Abstract
To determine the presence of viral pathogens in natural areas a survey was conducted on an opportunistic sample of fifty eight wild (Felis silvestris silvestris) and feral cats (F. s. catus). The biological materials included serum, lung tissue extract and stool. Feline leukemia virus p27 antigen was detected in 13/50 serum/lung tissue extract samples (26%), canine distemper virus antibodies were detected in 2/26 serum/lung tissue extract samples (7.7%), feline coronavirus RNA was present in 6/29 stool samples (20.7%) and feline parvovirus DNA in 2/29 stool samples (6.9%). Canine distemper virus RNA was not detected. Feline immunodeficiency virus and feline coronavirus antibodies were not detected. Evidence of exposure to feline leukemia virus, canine distemper virus, feline coronavirus and feline parvovirus was found in wild and feral cats raising the importance of performing a comprehensive survey to correctly evaluate the potential threat of infectious diseases to endangered species, namely to the wildcat and to the Iberian lynx, which is meant to be reintroduced after 2012 in Portugal.
Keywords: Wildcat, FeLV, FIV, CDV, FPV, FCoV, Lynx reintroduction, Felis silvestris
1. Introduction
Information concerning risk assessment of viral infectious pathogens to wild animal populations is currently an important issue in conservation biology, particularly when reintroduction is being planned (IUCN, 1998). Viral infectious diseases can act as threats to endangered species as already documented (Daszak et al., 2000, Meli et al., 2009).
Feline leukemia virus (FeLV), feline immunodeficiency virus (FIV), feline coronavirus (FCoV) and feline parvovirus (FPV) are important viral pathogens of the domestic cat and potentially of all wild felids. FeLV is responsible for a degenerative and proliferative disease leading to death and is transmitted by direct contact (Hartmann, 2011). FIV induces an acquired immunodeficiency syndrome similar to AIDS and is mainly transmitted by bites (Hartmann, 2011). FCoV is a ubiquitous virus transmitted by the fecal–oral route and although associated with a benign enteric disease is also the etiologic agent of feline infectious peritonitis, a severe disease affecting the Felidae (Addie et al., 2003). FPV is responsible for a viral panleukopenia frequently fatal in young kittens (Steinel et al., 2001). Several reports have confirmed that these viruses also infect the European wildcat (Felis silvestris silvestris) (Millán and Rodríguez, 2009), catalogued as vulnerable in Portugal (Queiroz et al., 2005), and the Iberian lynx (Lynx pardinus) (Meli et al., 2009), the most endangered felid species in the world (IUCN, 2007). Canine distemper virus is an important viral pathogen for members of the Panthera genus (Roelke et al., 2008) as well as to Mustelidae family (Thorne and Williams, 1988). Recently CDV was reported as the etiological agent of encephalitis in lynx (Lynx canadensis) in the USA (Daoust et al., 2009) and was also implicated as probable cause of death in an Iberian lynx in Spain (Meli et al., 2010b).
In 2008 the National Plan for Conservation of Iberian Lynx was legally approved in Portugal. Several projects to recover prey and habitat have been taking place in former historical areas for the species. In 2009 a captive breeding center in the south of Portugal received 16 lynxes contributing to the ex situ Iberian program. Reintroduction in areas of Portugal and Spain is being prepared and planned according to IUCN criteria which include sanitary concerns.
Recently a serological survey was conducted regarding the prevalence of canine distemper virus and canine parvovirus in wild carnivores, confirming viral seropositivity in several carnivore species (Santos et al., 2009), but little information is available regarding the prevalence of viral infectious agents in wild and free ranging cats. In view of future efforts towards conservation of threatened species, namely the reintroduction of L. pardinus, a virological survey was conducted to assess the presence of FeLV, FIV, FCoV, FPV and CDV infections in wildcats and feral cats. Due to their potential role as viral reservoirs data concerning the health status of these animal populations will be needed in a future risk analysis.
