pigs, increasing data indicate that PRRSV strains differ in virulence in infected animals and are antigenically and genetically heterogeneous. More recently, swine herds in the U.S. have experienced outbreaks of a severe form of PRRS characterized by abortion and high mortality in pregnant sows (Botner et al., 1997a, Bell, 1998, Mengeling et al., 1998, Lager et al., 1998, Osorio et al., 1998). This form of PRRS has been referred to as sow abortion and mortality syndrome, atypical PRRS, severe PRRS and acute PRRS. Surprisingly, many of the affected herds were vaccinated, suggesting that the current PRRS vaccines do not confer 100% protection and that a new generation of vaccines is needed.
This review will summarize published data describing the heterogeneity of PRRSV and discuss the potential implications for current vaccine efficacy and future vaccine development. Recent reviews on other aspects of PRRSV (Rossow, 1998, Albina, 1997, Botner, 1997b, Molitor et al., 1997, Meulenberg et al., 1997a, Zimmerman et al., 1997, Van Reeth, 1997) also discuss some of the work cited here.
2. Genomic organization
Meulenberg et al. (1993a) first cloned and sequenced the genome of LV, a European strain of PRRSV. Subsequently, partial sequences of another European strain (Conzelmann et al., 1993) and two North American strains of PRRSV (Mardassi et al., 1994, Meng et al., 1994) were reported. More recently, the complete genomes of two North American strains of PRRSV have been determined (Allende et al., 1999, Nelsen et al., 1999). The genome of PRRSV is an ∼15 kb positive strand RNA molecule that encodes eight overlapping open reading frames (ORFs) organized similarly to the ORFs of coronaviruses (Lai and Cavanagh, 1997, Meulenberg et al., 1993a, Allende et al., 1999, Nelsen et al., 1999). The overlaps between the ORFs of LV range from 1 bp (between the ORFs 4 and 5) to 253 bp (between the ORFs 3 and 4). The U.S. strains of PRRSV have a 10 bp noncoding region separating ORFs 4 and 5 (Meng et al., 1995b, Morozov et al., 1995). ORFs 1a and 1b comprise about 80% of the viral genome and are predicted to encode the viral RNA polymerase (Meulenberg et al., 1993a, Allende et al., 1999, Nelsen et al., 1999). The C-terminus of ORF 1a overlaps the N-terminus of ORF 1b by 16 nucleotides. A heptanucleotide slippery sequence, UUUAAAC, located just upstream of the UAG stop codon of ORF 1a, and a pseudo-knot structure downstream of the slippery sequence are believed to be essential for the expression of ORF 1b of PRRSV via a mechanism of ribosomal frame-shifting (Meulenberg et al., 1993a, Allende et al., 1999, Nelsen et al., 1999).
ORFs 2, 3 and 4 of PRRSV encode virion-associated proteins designated as GP2, GP3 and GP4, respectively (Meulenberg et al., 1995, Meulenberg et al., 1997b, Meulenberg and Petersen-den Besten, 1996, Van Nieuwstadt et al., 1996). However, the GP3 protein of a Canadian PRRSV isolate encodes a nonvirion-associated, soluble protein (Mardassi et al., 1998), which is similar to the ORF3 protein encoded by lactate dehydrogenase-elevating virus (LDV) (Faaberg and Plagemann, 1997). The reason for the discrepancy in whether GP3 protein of PRRSV is a structural protein is not clear, but genetic variation of the ORF3 gene might be responsible. Therefore, the GP3 protein of other diverse strains of PRRSV should also be evaluated. ORFs 5, 6 and 7 of PRRSV encode envelope (GP5), membrane (M) and nucleocapsid (N) proteins, respectively (Mardassi et al., 1996, Meulenberg et al., 1995, Meulenberg et al., 1997a). Monoclonal antibodies directed against GP4 and GP5 proteins are neutralizing (Meulenberg et al., 1997b, Pirzadeh and Dea, 1997). The M protein is an unglycosylated protein of 18 kDa (Meulenberg et al., 1995) which has the same hydrophobicity profile as the M proteins of equine arteritis virus (EAV) (de Vries et al., 1992) and LDV (Godeny et al., 1993). The N protein is not N-glycosylated, although it contains 1 or 2 potential N-glycosylation sites (Meulenberg et al., 1995). The order of PRRSV genes, 5′-viral polymerase (ORFs 1a/1b)-virion-associated proteins GP2 (ORF 2)-GP3 (ORF 3)-GP4 (ORF 4)-GP5 (ORF 5)-M (ORF 6)-N (ORF 7)-3′, is the same as in EAV (den Boon et al., 1991), LDV (Plagemann and Moennig, 1992) and simian hemorrhagic fever virus (SHFV) (Wang et al., 1998). Therefore, PRRSV, along with EAV, LDV and SHFV, is now classified within a single genus Arterivirus in the family Arteriviridae in the order Nidovirales (Cavanagh, 1997).
The expression and replication of PRRSV requires the production of at least six subgenomic mRNAs (sg mRNAs) (Conzelmann et al., 1993, Meulenberg et al., 1993a, Meulenberg et al., 1993b, Meulenberg et al., 1997a, Meng et al., 1994, Meng et al., 1996b, Snijder and Meulenberg, 1998). These sg mRNAs, together with the genomic virion RNA, form a 3′-coterminal nested set. Each of these sg mRNAs contains a 5′ common leader sequence of about 200 bp in size (Meulenberg et al., 1993a, Meulenberg et al., 1993b, Morozov et al., 1996, Nelsen et al., 1999, Allende et al., 1999, Oleksiewicz et al., 1999). The leader-mRNA junction sequence of PRRSV, in which the leader joins to the body of the sg mRNAs, is a conserved sequence motif of six nucleotides (UCAACC) or a highly similar sequence (Meulenberg et al., 1993a, Meulenberg et al., 1993b, Meng et al., 1995b, Meng et al., 1996b, Nelsen et al., 1999). The sg mRNAs of PRRSV are not packaged into the virions (Meng et al., 1996b), suggesting that the encapsidation signal of PRRSV is likely localized within the ORF 1 region that is unique to the viral genome, but not present in the sg mRNAs. Northern blot analysis with ORF-specific probes indicates that sg mRNAs are polycistronic (Meng et al., 1996b). It is generally believed that only the ORF at the 5′ end of each sg mRNA is translationally active and, thus, each of the sg mRNAs is functionally monocistronic. The precise mechanism of transcription and translation of PRRSV is not understood, although it is believed to be similar to that of coronaviruses (Lai and Cavanagh, 1997). The recently constructed infectious cDNA clone of PRRSV (Meulenberg et al., 1998) should facilitate investigation of the mechanism of PRRSV replication.
3. Biological and antigenic variation
Biological variations among PRRSV isolates have been reported. The European PRRSV isolates were preferentially propagated in PAM cultures (Wensvoort et al., 1991, Wensvoort, 1993), whereas the North American isolates were grown in PAM cultures as well as in three continuous cell lines, CL2621, MARC-145 and CRL11171 (Benfield et al., 1992, Collins et al., 1992, Kim et al., 1993, Meng et al., 1994, Meng et al., 1996a). Swine testis (ST) cells were also reported to support PRRSV replication (Plana et al., 1992). However, the ST cells cannot propagate a high virulence PRRSV isolate VR2385 (Meng et al., 1996a). Variations in the susceptibilities of CL2621 cells and PAM to PRRSV infection were reported. Not all PRRSV isolates growing in CL2621 cells replicated in PAM, and vice versa (Bautista et al., 1993a). Failure to propagate some strains of PRRSV in certain cell cultures indicates the existence of PRRSV variants and, thus, both PAM and other cell lines should be used when attempting virus isolation from clinical samples. It has been reported that field strains of PRRSV vary in their susceptibility to antibody-dependent enhancement (ADE) of infection (Yoon et al., 1996, Yoon et al., 1997). Therefore, the altered ability to infect may be due to the selection of variants that can facilitate infection of macrophages through ADE.
Antigenic variations among PRRSV isolates have been well documented. Wensvoort et al. (1992) and Wensvoort (1993) reported that, antigenically, four European isolates resembled each other closely, but differed from the U.S. isolates, and that three U.S. isolates differed from each other. Serologic survey of the field samples by immunofluorescence assay indicated that about 20% of the samples were positive for European LV, but negative for U.S. isolate VR2332, and that about 44% of the samples were positive for VR2332, but negative for LV (Bautista et al., 1993b). In another study, North American isolates were found to be more closely related serologically to each other than to the European isolates (Frey et al., 1992). Of 214 Canadian swine sera tested, 154 samples were positive for VR2332 antibody, but only 22 samples were positive for LV. When 50 swine sera from the Netherlands were tested, 44 samples were positive for LV antibody, but only 11 samples were positive for VR2332. Western blot analysis indicated that the ORF 4 protein of MN-1 isolate reacted with only 65% of PRRSV-infected pig sera tested (Kwang et al., 1994). Differential reactivity of monoclonal antibodies (MAbs) with different PRRSV isolates was also reported. Two MAbs to N protein recognized a conserved epitope in U.S. and European PRRSV isolates, but four other MAbs to N protein reacted with U.S. isolates only (Nelson et al., 1993). Six MAbs raised against a British isolate of PRRSV did not react with U.S. isolates tested (Drew et al., 1995). Five MAbs against GP5 protein of a Canadian isolate did not react with LV (Pirzadeh and Dea, 1997, Pirzadeh et al., 1998). An MAb to M protein reacted with all 148 North American PRRSV isolates tested, but failed to react with any of the 13 European isolates (Magar et al., 1997). The reactivity of MAbs against GP3, GP4 and N proteins with European and American PRRSV isolates also revealed antigenic differences not only between the U.S. and European isolates, but among different European or U.S. isolates as well (Katz et al., 1995, Wieczorek-Krohmer et al., 1996). In addition, antigenic variation was demonstrated between an isolate and its progeny recovered after in vivo passages (Le Gall et al., 1997), suggesting that a relatively high rate of mutations occurs during PRRSV replication in its natural host.
