Highlights
-
•
A comprehensive high-throughput viral sequencing strategy (ViroCap) was adapted for use with feline tumors.
-
•
Papillomavirus was not commonly associated with feline oral squamous cell carcinoma.
-
•
Feline oral squamous cell carcinoma is a good model for HPV-negative head and neck squamous cell carcinoma in people.
-
•
The virome of FOSCC and normal feline oral mucosa included feline foamy virus, torque teno virus, herpes and papillomavirus, FIV and EBV.
-
•
Co-occurrence of Epstein Barr Virus and feline papillomavirus-3 was found found in a feline oral squamous cell carcinoma sample.
Keywords: Virome, Sequencing, Feline oral squamous cell carcinoma, Epstein Barr virus, Metagenomic
Abstract
Feline oral squamous cell carcinoma (FOSCC) may be the best naturally-occurring model of human head and neck squamous cell carcinoma (HNSCC). HNSCC can be broadly divided into human papillomavirus (HPV)-negative cancers and HPV-positive cancers where HPV is the causative agent. Previous studies in FOSCC have used both species-specific and species-nonspecific PCR primers that may be insensitive to the detection of PVs and other viruses that may be divergent from known sequences. ViroCap is a targeted capture and next generation sequencing tool that was designed to identify all known vertebrate DNA and RNA viruses. In this study we used a metagenomic approach using ViroCap for DNA viruses in 20 FOSCC, 9 normal feline oral mucosal, and 8 suspected PV positive control samples. We tested the hypothesis that viruses would be enriched in FOSCC compared to normal oral mucosa. The virome of the FOSCC and normal feline oral mucosa consisted of feline foamy virus in 7/20 and 2/9 (35% and 22%), feline torque teno virus in 2/20 and 0/9 (10% and 0%), alphaherpesvirus in 2/10 and 0/9 (10% and 0%), FIV (0% and 22%), Epstein-Barr virus in 1/20 and 0/9 (5% and 0%) and feline papillomavirus in 1/20 and 0/9 samples (5% and 0% respectively). Felis catus papillomavirus-3 was found in 1 of 20 FOSCC samples. A virus was not associated consistently with FOSCC. If PVs have a role in FOSCC it is at most a supplementary or uncommon role. FOSCC appears most closely related to HPV-negative HNSCC. Future research on FOSCC should focus on identifying genetic and environmental causes.
1. Introduction
Feline oral squamous cell carcinoma (FOSCC) is the 4th most common feline neoplasm and there is no effective treatment for this disease. FOSCC has commonly been proposed as the best model for human head and neck squamous cell carcinomas (HNSCC). HNSCC can be divided into human papillomavirus (HPV) positive and HPV-negative cancers. The largest papillomavirus (PV) genera is the alpha-PVs, which includes subtypes HPV-16 and 18 that have the highest malignant transformation potential. PVs are non-enveloped circular double-stranded DNA (dsDNA) viruses with 5 or 6 early (E1, E2, E4, +/-E5, E6, E7) genes and two late genes (L1 and L2). All PVs contain a highly conserved L1 gene and thus family-specific consensus PCR primers can be used to amplify a region in this gene. There are currently only 5 Felis catus PV (FcaPV) species known to infect domestic cats. FcaPV DNA, especially FcaPV-2, and induced changes in cell regulation (increased p16 identified by IHC staining) have been detected in the majority of BISCs, Supplementary Table 1 (Lange et al., 2009; Munday, 2014; Munday et al., 2007). PV DNA and increased p16 have also been detected in 75% of UV-protected cutaneous SCC, and thus PV is likely a causative agent in feline BISCs and UV-protected cutaneous SCC, Supplementary Table 1(Munday, 2014; Munday et al., 2011a). Previous studies have used either PV consensus PCR primers or PV type specific PCR primers and have not found strong evidence to support a viral etiology, Supplementary Table 2. In summary, to date PV has only been found in 6 of 177 FOSCC samples in peer-reviewed journals and the association between PV and FOSCC thus remains weak. Contrary to these previous studies, an abstract presented at the 2015 Veterinary Cancer Society (VCS) conference detected PV with consensus PCR primers in all of the 12 FOSCC samples that were evaluated (Skor, 2015).
Next generation sequencing (NGS) can substantially increase the sensitivity and specificity of virus detection as it is not limited by primer specificity. Amplicon-based survey sequencing approaches, such as 16S rRNA gene sequencing, have been utilized to study bacterial diversity, but a similar method cannot be used for viruses due to the lack of universally conserved genes. ViroCap, a hybridization-based capture and NGS approach, has the ability to enrich nucleic acids from all currently known DNA and RNA viruses from vertebrate hosts (excluding endogenous retroviruses) for which probes are included (Wylie et al., 2015). Compared to PCR, ViroCap can detect viruses that are divergent from reference genome sequences (e.g. anellovirus family), and it has the ability to generate complete or nearly complete genome sequences because probes are tiled across the full length of the genomes. ViroCap has been utilized on human vaginal swabs (Wylie et al., 2018b), whole blood, plasma, cerebrospinal fluid, nasopharyngeal swabs, tracheal aspirates, skin swabs, and stool (Wylie et al., 2018a, 2015) and in a Coronavirus outbreak in Canada Geese (Papineau et al., 2019). To the author’s knowledge, the current study would be the first companion animal study to utilize a targeted capture and NGS strategy to study the virome. ViroCap is the most definitive method used to date to characterize the virome of the oral mucosa of cats and to find a viral cause of FOSCC.