2. Material and methods
2.1. Biological sampling
Between 1992 and 2007, 50 samples of LTE and/or serum and 28 stools samples were collected from 32 wildcats and 21 feral domestic cats in continental Portugal (Fig. 1 ), either from live captured animals or from carcasses, after a standard necropsy procedure. Animals were identified according to morphological characters (Kitchener et al., 2005), available genetic information (Oliveira et al., 2008, Pierpaoli et al., 2003; Driscoll and Fernandes, data not published) and lifestyle. No data was available regarding four samples.
Fig. 1.
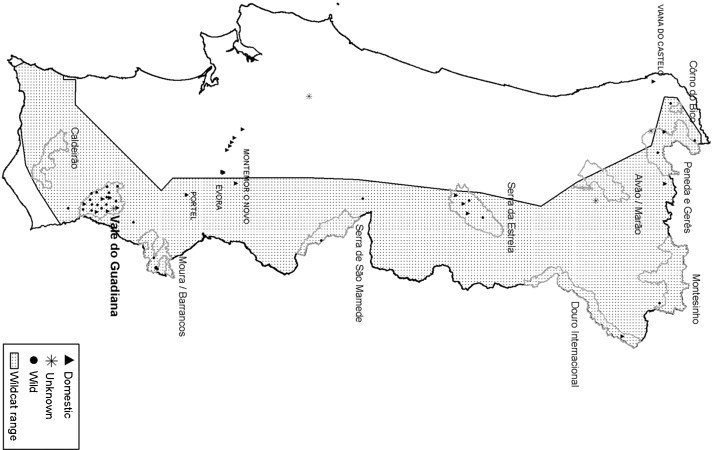
Geographic distribution of the samples collected from domestic; unknown and wild cats. A proposed area of occurrence for the wildcat in Portugal in shown based on nonsystematic data collected from 1996 to 2007 (Fernandes, 2007).
2.2. Serological screening
Serum and lung tissue extract (LTE) (Ferroglio et al., 2000), were used for FeLV p27, FIV antibodies (Ab) and FCoV Ab detection by Enzyme-linked immunoassay using commercial kits (Virachek Felv Aufel 1N; ViraCHEK™ FIV ACFIV3M, Synbiotic, France and Ingezim Corona Felino 16.FCV.K.1, Spain) following the manufacturer instructions; CDV Ab were also screened by a commercial enzyme-linked immunoassay (Ingezim Moquillo IgG 15.CDG.K.1) using Protein A-Peroxidase from Staphylococcus aureus/horseradish (Sigma–Aldrich), for detection of the primary Ab/Ag complex (Lindmark et al., 1983).
2.3. Detection of viral nucleic acids
Viral RNA and DNA were simultaneously extracted from stool using the QIAmp® Minielute® Virus spin Kit (Qiagen, Germany) following the manufacturer instructions. Detection of FCoV RNA was performed according to Herrewegh et al. (1995); FPV DNA was screened according to Desario et al. (2005) and CDV RNA according to Frisk et al. (1999). The amplification products were visualized after electrophoresis in a 2% agarose gel, stained by EtBr in an Image Master® VDS (Pharmacia Biotech).
3. Results
Considering the geographical distribution (Fig. 1), 16 animals were caught in Northern Portugal mainly in the natural areas of Peneda-Geres National Park; and also Montesinho and Douro Internacional natural parks; cats from the center of the country were mainly from Serra da Estrela Natural Park with the exception of one animal from Serra de S. Mamede Natural Park. The remaining samples (LTE/serum = 37; stool = 21) were from 41 animals caught in Alentejo and Algarve in the South, including the Vale do Guadiana Natural Park (n = 21) and the Natura 2000 area of Moura Barrancos (n = 5).
