Highlights
-
•
CH25H is not an interferon-stimulated gene (ISG) in Vero cells.
-
•
CH25H and 25HC inhibit PEDV infection through blocking viral penetration.
-
•
CH25H-M still restricts PEDV replication.
-
•
25HC has a broad-spectrum antiviral effect against porcine intestinal coronaviruses, including PEDV and TGEV.
Keywords: Cholesterol 25-hydroxylase, Porcine epidemic diarrhea virus, 25-hydroxycholesterol, Porcine transmissible gastroenteritis virus
Abstract
Cholesterol 25-hydroxylase (CH25H) has been shown lately to be a host restriction factor that encodes an enzyme, which catalyzes the oxidized form of cholesterol to 25-hydroxycholesterol (25HC). A series of studies have shown that 25HC activity in hosts plays a vital role in inhibiting viral infection. In this study, we explored the antiviral effect of CH25H and 25HC on porcine epidemic diarrhea virus (PEDV), which causes high mortality rates in newborn piglets with severe diarrhea, and considerable financial loss in the swine industry worldwide. Our results showed that PEDV infection downregulated the expression of CH25H in Vero cells. An overexpression and knockdown assay indicated that CH25H has significant antiviral action against PEDV, and a CH25H mutant (CH25H-M) that lacks hydroxylase activity also retains antiviral activity to a lesser extent. Furthermore, 25HC had a broad-spectrum antiviral effect against different PEDV strains by blocking viral entry. In addition, CH25H and 25HC inhibited the replication of porcine transmissible gastroenteritis virus (TGEV). Taken together, CH25H as a natural host restriction factor could inhibit PEDV and TGEV infection.
1. Introduction
Porcine epidemic diarrhea virus (PEDV), a member of the alphacoronaviruses (family Coronaviridae, order Nidovirales), is a single-stranded, positive-sense RNA virus that causes disease in swine with great financial losses (Chen et al., 2012). The PEDV genome comprises two overlapping open reading frames (ORFs) encoding two polyproteins (pp1a and pp1ab) and five other proteins, spike (S), ORF3, envelope (E), membrane (M), and nucleocapsid (N). Several mutations have been identified in the S (spike) 1 region in recent years. Small intestinal epithelial cells in vivo are primarily attacked by PEDV, which causes acute watery diarrhea, vomiting, dehydration, and anorexia in pigs of all ages (Li et al., 2012; Wang et al., 2014). After first being discovered in the 1970s in Europe, PEDV became widespread with significant mortality and morbidity, particularly in nursing piglets (Langel et al., 2016). Although PEDV vaccines have been available in China, the preventive effect is not evident and infectious cases still occur in the swine industry. Therefore, new anti-PEDV methods are urgently needed.
For over 50 years, lipid components, including cholesterol and its derivatives, have been studied as important structural components of physiological processes. Crucial cellular processes require the maintenance of sterol homeostasis. As a family of transcription factors, the sterol regulatory element-binding proteins (SREBPs) are considered modulators of lipid biosynthesis. One antiviral lipid effector, 25-hydroxycholesterol (25HC), is reportedly able to suppress cholesterol biosynthesis by repressing the activation of SREBPs (Holmes et al., 2011). Newly translated SREBPs bind the SREBP cleavage-activating protein (SCAP) at the endoplasmic reticulum (ER). Under conditions of sterol depletion, this SREBP–SCAP complex is transported to the Golgi apparatus, and site-1 protease (S1P) and site-2 protease (S2P) are used to cleave SREBP into its mature transcription factor form (Olsen et al., 2011). Under conditions of excess lipid, SCAP undergoes a conformational change that causes it to bind to insulin-induced genes (INSIGs). Retention of the SREBP–SCAP complex in the ER is accelerated by 25HC, not via direct action with SCAP, but through the overexpression of INSIGs that control the sterol biosynthesis pathway. Furthermore, a degenerative-feedback mechanism for the regulation of cholesterol biosynthesis has been recognized, which is associated with the expression of HMG-CoA reductase (HMGCR) (Singaravelu et al., 2015). Rapid proteasomal degradation can be effected by HMGCR through high cellular 25HC levels, independent of SREBPs (Singaravelu et al., 2015). Therefore, 25HC has been regarded as an inhibitor of cholesterol.
The host innate immune system is regarded as the principal factor of immune defense against viral infection. Interferons (IFNs) have been found to induce hundreds of IFN-stimulated genes (ISGs) that have been identified as a broader range of inhibitors against various viruses, such as protein kinase R, viperin, IFITM1, ribonuclease, and MX protein (Raniga and Liang, 2018). Cholesterol 25-hydroxylase (CH25H) encodes an ER-associated enzyme that catalyzes the oxidation of cholesterol to 25HC (Blanc et al., 2013; Hotter et al., 2013). Recent reports have revealed that CH25H inhibits many enveloped viruses, including vesicular stomatitis virus (VSV) (Liu et al., 2013), human immunodeficiency virus (HIV) (Moog et al., 1998), hepatitis C virus (HCV) (Anggakusuma et al., 2015), Ebola virus (EBOV) (Liu et al., 2013), porcine reproductive and respiratory syndrome virus (PRRSV) (Song et al., 2017), pseudorabies virus (PRV) (Wang et al., 2017) and Zika virus (Li et al., 2017a), as well as some non-enveloped viruses, such as human rotavirus and human rhinovirus (Fessler, 2016). However, the antiviral effects and mechanisms of CH25H and 25HC on PEDV are still unclear.
In this study, we investigated the effect of CH25H on PEDV replication. We demonstrated that CH25H is a host restriction factor that acts against PEDV in Vero cells, and its oxidation product, 25HC, also dramatically inhibits PEDV infection by blocking viral entry. Further study revealed that CH25H-M still inhibits PEDV replication. Furthermore, 25HC has a broad-spectrum antiviral effect against PEDV and CH25H, and plays an important role in TGEV infection.
2. Materials and methods
2.1. Cell culture and viruses
Vero cells and ST cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen Corporation, CA, USA) with 10% fetal bovine serum (Lonsera, Uruguay). The cells were incubated at 37 °C in a humidified incubator with 5% CO2. The PEDV CV777 strain, as a classical strain, was maintained in our laboratory and its titer was 106.66 TCID50 (50% tissue culture infective dose)/mL. The PEDV YZ and MS strains belong to G2b variant strains, and the SH strain is an absent strain in china, which lacks 36 bases in the N protein (Not registered). These viral strains were isolated from small intestinal samples of pigs with PED symptoms and maintained in our laboratory. The titer of the PEDV YZ strain and the SH strain were both 105.5 TCID50/mL, and that of the PEDV MS strain was 105 TCID50/mL. The TGEV strain was conserved in our laboratory and its titer was 107 TCID50/mL.
