Abstract
Aim:
To evaluate the antibacterial activity of fosfomycin–meropenem and fosfomycin–colistin combinations against carbapenem-resistant Klebsiella pneumoniae (CR-Kp).
Methods:
A total of 50 CR-Kp isolates recovered from blood cultures were included in this study. All the CR-Kp isolates were screened for the presence of carbapenem resistant genes blaIMP. blaVIM. blaNDM. blaOXA-48 like, blaKPC. blaGES.#x00A0;and blaSPM. Combination testing of fosfomycin–meropenem and fosfomycin–colistin were performed using time-kill assay.
Results:
Fosfomycin–meropenem combination showed synergy in 20% of the tested CR-Kp isolates. While, fosfomycin–colistin exhibited synergy against 16% of the isolates. A total of 68% (n = 34) of CR-Kp isolates were characterised as OXA-48-like producers and 22% (n = 11) as NDM producers. Synergistic activity of these combinations was observed against OXA-48, NDM and NDM + OXA-48 co-producers.
Conclusion:
Considerable synergistic antibacterial activity of fosfomycin–meropenem and fosfomycin–colistin was not observed against CR-Kp isolates. Therefore, these combinations may not be promising for infections associated with CR-Kp.
Keywords: : colistin, fosfomcyin, fosfomycin–colistin, fosfomycin–meropenem, Klebsiella pneumoniae, meropenem, NDM, OXA-48-like, time-kill assay
Lay abstract
Carbapenem-resistant Klebsiella pneumoniae (CR-Kp) infections are difficult to treat and are associated with a high mortality rate. This study aimed to evaluate the synergistic activity of fosfomycin–meropenem and fosfomycin–colistin combinations against CR-Kp. Synergistic activity of these combinations was observed against OXA-48, NDM and NDM + OXA-48 co-producers. However, synergism was not found to be significant. Therefore, these combinations may not be promising for infections associated with CR-Kp.
Carbapenem-resistant Enterobacteriaceae (CRE) infections represent an increasing global threat. CRE infections are highly endemic in India, but an estimate of the infection burden is lacking. Currently, double or triple combination therapy based on carbapenem, polymyxins and tigecycline is very often prescribed for the treatment of CRE infections. Monotherapy with either colistin, fosfomycin or tigecycline is insufficient, which contributes to a modest response in patients. Emergence and an increasing trend of colistin resistance among CRE are alarming. The Clinical and Laboratory Standards Institute (CLSI) guidelines do not recommend fosfomycin minimum inhibitory concentration (MIC) breakpoints for Enterobacteriaceae, except Escherichia coli [1]. However, the European Committee on Antimicrobial Susceptibility Testing guidelines have recommended fosfomycin MIC breakpoints for Enterobacteriaceae [2].
Fosfomycin is an alternative for the treatment of CRE infections, especially when combined with other antibiotics, due to its synergistic effect. Fosfomycin prevents the transpeptidation of peptidoglycan in bacteria [3]. Similar to other beta-lactams, meropenem inhibits peptidoglycan synthesis by binding to penicillin-binding protein 2, 3 and 4 [4]. Meanwhile, colistin binds to lipopolysaccharide and phospholipids in the outer cell membrane of Gram-negative bacteria [5]. This differentiated mechanism of action has been proposed to enhance killing when fosfomycin is combined with meropenem and colistin. Studies have documented the synergistic activity of fosfomycin with various antibacterial agents. However, limited information is available on the antibacterial activity of fosfomycin–meropenem and fosfomycin–colistin against extreme drug-resistant K. pneumoniae. The present study aimed to investigate the antibacterial activity of fosfomycin combined with meropenem and colistin against carbapenem-resistant K. pneumoniae (CR-Kp) using time-kill assay.
Material & methods
Bacterial strains
Nonduplicate isolates of CR-Kp (n = 50) recovered from blood cultures obtained during 2017–2018 were included. These blood cultures were received for routine laboratory diagnosis. All the data or samples were fully anonymized before accessing them for further processing. This study only utilized isolates from positive blood cultures. Moreover, the study does not involve patients’ direct participation or follow-up. The study was conducted at the 2600-bed tertiary care hospital, Christian Medical College in Vellore, India.