Given the degree of antigenic diversity observed among PRRSV strains, it is unlikely that a vaccine based on one strain of PRRSV will effectively protect against antigenically different enzootic field strains of PRRSV. In fact, Lager et al. (1999) recently showed that gilts inoculated with one strain of PRRSV did not completely protect against heterologous challenge with an antigenically distinct PRRSV strain. Therefore, the effectiveness of a vaccine against heterologous enzootic field strains of PRRSV will largely depend on the antigenic relatedness of the virus strain to which the vaccinated animals were exposed. The design of future vaccines will have to take into consideration the antigenic diversity. The author believes that a multivalent vaccine consisting of multiple antigenically distinct strains of PRRSV is the most promising candidate for the next generation of vaccines.
4. Pathogenesis and pathogenic variation
The mechanism of PRRSV pathogenesis is poorly understood. It is generally believed that PRRSV initiates an infection in pigs via entry through nasal epithelial, tonsillar, and pulmonary macrophages. PRRSV replicates in these cells, causes viremia and, subsequently, results in pneumonia, myocarditis, encephalitis, rhinitis, vasculitis, lymphadenopathy, etc. in target organs (Rossow et al., 1995, Rossow et al., 1996). It has been well documented that PRRSV causes persistent infections in pigs (Albina et al., 1994, Christopher-Hennings et al., 1995a, Christopher-Hennings et al., 1995b, Wills et al., 1997a, Wills et al., 1997b). In experimentally infected boars, PRRSV can be detected by PCR in semen samples at 92 days postinoculation (DPI) (Christopher-Hennings et al., 1995a, Christopher-Hennings et al., 1995b). Pigs persistently infected with PRRSV can transmit the virus to naive pigs by direct or indirect contact, and the transmission by direct contact occurs up to 22 weeks after infection (Albina et al., 1994, Wills et al., 1997a, Wills et al., 1997b). Bilodeau et al. (1994) showed that when specific-pathogen-free (SPF) pigs were introduced into a barn that had housed PRRSV-infected pigs as much as 4 months after clinical signs of infection had disappeared, the newly introduced SPF pigs became infected. This study indicates that subclinical PRRSV infection can persist in the animals. Wills et al., 1997a, Wills et al., 1997b demonstrated that PRRSV can be isolated from oropharyngeal samples for up to 157 DPI. Pigs persistently infected with PRRSV may appear clinically normal, but can still transmit virus to pigs in naive swine herds. Therefore, persistent infection of PRRSV plays an important role in PRRSV survival and transmission, and will likely pose a major obstacle in PRRS control programs. Despite the ample data documenting PRRSV persistence, little has been done to understand the mechanism of persistent infection or what clinical impact it may have. Clearly, more studies are needed in the future to determine the host and virus factors that lead to the persistent state and to fully elucidate the mechanism of PRRSV pathogenesis.
Marked differences in virulence among PRRSV strains have been observed in experimentally-infected pigs (Halbur et al., 1995b, Halbur et al., 1996b). Significant differences in severity of clinical respiratory disease, rectal temperatures, gross lung lesions and microscopic lung lesions were observed among nine different U.S. isolates of PRRSV. The European LV and the low-virulence U.S. PRRSV isolate VR2431 (ISU3927) induced mild transient pyrexia, dyspnea and tachypnea, but several high virulence U.S. isolates induced labored respiration, pyrexia, lethargy, anorexia and patchy dermal cyanosis. At 10 DPI, mean lung lesion scores estimating the percentage of lungs affected by pneumonia ranged from 6.8% for LV, 9.7% for VR2431 (ISU3927), 54.2% for VR2385, to 62.4% for ISU-28 (Halbur et al., 1995b, Halbur et al., 1996b). Despite the observed difference in virulence among PRRSV isolates, tissue tropism and distribution of PRRSV antigen or nucleic acid within tissues and organs were very similar in pigs inoculated with different strains of PRRSV (Halbur et al., 1994, Halbur et al., 1995a, Halbur et al., 1996a, Haynes et al., 1997). Strains of PRRSV also vary in virulence for their ability to cause reproductive failure (Mengeling et al., 1996). Mengeling et al. (1996) reported that the effects of PRRSV on reproductive performance are strain-dependent. In addition, apathogenic field isolates of PRRSV have been reported (Ohlinger et al., 1992, Van Alstine, 1992), indicating that field isolates of PRRSV differ in virulence. The recent outbreaks of severe atypical or acute PRRS further indicate that the recent atypical PRRSV strains circulating in the U.S. swine herds are more virulent than those strains isolated earlier (Botner et al., 1997a, Mengeling et al., 1998, Bell, 1998, Lager et al., 1998, Osorio et al., 1998). Mengeling et al. (1998) demonstrated that a field strain of atypical PRRSV produced especially severe clinical signs of disease and reproductive failure in experimentally infected gilts. Rossow et al. (1999) reported that marked neurovirulence in neonatal pigs was found to be associated with infection by some field isolates of PRRSV. PRRSV was identified in macrophages or microglia of brain lesions by immunohistochemical staining of brain sections. The replication of the virus in the brain was verified by in situ hybridization. The mechanism for the observed PRRSV neurovirulence in neonatal pigs is not known, but genetic changes in PRRSV genome may alter the tissue tropism of PRRSV.
The mechanism for pathogenic variation observed among PRRSV strains remains unknown, but the genetic make-up of a particular virus strain will likely determine the virulence of the virus in animals. Therefore, it is important to genetically characterize field strains of PRRSV with differing virulence. One recent breakthrough in PRRSV research is the construction of an infectious cDNA clone of PRRSV (Meulenberg et al., 1998). Using this infectious clone, one should be able to construct viruses that are chimeras of low and high virulence strains of PRRSV or to mutant genes of interest to study the structural and functional relationship of PRRSV genes. The availability of this infectious cDNA clone will eventually aid PRRSV researchers in identifying the genetic virulence determinant(s) of PRRSV.
5. Genetic variation and genotypes
PRRSV is genetically heterogeneous. Extensive sequence variation was found between the European and the U.S. isolates (Mardassi et al., 1994, Meng et al., 1994, Meng et al., 1995a, Meng et al., 1995b, Meng et al., 1996b, Morozov et al., 1995, Murtaugh et al., 1995, Nelsen et al., 1999, Allende et al., 1999). The nucleotide sequence identity between LV and the U.S. isolates is 65–67% in ORF 2, 61–64% in ORF 3, 63–66% in ORF 4, and 61–63% in ORF 5 (Meng et al., 1995a, Meng et al., 1995b). ORFs 6 and 7 genes are relatively conserved among the U.S. isolates or among the European isolates, but extensive genetic variation was observed in the ORFs 6 and 7 genes between European and U.S. isolates. It has been shown that the nucleotide sequence identity was 96–98% in ORF 2, 92–98% in ORF 3, 92–99% in ORF 4, and 90–98% in ORF 5 among six U.S. isolates (Meng et al., 1995b). Interestingly, the least virulent U.S. isolate, ISU3927 (ATCC VR2431), has the most divergent sequence compared to the other five U.S. isolates. The nucleotide sequence identity between ISU3927 and the other U.S. isolates was 93–94% in ORF 2, 89–90% in ORF 3, and 91–93% in ORF 4. The ORF 5 of ISU3927 has a three-nucleotide deletion and shares 91–93% nucleotide sequence identity compared to that of the other U.S. isolates. The sequence variation between the least virulent strain ISU3927 and other U.S. isolates appears to be randomly distributed throughout the genome (Meng et al., 1995b), thus it is difficult to speculate regarding any correlation between PRRSV virulence and a particular gene or sequence. Kapur et al. (1996) analyzed the nucleotide sequence of ORFs 2–7 of 10 U.S. PRRSV isolates, and found that the genetic distance ranges from 2.5–7.9% among these 10 U.S. isolates and is about 35% between LV and the U.S. isolates. Simple accumulation of random neutral mutations cannot explain the substantial nucleotide differences among PRRSV isolates, and the mechanism for generating the genetic heterogeneity remains unknown.