2.2. Plasmids and small interfering RNA (siRNA) assay
The pCAGGS-CH25H-Flag was constructed and maintained in our laboratory. In addition, pCAGGS-CH25H-M-Flag lacking catalytic activity converted histidine residues 242 and 243 to glutamines by site-directed mutagenesis and were constructed and conserved in our laboratory (Song et al., 2017). Vero cells or ST cells placed in 24-well plates were transfected with 1 μg of pCAGGS-CH25H-Flag, pCAGGS-CH25H-M-Flag, or pCAGGS using Lipmax as a transfection reagent (SUDGEN, Beijing, China), according to the manufacturer’s manual. Three siRNAs targeting CH25H were designed and synthesized by Invitrogen. The primer sequences used were as follows: siRNA1 (5′-CCUUCUUCCCGGUCAUCUUTT-3′, 5′-AAGAUGACCGGGAAGAAGGTT-3′); siRNA2 (5′-CCUGCAUCACUCUCACUUUTT-3′, 5′-AAAGUGAGAGUGAUGCAGGTT-3′); and siRNA3 (5′-GCAACUUCGCUCCGUACUUTT-3′, 5′-AAGUACGGAGCGAAGUUGCTT-3′). The Vero cells growing in 24-well plates were transfected with siRNAs or negative control (NC) using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen), following the manufacturer’s instructions. At 48 h post-transfection, the levels of CH25H were tested by quantitative real-time PCR (qRT-PCR).
2.3. Total RNA extraction and qRT-PCR
Total RNA was extracted from Vero cells using an OMEGA Total RNA Kit (Omega Bio-tek, USA) and reverse transcribed into cDNA using HiScript Q RT SuperMix for qPCR (Vazyme, China), both in accordance with the manufacturer’s recommendations. The following primers were used: PEDV N (F 5′-CGTACAGGTAAGTCAATTAC-3′, R 5′-GATGAAGCATTGACTGAA-3′); mCH25H (F 5′-CGCAGTATATGAGCGTCTGG-3′, R 5′-AAGGGAAGTTGTAGCCGGAG-3′); mGAPDH(F 5′-CCTTCCGTGTCCCTACTGCCAAC-3′, R 5′-GACGCCTGCTTCACCACCTTCT-3′) (Zhang et al., 2016); TGEV N (F 5′-CAATTCCCGTGGTCGGAAGA-3′, R 5′-TTTACGTTGCCCTTCACCA-3′). The qRT-PCR was carried out on the ABI QuantStudio 6 and 7 systems (Applied Biosystems) using SYBR Green Real-time PCR Master Mixes (Applied Biosystems, Foster City, CA, USA). The PCR procedure was as follows: 5 min at 95 °C for initial denaturation, followed by 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. The data were analyzed using the ΔΔCt method.
2.4. Immunofluorescence staining
Vero cells were fixed with 4% paraformaldehyde for 20 min, washed with PBS, and then permeabilized with 0.1% Triton X-100 for 20 min at 37 °C. The treated cells were labeled with a mouse anti-PEDV mAb (monoclonal antibody) made in our laboratory (1:200 dilution) for 2 h at 37 °C. The cells were then washed three times with PBS and incubated with FITC-labeled goat anti-mouse IgG (H + L) antibody (1:200 dilution) (Beyotime, China) at 37 °C for 1 h. After being washed with PBS, the cells were visualized using confocal laser-scanning microscopy (Axiovert 200, Zeiss, Germany).
2.5. Western blotting
Treated cells were washed with cold PBS twice and lysed with 100 μL of RIPA Lysis Buffer (Beyotime, China). The cell debris was removed from the lysates by centrifugation at 12,500 rpm for 10 min and the supernatant was collected. Equal concentrations of lysates were determined with a BCA Protein Assay Kit (CWBIO, China) and separated via 12% SDS-PAGE. The separated proteins were then blotted onto nitrocellulose membranes at 23 V for 35 min. Subsequently, the membranes were blocked in 10% nonfat milk at 4 °C for 2 h. After being washed with PBS, the membranes were incubated with primary antibody, including mouse mAbs (1:1000) and anti-β-actin antibody (1:1000) (Sigma-Aldrich, USA) at 4 °C for 2 h. The membranes were washed three times with PBS, after which horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (H + L) (Beyotime, China) was added. The membranes were then incubated for 1 h at 4 °C. After being washed three times with PBS, the proteins on the membranes were observed using the Chemistar High-sig ECL western blotting substrate (Tanon, China).
2.6. TCID50 assay for PEDV and TGEV
Vero cells and ST cells placed in 96-well plates were infected with 8-fold serial dilutions of PEDV or TGEV samples in four replicates. The plates were incubated for 48–72 h at 37 °C. The PEDV or TGEV titers were evaluated through the Reed–Muench method.
2.7. Statistical analysis
All data were analyzed by one-way analysis of variance using the GraphPad Prism 6.0 software (GraphPad, La Jolla, CA, USA). Differences between two groups were indicated as follows: * (P > 0.05), ** (P < 0.05), and *** (P < 0.01). Every experiment was performed with three biological replicates, and the results were recorded as mean ± SD.
3. Results
3.1. PEDV infection downregulates CH25H expression, and CH25H is not an ISG in Vero cells
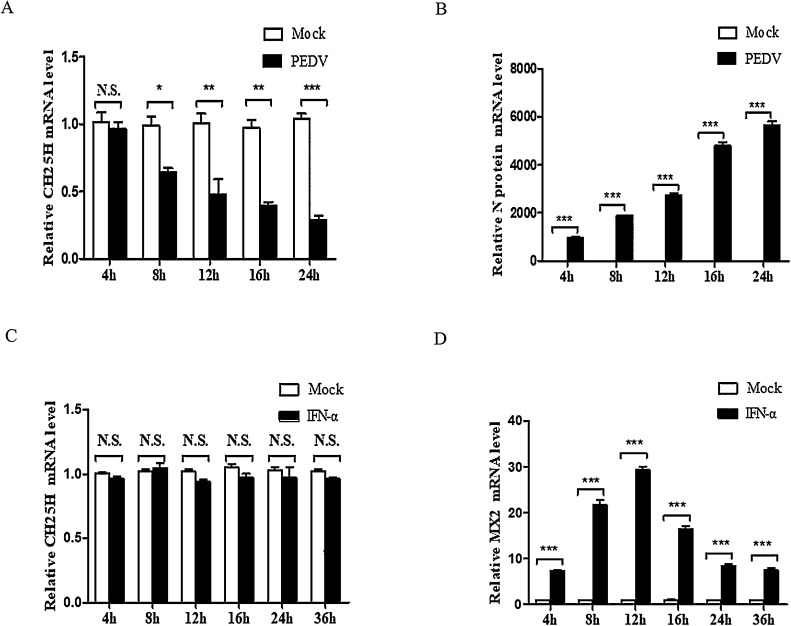
A number of studies have illustrated that CH25H can be upregulated by some viruses, including HCV, PRRSV, and PRV (Anggakusuma et al., 2015; Song et al., 2017; Wang et al., 2017). In the present study, we investigated whether PEDV infection could upregulate CH25H expression in Vero cells. Vero cells seeded in 24-well plates were infected with the PEDV YZ strain (at an MOI [multiplicity of infection] of 0.1) containing 8 μg/mL trypsin, and incubated for 4, 8, 12, 16, and 24 h at 37 °C. The mRNA levels of CH25H and PEDV N protein were measured by qRT-PCR. In contrast, CH25H mRNA levels in Vero cells were significantly downregulated by PEDV infection with increasing time (Fig. 1 A) and PEDV N protein mRNA levels were significantly increased with time (Fig. 1B). In addition, Vero cells were treated with 20,000 IU/mL IFN-α (Song et al., 2017) and incubated for 4, 8, 12, 16, 24, and 36 h at 37 °C. The CH25H and MX2 mRNA levels were measured by qRT-PCR. The results indicated that the CH25H mRNA levels apparently showed no fluctuations. In addition, CH25H was evidently not an ISG in Vero cells (Fig. 1C), and MX2 mRNA levels were induced by IFN-α (Fig. 1D).