2. Materials and methods
2.1. Patients
Formalin-fixed, paraffin embedded (FFPE) samples from 20 cats diagnosed with FOSCC in 2012–2013 were obtained from the University of Missouri Veterinary Medical Diagnostic Laboratory (MU VMDL), Table 1 . Banked FFPE samples from 8 presumed PV-positive control tumors were also obtained from the MU VMDL. Presumed PV-negative controls consisted of 9 fresh frozen (FF) tumor negative oral mucosal biopsy samples (combined tongue and gingival mucosa) from adult cats obtained from the University of Missouri Veterinary Health Center and Central Missouri Humane Society. None of the samples in this study have been used in any previous study.
Table 1.
Patient characteristics and read information for each sample that was sequenced by ViroCap.
| Patient | Species | Sexa | Age (yrs) | Lesion | Tissue Typeb | Location | Comments | Raw read count |
|---|---|---|---|---|---|---|---|---|
| OSCC1 | Feline | MC | 15 | FOSCC | FFPE | Gingiva | N/A | 5,739,653 |
| OSCC2 | Feline | MC | 17 | FOSCC | FFPE | Gingiva | N/A | 7,513,118 |
| OSCC3 | Feline | MC | 14 | FOSCC | FFPE | Tongue | N/A | 7,096,542 |
| OSCC4 | Feline | MC | 11 | FOSCC | FFPE | Gingiva | N/A | 6,687,626 |
| OSCC5 | Feline | MC | 15 | FOSCC | FFPE | Gingiva | N/A | 4,291,620 |
| OSCC6 | Feline | FS | 17 | FOSCC | FFPE | Gingiva | N/A | 6,882,619 |
| OSCC7 | Feline | FS | 16 | FOSCC | FFPE | N/A | N/A | 5,772,965 |
| OSCC8 | Feline | MC | 14 | FOSCC | FFPE | Gingiva | N/A | 24,800,351 |
| OSCC9 | Feline | MC | 18 | FOSCC | FFPE | Gingiva | N/A | 5,155,381 |
| OSCC10 | Feline | MC | 16 | FOSCC | FFPE | Gingiva | N/A | 6,325,271 |
| OSCC11 | Feline | MC | 14 | FOSCC | FFPE | Tongue | N/A | 6,165,815 |
| OSCC12 | Feline | FS | 19 | FOSCC | FFPE | N/A | N/A | 7,099,389 |
| OSCC13 | Feline | FS | 16 | FOSCC | FFPE | Tongue | N/A | 6,733,059 |
| OSCC14 | Feline | FS | 15 | FOSCC | FFPE | N/A | N/A | 6,403,217 |
| OSCC15 | Feline | MC | 18 | FOSCC | FFPE | Gingiva | N/A | 7,474,897 |
| OSCC16 | Feline | FS | 12 | FOSCC | FFPE | Gingiva | N/A | 9,158,237 |
| OSCC17 | Feline | FS | 14 | FOSCC | FFPE | Gingiva | N/A | 6,639,860 |
| OSCC18 | Feline | FS | 15 | FOSCC | FFPE | Gingiva | N/A | 3,732,410 |
| OSCC19 | Feline | FS | 11 | FOSCC | FFPE | Tongue | N/A | 6,354,314 |
| OSCC20 | Feline | FI | 14 | FOSCC | FFPE | Tongue | N/A | 7,086,469 |
| N1 | Feline | FS | Adult | Normal | FF | Gingiva and tongue | Surrendered. Euthanized due to complications associated with chronic kidney disease. FeLV/FIV negative on SNAP test. | 27,638,607 |
| N2 | Feline | FS | Adult | Normal | FF | Gingiva and tongue | Healthy stray cat. FeLV+/FIV- on SNAP test. Confirmatory testing not done. Euthanized due to diagnosis of FeLV. | 27,740,803 |
| N3 | Feline | MI | Adult | Stomatitis | FF | Gingiva and tongue | Healthy stray cat. FIV+/FeLV- on SNAP test. Confirmatory testing not done. Euthanized due to diagnosis of FIV. | 21,060,857 |
| N4 | Feline | FS | 6 | Normal | FF | Gingiva and tongue | Euthanized due to leukemia. FeLV/FIV - on SNAP test | 19,796,205 |
| N5 | Feline | N/A | Adult | Normal | FF | Gingiva and tongue | Stray cat. Death due to trauma. FeLV/FIV status unknown. | 20,603,477 |
| N6 | Feline | FS | Adult | Normal | FF | Gingiva and tongue | Euthanized due to endocarditis. FeLV/FIV - on SNAP test | 49,712,932 |
| N7 | Feline | FS | Adult | Normal | FF | Gingiva and tongue | Euthanized due to suspected FIP. Presumptive diagnosis based on macroscopic necropsy. FeLV/FIV status unknown. | 21,198,071 |
| N8 | Feline | MC | 5 | Normal | FF | Gingiva and tongue | Euthanized due to pulmonary hypertension from angiomatosis. FeLV/FIV - on SNAP test | 32,636,457 |
| N9 | Feline | N/A | Adult | Normal | FF | Gingiva and tongue | Stray cat. Euthanized due to large ulcerative lesion on nasal planum. Suspect squamous cell carcinoma. FeLV/FIV status unknown. | 20,714,975 |
| PV1 | Feline | MC | 12 | Sarcoid, less likely sarcoma | FFPE | Pinna | N/A | 5,695,092 |
| PV2 | Canine | FI | 2 | Papilloma | FFPE | Skin | Identified as CPV-6 on ViroCap | 24,972,777 |
| PV3 | Canine | MI | 5 | Papilloma | FFPE | Lip | Identified as CPV-1 on ViroCap | 23,334,663 |
| PV4 | Canine | MC | N/A | Papilloma | FFPE | Tongue | Identified as CPV-1 on ViroCap | 21,799,472 |
| PV5 | Equine | MC | 8 | Sarcoid | FFPE | Skin | Identified as BPV-1 on ViroCap | 9,292,084 |
| PV6 | Feline | FS | 2 | Papilloma | FFPE | Oral | N/A | 4,631,420 |
| PV7 | Feline | FS | 18 | Papilloma | FFPE | Oral | N/A | 5,848,794 |
| PV8 | Feline | MC | 13 | Sarcoid | FFPE | Skin | N/A | 6,658,664 |
FS (female spayed), FI (female intact), MC (male castrated) or MI (male intact).