The leader sequence of PRRSV strains also varies significantly. The 190 bp leader sequence of VR2332 strain is 31 bp shorter than that of LV, and has a sequence identity of 61% with that of LV (Nelsen et al., 1999). The leader sequence of another North American strain, 16244B, is 189 bp in length and also differs considerably in nucleotide sequence with that of LV (Allende et al., 1999). Like the leader sequence, the ORF1 gene sequence also differs extensively between the U.S. and the European strains (Allende et al., 1999, Nelsen et al., 1999). The ORF1a of VR2332 strain shares only about 55% nucleotide sequence identity with that of the LV strain. ORF1b is more conserved than ORF1a and shares about 63% nucleotide sequence identity with that of LV. However, a stretch of 151 amino acids at the carboxyl terminus of ORF1b of VR2332 has only 49% similarity with that of LV (Nelsen et al., 1999). Allende et al. (1999) also showed that North American strain 16244B shares only about 47% amino acid identity in the ORF1a polyprotein region with that of LV. The greatest divergence is found in the nonstructural protein 2 (NSP2), which shares only about 32% amino acid identity with the corresponding region of LV. Surprisingly, the NSP2 of strain 16244B is 120 amino acids longer than that of LV (Allende et al., 1999). Like strain VR2332, strain 16244B also exhibits greater divergence in the carboxyl terminal region of ORF1b (CP4 protein), with only 42% identity with the corresponding region of LV. Considering the striking differences in the leader sequence and in all ORFs between European and North American strains, it is surprising that both European and North American strains cause a similar disease. Although the origin of PRRSV remains unknown, these data strongly suggest that the European strains and the North American strains of PRRSV have undergone divergent evolution on separate continents from a common ancestor. Based on the sequence and evolution analyses of 10 U.S. isolates, Kapur et al. (1996) estimated that the time for generating the amount of nucleotide variation in the Midwestern U.S. isolates takes about 6–14 years of virus evolution, suggesting that PRRSV probably emerged as a swine pathogen approximately a decade ago. It has been speculated that European and North American PRRSV strains may have evolved from an LDV-like ancestor (Plagemann, 1996, Nelsen et al., 1999). However, the almost simultaneous emergence of PRRS in swine on two continents makes the theory of divergent evolution difficult to believe. More likely, the simultaneous emergence of PRRS on two continents might relate to global changes in commercial swine management and husbandry (Nelsen et al., 1999).
The sg mRNAs of PRRSV are heterogeneous (Meulenberg et al., 1993b, Meng et al., 1996b, Snijder and Meulenberg, 1998, Faaberg et al., 1998, Nelsen et al., 1999). Meng et al. (1996b) reported that, in addition to the genomic RNA, a nested set of six or seven sg mRNAs is present in cells infected with different isolates of U.S. PRRSV that differ in virulence. PRRSV isolates ISU55, ISU79 and ISU3927 produce seven sg mRNAs, whereas isolates ISU22 and ISU1894 produce only six sg mRNAs. The additional species of sg mRNA (designated as sg mRNA 3-1) is located between sg mRNAs 3 and 4, and is generated from the sequence upstream of ORF 4. However, there is no apparent correlation between virus virulence and the additional sg mRNA3-1 (Meng et al., 1996b). Interestingly, a small ORF with a coding capacity of 45 amino acid residues at the 5′-end of the sg mRNA 3-1 was identified. Thus, the sg mRNA 3-1 in some isolates is potentially bicistronic. However, whether this small ORF 3-1 is actually translated or has any biological functions remains to be studied. Morozov et al. (1996) reported that there are two leader-body junction sites for sg mRNAs 5 and 7. Nelsen et al. (1999) also demonstrated that VR2332 strain of PRRSV utilizes two leader-body junction sites for sg mRNA7 transcription: one site at 123 bp upstream of the ORF7 start codon for most mRNA7 transcripts, and the other site at 9 bp upstream of the ORF7 start codon for a minority of transcripts. In contrast, LV only utilizes the site at 9 bp upstream of the ORF7 start codon. Faaberg et al. (1998) has also shown that the sg mRNA 7 is transcribed with different leader-body junction sites. The differences in sg mRNAs and leader-body junction sites among PRRSV isolates further reflect the heterogeneous nature of the virus. Future studies are needed to elucidate the biological significance of the heterogeneity.
At least two distinct genotypes of PRRSV have been reported: the European and the North American (Meng et al., 1995a). Recently, an impressive amount of sequence data on PRRSV isolates has been generated. To gain a better understanding of the genetic relationship and evolution of PRRSV, phylogenetic analyses were performed based on the sequences of ORF5 (Fig. 1 a) and ORF7 (Fig. 1b) genes of 66 strains of PRRSV worldwide. These sequences were either published (Andreyev et al., 1997, Casal et al., 1998, Chueh et al., 1998, Conzelmann et al., 1993, Drew et al., 1997, Gagnon and Dea, 1998, Kapur et al., 1996, Le Gall et al., 1998, Madsen et al., 1998, Mardassi et al., 1994, Meulenberg et al., 1993a, Murtaugh et al., 1995, Nelsen et al., 1999, Pirzadeh et al., 1998, Plana et al., 1992, Rodriguez et al., 1997, Saito et al., 1996, Suarez et al., 1996, Sur et al., 1997, Valicek et al., 1997, Wesley et al., 1998, Wootton et al., 1998, Yang et al., 1998) or are available in GenBank (AF046869, AF090173, AF066066, AF059352, AF035409, AF030306, X92942, AF046869, U87392, U00153). Phylogenetic analysis was also performed on the basis of the complete sequences of the structural genes (ORFs 2–7), which are available for 22 isolates of PRRSV (Fig. 1c). All three phylogenetic trees, based on different regions of the genome, indicate that the North American and European isolates of PRRSV represent two distinct genotypes as reported previously. However, within each of the two major genotypes, several minor genotypes (or variants) of PRRSV were also identified (Fig. 1a and c). The N gene of PRRSV is relatively conserved within each of the two major genotypes (Fig. 1b). In contrast, the major envelope protein gene (GP5) of PRRSV exhibits greater genetic diversity within each major genotype (Fig. 1a). Similarly, the phylogenetic tree based on the complete sequence of the structural genes (Fig. 1c) displays greater genetic diversity than the N gene within each major genotype. Interestingly, the strains from Japan, China, Taiwan, Guatemala and three Danish strains of PRRSV are all clustered within the North American genotype. The three Danish strains, the Chinese strain (S1), a Canadian strain (PA8), and a strain from Nebraska (NE16244B) are all found to be closely related to the modified-live vaccine (MLV) virus and the vaccine strain VR2332. The three Danish strains of PRRSV are believed to originate from the MLV vaccine virus (VR2332) that was used in Danish swine herds (Madsen et al., 1998). Phylogenetic trees (Fig. 1a and c) confirm that these three Danish strains are most closely related to the MLV RespPRRS vaccine virus, but are less related to other North American strains, further indicating that the introduction of North American type of PRRSV in Denmark was due to the spread of vaccine virus VR2332 (Madsen et al., 1998). The origin of other strains that are closely related to the MLV and strain VR2332 is not known. It is possible that these strains isolated from different geographic regions also evolved from the MLV vaccine virus VR2332 as a result of large-scale vaccination programs in swine herds around the world.
Fig. 1.
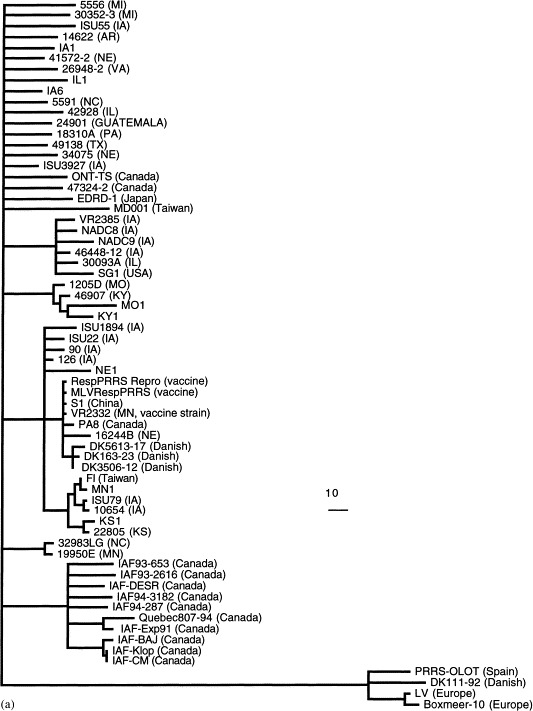

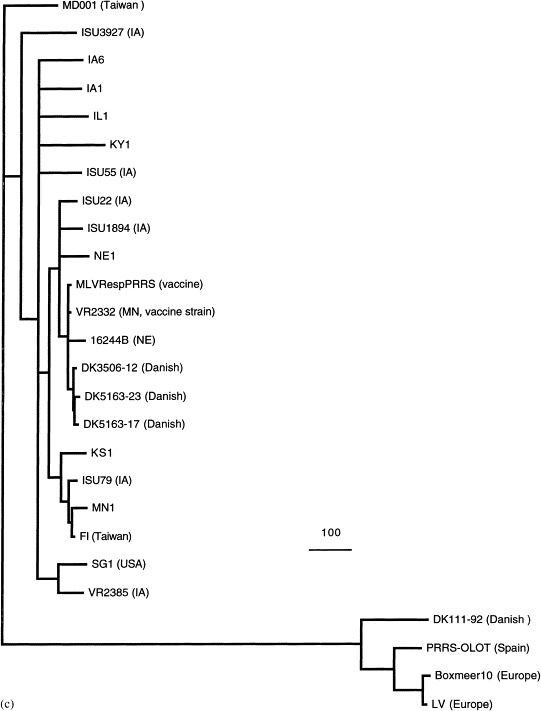
Phylogenetic trees based on the nucleotide sequence of ORF5 (a), ORF7 (b), and the complete structural genes ORFs 2–7 (c) of PRRSV. The trees were constructed by maximum parsimony methods with the aid of the PAUP program (GCG version 9.1, David L. Swofford, Smithsonian Institute, Washington, DC). Bootstrap (100 replications) with heuristic searching and midpoint rooting options was used to construct the tree. The scale bar representing the numbers of character state changes is shown in each tree. Branch lengths are proportional to the numbers of character state changes. The references for the sequences of PRRSV isolates used in the phylogenetic analyses are cited in the text.