Fig. 1.
Porcine epidemic diarrhea virus (PEDV) infection downregulates cholesterol 25-hydroxylase (CH25 H) expression, and CH25H is not an interferon (IFN)-stimulated gene (ISG) in Vero cells. (A–B) Vero cells were infected with the PEDV YZ strain at a MOI of 0.1, containing 8 μg/mL trypsin and maintained at 37 °C. The cell samples were harvested at 4, 8, 12, 16, and 24 h, and CH25H and PEDV N protein mRNA levels were detected by qRT-PCR. (C–D) Vero cells cultured in 24-well plates were treated with 20,000 IU/mL IFN-α and incubated for 4, 8, 12, 16, 24, and 36 h at 37 °C. The CH25H and MX2 mRNA levels were measured by qRT-PCR. Results are presented as the mean ± SD of data from three independent experiments. *, (P > 0.05); **, P < 0.05; ***, P < 0.01.
3.2. Overexpression of CH25H inhibits PEDV replication
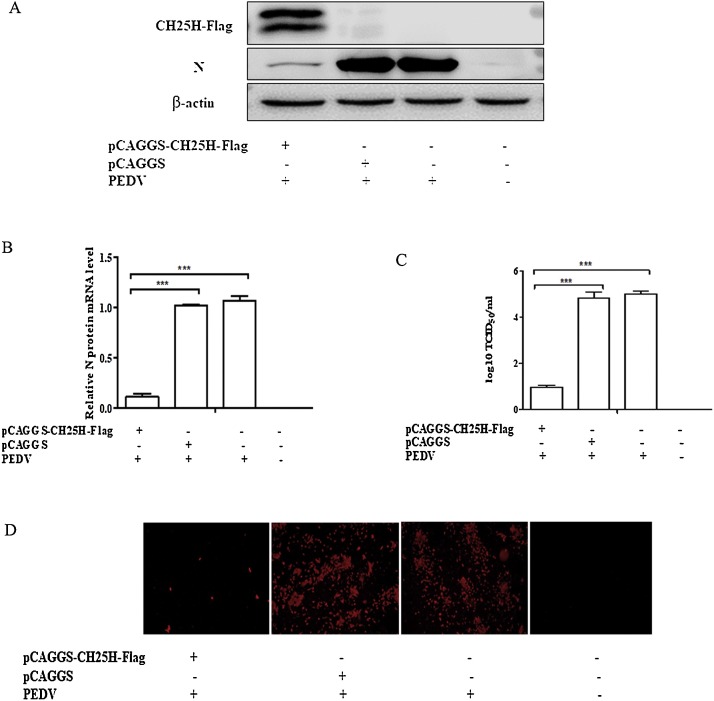
To investigate whether CH25H could suppress PEDV replication, pCAGGS or pCAGGS-CH25H-Flag was used to transiently transfect Vero cells. Subsequently, the cells were infected with the PEDV YZ strain (at a MOI of 0.01) containing 8 μg/mL trypsin. After 24 h, the cell samples were analyzed by western blotting (Fig. 2 A), qRT-PCR (Fig. 2B), and immunofluorescent assay (IFA) (Fig. 2D). The supernatant was used to measure the TCID50 (Fig. 2C). The results showed that the PEDV N protein and mRNA levels of the treatment group were significantly lower than those of the empty vector control group. The IFA assay demonstrated that PEDV was significantly repressed. In addition, the viral titers of the treatment group were also less than those of the control group. The overexpression of CH25H can repress PEDV infection.
Fig. 2.
Cholesterol 25-hydroxylase (CH25 H) overexpression inhibits porcine epidemic diarrhea virus (PEDV) replication. (A) Vero cells were transfected with a plasmid expressing CH25H or an empty vector. After 24 h, cells were infected with the PEDV YZ strain (at a MOI of 0.01) containing 8 μg/mL trypsin. Cell lysates were then harvested using lysis buffer. Western blotting was used to detect the PEDV N protein, CH25H, and β-actin. (B–D) Vero cells transfected with pCAGGS-CH25H-Flag or pCAGGS were infected with the PEDV YZ strain at a MOI of 0.01 at 24 h post-transfection. (B) The PEDV mRNA levels were evaluated by qRT-PCR. (C) The supernatant was used to measure viral titers by TCID50 analysis. (D) The amount of PEDV was measured by IFA. Results are presented as mean ± SD of data from three independent experiments. ***, P < 0.01.
3.3. CH25H mutant lacking hydroxylase activity (CH25H-M) also suppresses PEDV replication
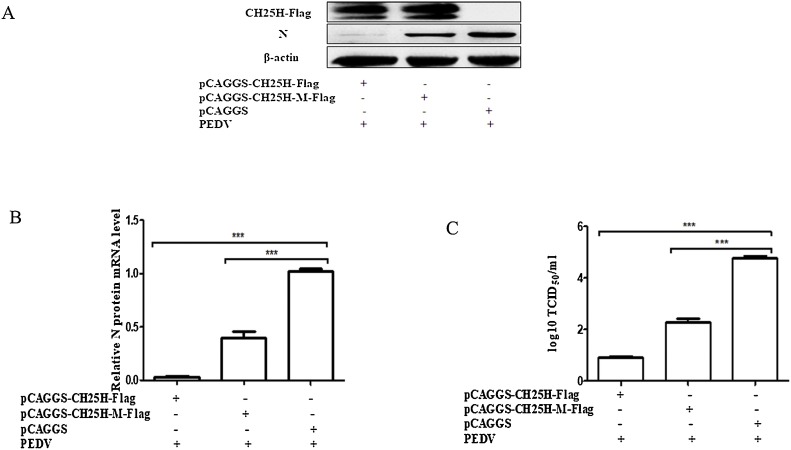
The CH25H-M, which lacks catalytic activity owing to the conversion of histidine residues 242 and 243 to glutamines, and is incapable of producing 25HC, was created by site-directed mutagenesis and maintained in our laboratory (Song et al., 2017). To investigate whether the antiviral effect of CH25H was associated with enzymatic activity, Vero cells were transfected with plasmids containing pCAGGS-CH25H-Flag, pCAGGS-CH25H-M-Flag, and an empty vector, and then infected with the PEDV YZ strain (MOI of 0.01) containing 8 μg/mL trypsin at 24 h post-transfection. Western blotting (Fig. 3 A) and qRT-PCR (Fig. 3B) were used to examine protein expression and PEDV mRNA levels. The supernatant was harvested and the TCID50 analyzed (Fig. 3C). The PEDV N protein expression and PEDV N protein mRNA levels of the CH25H-M group were significantly less than those of the control group, but greater than those of the CH25H group. The overexpression of CH25H-M evidently reduced PEDV titers. These results indicate that CH25H lacking enzymatic activity can also suppress PEDV replication.