Formalin fixed paraffin embedded (FFPE) or fresh frozen (FF).
2.2. DNA extraction, library preparation and capture
Dual indexed libraries from the fresh frozen and FFPE samples were pooled separately and hybridized to the ViroCap probe set as 2 separate reactions. Details can be found in the Supplementary files.
2.3. Sequencing and analysis
Captured reads were sequenced on one lane of the HiSeq2500 1 T as 2 × 126 bp reads generating on average 1.3Gb of data per sample. The analysis workflow is similar to the one that was used in a study of the vaginal virome in preterm birth (Wylie et al., 2018b). Details can be found in the Supplementary files. Average genomic coverage (depth of coverage or DoC) was estimated from base representation by the extracted, deduplicated and aligned reads for each base of the genome. Breadth of coverage (BoC) was estimated in SAMtools and was defined as the percent of bases of the reference viral genome that was covered by sequence reads at a level of 5x or higher. The detection of false positives (e.g. by index swapping or contamination from sample handling) were minimized with the definition of strict coverage criteria. Positive samples were defined as any sample that had ≥0.5x DoC. Exceptions to this rule included samples that were manually reviewed for the presence of reads that were distributed across the viral genome to confirm presence of a virus
2.4. Papillomavirus and Epstein Barr virus validation
The FAP59/FAP64 and BamHI W primers were used for papillomavirus and EBV respectively (Chiou et al., 2005; Forslund et al., 1999). Details of the PCR conditions can be found in Supplementary Table 3. Each sample was amplified in duplicate and a no template control was included in each assay. The EBV amplicon was sequenced.
3. Results
Of the 20 FOSCC samples, 12 (60%) were from the gingiva, 5 (25%) were from the tongue and the location of 3 (15%) samples was unknown. Sequence data for the 37 samples were deposited into the Sequence Read Archive (SRA) under BioProject PRJNA553834.
3.1. The virome of the oral mucosa of cats consisted of foamy virus, torque teno virus, alphaherpesvirus, feline papillomavirus, FeLV, FIV and EBV but these viruses were not associated consistently with FOSCC
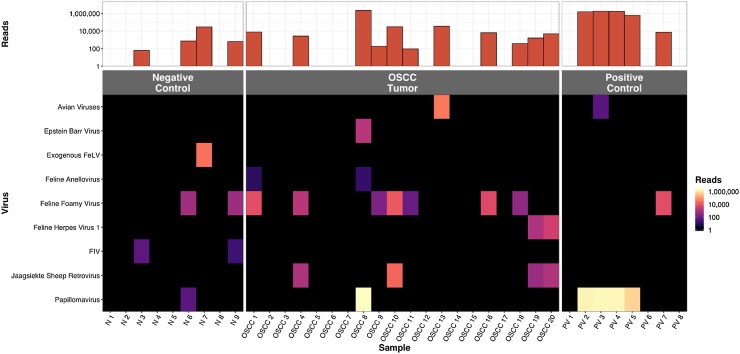
FcaPV was found in 1/20 and 0/9 (p = 1.00, Fisher’s exact test, 2-tailed), feline foamy virus in 7/20 and 2/9 (p = 0.67), feline torque teno virus in 2/20 and 0/9 (p = 1.00), alphaherpesvirus in 2/20 and 0/9 (p = 1.00), FIV in 0/20 and 2/9, exogenous FeLV (exFeLV) in 0/20 and 1/9, Epstein-Barr virus in 1/20 and 0/9 (p = 1.00) respectively in FOSCC and normal oral mucosa samples (Fig. 1 , Supplementary Fig. 1). Probability values were not evaluated for FIV and FeLV since these viruses can be higher in the group of cats in the normal mucosa group that consisted of largely unowned cats that were euthanized for various reasons.
Fig. 1.
Summary of the viruses identified by ViroCap from each patient in the FOSCC, positive and negative control groups. The top bar graph shows the number of viral reads in each sample. The heatmap shows the number of reads of each virus in each sample. Papillomavirus for each genus were combined into one papillomavirus category. Avian viruses included, avian leukosis virus, Gallid herpesvirus 3 virus and siadenovirus.
3.2. Papillomavirus was not associated with FOSCC
The results of conventional PCR, and ViroCap were concordant for all samples except PV2, Supplementary Table 4. PV2 was a papilloma on the skin of a dog and contained CPV-6 by ViroCap. PV2 was negative on PCR. Even though the FAP59/64 primers were created for cutaneous adapted PVs, they have never been shown to amplify CPV-6 and this may explain PCR failure. The 1 FOSCC sample that had PV, OSCC8, had a high viral read count, >2.1 million reads, with feline PV (FcaPV-3), likely indicative of a true infection. A normal mucosal sample, N6, had a very low PV read count, 56 reads, with a human PV (HPV-28) and likely represented contamination (Supplementary Table 5, Supplementary Fig. 2). PV was not detected by any method for the following positive control samples, PV1, PV6, PV7 and PV8 (Supplementary Table 4). These samples were from feline lesions that were diagnosed as sarcoids (fibropapillomas) and oral papillomas via histopathology with hematoxylin and eosin staining. Five novel variants of PV were discovered with ViroCap (Table 2 , Fig. 2 , Supplementary Table 6, and Supplementary Figures 3–6). GenBank accessions for the new variants are KY802017, KY825186, KY825187, KY825188, and KY886226. All SNPs in the L1 gene in the novel variants were synonymous. The full list of variants can be found in Supplementary Table 7. For the 5 novel PV variants, the reads that aligned to the ends of the reference genome contained overhangs that mapped to the other end of the genome. This is consistent with a circularized virus and maintenance of the virus as an episome.