FeLV p27 antigen was detected in 13/50 samples (26%), FCoV RNA was present in 6/28 samples (21.4%) and FPV DNA in 2/28 samples (7.1%) (Table 1 ). CDV RNA was not detected (n = 13). CDV antibodies were detected in 2/26 samples (7.7%); FIV (n = 50) and FCoV (n = 26) antibodies were not detected. Feral cats (F. s. catus) showed a higher frequency of FeLV antigen (33.3%) and FPV DNA (20%), and wild cats (F. s. silvestris) of FCoV RNA (33.3%).
Table 1.
Frequencies of Ag (FeLV), Ab (FIV; CDV, FCoV) and FCoV, CDV and FPV nucleic acid in biological materials from wild (Felis silvestris) and feral cats (Felis catus) with 95% confidence intervals.
| F. s. catus | F. s. silvestris | Unknown | Total | |
|---|---|---|---|---|
| FeLV Ag | 6/18 (33.3%) (CI95% 16.3–56.3) | 7/28 (25%) (CI95% 12.7–43.4) | 0/4 (0%) (CI95% 0–49.0) | 13/50 (26%) (CI95% 15.9–39.6) |
| FIV Ab | 0/18 (0%) (CI95% 0–17.6) | 0/28 (0%) (CI95% 0–12.1) | 0/4 (0%) (CI95% 0–49.0) | 0/50 (0%) (CI95% 0–7.1) |
| FCoV Ab | 0/26 (0%) (CI95% 0–12.9) | 0/26 (0%) (CI95% 0–12.9) | ||
| CDV Ab | 2/26 (7.7%) (CI95% 2.1–24.1) | 2/26 (7.7%) (CI95% 2.1–24.1) | ||
| FCoV RNA | 1/10 (10%) (CI95% 1.8–40.4) | 5/15 (33.3%) (CI95% 15.2–58.3) | 0/3 (0%) (CI95% 0–56.2) | 0/3 (0%) (CI95% 0–56.2) |
| CDV RNA | 0/13 (0%) (CI95% 0–22.8) | |||
| FPV DNA | 2/10 (20%) (CI95%5.7–51.0) | 0/15 (0%) (CI95% 0–20.4) | 0/3 (0%) (CI95% 0–56.2) | 2/28 (7.1%) (CI95% 2.0–22.6) |
Among the 16 samples analyzed from North and Central Portugal (12 LTE/serum and 7 stool samples), one sample from a feral cat (n = 4) and one sample of a wildcat (n = 6) were positive for FeLV Ag. FCoV RNA was found in one sample (1/4) from a wild cat and one sample from a feral cat (1/2) was positive for FPV DNA. FeLV was detected throughout the country including all the natural parks sampled. Presence of FPV was confirmed in Vale do Guadiana and Serra da Estrela. The priority areas for Iberian lynx included in the survey – S. Mamede, Moura Barrancos and Guadiana – showed presence of FeLV, FPV and FCoV. CDV seropositive samples were also from this area (2/17 of wild cats). Southern districts (Alentejo and Algarve) contributed with 41 animals (36 LTE/serum samples; 19 stool samples). FeLV antigen was found in 5/15 of feral cats and 6/21 of wild cats. FCoV RNA was found in 1/9 samples from feral cats and in 4/10 samples of wild cats. FPV DNA was only found in 1/9 samples of feral cats.
4. Discussion
In this study we confirmed the presence of FeLV antigen in wild and feral cats with higher frequencies (25–33.3%) than previously reported in France (23.7%) (Fromont et al., 2000), Scotland, UK (10%) (Daniels et al., 1999) and recently in Spain (15%) (Millán et al., 2009). Due to FeLV pathogenesis, detection of antigenemic samples confirmed the presence of the disease in the sampled populations. Considering that this study was performed on an opportunist sample, a bias could have occurred, which would partially explain the high FeLV frequency both in wild (25%) and feral cats (33.3%). Although an uneven distribution of the samples was evident, results indicate a higher frequency of FeLV in feral cats, suggesting that these populations might have a greater responsibility in the spread of the virus in wild populations, contrary to what has been suggested by others (Leutenegger et al., 1999, Millán and Rodríguez, 2009).