Fig. 3.
The cholesterol 25-hydroxylase mutant (CH25H-M) lacking hydroxylase activity also suppresses porcine epidemic diarrhea virus (PEDV) replication. (A–C) Vero cells cultured in a 24-well plate were transfected with pCAGGS-CH25H-Flag, pCAGGS-CH25H-M-Flag, and an empty vector for 24 h and then infected with the PEDV YZ strain at a MOI of 0.01, to which 8 μg/mL trypsin was added. After 18 h, the cell samples were collected and analyzed by (A) western blotting, (B) qRT-PCR, and (C) TCID50. Results are presented as mean ± SD of data from three independent experiments. ***, P < 0.01.
3.4. Knockdown of CH25H by siRNAs can enhance PEDV replication
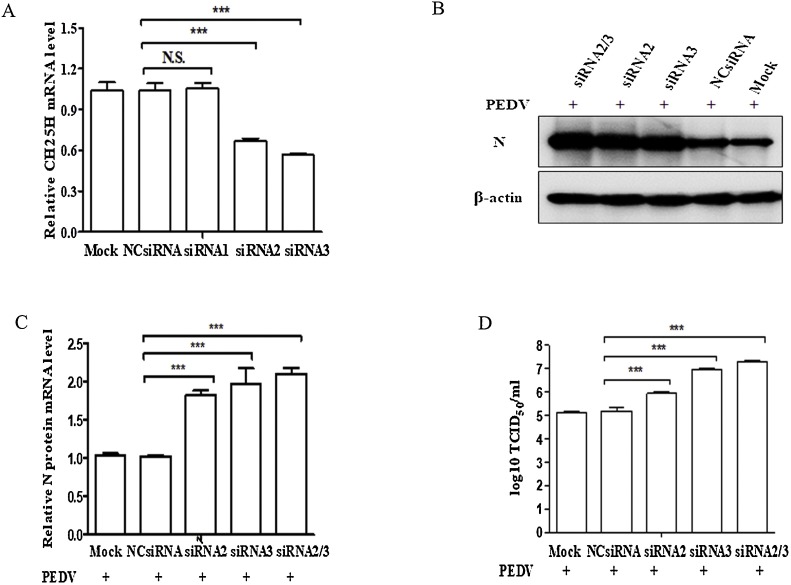
We investigated whether CH25H affects the replication of PEDV by using siRNA targeting CH25H. We first designed three siRNAs targeting CH25H and selected an appropriate concentration to suppress the expression of CH25H. Vero cells were transfected with the three siRNAs and negative control siRNA (NCsiRNA) separately. After 48 h, the CH25H mRNA levels were detected by qRT-PCR (Fig. 4 A). The results showed that siRNA2 and siRNA3 significantly downregulated the CH25H mRNA levels in Vero cells. Subsequently, we chose siRNA2 and siRNA3 for interference experiments. Vero cells were transfected with siRNA2, siRNA3, siRNA2/3, and NC, and infected with the PEDV YZ strain (MOI of 0.01) containing 8 μg/mL trypsin at 48 h post-transfection. After CH25H was depleted, PEDV replication was determined by western blotting (Fig. 4B), qRT-PCR (Fig. 4C), and TCID50 values (Fig. 4D) in Vero cells. As shown in Fig. 4B–C, PEDV N protein expression and mRNA levels were increased in response to the downregulation of CH25H, compared to cells transfected with NC. The TCID50 assays also demonstrated that knockdown of CH25H significantly increased viral titers (Fig. 4D).
Fig. 4.
Knockdown of cholesterol 25-hydroxylase (CH25 H) by siRNAs can enhance replication of porcine epidemic diarrhea virus (PEDV). (A) Vero cells were transfected with siRNAs or negative control (NC). At 48 h post-transfection, the knockdown efficiency of CH25H was determined by qRT-PCR. (B–D) Vero cells placed in 24-well plates were transfected with siRNAs (siRNA2, siRNA3, and siRNA2/3) for 48 h and infected with the PEDV YZ strain (MOI of 0.01) containing 8 μg/mL trypsin. Cells were collected to measure levels of the PEDV N protein by western blotting with anti-N mouse mAb (B), and PEDV mRNA levels by qRT-PCR (C). The viruses in cells were frozen and thawed twice and tested by the TCID50 assay (D). Results are presented as mean ± SD of data from three independent experiments. ***, P < 0.01.