6. Quasispecies and RNA recombination
Quasispecies is defined as a population of closely related, yet heterogeneous sequences that are variants of one dominant sequence (Bukh et al., 1995). Viral quasispecies are closely related mutant and recombinant viral genomes subjected to continuous genetic variation, competition and selection (Domingo et al., 1998). Within a single infected animal, many RNA viruses have been found to exist as quasispecies. This heterogeneity can cause persistent infection resulting from selection of mutants that escape neutralizing antibody or cytotoxic T lymphocytes (CTL) or as a result of the presence of defective particles (Duarte et al., 1994, Ahmed et al., 1996, Domingo et al., 1998). Extensive genetic variations have been observed among different strains of PRRSV; however, little is known about the mechanism of generating genetic diversity. Recently, Rowland et al. (1999) reported evidence for quasispecies evolution and emergence of a virus subpopulation during in utero infection of pigs with a PRRSV isolate. A single nucleotide change in the ectodomain of GP5 protein was identified during infection of pigs with PRRSV strain VR2332. This finding suggests that the genetic variability in the ectodomain of the ORF5 may be due to positive or negative selection forces, such as selection by antibody or other host defenses. Other factors such as RNA secondary structure, especially in ORF1, the diverse leader-body junction sites, and the size and sequence difference of the 5′ leader sequence among PRRSV strains should also be considered as potential driving forces for heterogeneity and quasispecies evolution of PRRSV. Quasispecies has been well documented in other viruses that cause persistent infections including LDV, an arterivirus closely related to PRRSV (Duarte et al., 1994, Plagemann et al., 1995, Plagemann, 1996, Bukh et al., 1995, Ahmed et al., 1996, Domingo et al., 1998). The extent and nature of PRRSV quasispecies evolution and whether quasispecies evolution are related to PRRSV persistency remain to be determined. In LDV, quasispecies have been identified and biologically characterized (Chen et al., 1997, Chen et al., 1998a, Chen et al., 1998b, Chen et al., 1999). The LDV quasispecies were found to differ in neuropathogenicity, and the neuropathogenic LDVs are incapable of establishing persistent infection in mice. The existence of a quasispecies population during virus infection will affect vaccine efficacy and may lead to vaccine failure (Domingo and Holland, 1992, Duarte et al., 1994, Domingo et al., 1998). The first report of PRRSV quasispecies evolution by Rowland et al. (1999) should stimulate further study of the nature of PRRSV quasispecies evolution and its clinical implications.
RNA recombination can provide a powerful and effective mechanism for evolution of an RNA virus. The ability to exchange genetic information may allow RNA viruses to adapt a changing environment and to escape a selection pressure (such as neutralizing antibodies), thus providing the recombinant virus with an evolutionary advantage (Lai and Cavanagh, 1997). Recently, Yuan et al. (1999) provided evidence for homologous RNA recombination between PRRSV isolates propagated in cell culture. Recombinant viral particles containing chimeric ORF 3 and ORF 4 proteins were identified in MA-104 cells co-infected with two PRRSV isolates. Nucleotide sequence analyses confirmed independent recombination events. The frequency of recombination was estimated from <2% up to 10% within the 1182-bp fragment analyzed. Sequence analyses of field isolates of PRRSV suggest that RNA recombination of PRRSV may also occur in nature (Yuan et al., 1999). By analyzing 10 field isolates of U.S. PRRSV, Kapur et al. (1996) also provided evidence for intragenic recombination in ORFs 2–5 and in ORF 7 among PRRSV isolates. Whether RNA recombination plays any roles in generating genetic heterogeneity of PRRSV is not known. Direct experimental evidence for in vivo RNA recombination of PRRSV is still lacking. Most RNA virus recombination studies have been performed in cell cultures, although RNA recombination has also been demonstrated in the natural host of picornaviruses, coronavirus and LDV (Lai and Cavanagh, 1997, Minor et al., 1986, Li et al., 1999). For example, a case of poliovirus vaccine-associated poliomyelitis in a human was caused by recombination between two poliovirus vaccine strains (Minor et al., 1986). High frequency homologous genetic recombination was reported in mice dually infected with two strains of LDV (Li et al., 1999). Therefore, future studies are warranted to determine whether RNA recombination of PRRSV is a phenomenon unique to cell culture or whether this actually occurs in vivo. The frequency of recombination, crossover sites and the clinical implications of PRRSV RNA recombination also need to be studied. The sg mRNA species may also be involved in RNA recombination events during infection. Thus, future studies are needed to examine RNA recombination not only at genomic RNA level, but at the sg mRNA level as well.
7. Implications on vaccine efficacy and development
Several PRRS vaccines are currently available; however, there are mixed results regarding the efficacy of these vaccines against the genetically diverse field strains of PRRSV (Lager and Mengeling, 1997, Plana-Duran et al., 1997, Christopher-Hennings et al., 1997, Van Woensel et al., 1998, Osorio et al., 1998, Madsen et al., 1998, Mengeling et al., 1999a, Mengeling et al., 1999b, Mengeling et al., 1999c, Wesley et al., 1999). RespPRRS/Repro™ (Boehringer Ingelheim), an MLV, is recommended for use in 3–18 week-old pigs and in nonpregnant females (Lager and Mengeling, 1997, Dee and Joo, 1997). The Prime Pac PRRS vaccine (Schering Plough Animal Health Corporation) (Hesse et al., 1997) is also an MLV which has been shown to reduce the severity and duration of disease following challenge. However, it did not prevent infection of vaccinated pigs by a virulent heterologous strain. A live vaccine based on a European isolate of PRRSV (Porcilis PRRS) was found to protect fattening pigs against the respiratory manifestations of PRRS (Mavromatis et al., 1999). Osorio et al. (1998) compared three commercial vaccines in their ability to induce protection against PRRSV strains of high virulence, and found that these vaccines confer protection against clinical disease, but not against infection. The use of MLVs in boars resulted in vaccine virus shedding in semen and reduced semen quality, but there was reduced or no shedding of wild-type PRRSV after challenge (Christopher-Hennings et al., 1997). In another study, vaccination with a MLV in boars resulted in marked reduction in viremia and shedding of virus in semen, but vaccination with inactivated vaccine did not change the onset, duration or level of viremia or shedding of virus in semen (Nielsen et al., 1997).
By using a restriction-site marker that is present in the vaccine virus (VR2332), Mengeling et al. (1999b) demonstrated that the marker was not detected in any of the 25 field strains of PRRSV isolated before use of the vaccine. However, the restriction-site marker was detected in 24 of 25 field strains isolated after the introduction of the vaccine, and these field strains were believed to be direct-line descendants of the vaccine virus (Mengeling et al., 1999b). More importantly, these putative vaccine-related strains produced more pronounced pathological changes than did the vaccine virus alone (Mengeling et al., 1999b). Wesley et al. (1999) also showed that the restriction fragment length polymorphism (RFLP) patterns change as the vaccine virus spreads among a swine population. A glycine marker in the ORF5 gene of the vaccine virus is rapidly lost and replaced with arginine. The use of MLVs in herds may lessen the clinical signs of PRRS following infection. However, the potential risk for reversion of MLVs to virulent phenotypes cannot be overlooked. In the absence of a new generation of vaccines, more studies are needed to fully evaluate the safety and efficacy of the current MLVs.