Table 2.
Complete papillomavirus variant genomes identified by ViroCap in FOSCC samples.
| Patient | New Complete Genome Name | GenBank Accession | Genome Size (bp) | Closest relative (GenBank Accession) | Genera | % Analogous (genome)a | SBSb | Indelsc | Read countd | DoCe | BoCf | Physical form |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OSCC8 | Felis catus papillomavirus 3, isolate Missouri | KY825188.1 | 7583 | FcaPV-3 (NC_021472.1) | TauPV | 99.93% | 4 | None | 2,188,150 | 34,636x | 100% | Circularized |
Percentage analogous was calculated with Clustal Omega.
Single base substitutions.
IN (insertion) or DEL (deletion).
Reads that primarily mapped to the specified virus. Counts reported after deduplication with Picard.
DoC: depth of coverage.
BoC: breadth of coverage.
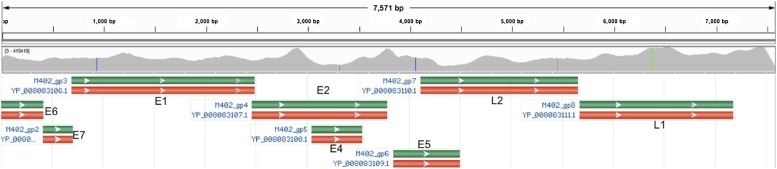
Fig. 2.
Read alignment from the FOSCC sample OSCC8 to FcaPV-3 (GenBank NC_021472.1) are shown in the top coverage track. Red, green, brown and blue vertical lines on the coverage track indicate SNPs. Gene annotations from GenBank are shown in the lower track (red and green boxes). This variant was deposited into GenBank as KY825188, Felis catus papillomavirus 3, isolate Missouri, complete genome. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.3. Foamy virus was the most common virus in feline oral mucosa
Foamy virus that was integrated into the host genome was found in 10 feline samples, N6, N9, PV7, OSCC1, 4, 9, 10, 11, 16, 18 (Supplementary Table 8). The difficulties of mapping short reads to repeat regions (e.g. long terminal repeat) prevented prediction of the whole genome sequence and location of genomic integration sites. Many SNPs were detected throughout the foamy virus genomes when compared to the closest relative (Supplementary Figures 7 and 8).
3.4. Anelloviruses or torque teno viruses were present at low read counts
Anellovirus was detected in 8 of 33 cats (23% in non-FOSCC patients and 25% in FOSCC patients) in the current study but the coverage was <0.5x in all but 2 of the samples, OSCC1 and OSCC8, and thus insufficient to confidently conclude the presence of anellovirus, Supplementary Table 9.
3.5. Epstein Barr Virus was present in a FOSCC sample
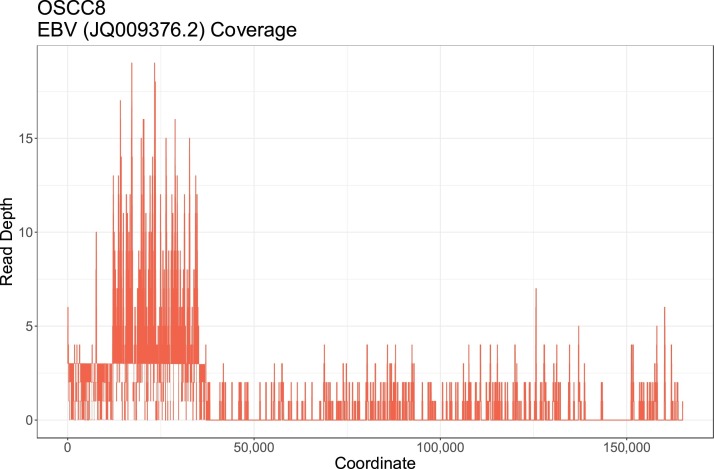
Only 2 cats, OSCC19 and OSCC20, had evidence of felid herpes virus 1 (FHV-1), which is an alphaherpesvirus that causes upper respiratory tract infections in cats (Supplementary Table 9). Epstein Barr Virus (EBV; Human herpesvirus-4; HHV-4), a gammaherpesvirus, was detected in 1 cat, OSCC8. None of the EBV reads aligned to FcaGHV-1, feline gammaherpesvirus, (GenBank accession NC_028099.1). The DoC and BoC of EBV were approximately 2x and 7.1% (Fig. 3 and Supplementary Table 5). The presence of EBV was confirmed with PCR (Supplementary Figure 9a). EBV was the sole match with BLAST analysis and the sequence was identical to >100 deposited EBV isolates with complete genomes (Supplementary Figure 9b).
Fig. 3.
Read alignment with the 1544 deduplicated reads from the FOSCC sample OSCC8 to EBV are shown. Due to the large size of the EBV genome SNPs cannot be seen with a whole genome scale. Coverage was seen throughout the EBV genome. The peak in coverage around 25,000 bp in the EBV genome corresponds to a poorly mapped repeat region in the EBNA gene.