3.5. 25HC inhibits PEDV infection
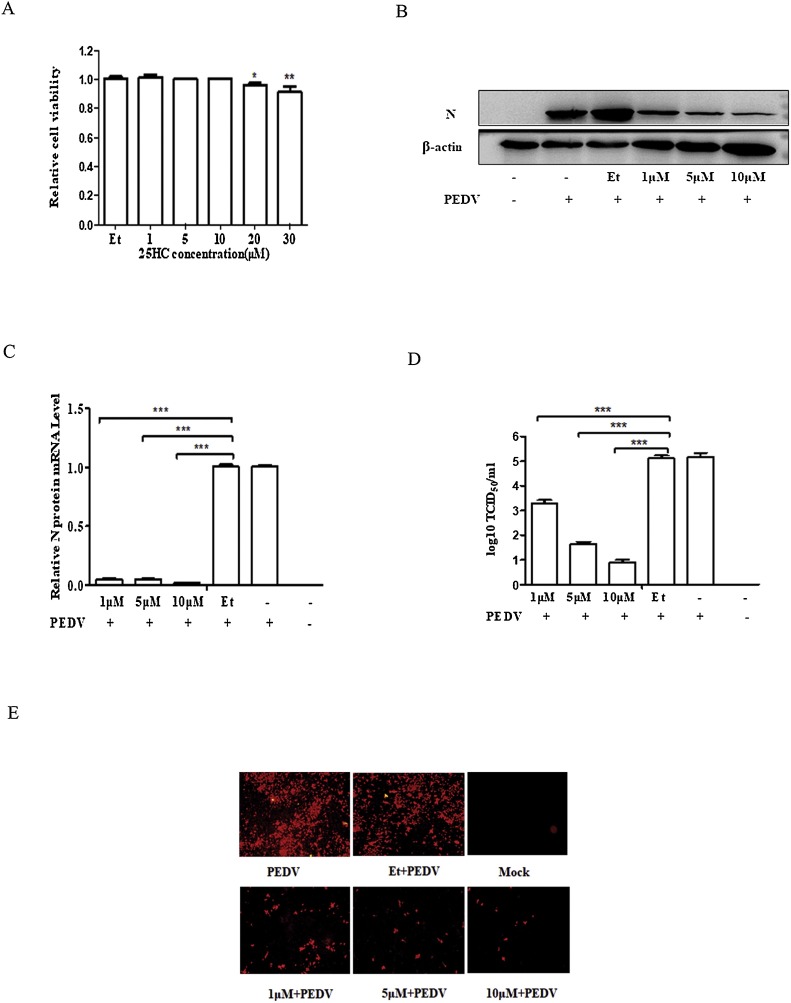
We further investigated the suppressive effect of 25HC against PEDV infection. We explored the optimal concentration of 25HC and performed a cytotoxicity assay with a Sigma-Aldrich Cell Counting Kit 8. Vero cells cultured in 24-well plates were mixed with various concentrations of 25HC (1, 5, 10, 20, and 30 μM) and Ethanol (Et) for 24 h. The cells were then harvested to determine the cytotoxicity effect (Fig. 5 A). The results demonstrated that a 25HC concentration of less than 20 μM was the optimal concentration for Vero cells. The antiviral activity of 25HC against PEDV was then analyzed. Vero cells were pretreated with 1, 5, and 10 μM 25HC and Et for 1 h, and then infected with the PEDV YZ strain (MOI of 0.01) containing 8 μg/mL trypsin. Western blotting (Fig. 5B), qRT-PCR (Fig. 5C), TCID50 assay (Fig. 5D), and IFA (Fig. 5E) were used to measure viral replication levels. As depicted in Fig. 5B–E, treatment with 25HC can significantly inhibit PEDV infection in a dose-dependent manner.
Fig. 5.
25-Hydroxycholesterol (25HC) inhibits porcine epidemic diarrhea virus (PEDV) infection. (A) Cytotoxicity assay of 25HC against Vero cells. Vero cells were incubated with different concentrations of 25HC or Et for 24 h, and were tested by a Sigma-Aldrich Cell Counting Kit 8. (B–E) Vero cells were pretreated with 25HC at the indicated concentrations for 1 h, and were then infected with the PEDV YZ strain (MOI of 0.01) to which 8 μg/mL trypsin was added. The various concentrations of 25HC were again added to infected cells at 37 °C for 18 h. Cell samples were collected and examined by western blotting (B), qRT-PCR (C), and IFA (E). Viral titers were tested by the TCID50 assay (D). Results are presented as mean ± SD of data from three independent experiments. * (P > 0.05); **, P < 0.05; ***, P < 0.01.
3.6. 25HC inhibits PEDV infection by blocking viral entry
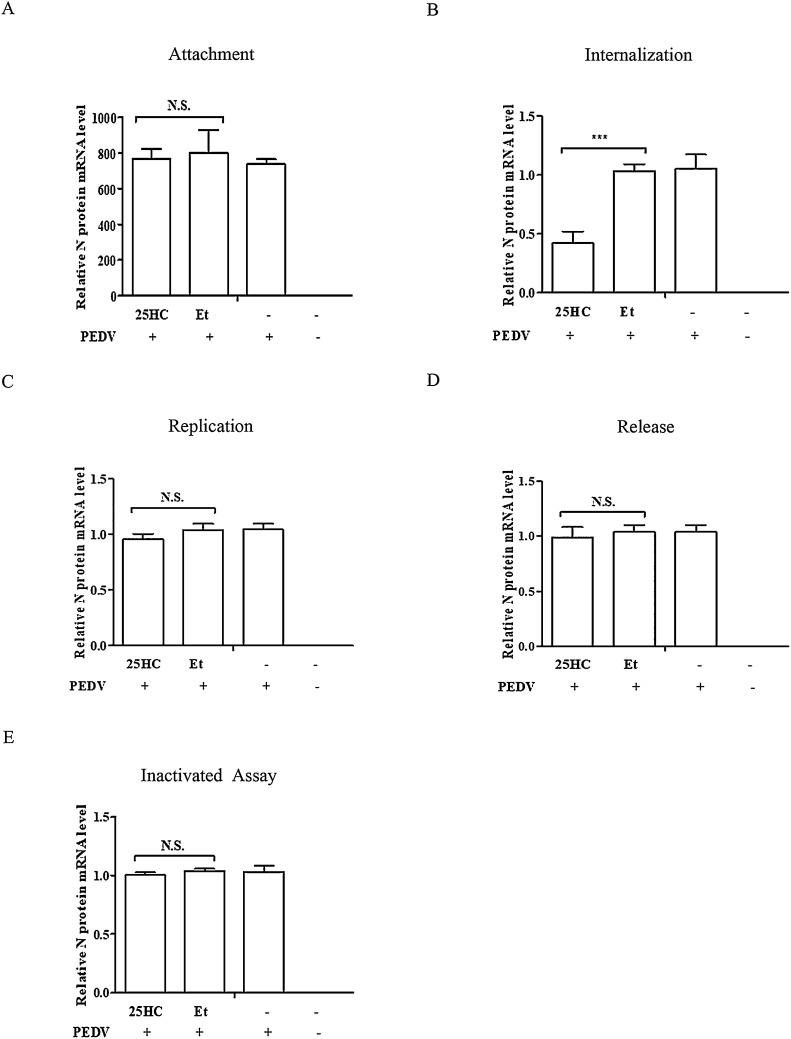
Our next experiment was undertaken to determine which stage of PEDV infection was inhibited by 25HC. We examined the effect of 25HC on PEDV at the adsorption, internalization, replication, and release stages of the viral life cycle. For the attachment assay, Vero cells were pretreated using 25HC (10 μM) or Et for 1 h at 37 °C, and then infected with the PEDV YZ strain (MOI of 0.1) containing 8 μg/mL trypsin for 1 h at 4 °C. The mRNA levels of the PEDV N protein were analyzed by qRT-PCR. As shown in Fig. 6 A, 25HC evidently did not affect viral adsorption. Subsequently, the penetration assay was performed in Vero cells. The cells were infected with the PEDV YZ strain (MOI of 0.1) containing 8 μg/mL trypsin at 4 °C for 1 h, after which 25HC (10 μM) or Et was added and the mixture was incubated at 37 °C for 2 h. The cell samples were tested by qRT-PCR (Fig. 6B). The results showed that 25HC inhibits PEDV internalization.
Fig. 6.