The emergence and re-emergence of viral infectious diseases is often influenced by the genetics of the viruses (Domingo and Holland, 1992, Duarte et al., 1994, Domingo et al., 1998). Genetic heterogeneity of PRRSV, due to quasispecies evolution and RNA recombination, could lead to the selection of virulent viruses and to the emergence or re-emergence of new forms of PRRS. Quasispecies evolution of PRRSV in response to positive or negative selection pressures may significantly change the genomic sequence of MLVs over time as the vaccine virus spreads among swine herds, and ultimately, these genetic changes may revert MLVs to virulent phenotypes. It is also possible that virulent strains of PRRSV could be generated through RNA recombination between MLVs used in the vaccination programs and enzootic field strains of PRRSV. The recent outbreaks of the atypical or acute PRRS reflect the need to further study this virus to better understand its biology and develop more effective vaccines. Most of the herds affected by the atypical PRRS had been vaccinated with the current vaccines (Bell, 1998, Lager et al., 1998). It is possible that a mutant strain(s) of PRRSV may be responsible for the recent outbreaks of acute PRRS. The heterogeneous nature of PRRSV suggests that complete elimination of the virus from the environment is unlikely. The observed genetic diversity among field isolates of PRRSV will continue to be the major obstacle for PRRS control. Therefore, the design for the next generation of vaccines will have to take into consideration the genetic heterogeneity of PRRSV, or PRRS will remain difficult to control. Intensive research is required to answer the many questions that remain. The recently constructed infectious cDNA clone of PRRSV (Meulenberg et al., 1998) should enable us to study the basic biology of PRRSV. Using the infectious cDNA clone, one can monitor the nature of quasispecies evolution of PRRSV in pigs infected by a homogeneous virus derived from the infectious clone. One can also genetically engineer the virus to produce a modified avirulent strain that could be used as an MLV. The immunogenic gene(s) of the genetically-engineered avirulent strain of PRRSV can be replaced with that of other antigenically and genetically distinct PRRSV strains to produce avirulent virus strains that can be used as a multivalent MLV.
Acknowledgements
I wish to thank Drs. Roger Avery and Thomas Toth of Virginia–Maryland Regional College of Veterinary Medicine, and Dr. Jill Sible of Department of Biology at Virginia Tech for their critic reviews of the manuscript; Drs. Prem Paul and Patrick Halbur of Iowa State University’s College of Veterinary Medicine for their continuous support and collaboration, and Mr. Denis Guenette for editorial assistance. I apologize to the authors of important papers not cited here because of the narrow scope of this review and of space limitations.
References
- Albina E., Madec F., Cariolet R., Torrison J. Immune response and persistence of the porcine reproductive and respiratory syndrome virus in infected pigs and farm units. Vet. Rec. 1994;134:567–573. doi: 10.1136/vr.134.22.567. [DOI] [PubMed] [Google Scholar]
- Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet. Microbiol. 1997;55:309–316. doi: 10.1016/s0378-1135(96)01322-3. [DOI] [PubMed] [Google Scholar]
- Allende R., Lewis T.L., Lu Z., Rock D.L., Kutish G.F., Ali A., Doster A.R., Osorio F.A. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. 1999;80:307–315. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- Ahmed, R., Morrison, L.A., Knipe, D.M., 1996. Persistence of viruses. In: Fields, B.N., Knipe, D.M., Howley, P.M., et al. (Eds.), Field Virology, Lippincott-Raven, Philadelphia, PA, pp. 219–249.
- Andreyev V.G., Wesley R.D., Mengling W.L., Vorwald A.C., Lager K.M. Genetic variation and phylogenetic relationships of 22 porcine reproductive and respiratory syndrome virus (PRRSV) field strains based on sequence analysis of open reading frame 5. Arch. Virol. 1997;142:993–1001. doi: 10.1007/s007050050134. [DOI] [PubMed] [Google Scholar]
- Bautista E.M., Goyal S.M., Yoon I.J., Joo H.S., Collins J.E. Comparison of porcine alveolar macrophages and CL 2621 for the detection of porcine reproductive and respiratory syndrome (PRRS) virus and anti-PRRS antibody. J. Vet. Diagn. Invest. 1993;5:163–165. doi: 10.1177/104063879300500204. [DOI] [PubMed] [Google Scholar]
- Bautista E.M., Goyal S.M., Collins J.E. Serologic survey for Lelystad and VR-2332 strains of porcine respiratory and reproductive syndrome (PRRS) virus in U.S. swine herds. J. Vet. Diagn. Invest. 1993;5:612–614. doi: 10.1177/104063879300500418. [DOI] [PubMed] [Google Scholar]
- Bell, A., 1998. Hot PRRS is still hot. Pork (February), pp. 32–33.
- Benfield D.A., Nelson E., Collins J.E., Harris L., Goyal S.M., Bobinson D., Christianson W.T., Morrison R.B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J. Vet. Diagn. Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Bilodeau R., Archambault D., Vezina S.A., Sauvageau R., Fournier M., Dea S. Persistence of porcine reproductive and respiratory syndrome virus infection in a swine operation. Can. J. Vet. Res. 1994;58:291–298. [PMC free article] [PubMed] [Google Scholar]
- Botner A., Strandbygaard B., Sorensen K.J., Have P., Madsen K.G., Madsen E.S., Alexandersen S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 1997;141:497–499. doi: 10.1136/vr.141.19.497. [DOI] [PubMed] [Google Scholar]
- Botner A. Diagnosis of PRRS. Vet. Microbiol. 1997;55:295–301. doi: 10.1016/s0378-1135(96)01333-8. [DOI] [PubMed] [Google Scholar]
- Bukh J., Miller R.H., Purcell R.H. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin. Liver Dis. 1995;15:41–63. doi: 10.1055/s-2007-1007262. [DOI] [PubMed] [Google Scholar]
- Casal J.I., Rodriguez M.J., Sarraseca J., Garcia J., Plana-Duran J., Sanz A. Identification of a common antigenic site in the nucleocapsid protein of European and North American isolates of porcine reproductive and respiratory syndrome virus. Adv. Exp. Med. Biol. 1998;440:469–477. doi: 10.1007/978-1-4615-5331-1_60. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising coronaviridae and arteriviridae. Arch. Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- Chen Z., Rowland R.R., Anderson G.W., Palmer G.A., Plagemann P.G.W. Coexistence in lactate dehydrogenase-elevating virus pools of variants that differ in neuropathogenicity and ability to establish a persistent infection. J. Virol. 1997;71:2913–2920. doi: 10.1128/jvi.71.4.2913-2920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Li K., Rowland R.R., Plagemann P.G.W. Neuropathogenicity and susceptibility to immune response are interdependent properties of lactate dehydrogenase-elevating virus (LDV) and correlate with the number of N-linked polylactosaminoglycan chains on the ectodomain of the primary envelope glycoprotein. Adv. Exp. Med. Biol. 1998;440:583–592. doi: 10.1007/978-1-4615-5331-1_76. [DOI] [PubMed] [Google Scholar]
- Chen Z., Li K., Rowland R.R., Anderson G.W., Plagemann P.G.W. Lactate dehydrogenase elevating virus variants: cosegregation of neuropathogenicity and impaired capability for high viremic persistent infection. J. Neurovirol. 1998;4:560–568. doi: 10.3109/13550289809113501. [DOI] [PubMed] [Google Scholar]
- Chen Z., Li K., Rowland R.R., Plagemann P.G.W. Selective antibody neutralization prevents neuropathogenic LDV from causing paralytic disease in immunocompetent mice. J. Neurovirol. 1999;5:200–208. doi: 10.3109/13550289909022003. [DOI] [PubMed] [Google Scholar]
- Chueh L.L., Lee K.H., Wang F.I., Pang V.F., Weng C.N. Sequence analysis of the nucleocapsid protein gene of the porcine reproductive and respiratory syndrome virus Taiwan MD-001 strain. Adv. Exp. Med. Biol. 1998;440:795–799. doi: 10.1007/978-1-4615-5331-1_103. [DOI] [PubMed] [Google Scholar]
- Christopher-Hennings J., Nelson E.A., Hines R.J., Nelson J.K., Swenson S.L., Zimmerman J.J., Chase C.L., Yaeger M.J., Benfield D.A. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J. Vet. Diagn. Invest. 1995;7:456–464. doi: 10.1177/104063879500700406. [DOI] [PubMed] [Google Scholar]