Regarding the prevalence of FeLV and FIV our results showed high frequencies for FeLV (25–33.3%) but no occurrence of FIV (0%). FeLV is transmitted by direct contact, mainly by saliva and also by vertical transmission. Caring for offspring and territory marking are behaviors that may favor FeLV transmission in wildcats and feral rural populations. On the other hand FIV is mainly transmitted by bites frequent in territorial struggles and mating, with a direct correlation between the animal's behavior and the prevalence of FIV infection (Ishida et al., 1989). Our samples were collected from wild and rural feral cats roaming over territories which are known to be relatively large in these species (Fernandes, 1992, Monterroso et al., 2009), thus minimizing interactions. Interestingly FeLV infection is more aggressive from the clinical point of view, causing anemia, and a severe immunosuppression rendering the animals more susceptible to secondary infections (Hartmann, 2011). In 2007 an important FeLV outbreak occurred in Donana (Spain) causing the death of several lynxes (Meli et al., 2010a). This disease can be considered to be an important threat to endangered species with small restricted populations. This may already be the case for wildcat populations in certain areas of Portugal and would also apply to lynx nuclei in a future reintroduction scenario. The prevalence found in the present study is an important risk indicator and should be confirmed for local areas with a wider sample. The expected values of the disease can be used to estimate sample size. For instance in Vale do Guadiana, presently a potential area for reintroduction of lynx and where an important wildcat population persists (Monterroso et al., 2009), 35% (7/20) LTE samples were positive for FeLV antigen, a remarkably high value. A previous survey conducted in 2007 in SE Portugal, including Vale do Guadiana and Moura Barrancos with samples from 19 rural domestic cats (Duarte et al., 2007) also revealed 21% FeLV Ag positivity, thus confirming high FeLV frequency in wild and feral feline populations in potential reintroduction areas for L. pardinus, reinforcing the need for a thorough evaluation of disease prevalence.
Regarding FIV, no animal was seropositive. Similar surveys had shown null or low frequency of FIV infection in wild cat populations (Leutenegger et al., 1999, Millán et al., 2009) and FIV infection is uncommon in low-density populations (Artois and Remond, 1994).
Detection of FCoV and FPV nucleic acids was also confirmed in both populations. Wild cats displayed a higher frequency of FCoV RNA (33.3%) and feral cats of FPV DNA (18.2%), confirming the presence of carrier animals within wild populations as already reported (Leutenegger et al., 1999). Considering the pattern of FCoV infection carrier animals are responsible for viral availability in the environment, leading to exposure of susceptible animals whenever they share the same habitat, potentially constituting a disease threat (Addie et al., 2003). FCoV antibodies were not detected in the sampled population of 26 wild cats, including 3 positive viral RNA samples. This finding may reflect the use of serum and LTE mainly collected from decomposed carcasses for the serological screening, raising the possibility of an underestimated frequency of FCoV Ab due to protein denaturation and low sensitivity of LTE for serological testing (Tryland et al., 2006). On the other hand FCoV tend to establish recurrent infections in the cat and antibody detection does not always correlate with virus shedding neither in carrier animals (Foley et al., 1997), nor in Feline Infectious Peritonitis affected animals (Addie et al., 2009).
FPV was only detected in feral cats confirming previous information on FPV detection in domestic cats in Portugal (Duarte et al., 2007). Detection of positive samples in wild populations although expected, should be monitored due to the rapid evolutionary rate of carnivore parvovirus (Shackelton et al., 2005) enhancing the risk of spreading its host range.
5. CDV is presently considered an emerging pathogen due to its proneness
Although usually not recognized as a cat pathogen, CDV displays a wide host range and was already implicated in disease outbreaks in several animal species (Kapil and Yeary, 2011). In the present study we detected 7.7% (2/26) of CDV seropositivity in wild cat samples pointing to viral exposure in the sampled population. Domestic dogs are the main CDV source (Kapil and Yeary, 2011) although foxes may also spread the virus to other carnivore species as already reported in Spain (Sobrino et al., 2008) and CDV exposure was already confirmed in foxes and wolves in northern Portugal (Santos et al., 2009).