3.6. Exogenous FeLV and FIV were detected in normal oral mucosa
The FIV-positive cats were the FIV SNAP positive cat, N3, and a stray cat N9 where FIV testing was not done. Integrated FIV was detected at low DoC, 0.8x and 0.3x in N3 and N9 respectively (Supplementary Table 8 and Supplementary Figure 10). Exogenous FeLV (exFeLV) was not detected by ViroCap in the cat that was FeLV positive on SNAP test, N2. In addition, the region of the U3 long terminal repeat that contains the CCAAT enhancer and TATA boxes found in exFeLV did not contain aligned reads in any patient except N7 and there were gaps in coverage in ENV and the 5′ end of the gap-pro-pol polyprotein (Supplementary Figure 11a and b). In contrast, cat N7 had a high BoC, 95.3%, and was thus suspected to be infected with exFeLV. Cat N7 was not FeLV/FIV tested but was diagnosed with feline infectious peritonitis (FIP) on necropsy. FIP is caused by infection with a mutated feline coronavirus, a ssRNA virus, and immunosuppression by FeLV has been associated with increased risk of FIP.
3.7. Contamination with ovine and avian viruses
A turkey hemorrhagic enteritis like virus, in the siadenovirus genus, was detected in sample PV3, 1 of 3 canine samples. The DoC and BoC were low, 0.3x and 0% respectively (Supplementary Figure 12). BLAST of the reads that mapped to the hexon and fiber genes aligned solely to the turkey hemorrhagic enteritis viruses. A canine siadenovirus has not been previously reported. Gallid herpesvirus 3 (GaHV-3) was present in one sample, OSCC13 (Supplementary Table 5). BLAST of representative aligned reads were a match only to the Marek’s disease viruses (MDV-2 and MDV-3) that infect chickens. This sample also contained avian leucosis virus (ALV). To determine if these avian viruses were present as a contaminant from chicken tissues, all of the sequenced reads from OSCC13 were aligned to the Gallus gallus genome (Galgal4). OSCC13 had 98,146 reads (deduplicated with a mapping quality of ≥60) that mapped to Galgal4 with a BoC of 0.011%. In comparison, representative FOSCC FFPE samples that did not contain avian viruses, OSCC12-14, had 9144-16,699 reads with a BoC of 0.001-0.002%. GaHV-3 and ALV were thus likely contaminants in sample OSCC13. A Jaagsiekte retrovirus (ovine pulmonary carcinoma virus) like virus was present in 4 samples, OSCC4, OSCC10 OSCC19 and OSCC20 (Supplementary Table 5). The pattern of SNPs was identical in all samples and thus the virus in all samples came from the same source (Supplementary Figure 13). The viral load was highest in OSCC10. OSCC10 could have cross-contaminated the other samples or all of these samples could have been contaminated by the same sheep tissue source.
4. Discussion
This is the first study to use a comprehensive capture approach to characterize the virome of the oral mucosa of domestic cats. To demonstrate that PV causes FOSCC, ideally all 3 of the following criteria would be met: (1) PV DNA in the lesion, (2) expression of oncogenes such as E6 and E7, (3) disruption of tumor suppressor gene, TP53 and RB1, function by viral oncoproteins. The first criterion was specifically evaluated in this study and was not met. FcaPV was only detected in 1 of 20 FOSCC samples. These results are consistent with all published literature that includes >1 FOSCC patient, where PV infection was detected in only 0–6.9% of patients (Munday and French, 2015; Munday et al., 2019, 2009; Munday et al., 2011b; O’Neill et al., 2011; Supsavhad et al., 2016; Yamashita-Kawanishi et al., 2018). It is possible that PV was not detected but causes FOSCC via a ‘hit and run’ mechanism, where E6 and E7 contribute to tumor initiation, the virus is lost in overt neoplasms, and it is not required to maintain the transformed phenotype. The hit and run hypothesis is unlikely in FOSCC due to the rarity of detecting FcaPV in FOSCC and in the oral mucosa of cats, lack of in vivo evidence that FcaPV causes premalignant changes in the oral mucosa, and the fact that PV is usually detectable with sensitive methods such as NGS in HNSCC lesions that have a genetic signature consistent with PV induced lesions (Parfenov et al., 2014). The only PV virus that was isolated from a FOSCC lesion was FcaPV-3. FcaPV-3 is not as commonly isolated as FcaPV-2 in cats (Supplementary Table 1). FcaPV-3 infection has been found in cutaneous SCC, nasal planum SCCs and BISC lesions (Munday et al., 2013a, 2016; Munday et al., 2013b, a; Yamashita-Kawanishi et al., 2018). FcaPV-3 could have been an incidental infection. The authors plan to further investigate PV as a cause of FOSCC in patient OSCC8 by demonstrating evidence of criteria 2 and 3 through analysis of expression of oncogenes and interaction with host tumor suppressor genes in a future study. Increased expression of p16, E6 and E7 and decreased expression of pRb and p53 would be expected in high-risk PV induced lesions. Decreased p53 and pRB expression and increased p16 expression was found in only 2.3% of FOSCC samples and all samples with high p16 expression were negative for PV (Supsavhad et al., 2016). Increased p16 expression has been identified as a positive prognostic factor in FOSCC but has only been seen in a minority of samples, 6.7% to 19%, and has not been associated with PV infection (Munday et al., 2019, 2011b). High-risk PVs encode an E6 protein that binds to p53 sufficiently to induce ubiquitin proteolysis (Faraji et al., 2017). E7 similarly induces pRb degradation. However, E7 of FcaPV-3 is not expected to behave like in high-risk HPVs. The critical cysteine amino acid in the LXCXE motif on E7, that is conserved in HPV-16, FcaPV-1 and FcaPV-2, and which is responsible for pRb binding, was not present in FcaPV-3 (Supplementary Figure 14). FcaPV-3 either does not bind to pRb and cause its degradation or binds pRb in a yet to be discovered novel mechanism.