Antimicrobial susceptibility testing
The MIC of meropenem and colistin was determined using the broth microdilution method (CLSI, 2015) [6]. The agar dilution method was used for the determination of fosfomycin MIC (CLSI, 2015) [6]. Cation-adjusted Mueller-Hinton broth-containing 25 μg/ml of glucose-6-phosphate was used for fosfomycin susceptibility testing. A meropenem MIC of ≥4 μg/ml was considered as resistant [6]. The European Committee on Antimicrobial Susceptibility Testing-recommended breakpoints were used for the interpretation of fosfomycin and colistin susceptibility [2]. An isolate which had fosfomycin MIC of >32 μg/ml and colistin MIC of >2 μg/ml was considered as resistant. For fosfomycin susceptibility testing, E.coli ATCC 25922 was used as the quality control strain. For colistin susceptibility testing, E. coli ATCC 25922, P. aeruginosa ATCC 27853 and colistin-resistant E. coli (mcr-1-positive) were used as the control strains.
Molecular detection of resistance genes
Multiplex PCR was performed for the detection of carbapenem-resistant genes blaIMP. blaVIM. blaNDM. blaOXA-48 like, blaKPC. blaGES.#x00A0;and blaSPM [7–9].
Time kill assays
Time kill assay (TKA) was performed as previously described [10]. The combinations of fosfomycin plus meropenem and fosfomycin plus colistin were tested against CR-Kp isolates. The Mueller-Hinton broth-containing 25 μg/ml of glucose-6-phosphate was used for combination testing. For TKA assays, the initial inoculum was targeted at 1 × 106 colony forming unit (CFU)/ml. A sampling of the inoculum incubated with fosfomycin, meropenem, colistin, fosfomycin plus meropenem and fosfomycin plus colistin were performed at times of 0, 3, 6 and 24 h of postincubation at 35 ± 2°C. The inoculum was diluted 1 in 100 using a 0.85% saline solution. A volume of 100 μl was plated directly onto nutrient agar plates and incubated at 37°C for 24 h. After incubation, colonies were counted and expressed as log10 CFU/ml. The viable count was plotted over time to create time-kill curves. For each batch of testing, sterility and growth control (without antibiotic) were included. TKA interpretation was performed as described earlier by Isenberg [10]. Synergy was defined as a ≥2 log10 CFU/ml decrease in combination compared with the most active single agent; indifference was defined as a <2-log10 CFU/ml increase or decrease in colony count at 6 or 24 h in combination compared with the most active single agent; antagonism was defined as a ≥2 log10 CFU/ml increase in combination compared with the most active single agent; bactericidal activity was defined as a ≥3 log10 CFU/ml reduction from the initial inoculum; regrowth was defined as an initial decrease of ≥3 log10 CFU/ml followed by ≥2 log10 CFU/ml increase at 24 h. For each batch of testing, Escherichia coli ATCC 25922 was used as the quality control strains.
Results
All the tested K. pneumoniae isolates were resistant to meropenem with the MICs ranging from 4 to 512 μg/ml. Colistin MICs ranges from 0.5 to 128 μg/ml, while fosfomycin MICs ranges from 2 to 256 μg/ml. Among the tested CR-Kp isolates, 48% (n = 24) were resistant to fosfomycin and 30% (n = 14) were resistant to colistin. Eight CR-Kp isolates were resistant to both colistin (16–128 μg/ml) and fosfomycin (64–256 μg/ml). For each batch of testing, mcr-1-positive E. coli strain produced a consistent colistin MIC of 4 μg/ml. All CR-Kp isolates were screened for the presence of carbapenem-resistant genes. Majority, 68% (n = 34) of CR-Kp isolates carried OXA-48-like gene and 22% (n = 11) was found with NDM gene. Co-existence of OXA-48-like and NDM genes were seen in five K.pneumoniae isolates.