In conclusion our survey of feline viruses in free ranging feline population revealed the presence of FeLV, FCoV and FPV, but not FIV. CDV seropositive samples were also identified. Due to the values detected for FeLV and FCoV and the potential pathogenic impact of viral diseases in endangered susceptible species such as the Iberian Lynx (Meli et al., 2009, Roelke et al., 2008) a more thorough survey will be required to correctly evaluate their prevalence in Portugal, particularly in future reintroduction areas. Information regarding pathogen epidemiology is critical to attempt to decrease the infectious pressure exerted by neighbor species over Iberian Lynx populations and will be taken into account in future reintroduction areas.
Conflicts of interest
None.
Acknowledgments
This work was sponsored by CIISA (Centre for Interdisciplinary Research in Animal Health), Faculty of Veterinary Medicine, Technical University of Lisbon.
We acknowledge the BTVS-ICN (Wild Animal Tissue Bank, Institute for Nature Conservation in Portugal) for most of the samples and all the colleagues and collaborators who collected cats in the field in particular to Carlos Carrapato; Ana Coito from ICNB kindly helped constructing the geographical map and Luka Clarke for the English revision of the manuscript.
References
- Addie D., Belak S., Boucraut-Baralon C., Egberink H., Frymus T., Gruffydd-Jones T., Hartmann K., Hosie M.J., Lloret A., Lutz H., Marsilio F., Pennisi M.G., Radford A.D., Thiry E., Truyen U., Horzinek M.C. Feline infectious peritonitis. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009;11:594–604. doi: 10.1016/j.jfms.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addie D.D., Schaap I.A., Nicolson L., Jarrett O. Persistence and transmission of natural type I feline coronavirus infection. J. Gen. Virol. 2003;84:2735–2744. doi: 10.1099/vir.0.19129-0. [DOI] [PubMed] [Google Scholar]
- Artois M., Remond M. Viral diseases as a threat to free-living wild cats (Felis silvestris) in continental Europe. Vet. Rec. 1994;134:651–652. doi: 10.1136/vr.134.25.651. [DOI] [PubMed] [Google Scholar]
- Daniels M.J., Golder M.C., Jarrett O., MacDonald D.W. Feline viruses in wildcats from Scotland. J. Wildl. Dis. 1999;35:121–124. doi: 10.7589/0090-3558-35.1.121. [DOI] [PubMed] [Google Scholar]
- Daoust P.-Y., McBurney S.R., Godson D.L., van de Bildt M.W.G., Osterhaus A.D.M.E. Canine distemper virus-associated ancephalitis in free-living Lynx (Lynx Canadensis) nnd Bobcats (Lynx Rufus) of eastern Canada. J. Wildl. Dis. 2009;45:611–624. doi: 10.7589/0090-3558-45.3.611. [DOI] [PubMed] [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Desario C., Decaro N., Campolo M., Cavalli A., Cirone F., Elia G., Martella V., Lorusso E., Camero M., Buonavoglia C. Canine parvovirus infection: which diagnostic test for virus? J. Virol. Methods. 2005;126:179–185. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Duarte A., Ferreira J.P., Veiga I., Mota P., Leitão I., Rosado R., Santos-Reis M., Tavares L. Virological survey of rural cats in South-East Portugal. Proceedings of the 4th Congress of the European Society for Emerging Infections, vol. 64; Hotel Tivoli Tejo, Lisboa, Portugal, 30 September–3 October; 2007. [Google Scholar]
- Fernandes M. Seminar on the biology and conservation of the wildcat (Felis silvestris). Environmental encounters, no. 16. Press, CoE; Nancy, France: 1992. Some aspects of the ecology and systematics of the wildcat in Portugal. pp. 89–93. [Google Scholar]