There is thus currently no evidence that PV is associated with changes in oncoproteins or tumor suppressor proteins in FOSCC analogous to high-risk HPVs. Three other characteristics of FOSCC decrease the likelihood that FOSCC is a good model for high-risk PV induced SCC in people: (1) PV was maintained as a full-length episome compared to integration into the host genome in high-risk HPVs (Cancer Genome Atlas, 2015; Faraji et al., 2017; Parfenov et al., 2014); (2) FOSCC is almost exclusively a cancer of geriatric patients in contrast to PV-positive HNSCC being most prevalent in younger patients compared to carcinogen induced HNSCC; (3) FOSCC most commonly occurs in the oral cavity proper compared to HPV-positive tumors that are the most common in the oropharynx (e.g. tonsils) (Kang et al., 2015). There were no tonsillar FOSCC lesions included in this study, due to the rarity of this lesion at the MU VMTH. None of the PV-positive FOSCC lesions from previous studies were classified as oropharyngeal (Supplementary Table 2). To the author’s knowledge PV status of oropharyngeal FOSCC has been evaluated in only 15 cats to date (Munday and French, 2015; Munday et al., 2019, 2009; Munday et al., 2011b; O’Neill et al., 2011). Tonsillar FOSCC lesions have been associated with a better prognosis, than FOSCC lesions located in the oral cavity, when treated with an accelerated radiation therapy and carboplatin protocol (Fidel et al., 2011). If there is a feline correlate with HPV-positive OPSCC, tonsillar FOSCC would likely be the best candidate. Non-viral causes of FOSCC may include age, microbiota, genetics and exposure to carcinogens which can affect the oral cavity via ingestion or grooming. Carcinogenic agents in HNSCC commonly cause oxidative damage (Altieri et al., 2004; Freedman et al., 2007; Guha et al., 2014). Future studies on the etiology of FOSCC should focus on carcinogen exposure.
PV was also not found in some lesions that commonly have a PV cause. Based on these results, it appears that at least a portion of histologically diagnosed feline sarcoids and oral papillomas are not virally induced and histology alone may be insufficient to diagnose these lesions. Foamy viruses are continuously shed from the oral mucosa in infected cats and it was the most prevalent virus in normal and FOSCC oral mucosa. A prevalence of 36% was seen in homeless cats from South Australia (Winkler et al., 1998), similar to the 30% observed in this study. Foamy viruses have been used in gene therapy due to their preferential integration into transcriptionally inactive regions and resulting apparent lack of association with clinical disease. Torque teno virus was the second most prevalent virus but was detected at very low coverage. Anelloviruses have been detected in cat feces, saliva and serum (Jarosova et al., 2015; Zhang et al., 2014). Anelloviruses are not known to be pathogenic in cats and pathogenicity in other species is controversial with the exception of chicken anemia virus and porcine circovirus, which are closely related to anelloviruses. Prevalence in feline serum of 12.5–43% has been seen worldwide, which is similar to the suspected prevalence in this study (Jarosova et al., 2015; Okamoto et al., 2002; Zhu et al., 2011). To confirm the suspected higher prevalence a targeted PCR approach could be done. Studies evaluating FHV-1 with PCR in conjunctival and gingival samples of clinical and non-clinical cats reported that only 1–6% cats were positive for FHV-1 (Sjodahl-Essen et al., 2008). In the current study, a similarly low prevalence of 6% was found. EBV was detected at low levels in a cat with FOSCC and concurrent FcaPV-3 infection. Contamination was deemed an unlikely source since EBV is shed via human saliva, plasma and blood and personal protective equipment was worn during surgery or necropsy. Cross contamination was unlikely since the MU VMDL does not process human samples. The oral mucosal sampling route used in this study would have been appropriate for detection of an active EBV infection since EBV replicates in the B-lymphocytes and epithelial cells in the oropharynx and is transmitted via oral secretions. EBV or an EBV-like virus has been detected previously in cats with serology (Milman et al., 2011). False positives due to cross-reactivity with other herpes viruses are possible with serology (Heller et al., 1982; Milman et al., 2011). The presence of circulating EBV DNA in the blood pre and post treatment and antibody titers have been shown to be independent negative prognostic indicators in nasopharyngeal carcinomas (Kang et al., 2015). EBV infection has also been shown to increase the risk of HOSCC (She et al., 2017). This is the first study to find EBV DNA in a feline tumor. The SNAP FeLV positive but ViroCap exFeLV negative cat could have been a false positive since a confirmatory test was not done and the screening test was not repeated. Alternatively, if N2 was truly antigen positive, explanations for negative proviral DNA include focal/atypical infection, regressive FeLV infection after initial antigen testing or limitations to detect exFeLV from the methodologies used in this study. For FIV, the ViroCap result was consistent with the SNAP test for N3. The FeLV/FIV status of the stray cat, N9, was not known and thus the FIV positive ViroCap result cannot be confirmed. Similarly with FeLV, increased read counts and thus sensitivity for FIV provirus detection would likely have been obtained if a lymphocyte rich sample type was tested. Alternatively, fresh frozen oral mucosal tissue with cDNA conversion would be a more suitable methodology since both of these viruses are shed in the saliva during active infection. One sample, OSCC13, contained two chicken viruses, GaHV-3 and ALV. GaHV-3 also known as MDV-2, is not oncogenic in chickens, and is used in the bivalent vaccine for protection against Marek’s disease (MDV-1). It is likely that this sample was contaminated with materials from chickens during sample preparation. Similarly, PV3 contained an adenovirus possibly from contamination from turkeys and OSCC4, OSCC10, OSCC19 and OSCC20 contained retrovirus possibly from contamination from sheep. HPV-28 causes benign flat plane warts in humans and was likely present in N6 due to contamination from sample handling. Forensic studies have shown that wiping down surfaces with ethanol are not sufficient to remove contamination. In future studies, where sensitive genomic work is involved cleaning techniques with a dilute (0.9–6%) hypochlorite solution or commercial decontamination solution should be requested.