- Christopher-Hennings J., Nelson E.A., Nelson J.K., Hines R.J., Swenson S.L., Hill H.T., Zimmerman J.J., Katz J.B., Yaeger M.J., Chase C.C. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J. Clin. Microbiol. 1995;33:1730–1734. doi: 10.1128/jcm.33.7.1730-1734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher-Hennings J., Nelson E.A., Nelson J.K., Benfield D.A., Hennings J.C. Effects of a modified-live virus vaccine against porcine reproductive and respiratory syndrome in boars. Am. J. Vet. Res. 1997;58:40–45. [PubMed] [Google Scholar]
- Collins J.E., Benfield D.A., Christianson W.T., Harris L., Hennings J.C., Shaw D.P., Goyal S.M., McCullough S., Morrison R.B., Joo H.S. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J. Vet. Diagn. Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- Conzelmann K.K., Visser N., Van Woensel P., Thiel H.J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993;193:329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee S.A., Joo H. Strategies to control PRRS: a summary of field and research experiences. Vet. Microbiol. 1997;55:347–353. doi: 10.1016/S0378-1135(96)01319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries A.A., Chirnside E.D., Horzinek M.C., Rottier P.J. Structural proteins of equine arteritis virus. J. Virol. 1992;66:6294–6303. doi: 10.1128/jvi.66.11.6294-6303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boon J.A., Snijder E.J., Chirnside E.D., de Vries A.A., Horzinek M.C., Spaan W.J. Equine arteritis virus is not a togavirus but belongs to the coronaviruslike superfamily. J. Virol. 1991;65:2910–2920. doi: 10.1128/jvi.65.6.2910-2920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo, E., Holland, J.J., 1992. Complications of RNA heterogeneity for the engineering of virus vaccines and antiviral agents. In: Setlow, J.K. (Ed.), Genetic Engineering, Principles and Methods, Vol. 14, Plenum, New York, pp. 18–82. [DOI] [PubMed]
- Domingo E., Baranowski E., Ruiz-Jarabo C.M., Martin-Hernandez A.M., Saiz J.C., Escarmis C. Quasispecies structure and persistence of RNA virus. Emerging Infect. Dis. 1998;4:521–527. doi: 10.3201/eid0404.980402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T.W., Meulenberg J.J., Sands J.J., Paton D.J. Production, characterization and reactivity of monoclonal antibodies to porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1995;76:1361–1369. doi: 10.1099/0022-1317-76-6-1361. [DOI] [PubMed] [Google Scholar]
- Drew T.W., Lowings J.P., Yapp F. Variation in open reading frames 3, 4 and 7 among porcine reproductive and respiratory syndrome virus isolates in the UK. Vet. Microbiol. 1997;55:209–221. doi: 10.1016/s0378-1135(96)01328-4. [DOI] [PubMed] [Google Scholar]
- Duarte E.A., Novella I.S., Weaver S.C., Domingo E., Wain-Hobson S., Clarke D.K., Moya A., Elena S.F., de la Torre J.C., Holland J.J. RNA virus quasispecies: significance for viral disease and epidemiology. Infect. Agents Dis. 1994;3:201–214. [PubMed] [Google Scholar]
- Faaberg K.S., Plagemann P.G. ORF 3 of lactate dehydrogenase-elevating virus encodes a soluble, nonstructural, highly glycosylated, and antigenic protein. Virology. 1997;227:245–251. doi: 10.1006/viro.1996.8310. [DOI] [PubMed] [Google Scholar]
- Faaberg K.S., Elam M.R., Nelsen C.J., Murtaugh M.P. Subgenomic RNA7 is transcribed with different leader-body junction sites in PRRSV (strain VR2332) infection of CL2621 cells. Adv. Exp. Med. Biol. 1998;440:275–279. doi: 10.1007/978-1-4615-5331-1_36. [DOI] [PubMed] [Google Scholar]
- Frey M., Eernisse K., Landgraf J., Pearson J., Chladek D. Diagnostic testing for SIRS virus at the National Veterinary Service Laboratory (NVSL) Am. Assoc. Swine Pract. Newsl. 1992;4:31. [Google Scholar]
- Gagnon C.A., Dea S. Differentiation between porcine reproductive and respiratory syndrome virus isolates by restriction fragment length polymorphism of their ORFs 6 and 7 genes. Can. J. Vet. Res. 1998;62:110–116. [PMC free article] [PubMed] [Google Scholar]
- Godeny E.K., Chen L., Kumar S.N., Methven S.L., Koonin E.V., Brinton M.A. Complete genomic sequence and phylogenetic analysis of the lactate dehydrogenase-elevating virus (LDV) Virology. 1993;194:585–596. doi: 10.1006/viro.1993.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbur P.G., Andrews J.J., Huffman E.L., Paul P.S., Meng X.J., Niyo Y. Development of a streptavidin-biotin immunoperoxidase procedure for the detection of porcine reproductive and respiratory syndrome virus antigen in porcine lung. J. Vet. Diagn. Invest. 1994;6:254–257. doi: 10.1177/104063879400600219. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Miller L.D., Paul P.S., Meng X.J., Huffman E.L., Andrews J.J. Immunohistochemical identification of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the heart and lymphoid system of three-week-old colostrum-deprived pigs. Vet. Pathol. 1995;32:200–204. doi: 10.1177/030098589503200218. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Paul P.S., Frey M.L., Landgraf J., Eernisse K., Meng X.J., Lum M.A., Andrews J.J., Rathje J.A. Comparison of the pathogenicity of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Paul P.S., Frey M.L., Landgraf J., Eernisse K., Meng X.J., Andrews J.J., Lum M.A., Rathje J.A. Comparison of the antigen distribution of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1996;33:159–170. doi: 10.1177/030098589603300205. [DOI] [PubMed] [Google Scholar]
- Halbur P.G., Paul P.S., Meng X.J., Lum M.A., Andrews J.J., Rathje J.A. Comparative pathogenicity of nine U.S. porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a five-week-old cesarean-derived, colostrum-deprived pig model. J. Vet. Diagn. Invest. 1996;8:11–20. doi: 10.1177/104063879600800103. [DOI] [PubMed] [Google Scholar]
- Haynes J.S., Halbur P.G., Sirinarumitr T., Paul P.S., Meng X.J., Huffman E.L. Temporal and morphologic characterization of the distribution of porcine reproductive and respiratory syndrome virus (PRRSV) by in situ hybridization in pigs infected with isolates of PRRSV that differ in virulence. Vet. Pathol. 1997;34:39–43. doi: 10.1177/030098589703400106. [DOI] [PubMed] [Google Scholar]
- Hesse, R.A., Couture, L.P., Lau, M.L., Wasmoen, T.L., 1997. Efficacy of Prime Pac PRRS in controlling PRRS respiratory disease: homologous and heterologous challenge. In: 28th Annual Meeting of Am. Assoc. Swine Pract. Quebec City, Que, pp. 137–141.
- Hill, H., 1990. Overview and history of mystery swine disease (swine infertility and respiratory syndrome). In Mystery Swine Disease Communication Meeting. Denver, CO, pp. 29–31.
- Kapur V., Elam M.R., Pawlovich T.M., Murtaugh M.P. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the Midwestern United States. J. Gen. Virol. 1996;77:1271–1276. doi: 10.1099/0022-1317-77-6-1271. [DOI] [PubMed] [Google Scholar]
- Katz J.B., Shafer A.L., Eernisse K.A., Landgraf J.G., Nelson E.A. Antigenic differences between European and American isolates of porcine reproductive and respiratory syndrome virus (PRRSV) are encoded by the carboxyterminal portion of viral open reading frame 3. Vet. Microbiol. 1995;44:65–76. doi: 10.1016/0378-1135(94)00113-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffaber K.K. Reproductive failure of unknown etiology. Am. Assoc. Swine Pract. Newsl. 1989;1:1–9. [Google Scholar]
- Kim H.S., Kwang J., Yoon I.J., Joo H.S., Frey M.L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- Kwang J., Kim H.S., Joo H.S. Cloning, expression and sequence analysis of the ORF4 gene of porcine reproductive and respiratory syndrome virus MN-1b. J. Vet. Diagn. Invest. 1994;6:293–296. doi: 10.1177/104063879400600302. [DOI] [PubMed] [Google Scholar]
- Lager, K.M., Mengeling, W.L., 1997. Current status of vaccines and vaccination for porcine reproductive and respiratory syndrome. In: 28th Annual Meeting of Am. Assoc. of Swine Pract. Quebec City, Que, pp. 443–446.
- Lager, K.M., Mengeling, W.L., Wesley, R.D., Halbur, P.G., Sorden, S.D., 1998. Acute PRRS. In: 29th Annual Meeting of American Association of Swine Practitioners. Des Moines, IA, pp. 449–453.