- Ferroglio E., Rossi L., Gennero S. Lung-tissue extract as an alternative to serum for surveillance for brucellosis in chamois. Prev. Vet. Med. 2000;43:117–122. doi: 10.1016/s0167-5877(99)00090-2. [DOI] [PubMed] [Google Scholar]
- Foley J.E., Poland A., Carlson J., Pedersen N.C. Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments. J. Am. Vet. Med. Assoc. 1997;210:1307–1312. [PubMed] [Google Scholar]
- Frisk A.L., Konig M., Moritz A., Baumgartner W. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 1999;37:3634–3643. doi: 10.1128/jcm.37.11.3634-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont E., Sager A., Leger F., Bourguemestre F., Jouquelet E., Stahl P., Pontier D., Artois M. Prevalence and pathogenicity of retroviruses in wildcats in France. Vet. Rec. 2000;146:317–319. doi: 10.1136/vr.146.11.317. [DOI] [PubMed] [Google Scholar]
- Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet. Immunol. Immunopathol. 2011;143:190–201. doi: 10.1016/j.vetimm.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., de Groot R.J., Cepica A., Egberink H.F., Horzinek M.C., Rottier P.J. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR. J. Clin. Microbiol. 1995;33:684–689. doi: 10.1128/jcm.33.3.684-689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Washizu T., Toriyabe T., Motoyochi S., Tomoda I., Pedersen N.C. Feline immunodeficiency virus infection in cats of Japan. J. Am. Vet. Med. Assoc. 1989;194:221–225. [PubMed] [Google Scholar]
- IUCN, 1998. IUCN Guidelines for re-introductions prepared by the IUCN/SSC Re-introduction Specialist group, (IUCN, Gland, Switzerland and Cambridge, UK) 20 p.
- IUCN. 2007 http://ec.europa.eu/environment/nature/conservation/species/redlist/mammals/status.htm, downloaded on June 2010.
- Kapil S., Yeary T.J. Canine distemper spillover in domestic dogs from urban wildlife. Vet. Clin. North Am. Small Anim. Pract. 2011;41:1069–1086. doi: 10.1016/j.cvsm.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener A.C., Yamaguchi N., Ward J.M., Macdonald D.W. A diagnosis for the Scottish wildcat (Felis silvestris): a tool for conservation action for a critically endangered felid. Anim. Conserv. 2005;8:223–237. [Google Scholar]
- Leutenegger C.M., Hofmann-Lehmann R., Riols C., Liberek M., Worel G., Lups P., Fehr D., Hartmann M., Weilenmann P., Lutz H. Viral infections in free-living populations of the European wildcat. J. Wildl. Dis. 1999;35:678–686. doi: 10.7589/0090-3558-35.4.678. [DOI] [PubMed] [Google Scholar]
- Lindmark R., Thorén-Tolling K., Sjöquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J. Immunol. Methods. 1983;62:1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- Meli M.L., Cattori V., Martínez F., López G., Vargas A., Palomares F., López-Bao J.V., Hofmann-Lehmann R., Lutz H. Feline leukemia virus infection: a threat for the survival of the critically endangered Iberian lynx (Lynx pardinus) Vet. Immunol. Immunopathol. 2010;134:61–67. doi: 10.1016/j.vetimm.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli M.L., Cattori V., Martinez F., López G., Vargas A., Simón M.A., Zorrilla I., Muñoz A., Palomares F., López-Bao J.V., Pastor J., Tandon R., Willi B., Hofmann-Lehmann R., Lutz H. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus) PLoS ONE. 2009;4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli M.L., Simmler P., Cattori V., Martínez F., Vargas A., Palomares F., López-Bao J.V., Simón M.A., López G., León-Vizcaino L., Hofmann-Lehmann R., Lutz H. Importance of canine distemper virus (CDV) infection in free-ranging Iberian lynxes (Lynx pardinus) Vet. Microbiol. 2010;146:132–137. doi: 10.1016/j.vetmic.