A limitation of the current study is that RNA viruses that had not integrated into the host genome would not have been detected. A future metagenomic study that includes cDNA conversion would be required to completely rule out a viral cause of FOSCC, but RNA viruses would be considered a highly unlikely cause of FOSCC since a non-integrated RNA virus has not caused oral squamous cell carcinoma in any species to date. Another limitation is that differences in the tissue sample preparation methods could have led to a higher viral capture efficiency in the fresh frozen negative control samples compared to the FFPE positive control and FOSCC samples. Ideally future studies should utilize fresh frozen samples and include a larger number of samples.
Funding information
This work was supported by a University of Missouri Phi Zeta Veterinary Honor Society resident grant.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.vetmic.2019.108491.
Appendix A. Supplementary data
The following is supplementary data to this article:
References
- Altieri A., Bosetti C., Gallus S., Franceschi S., Dal Maso L., Talamini R., Levi F., Negri E., Rodriguez T., La Vecchia C. Wine, beer and spirits and risk of oral and pharyngeal cancer: a case-control study from Italy and Switzerland. Oral Oncol. 2004;40:904–909. doi: 10.1016/j.oraloncology.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou S.H., Chow K.C., Yang C.H., Chiang S.F., Lin C.H. Discovery of Epstein-Barr virus (EBV)-encoded RNA signal and EBV nuclear antigen leader protein DNA sequence in pet dogs. J. Gen. Virol. 2005;86:899–905. doi: 10.1099/vir.0.80792-0. [DOI] [PubMed] [Google Scholar]
- Faraji F., Zaidi M., Fakhry C., Gaykalova D.A. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 2017;19:464–475. doi: 10.1016/j.micinf.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel J., Lyons J., Tripp C., Houston R., Wheeler B., Ruiz A. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. J. Vet. Intern. Med. 2011;25:504–510. doi: 10.1111/j.1939-1676.2011.0721.x. [DOI] [PubMed] [Google Scholar]
- Forslund O., Antonsson A., Nordin P., Stenquist B., Hansson B.G. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999;80(Pt 9):2437–2443. doi: 10.1099/0022-1317-80-9-2437. [DOI] [PubMed] [Google Scholar]
- Freedman N.D., Abnet C.C., Leitzmann M.F., Hollenbeck A.R., Schatzkin A. Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer. 2007;110:1593–1601. doi: 10.1002/cncr.22957. [DOI] [PubMed] [Google Scholar]
- Guha N., Warnakulasuriya S., Vlaanderen J., Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int. J. Cancer. 2014;135:1433–1443. doi: 10.1002/ijc.28643. [DOI] [PubMed] [Google Scholar]
- Heller M., Gerber P., Kieff E. DNA of herpesvirus pan, a third member of the Epstein-Barr virus-Herpesvirus papio group. J. Virol. 1982;41:931–939. doi: 10.1128/jvi.41.3.931-939.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosova V., Hrazdilova K., Filipejova Z., Schanilec P., Celer V. Whole genome sequencing and phylogenetic analysis of feline anelloviruses. Infect. Genet. Evol. 2015;32:130–134. doi: 10.1016/j.meegid.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Kang H., Kiess A., Chung C.H. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2015;12:11–26. doi: 10.1038/nrclinonc.2014.192. [DOI] [PubMed] [Google Scholar]
- Lange C.E., Tobler K., Markau T., Alhaidari Z., Bornand V., Stockli R., Trussel M., Ackermann M., Favrot C. Sequence and classification of FdPV2, a papillomavirus isolated from feline Bowenoid in situ carcinomas. Vet. Microbiol. 2009;137:60–65. doi: 10.1016/j.vetmic.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Milman G., Smith K.C., Erles K. Serological detection of Epstein-Barr virus infection in dogs and cats. Vet. Microbiol. 2011;150:15–20. doi: 10.1016/j.vetmic.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Munday J.S. Papillomaviruses in felids. Vet. J. 2014;199:340–347. doi: 10.1016/j.tvjl.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Munday J.S., Dunowska M., Hills S.F., Laurie R.E. Genomic characterization of Felis catus papillomavirus-3: a novel papillomavirus detected in a feline Bowenoid in situ carcinoma. Vet. Microbiol. 2013;165:319–325. doi: 10.1016/j.vetmic.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Munday J.S., Fairley R., Atkinson K. The detection of Felis catus papillomavirus 3 DNA in a feline bowenoid in situ carcinoma with novel histologic features and benign clinical behavior. J. Vet. Diagn. Invest. 2016;28:612–615. doi: 10.1177/1040638716658930. [DOI] [PubMed] [Google Scholar]
- Munday J.S., French A.F. Felis catus papillomavirus types 1 and 4 are rarely present in neoplastic and inflammatory oral lesions of cats. Res. Vet. Sci. 2015;100:220–222. doi: 10.1016/j.rvsc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Munday J.S., French A.F., Gibson I.R., Knight C.G. The presence of p16 CDKN2A protein immunostaining within feline nasal planum squamous cell carcinomas is associated with an increased survival time and the presence of papillomaviral DNA. Vet. Pathol. 2013;50:269–273. doi: 10.1177/0300985812452582. [DOI] [PubMed] [Google Scholar]
- Munday J.S., Gibson I., French A.F. Papillomaviral DNA and increased p16(CDKN2A) protein are frequently present within feline cutaneous squamous cell carcinomas in ultraviolet-protected skin. Vet. Dermatol. 2011;22:360–366. doi: 10.1111/j.1365-3164.2011.00958.x. [DOI] [PubMed] [Google Scholar]