- Lager K.M., Mengeling W.L., Brockmeier S.L. Evaluation of protective immunity in gilts inoculated with the NADC-8 isolate of porcine reproductive and respiratory syndrome virus (PRRSV) and challenge-exposed with an antigenically distinct PRRSV isolate. Am. J. Vet. Res. 1999;60:1022–1027. [PubMed] [Google Scholar]
- Lai M.M.C., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall A.L., Albina E., Magar R., Gauthier J.P. Antigenic variability of porcine reproductive and respiratory syndrome (PRRS) virus isolates: influence of virus passage in pig. Virus Res. 1997;28:247–257. [PubMed] [Google Scholar]
- Le Gall A., Legeay O., Bourhy H., Arnauld C., Albina E., Jestin A. Molecular variation in the nucleoprotein gene (ORF7) of the porcine reproductive and respiratory syndrome virus (PRRSV) Virus Res. 1998;54:9–21. doi: 10.1016/s0168-1702(97)00146-9. [DOI] [PubMed] [Google Scholar]
- Li K., Chen Z., Plagemann P.G.W. High-frequency homologous genetic recombination of an arterivirus, lactate dehydrogenase-elevating virus, in mice and evolution of neuropathogenic variants. Virology. 1999;258:73–83. doi: 10.1006/viro.1999.9660. [DOI] [PubMed] [Google Scholar]
- Madsen K.G., Hansen C.M., Madsen E.S., Strandbygaard B., Botner A., Sorensen K.J. Sequence analysis of porcine reproductive and respiratory syndrome virus of the American type collected from Danish swine herds. Arch. Virol. 1998;143:1683–1700. doi: 10.1007/s007050050409. [DOI] [PubMed] [Google Scholar]
- Magar R., Larochelle R., Nelson E.A., Charreyre C. Differential reactivity of a monoclonal antibody directed to the membrane protein of porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 1997;61:69–71. [PMC free article] [PubMed] [Google Scholar]
- Mardassi H., Mounir S., Dea S. Identification of major differences in the nucleocapsid protein genes of a Quebec strain and European strains of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1994;75:681–685. doi: 10.1099/0022-1317-75-3-681. [DOI] [PubMed] [Google Scholar]
- Mardassi H., Massie B., Dea S. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology. 1996;221:98–112. doi: 10.1006/viro.1996.0356. [DOI] [PubMed] [Google Scholar]
- Mardassi H., Gonin P., Gagnon C.A., Massie B., Dea S. A subset of porcine reproductive and respiratory syndrome virus GP3 glycoprotein is released into the culture medium of cells as a non-virion-associated and membrane-free (soluble) form. J. Virol. 1998;72:6298–6306. doi: 10.1128/jvi.72.8.6298-6306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavromatis I., Kritas S.K., Alexopoulos C., Tsinas A., Kyriakis S.C. Field evaluation of a live vaccine against porcine reproductive and respiratory syndrome in fattening pigs. Zentralbl Veterinarmed [B] 1999;46:603–612. doi: 10.1046/j.1439-0450.1999.00282.x. [DOI] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G. Molecular cloning and nucleotide sequencing of the 3′ terminal genomic RNA of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1994;75:1795–1801. doi: 10.1099/0022-1317-75-7-1795. [DOI] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G., Lum M.A. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the USA and Europe. Arch. Virol. 1995;140:745–755. doi: 10.1007/BF01309962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G., Morozov I. Sequence comparison of open reading frames 2 to 5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1995;76:3181–3188. doi: 10.1099/0022-1317-76-12-3181. [DOI] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Halbur P.G., Lum M.A. Characterization of a high-virulence U.S. isolate of porcine reproductive and respiratory syndrome virus in a continuous cell line, ATCC CRL11171. J. Vet. Diagn. Invest. 1996;8:374–381. doi: 10.1177/104063879600800317. [DOI] [PubMed] [Google Scholar]
- Meng X.J., Paul P.S., Morozov I., Halbur P.G. A nested set of six or seven subgenomic mRNAs is formed in cells infected with different isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1996;77:1265–1270. doi: 10.1099/0022-1317-77-6-1265. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L., Vorwald A.C., Lager K.M., Brockmeier S.L. Comparison among strains of porcine reproductive and respiratory syndrome virus for their ability to cause reproductive failure. Am. J. Vet. Res. 1996;57:834–839. [PubMed] [Google Scholar]
- Mengeling W.L., Lager K.M., Vorwald A.C. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of atypical PRRS. Am. J. Vet. Res. 1998;59:1540–1544. [PubMed] [Google Scholar]
- Mengeling W.L., Lager K.M., Vorwald A.C. Safety and efficacy of vaccination of pregnant gilts against porcine reproductive and respiratory syndrome. Am. J. Vet. Res. 1999;60:796–801. [PubMed] [Google Scholar]
- Mengeling W.L., Vorwald A.C., Lager K.M., Clouser D.F., Wesley R.D. Identification and clinical assessment of suspected vaccine-related field strains of porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 1999;60:334–340. [PubMed] [Google Scholar]
- Mengeling W.L., Lager K.M., Wesley R.D., Clouser D.F., Vorwald A.C., Roof M.B. Diagnostic implications of concurrent inoculation with attenuated and virulent strains of porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 1999;60:119–122. [PubMed] [Google Scholar]
- Meulenberg J.J., Hulst M.M., De Meijer E.J., Moonen P.L., Den Besten A., De Kluyver E.P., Wensvoort G., Moormann R.J. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J., de Meijer E.J., Moormann R.J. Subgenomic RNAs of Lelystad virus contain a conserved leader–body junction sequence. J. Gen. Virol. 1993;74:1697–1701. doi: 10.1099/0022-1317-74-8-1697. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J., Petersen-Den Besten A., De Kluyver E.P., Moormann R.J., Schaaper W.M.M., Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995;206:155–163. doi: 10.1016/S0042-6822(95)80030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J., Petersen-den Besten A. Identification and characterization of a sixth structural protein of Lelystad virus: the glycoprotein GP2 encoded by ORF2 is incorporated in virus particles. Virology. 1996;225:44–51. doi: 10.1006/viro.1996.0573. [DOI] [PubMed] [Google Scholar]
- Meulenberg J.J., Petersen-den Besten A., de Kluyver E., van Nieuwstadt A., Wensvoort G., Moormann R.J. Molecular characterization of Lelystad virus. Vet. Microbiol. 1997;55:197–202. doi: 10.1016/S0378-1135(96)01335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J., van Nieuwstadt A.P., van Essen-Zandbergen A., Langeveld J.P. Posttranslational processing and identification of a neutralization domain of the GP4 protein encoded by ORF4 of Lelystad virus. J. Virol. 1997;71:6061–6067. doi: 10.1128/jvi.71.8.6061-6067.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J.M., Bos-De Ruijter J.N., Van De Graaf R., Wensvoort G., Moormann R.J.M. Infectious transcripts from cloned genome-length cDNA of porcine reproductive and respiratory syndrome virus. J. Virol. 1998;72:380–387. doi: 10.1128/jvi.72.1.380-387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P.D., John A., Ferguson M., Icenogle J.P. Antigenic and molecular evolution of the vaccine strain of type 3 poliovirus during the period of excretion by a primary vaccine. J. Gen. Virol. 1986;67:693–706. doi: 10.1099/0022-1317-67-4-693. [DOI] [PubMed] [Google Scholar]
- Molitor T.W., Bautista E.M., Choi C.S. Immunity to PRRSV: double-edged sword. Vet. Microbiol. 1997;55:265–276. doi: 10.1016/S0378-1135(96)01327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov I., Meng X.J., Paul P.S. Sequence analysis of open reading frames (ORFs) 2 to 4 of a U.S. isolate of porcine reproductive and respiratory syndrome virus. Arch. Virol. 1995;140:1313–1319. doi: 10.1007/BF01322758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov, I., Paul, P.S., Meng, X.J., 1996. Characterization of leader-body junction sites in subgenomic mRNAs of a U.S. PRRSV isolate. 15th Annual Meeting of the AM. Soc. For Virol. London, Ontario, Canada, pp. 150.
- Murtaugh M.P., Elam M.R., Kakach L.T. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch. Virol. 1995;140:1451–1460. doi: 10.1007/BF01322671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.A., Christopher-Hennings J., Drew T., Wensvoort G., Collins J.E., Benfield D.A. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J. Clin. Microbiol. 1993;31:3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T.L., Nielsen J., Have P., Baekbo P., Hoff Jorgensen R., Botner A. Examination of virus shedding in semen from vaccinated and from previously infected boars after experimental challenge with porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 1997;54:101–112. doi: 10.1016/s0378-1135(96)01272-2. [DOI] [PubMed] [Google Scholar]
- Nelsen C.J., Murtaugh M.P., Faaberg K.S. Porcine reproductive and respiratory syndrome virus comparison: Divergent evolution on two continents. J. Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlinger V., Weiland F., Weiland E., Mettenleiter T., Haas B., Visser N., Ahl R. Some aspects of the virus causing PRRS in Germany. Am. Assoc. Swine Pract. Newsl. 1992;4:16. [Google Scholar]
- Oleksiewicz M.B., Botber A., Nelsen J., Storgaard T. Determination of 5′-leader sequences from radically disparate strains of porcine reproductive and respiratory syndrome virus reveals the presence of highly conserved sequence motifs. Arch. Virol. 1999;144:981–987. doi: 10.1007/s007050050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio, F.A., Zuckermann, F., Wills, R., Meier, W., Christian, S., Galeota, J., Doster, A., 1998. PRRSV: comparison of commercial vaccines in their ability to induce protection against current PRRSV strains of high virulence. 1998 Allen D. Leman Swine Conference. Vol. 25, pp. 176–182.
- Pirzadeh B., Dea S. Monoclonal antibodies to the ORF5 product of porcine reproductive and respiratory syndrome virus define linear neutralizing determinants. J. Gen. Virol. 1997;78:1867–1873. doi: 10.1099/0022-1317-78-8-1867. [DOI] [PubMed] [Google Scholar]
- Pirzadeh B., Gagnon C.A., Dea S. Genetic and antigenic variations of porcine reproductive and respiratory syndrome virus major envelope GP5 glycoprotein. Can. J. Vet. Res. 1998;62:170–177. [PMC free article] [PubMed] [Google Scholar]
- Plagemann P.G.W., Moennig V. Lactate dehydrogenase-elevating virus, equine arteritis virus and simian hemorrhagic fever virus, a new group of positive strand RNA virus. Adv. Virus Res. 1992;41:99–192. doi: 10.1016/S0065-3527(08)60036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P.G.W., Rowland R.R., Even C., Faaberg K.S. Lactate dehydrogenase-elevating virus: an ideal persistent virus? Springer Semin. Immunopathol. 1995;17:167–186. doi: 10.1007/BF00196164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann, P.G.W., 1996. Lactate dehydrogenase-elevating virus and related viruses. In: Fields, B.N., Knipe, D.M., Howley, P.M., et al. (Ed.), Fields Virology. Lippincott-Raven, Philadelphia, PA, pp. 1105–1120.