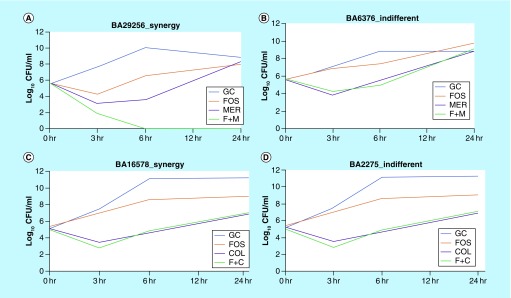
TKA experiments were performed for all CR-Kp (n = 50) isolates. Fosfomycin–meropenem combination showed synergistic antibacterial activity in 20% (n = 10) of the isolates (Table 1). Meanwhile, fosfomycin–colistin showed synergistic activity in 16% (n = 8) of CR-Kp isolates. A representative time-kill curves of these two combinations are shown in Figure 1. At 24 h of postincubation, regrowth of the strains was observed with both the combinations (Table 1). Retesting of MIC in these isolates showed a two to sixfold increase in fosfomycin MICs, while colistin MICs remains unchanged.
Table 1. . Synergy, indifference, bactericidal effect and regrowth of fosfomcyin–meropenem and fosfomycin–colistin combination against carbapenem resistant K. pneumoniae in time kill assay.
| Tested antibiotic combinations | n (%) | |||||
|---|---|---|---|---|---|---|
| Synergy | Indifference | Bactericidal effect | Regrowth | |||
| Positive | Negative | Positive | Negative | |||
| Fosfomycin–meropenem | 10 (20) | 40 (80) | Syn – 5 (50); Ind – 16 (40) |
Syn – 5 (50); Ind – 24 (60) |
Syn – 3 (30); Ind – 22 (55) |
Syn – 7 (70); Ind – 18 (45) |
| Fosfomycin–colistin | 8 (16) | 42 (84) | Syn – 4 (50); Ind – 15 (36) |
Syn – 4 (50); Ind – 27 (64) |
Syn – 0 (0); Ind – 25 (60) |
Syn – 8 (100); Ind – 17 (40) |
Figure 1. . Time-kill curves of fosfomycin–meropenem and fosfomycin–colistin combination.
(A) CR-Kp isolate showing synergy with fosfomcyin–meropenem combination; (B) isolate exhibits indifferent activity against fosfomcyin–meropenem; (C) and (D) isolate showing synergy and indifferent activity against fosfomycin–colistin combination.
C: Colistin; col: Colistin; CR-Kp: Carbapenem-resistant Klebsiella pneumoniae; Fos: Fosfomycin; F: Fosfomycin; GC: Growth control; Mer: Meropenem.
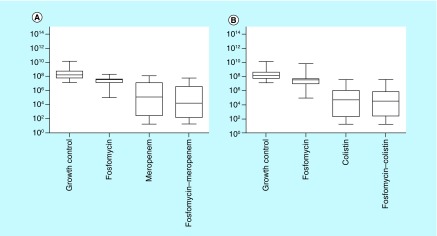
Considerable reduction in log10 CFU/ml was observed with the fosfomycin–meropenem combination than fosfomycin or meropenem alone (Figure 2). However, such a difference in the median log10 CFU/ml was not seen between fosfomycin–colistin combination and colistin alone. Antagonism was not observed in either of these tested combinations.
Figure 2. . Box plot showing median log10 CFU/ml of carbapenem resistant K. pneumoniae.
(A) Box plot showing median log10 CFU/ml of CR-Kp observed with fosfomycin, meropenem and fosfomycin–meropenem combination. (B) Box plot showing median log10 CFU/ml of CR-Kp observed with fosfomycin, colistin and fosfomycin–colistin combination.
CFU: Colony-forming unit; CR-Kp: Carbapenem-resistant Klebsiella pneumoniae.
Synergistic activity of fosfomycin–meropenem and fosfomycin–colistin was observed against NDM, OXA-48-like and NDM plus OXA-48-like co-producers (Table 2). However, this effect was not significantly seen.