- Munday J.S., He Y., Aberdein D., Klobukowska H.J. Increased p16(CDKN2A), but not p53, immunostaining is predictive of longer survival time in cats with oral squamous cell carcinomas. Vet. J. 2019;248:64–70. doi: 10.1016/j.tvjl.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Munday J.S., Howe L., French A., Squires R.A., Sugiarto H. Detection of papillomaviral DNA sequences in a feline oral squamous cell carcinoma. Res. Vet. Sci. 2009;86:359–361. doi: 10.1016/j.rvsc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Munday J.S., Kiupel M., French A.F., Howe L., Squires R.A. Detection of papillomaviral sequences in feline Bowenoid in situ carcinoma using consensus primers. Vet. Dermatol. 2007;18:241–245. doi: 10.1111/j.1365-3164.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Munday J.S., Knight C.G., French A.F. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Res. Vet. Sci. 2011;90:280–283. doi: 10.1016/j.rvsc.2010.06.014. [DOI] [PubMed] [Google Scholar]
- O’Neill S.H., Newkirk K.M., Anis E.A., Brahmbhatt R., Frank L.A., Kania S.A. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Vet. Dermatol. 2011;22:68–74. doi: 10.1111/j.1365-3164.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takahashi M., Nishizawa T., Tawara A., Fukai K., Muramatsu U., Naito Y., Yoshikawa A. Genomic characterization of TT viruses (TTVs) in pigs, cats and dogs and their relatedness with species-specific TTVs in primates and tupaias. J. Gen. Virol. 2002;83:1291–1297. doi: 10.1099/0022-1317-83-6-1291. [DOI] [PubMed] [Google Scholar]
- Papineau A., Berhane Y., Wylie T.N., Wylie K.M., Sharpe S., Lung O. Genome organization of canada goose coronavirus, a novel species identified in a mass die-off of Canada geese. Sci. Rep. 2019;9:5954. doi: 10.1038/s41598-019-42355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenov M., Pedamallu C.S., Gehlenborg N., Freeman S.S., Danilova L., Bristow C.A., Lee S., Hadjipanayis A.G., Ivanova E.V., Wilkerson M.D., Protopopov A., Yang L., Seth S., Song X., Tang J., Ren X., Zhang J., Pantazi A., Santoso N., Xu A.W., Mahadeshwar H., Wheeler D.A., Haddad R.I., Jung J., Ojesina A.I., Issaeva N., Yarbrough W.G., Hayes D.N., Grandis J.R., El-Naggar A.K., Meyerson M., Park P.J., Chin L., Seidman J.G., Hammerman P.S., Kucherlapati R., Genome C., Atlas N. Characterization of HPV and host genome interactions in primary head and neck cancers. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15544–15549. doi: 10.1073/pnas.1416074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She Y., Nong X., Zhang M., Wang M. Epstein-Barr virus infection and oral squamous cell carcinoma risk: a meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodahl-Essen T., Tidholm A., Thoren P., Persson-Wadman A., Bolske G., Aspan A., Berndtsson L.T. Evaluation of different sampling methods and results of real-time PCR for detection of feline herpes virus-1, Chlamydophila felis and Mycoplasma felis in cats. Vet. Ophthalmol. 2008;11:375–380. doi: 10.1111/j.1463-5224.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Skor O. Presence of papillomavirus DNA in feline squamous cell carcinoma and injection site-sarcoma. Veterinary Cancer Society Conference, Fairfax County, Virginia, October 15-17, 2015. 2015:41. [Google Scholar]
- Supsavhad W., Dirksen W.P., Hildreth B.E., Rosol T.J. p16, pRb, and p53 in feline oral squamous cell carcinoma. Vet. Sci. 2016:3. doi: 10.3390/vetsci3030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I.G., Flugel R.M., Lochelt M., Flower R.L.P. Detection and molecular characterisation of feline foamy virus serotypes in naturally infected cats. Virology. 1998;247:144–151. doi: 10.1006/viro.1998.9232. [DOI] [PubMed] [Google Scholar]
- Wylie K.M., Wylie T.N., Buller R., Herter B., Cannella M.T., Storch G.A. Detection of viruses in clinical samples using metagenomic sequencing and targeted sequence capture. J. Clin. Microbiol. 2018 doi: 10.1128/JCM.01123-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie K.M., Wylie T.N., Cahill A.G., Macones G.A., Tuuli M.G., Stout M.J. The vaginal eukaryotic DNA virome and preterm birth. Am. J. Obstet. Gynecol. 2018;219 doi: 10.1016/j.ajog.2018.04.048. 189 e181-189 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie T.N., Wylie K.M., Herter B.N., Storchl G.A. Enhanced virome sequencing using targeted sequence capture. Genome Res. 2015;25:1910–1920. doi: 10.1101/gr.191049.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita-Kawanishi N., Sawanobori R., Matsumiya K., Uema A., Chambers J.K., Uchida K., Shimakura H., Tsuzuki M., Chang C.Y., Chang H.W., Haga T. Detection of felis catus papillomavirus type 3 and 4 DNA from squamous cell carcinoma cases of cats in Japan. J. Vet. Med. Sci. 2018;80:1236–1240. doi: 10.1292/jvms.18-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Li L., Deng X., Kapusinszky B., Pesavento P.A., Delwart E. Faecal virome of cats in an animal shelter. J. Gen. Virol. 2014;95:2553–2564. doi: 10.1099/vir.0.069674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.X., Shan T.L., Cui L., Luo X.N., Liu Z.J., Tang S.D., Liu Z.W., Yuan C.L., Lan D.L., Zhao W., Hua X.G. Molecular detection and sequence analysis of feline Torque teno virus (TTV) in China. Virus Res. 2011;156:13–16. doi: 10.1016/j.virusres.2010.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.