- Plana J., Vayreda M., Vilarrasa J., Bastons M., Rosell R., Martinez M., San Gabriel A., Pujols J., Badiola J.L., Ramos J.A., Damingo M. Porcine epidemic abortion and respiratory syndrome (mystery swine disease). Isolation in Spain of the causative agent and experimental reproduction of the disease. Vet. Microbiol. 1992;33:203–211. doi: 10.1016/0378-1135(92)90048-x. [DOI] [PubMed] [Google Scholar]
- Plana-Duran J., Bastons M., Urniza A., Vayreda M., Vila X., Mane H. Efficacy of an inactivated vaccine for prevention of reproductive failure induced by porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 1997;55:361–370. doi: 10.1016/s0378-1135(96)01317-x. [DOI] [PubMed] [Google Scholar]
- Polson D.D., Marsh W.E., Dial G.D. Financial evaluation and decision making in the swine breeding herd. Vet. Clin. North Am. Food Anim. Pract. 1992;8:725–747. doi: 10.1016/s0749-0720(15)30713-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.J., Sarraseca J., Garcia J., Sanz A., Plana-Duran J., Casal J.I. Epitope mapping of the nucleocapsid protein of European and North American isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 1997;78:2269–2278. doi: 10.1099/0022-1317-78-9-2269. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Collins J.E., Goyal S.M., Nelson E.A., Christopher-Hennings J., Benfield D.A. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in gnotobiotic pigs. Vet. Pathol. 1995;32:361–373. doi: 10.1177/030098589503200404. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Benfield D.A., Goyal S.M., Nelson E.A., Christopher-Hennings J., Collins J.E. Chronological immunohistochemical detection and localization of porcine reproductive and respiratory syndrome virus in gnotobiotic pigs. Vet. Pathol. 1996;33:551–556. doi: 10.1177/030098589603300510. [DOI] [PubMed] [Google Scholar]
- Rossow K.D. Porcine reproductive and respiratory syndrome. Vet. Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Rossow K.D., Shivers J.L., Yeske P.E., Polson D.D., Rowland R.R., Lawson S.R., Murtaugh M.P., Nelson E.A., Collins J.E. Porcine reproductive and respiratory syndrome virus infection in neonatal pigs characterized by marked neurovirulence. Vet. Rec. 1999;144:444–448. doi: 10.1136/vr.144.16.444. [DOI] [PubMed] [Google Scholar]
- Rowland R.R.R., Steffen M., Ackerman T., Benfield D.A. The evolution of porcine reproductive and respiratory syndrome virus: quasispecies and emergence of a virus subpopulation during infection of pigs with VR-2332. Virology. 1999;259:262–266. doi: 10.1006/viro.1999.9789. [DOI] [PubMed] [Google Scholar]
- Saito A., Kanno T., Murakami Y., Muramatsu M., Yamaguchi S. Characteristics of major structural protein coding gene and leader-body sequence in subgenomic mRNA of porcine reproductive and respiratory syndrome virus isolated in Japan. J. Vet. Med. Sci. 1996;58:377–380. doi: 10.1292/jvms.58.377. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., Meulenberg J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- Suarez P., Zardoya R., Martin M.J., Prieto C., Dopazo J., Solana A., Castro J.M. Phylogenetic relationships of European strains of porcine reproductive and respiratory syndrome virus (PRRSV) inferred from DNA sequences of putative ORF-5 and ORF-7 genes. Virus Res. 1996;42:159–165. doi: 10.1016/0168-1702(95)01305-9. [DOI] [PubMed] [Google Scholar]
- Sur J.H., Doster A.R., Christian J.S., Galeota J.A., Wills R.W., Zimmerman J.J., Osorio F.A. Porcine reproductive and respiratory syndrome virus replicates in testicular germ cells, alters spermatogenesis, and induces germ cell death by apoptosis. J. Virol. 1997;71:9170–9179. doi: 10.1128/jvi.71.12.9170-9179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valicek L., Pasikal I., Smid B., Rodak L., Kubalikova R., Kosinova E. Isolation and identification of porcine reproductive and respiratory syndrome virus in cell cultures. Vet. Med. (Praha.) 1997;42:281–287. [PubMed] [Google Scholar]
- Van Alstine W. Isolation of SIRS virus from nursery pigs of two herds without current reproductive failure. Proc. Annu. Meet. Livest. Conserv. Inst. 1992;1:253–259. [Google Scholar]
- Van Nieuwstadt A.P., Meulenberg J.J.M., Essen-Zandbergen A., Besten A.P., Bende R.J., Moormann R.J.M., Wensvoort G. Proteins encoded by open reading frames 3 and 4 of the genome of Lelystad virus (Arteriviridae) are structural proteins of the virion. J. Virol. 1996;70:4767–4772. doi: 10.1128/jvi.70.7.4767-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K. Pathogenesis and clinical aspects of a respiratory porcine reproductive and respiratory syndrome virus infection. Vet. Microbiol. 1997;55:223–230. doi: 10.1016/S0378-1135(96)01331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Woensel P.A., Liefkens K., Demaret S. European serotype PRRSV vaccine protects against European serotype challenge whereas an American serotype vaccine does not. Adv. Exp. Med. Biol. 1998;440:713–718. doi: 10.1007/978-1-4615-5331-1_92. [DOI] [PubMed] [Google Scholar]
- Wang X.C., Smith S.L., Godeny E.K. Organization of the simian hemorrhagic fever virus genome and identification of the sgRNA junction sequences. Adv. Exp. Med. Biol. 1998;440:281–287. doi: 10.1007/978-1-4615-5331-1_37. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M., Ter Laak E.A., Bloemraad M., De Kluyver E.P., Kragten C., Van Buiten L., Den Besten A., Wagenaar F. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., De Kluyver E.P., Luijtze E.A., Den Besten A., Harris L., Collins J.E., Christianson W.T., Chladek D. Antigenic comparison of Lelystad virus and swine infertility and respiratory syndrome virus. J. Vet. Diagn. Invest. 1992;4:134–138. doi: 10.1177/104063879200400203. [DOI] [PubMed] [Google Scholar]
- Wensvoort G. Lelystad virus and the porcine epidemic abortion and respiratory syndrome. Vet. Res. 1993;24:117–124. [PubMed] [Google Scholar]
- Wesley R.D., Mengeling W.L., Lager K.M., Clouser D.F., Landgraf J.G., Frey M.L. Differentiation of a porcine reproductive and respiratory syndrome virus vaccine strain from North American field strains by restriction fragment length polymorphism analysis of ORF5. J. Vet. Diagn. Invest. 1998;10:140–144. doi: 10.1177/104063879801000204. [DOI] [PubMed] [Google Scholar]
- Wesley R.D., Mengeling W.L., Lager K.M., Vorwald A.C., Roof M.B. Evidence for divergence of restriction fragment length polymorphism patterns following in vivo replication of porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 1999;60:463–467. [PubMed] [Google Scholar]
- Wieczorek-Krohmer M., Weiland F., Conzelmann K., Kohl D., Visser N., Van Woensel P., Thiel H.J., Weiland E. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and U.S. isolates. Vet. Microbiol. 1996;51:257–266. doi: 10.1016/0378-1135(96)00047-8. [DOI] [PubMed] [Google Scholar]
- Wills R.W., Zimmerman J.J., Swenson S.L., Yoon K.J., Hill H.T., Bundy D.S., McGinley M.J. Transmission of PRRSV by direct, close or indirect contact. Swine Health Prod. 1997;5:213–218. [Google Scholar]
- Wills R.W., Zimmerman J.J., Yoon K.J., Swenson S.L., McGinley M.J., Hill H.T., Platt K.B., Christopher-Hennings J., Nelson E.A. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet. Microbiol. 1997;55:231–240. doi: 10.1016/s0378-1135(96)01337-5. [DOI] [PubMed] [Google Scholar]
- Wootton S.K., Nelson E.A., Yoo D. Antigenic structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus. Clin. Diagn. Lab. Immunol. 1998;5:773–779. doi: 10.1128/cdli.5.6.773-779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.X., Kwang J., Laegreid W. Comparative sequence analysis of open reading frames 2 to 7 of the modified live vaccine virus and other North American isolates of the porcine reproductive and respiratory syndrome virus. Arch. Virol. 1998;143:601–612. doi: 10.1007/s007050050316. [DOI] [PubMed] [Google Scholar]
- Yoon K.J., Wu L.L., Zimmerman J.J., Hill H.T., Platt K.B. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral. Immunol. 1996;9:51–63. doi: 10.1089/vim.1996.9.51. [DOI] [PubMed] [Google Scholar]
- Yoon K.J., Wu L.L., Zimmerman J.J., Platt K.B. Field isolates of porcine reproductive and respiratory syndrome virus (PRRSV) vary in their susceptibility to antibody-dependent enhancement (ADE) of infection. Vet. Microbiol. 1997;55:277–287. doi: 10.1016/s0378-1135(96)01338-7. [DOI] [PubMed] [Google Scholar]
- Yuan S., Nelsen C.J., Murtaugh M.P., Schmitt B.J., Faaberg K.S. Recombination between North American strains of porcine reproductive and respiratory syndrome virus. Virus Res. 1999;61:87–98. doi: 10.1016/S0168-1702(99)00029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J.J., Yoon K.J., Wills R.W., Swenson S.L. General overview of PRRSV: a perspective from the United States. Vet. Microbiol. 1997;55:187–196. doi: 10.1016/s0378-1135(96)01330-2. [DOI] [PubMed] [Google Scholar]