25-Hydroxycholesterol (25HC) inhibits porcine epidemic diarrhea virus (PEDV) infection by blocking viral entry. (A) Adsorption assay. Vero cells cultured in 24-well plates were pretreated using 25HC (10 μM) or Et for 1 h at 37 °C. The media were then replaced by a mixture of 25HC (10 μM) or Et and PEDV (MOI of 0.1) containing 8 μg/mL trypsin for 1 h at 4 °C. After washing three times with PBS, the mRNA levels of the PEDV N protein were measured by qRT-PCR. (B) Penetration assay. Vero cells cultured in 24-well plates were infected with the PEDV YZ strain (MOI of 0.1) containing 8 μg/mL trypsin for 1 h at 4 °C. They were then treated with 25HC (10 μM) or Et for 2 h at 37 °C, after which they were washed three times with PBS. The cell samples were washed with sodium citrate buffer and tested by qRT-PCR. (C) Replication assay. Cells infected with the PEDV YZ strain (MOI of 0.1) containing 8 μg/mL trypsin were incubated at 37 °C for 6 h and washed three times with sodium citrate buffer. Cells were then treated with 25HC (10 μM) or Et for 10 h. The cells were harvested and examined by qRT-PCR. (D) Release assay. Vero cells were infected with the PEDV YZ strain (MOI of 0.1) containing 8 μg/mL trypsin for 16 h and 25HC (10 μM) or Et was added to the cells for 1 h. The mRNA levels of the virus were evaluated by qRT-PCR. (E) Inactivated assay. 25HC (10 μM) or Et and PEDV (MOI of 0.1) containing 8 μg/mL trypsin were incubated at 37 °C for 3 h. Vero cells cultured in 24-well plates were pre-chilled for 1 h at 4 °C, and then replaced by a mixture of 25HC or Et and PEDV at 4 °C for 2 h. After being washed three times with PBS, DMEM containing 8 μg/mL trypsin was added to the media and the mixture was incubated for 16 h at 37 °C. The mRNA levels of the PEDV N protein were tested by qRT-PCR. Results are presented as mean ± SD of data from three independent experiments. ***, P < 0.01.
A replication assay was also conducted in Vero cells. The cells were infected with the PEDV YZ strain (MOI of 0.1) containing 8 μg/mL trypsin, and incubated at 37 °C for 6 h, after which 25HC (10 μM) or Et was added and the mixture was incubated further at 37 °C for 10 h. The cells were harvested and examined by qRT-PCR (Fig. 6C). The replication assay illustrated that viral replication was not disturbed by 25HC. Furthermore, we investigated the effect of 25HC on viral release. Vero cells were infected with the PEDV YZ strain (MOI of 0.1) containing 8 μg/mL trypsin for 16 h and treated with 25HC (10 μM) or Et for 1 h. The qRT-PCR was used to test the mRNA levels of the virus. As shown in Fig. 6D, viral release was not influenced by 25HC.
The inactivation assay was performed to investigate whether 25HC directly kills PEDV particles. The results showed that 25HC did not exert a direct virucidal effect on PEDV (Fig. 6E). These assays demonstrated that 25HC inhibits PEDV infection by blocking viral entry.
3.7. 25HC has a broad-spectrum antiviral effect against porcine intestinal coronaviruses, including PEDV and TGEV
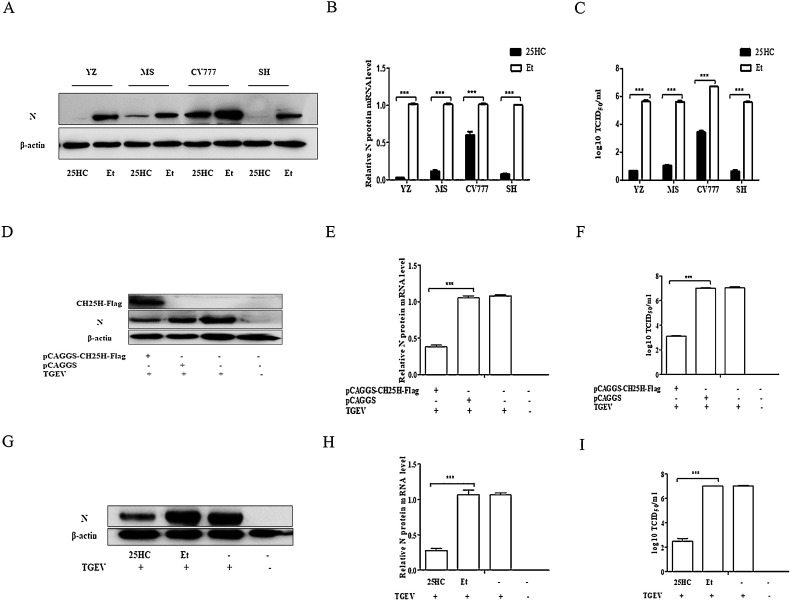
An experiment was carried out to investigate whether 25HC has a broad-spectrum antiviral effect against PEDV. Four viral strains of PEDV were used, including the YZ, MS, CV777, and SH strains. The PEDV YZ and MS strains are variant strains, CV777 is a classic strain, and SH is a variant strain with a partly missing N genome. Vero cells were treated with 25HC (10 μM) and Et for 1 h. The cells were then infected individually with the PEDV YZ strain, MS strain, CV777 strain, and SH strain (MOI of 0.01) for 18 h, all of which contained 8 μg/mL trypsin. The cell samples were harvested and measured by western blotting (Fig. 7 A) and qRT-PCR (Fig. 7B). The supernatant was used to assess the TCID50 (Fig. 7C). The results showed that 25HC can significantly repress infection with PEDV YZ, MS, and SH strains. Although CV777 infection was suppressed by 25HC, the inhibitory effect was inferior to that shown by the other three viral strains.
Fig. 7.
25-Hydroxycholesterol (25HC) has a broad-spectrum antiviral effect against porcine intestinal coronaviruses, including porcine epidemic diarrhea virus (PEDV) and transmissible gastroenteritis virus (TGEV). (A–C) Vero cells cultured in 24-well plates were pretreated with 25HC (10 μM) and Et for 1 h, and cells were then separately infected with the PEDV YZ strain (MOI of 0.01), MS strain, CV777 strain, and SH strain (all containing 8 μg/mL trypsin) for 18 h. (A) The N protein level of PEDV in the cell samples was tested by western blotting, and (B) the PEDV mRNA levels were measured by qRT-PCR. (C) Viral titers were detected using TCID50 analysis. (D–F) ST cells cultured in 24-well plates were transiently transfected with pCAGGS-CH25H-Flag or pCAGGS for 24 h, the cells were infected with TGEV (MOI of 0.01). At 18 h post infection, western blotting (D) and qRT-PCR (E) were used to detect the TGEV N protein, and viral titer was measured by TCID50 (F). (G–I) ST cells cultured in 24-well plates were pretreated with 10 μM of 25HC or Et for 1 h, and then infected with TGEV (MOI of 0.01) for 18 h. Western blotting (G), qRT-PCR (H), and TCID50 (I) were used to measure levels of the TGEV N protein and viral titer. Results are presented as mean ± SD of data from three independent experiments. **, P < 0.05; ***, P < 0.01.