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Millán J., Candela M.G., Palomares F., Cubero M.J., Rodríguez A., Barral M., de la Fuente J., Almería S., León-Vizcaíno L. Disease threats to the endangered Iberian lynx (Lynx pardinus) Vet. J. 2009;182:114–124. doi: 10.1016/j.tvjl.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J., Rodríguez A. A serological survey of common feline pathogens in free-living European wildcats (Felis silvestris) in central Spain. Eur. J. Wildl. Res. 2009;55:285–291. doi: 10.1007/s10344-008-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterroso P., Brito J.C., Ferreras P., Alves P.C. Spatial ecology of the European wildcat in a Mediterranean ecosystem: dealing with small radio-tracking datasets in species conservation. J. Zool. 2009;279:27–35. [Google Scholar]
- Oliveira R., Godinho R., Randi E., Ferrand N., Alves P. Molecular analysis of hybridisation between wild and domestic cats (Felis silvestris) in Portugal: implications for conservation. Conserv. Genet. 2008;9:1–11. [Google Scholar]
- Pierpaoli M., Biro Z.S., Herrmann M., Hupe K., Fernandes M., Ragni B., Szemethy L., Randi E. Genetic distinction of wildcat (Felis silvestris) populations in Europe, and hybridization with domestic cats in Hungary. Mol. Ecol. 2003;12:2585–2598. doi: 10.1046/j.1365-294x.2003.01939.x. [DOI] [PubMed] [Google Scholar]
- Queiroz, A.I. (coord), Alves P.C., Barroso I., Beja P., Fernandes M., Freitas L., Mathias M.L., Mira A., Palmeirim J.M., Preito, R., Rainho, A., Rodrigues, L., Santos-Reis, M., Sequeira, M., 2005. Felis silvestris. In: Cabral, M.J., Almeida, J., Almeida, P.R., Dellinger, T., Ferrand de Almeida, N., Oliveira, M.E., Palmeirim, J.M., Queiroz, A.I., Rogado, L., Santos-Reis, M. (Eds.), Livro Vermelho dos Vertebrados de Portugal, Instituto da Conservação da Natureza, Lisboa, pp. 525–526.
- Roelke M., Johnson W., Millán J., Palomares F., Revilla E., Rodríguez A., Calzada J., Ferreras P., León-Vizcaíno L., Delibes M., O’Brien S. Exposure to disease agents in the endangered Iberian lynx (Lynx pardinus) Eur. J. Wildl. Res. 2008;54:171–178. doi: 10.1007/s10344-007-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos N., Almendra C., Tavares L. Serologic survey for canine distemper virus and canine parvovirus in free-ranging wild carnivores from Portugal. J. Wildl. Dis. 2009;45:221–226. doi: 10.7589/0090-3558-45.1.221. [DOI] [PubMed] [Google Scholar]
- Shackelton L.A., Parrish C.R., Truyen U., Holmes E.C. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc. Natl. Acad. Sci. U.S.A. 2005;102:379–384. doi: 10.1073/pnas.0406765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino R., Arnal M.C., Luco D.F., Gortazar C. Prevalence of antibodies against canine distemper virus and canine parvovirus among foxes and wolves from Spain. Vet. Microbiol. 2008;126:251–256. doi: 10.1016/j.vetmic.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Steinel A., Parrish C.R., Bloom M.E., Truyen U. Parvovirus infections in wild carnivores. J. Wildl. Dis. 2001;37:594–607. doi: 10.7589/0090-3558-37.3.594. [DOI] [PubMed] [Google Scholar]
- Thorne E.T., Williams E.S. Disease and endangered species: the black-footed ferret as a recent example. Conserv. Biol. 1988;2:66–74. [Google Scholar]
- Tryland M., Handeland K., Bratberg A., Solbakk I., Oksanen A. Persistence of antibodies in blood and body fluids in decaying fox carcasses, as exemplified by antibodies against Microsporum canis. Acta Vet. Scand. 2006;48:10. doi: 10.1186/1751-0147-48-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