Both TGEV and PEDV belong to porcine coronavirus family. To investigate whether CH25H and 25HC can repress TGEV replication, ST cells were transiently transfected with pCAGGS- CH25H-Flag or pCAGGS, following which, they were infected with TGEV (MOI of 0.01) for 18 h. Western blotting (Fig. 7D), qRT-PCR (Fig. 7E), and the TCID50 assay (Fig. 7F) were performed to analyze the replication of TGEV. The results illustrated that TGEV replication was significantly suppressed by overexpression of CH25H. The ST cells were pretreated with 10 μM 25HC and Et for 1 h, and then infected with TGEV (MOI of 0.01) for 18 h. Western blotting (Fig. 7G), qRT-PCR (Fig. 7H), and the TCID50 assay (Fig. 7I) were used to detect the inhibitory effects against TGEV. As expected, 25HC significantly repressed TGEV replication.
4. Discussion
Host restriction factors play a crucial role against a variety of viruses, with which they generally interact during the specific period of viral replication to block infection; these interactions provide opportunities for potential new antiviral therapies (Liu et al., 2012). Infection with PEDV is characterized by acute enteritis, vomiting, diarrhea, and dehydration. It is a major pathogen that causes considerable economic loss in the swine industry (Zeng et al., 2015). At present, many anti-PEDV therapies are in general use, including recombinant Lactobacillus casei expressing dendritic cell-targeting peptide fused with the COE protein of PEDV (Langel et al., 2016), and IFN-lambda, which preferably inhibits PEDV infection (Li et al., 2017b). Previous studies revealed that CH25H can be induced by several viruses, including HCV, PRRSV, and PRV (Anggakusuma et al., 2015; Song et al., 2017; Wang et al., 2017). In contrast, the present study demonstrates that CH25H expression can be downregulated by PEDV infection, and CH25H cannot be induced by IFN-α in Vero cells. The results also indicate that CH25H is not an ISG in Vero cells.
Previous studies have indicated that CH25H has an antiviral effect against some viruses and generates antiviral activities through the production of 25HC. For example, CH25H restricts HCV replication through the obstruction of membranous web formation (Anggakusuma et al., 2015); CH25H inhibits PRV by blocking viral attachment and internalization (Wang et al., 2017); 25HC reduces PRRSV infection by inhibiting viral penetration but does not affect adsorption, replication, or release of PRRSV (Ke et al., 2017); and 25HC suppresses Lassa virus infection through aberrant GP1 glycosylation (Shrivastava-Ranjan et al., 2016). In the present study, the overexpression of CH25H or 25HC inhibited PEDV infection by impeding virion entry, which is similar to that observed in PRRSV. In addition, the catalytically inactive form of CH25H retains antiviral action against HCV and PRRSV (Chen et al., 2014). We further investigated the antiviral activities of CH25H-M on PEDV. Similar results were observed, such that CH25H-M still restricted PEDV infection, although the antiviral effect was attenuated. These findings demonstrate that CH25H does not depend entirely on enzymatic activity against PEDV.
Moreover, 25HC plays an important role in the regulation of inflammation (Reboldi et al., 2014). Gold et al. (2014) have indicated that 25HC in macrophages is deemed an amplifier of inflammatory signaling. The activation is mediated by jun proto-oncogene (JUN) and the recruitment of activator protein 1 to the boosters of a subset of Toll-like receptor-responsive genes (Gold et al., 2014). The inflammatory effects are mediated by 25HC via activation of NF-κB signaling, leading to the increased release of interleukin 6 and interleukin 8 (Rydberg et al., 2003). In addition, LXR signaling may significantly affect inflammation via LXR-mediated trans-repression (Joseph et al., 2003).
A specific lipid microenvironment is necessary to facilitate the different stages of the viral life cycle (Park and Scott, 2010). As an endogenous oxysterol, 25HC inhibits cholesterol and fatty acid biosynthesis, thereby exerting antiviral effects against some viruses (Koarai et al., 2012). In this study, we found that 25HC has antiviral effects against PEDV, which could be attributed to the disruption of lipid metabolism when 25HC was used to treat Vero cells. More importantly, in the present study, we found that both CH25H and 25HC could inhibit PEDV and TGEV infection. These findings highlight opportunities for new antiviral therapies against members of the Coronaviridae family.
5. Conclusion
In general, our study demonstrates that CH25H, as a host restriction factor, has a repressive effect against PEDV infection. We found that PEDV infection downregulated CH25H expression in a time-dependent manner. However, CH25H could not have been validated as an ISG in Vero cells. Overexpression and knockdown of CH25H indicated that it has superior antiviral activity against PEDV. Further study revealed that CH25H-M retained the effect of restriction on PEDV replication and 25HC inhibited different viral strains of PEDV. In addition, we found that replication of TGEV, another coronavirus, was also suppressed by CH25H and 25HC in ST cells. These findings provide novel research insights for the future control of porcine intestinal coronaviruses, including PEDV and TGEV, and even other members of the Coronaviridae family.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (2016YFD0500104), grants from the National Natural Science Foundation of China (31502086), the Priority Academic Program Development of Jiangsu higher education institutions (PAPD), Ministry of Agriculture (CARS-35) and the Fundamental Research Funds for the Central Universities (KJQN201619).
References
- Anggakusuma, Romero-Brey I., Berger C., Colpitts C.C., Boldanova T., Engelmann M., Todt D., Perin P.M., Behrendt P., Vondran F.W., Xu S., Goffinet C., Schang L.M., Heim M.H., Bartenschlager R., Pietschmann T., Steinmann E. Interferon-inducible cholesterol-25-hydroxylase restricts hepatitis C virus replication through blockage of membranous web formation. Hepatology. 2015;62:702–714. doi: 10.1002/hep.27913. [DOI] [PubMed] [Google Scholar]
- Blanc M., Hsieh W.Y., Robertson K.A., Kropp K.A., Forster T., Shui G., Lacaze P., Watterson S., Griffiths S.J., Spann N.J., Meljon A., Talbot S., Krishnan K., Covey D.F., Wenk M.R., Craigon M., Ruzsics Z., Haas J., Angulo A., Griffiths W.J., Glass C.K., Wang Y., Ghazal P. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38:106–118. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Liu X., Shi D., Shi H., Zhang X., Feng L. Complete genome sequence of a porcine epidemic diarrhea virus variant. J. Virol. 2012;86:3408. doi: 10.1128/JVI.07150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang S., Yi Z., Tian H., Aliyari R., Li Y., Chen G., Liu P., Zhong J., Chen X., Du P., Su L., Qin F.X., Deng H., Cheng G. Interferon-inducible cholesterol-25-hydroxylase inhibits hepatitis C virus replication via distinct mechanisms. Sci. Rep. 2014;4:7242. doi: 10.1038/srep07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler M.B. The intracellular cholesterol landscape: dynamic integrator of the immune response. Trends Immunol. 2016;37:819–830. doi: 10.1016/j.it.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E.S., Diercks A.H., Podolsky I., Podyminogin R.L., Askovich P.S., Treuting P.M., Aderem A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10666–10671. doi: 10.1073/pnas.1404271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R.S., Vandeberg J.L., Cox L.A. Genomics and proteomics of vertebrate cholesterol ester lipase (LIPA) and cholesterol 25-hydroxylase (CH25H) 3 Biotech. 2011;1:99–109. doi: 10.1007/s13205-011-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotter D., Sauter D., Kirchhoff F. Emerging role of the host restriction factor tetherin in viral immune sensing. J. Mol. Biol. 2013;425:4956–4964. doi: 10.1016/j.jmb.2013.09.029. [DOI] [PubMed] [Google Scholar]
- Joseph S.B., Castrillo A., Laffitte B.A., Mangelsdorf D.J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- Ke W., Fang L., Jing H., Tao R., Wang T., Li Y., Long S., Wang D., Xiao S. Cholesterol 25-Hydroxylase inhibits porcine reproductive and respiratory syndrome virus replication through enzyme activity-dependent and -Independent mechanisms. J. Virol. 2017:91. doi: 10.1128/JVI.00827-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koarai A., Yanagisawa S., Sugiura H., Ichikawa T., Kikuchi T., Furukawa K., Akamatsu K., Hirano T., Nakanishi M., Matsunaga K., Minakata Y., Ichinose M. 25-Hydroxycholesterol enhances cytokine release and Toll-like receptor 3 response in airway epithelial cells. Respir. Res. 2012;13:63. doi: 10.1186/1465-9921-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel S.N., Paim F.C., Lager K.M., Vlasova A.N., Saif L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): historical and current concepts. Virus Res. 2016;226:93–107. doi: 10.1016/j.virusres.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Li H., Liu Y., Pan Y., Deng F., Song Y., Tang X., He Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012;18:1350–1353. doi: 10.3201/eid1808.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Deng Y.Q., Wang S., Ma F., Aliyari R., Huang X.Y., Zhang N.N., Watanabe M., Dong H.L., Liu P., Li X.F., Ye Q., Tian M., Hong S., Fan J., Zhao H., Li L., Vishlaghi N., Buth J.E., Au C., Liu Y., Lu N., Du P., Qin F.X., Zhang B., Gong D., Dai X., Sun R., Novitch B.G., Xu Z., Qin C.F., Cheng G. 25-Hydroxycholesterol protects host against zika virus infection and its associated microcephaly in a mouse model. Immunity. 2017;46:446–456. doi: 10.1016/j.immuni.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Fu F., Xue M., Chen W., Liu J., Shi H., Chen J., Bu Z., Feng L., Liu P. IFN-lambda preferably inhibits PEDV infection of porcine intestinal epithelial cells compared with IFN-alpha. Antiviral Res. 2017;140:76–82. doi: 10.1016/j.antiviral.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Sanchez D.J., Aliyari R., Lu S., Cheng G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl. Acad. Sci. U. S. A. 2012;109:4239–4244. doi: 10.1073/pnas.1114981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Aliyari R., Chikere K., Li G., Marsden M.D., Smith J.K., Pernet O., Guo H., Nusbaum R., Zack J.A., Freiberg A.N., Su L., Lee B., Cheng G. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog C., Aubertin A.M., Kirn A., Luu B. Oxysterols, but not cholesterol, inhibit human immunodeficiency virus replication in vitro. Antivir. Chem. Chemother. 1998;9:491–496. doi: 10.1177/095632029800900605. [DOI] [PubMed] [Google Scholar]
- Olsen B.N., Schlesinger P.H., Ory D.S., Baker N.A. 25-Hydroxycholesterol increases the availability of cholesterol in phospholipid membranes. Biophys. J. 2011;100:948–956. doi: 10.1016/j.bpj.2010.12.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Scott A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 2010;88:1081–1087. doi: 10.1189/jlb.0610318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raniga K., Liang C. Interferons: reprogramming the metabolic network against viral infection. Viruses. 2018:10. doi: 10.3390/v10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A., Dang E.V., McDonald J.G., Liang G., Russell D.W., Cyster J.G. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydberg E.K., Salomonsson L., Hulten L.M., Noren K., Bondjers G., Wiklund O., Bjornheden T., Ohlsson B.G. Hypoxia increases 25-hydroxycholesterol-induced interleukin-8 protein secretion in human macrophages. Atherosclerosis. 2003;170:245–252. doi: 10.1016/s0021-9150(03)00302-2. [DOI] [PubMed] [Google Scholar]
- Shrivastava-Ranjan P., Bergeron E., Chakrabarti A.K., Albarino C.G., Flint M., Nichol S.T., Spiropoulou C.F. 25-hydroxycholesterol inhibition of lassa virus infection through aberrant GP1 glycosylation. MBio. 2016;7(6) doi: 10.1128/mBio.01808-16. e01808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelu R., Srinivasan P., Pezacki J.P. Armand-Frappier Outstanding Student Award--The emerging role of 25-hydroxycholesterol in innate immunity. Can. J. Microbiol. 2015;61:521–530. doi: 10.1139/cjm-2015-0292. [DOI] [PubMed] [Google Scholar]
- Song Z., Zhang Q., Liu X., Bai J., Zhao Y., Wang X., Jiang P. Cholesterol 25-hydroxylase is an interferon-inducible factor that protects against porcine reproductive and respiratory syndrome virus infection. Vet. Microbiol. 2017;210:153–161. doi: 10.1016/j.vetmic.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg Infect Dis. 2014;20:917–919. doi: 10.3201/eid2005.140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zeng L., Zhang L., Guo Z.Z., Lu S.F., Ming S.L., Li G.L., Wan B., Tian K.G., Yang G.Y., Chu B.B. Cholesterol 25-hydroxylase acts as a host restriction factor on pseudorabies virus replication. J. Gen. Virol. 2017;98:1467–1476. doi: 10.1099/jgv.0.000797. [DOI] [PubMed] [Google Scholar]
- Zeng S., Zhang H., Ding Z., Luo R., An K., Liu L., Bi J., Chen H., Xiao S., Fang L. Proteome analysis of porcine epidemic diarrhea virus (PEDV)-infected Vero cells. Proteomics. 2015;15:1819–1828. doi: 10.1002/pmic.201400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Jiang P., Song Z., Lv L., Li L., Bai J. Pathogenicity and antigenicity of a novel NADC30-like strain of porcine reproductive and respiratory syndrome virus emerged in China. Vet. Microbiol. 2016;197:93–101. doi: 10.1016/j.vetmic.2016.11.010. [DOI] [PubMed] [Google Scholar]