Table 2. . Combination testing of fosfomcyin–meropenem and fosfomcyin–colistin against carbapenemase producing K. pneumoniae using time-kill assay.
| Carbapenem resistant genes (n) | Fosfomcyin–meropenem | Fosfomcyin–colistin | ||
|---|---|---|---|---|
| Synergy, n (%) | Indifference, n (%) | Synergy, n (%) | Indifference, n (%) | |
| blaOXA-48.like (n = 34) | 8 (24) | 26 (76) | 5 (15) | 29 (85) |
| blaNDM (n = 11) | 1 (9) | 10 (91) | 2 (18) | 9 (82) |
| blaOXA-48.like + blaNDM (n = 5) | 1 (20) | 4 (80) | 1 (20) | 4 (80) |
Discussion
Infections associated with CRE are of great concern and associated with poor outcome. However, optimal management of CRE infections remains unclear. In recent years, fosfomycin and colistin have gained attention in treating multidrug-resistant and extreme drug-resistant Gram-negative infections. Suboptimal dose and prolonged monotherapy with colistin contribute to the development of colistin resistance [11]. Fosfomycin has a unique chemical structure and mechanism of action and thus cross-resistance is uncommon. The present study aimed to evaluate the antibacterial activity of fosfomycin–meropenem and fosfomycin–colistin combinations against CR-Kp isolates.
In intensive care units, an optimal colistin dosing regimen against CRE infections remains uncertain. Higher doses of colistin are associated with the potential risk of developing nephrotoxicity in patients [12]. Colistin penetrates poorly into the lung tissues and achieves lower concentration in the pleural cavity [13,14]. In contrast, intravenous infusion of fosfomycin has been reported with a high plasma concentration and greater tissue penetration [15]. In the infected lung tissue mouse model, intravenous administration of fosfomycin showed good penetration and achieves higher concentration in the pleural fluid [16]. Fosfomycin is not associated with the risk of nephrotoxicity [17].
In recent years, colistin and fosfomycin are being frequently used to treat carbapenem-resistant Gram-negative infections. An in vitro study has demonstrated that 78% of CRE isolates were susceptible to fosfomycin [18]. In the present study, 58% of CR-Kp isolates were susceptible to fosfomycin. In addition, co-existence of resistance to colistin and fosfomycin was noticed in 16% of the tested isolates. Further, these isolates were not investigated for molecular determinants. Presence of fosA gene and mutation in mgrB has been described for the co-occurence of fosfomycin and colistin resistance in CR-Kp [19].
Studies have reported an in vitro synergy of fosfomycin with β-lactams, carbapenems and colistin against CRE infections [20–22]. A higher percentage of fosfomycin synergy has been described with carbapenems (70%) than colistin (36%) against CR-Kp isolates [23]. It is perhaps notable that synergy with fosfomycin–meropenem and fosfomycin–colistin combinations was not observed in our study. This could be due to the predominance of OXA-48-like producers, showing resistance to fosfomycin and colistin in K. pneumoniae. A study has demonstrated the synergistic activity of fosfomycin–meropenem combination against fosfomycin-resistant CR-Kp [24]. Similarly, in the present study, synergistic activity of fosfomycin–meropenem combination was noted against colistin- and fosfomycin-resistant CR-Kp.
Synergistic antibacterial activity of colistin and fosfomycin has been reported against K. pneumoniae carbapenemase (KPC)-producing K. pneumoniae and NDM-1 producing Enterobacteriaceae [25,26] while fosfomycin plus colistin combination has been reported with antagonistic activity against OXA-48 producing K. pneumoniae [27]. In contrast, in our study, synergistic activity of fosfomcyin-colistin combination was observed against OXA-48-like producers.
The present study showed synergistic activity of fosfomycin–colistin and fosfomycin–meropenem combinations against OXA-48-like, NDM and co-producers. However, remarkable synergy is not observed against these carbapenemase-producing K. pneumoniae. This could be due to the fact that the fosA gene is chromosomally encoded in K. pneumoniae [28] and mutations in GlpT (glycerol-3-phosphate [G3P] transporter) and UhpT (glucose-6-phosphate [G6P] transporter) are the most frequent events leading to lowered fosfomycin susceptibility in K. pneumoniae [29].
In pharmacodynamic studies, resistant subpopulations suppression with fosfomycin–meropenem and fosfomycin–colistin combinations has been reported [25,30]. A clinical cure rate of 70% was reported in patients treated with fosfomycin–meropenem combination, who had colistin-resistant Gram-negative infections [31]. Similarly, in the present study, fosfomycin–meropenem had better synergistic activity against colistin-resistant CR-Kp than fosfomycin–colistin combination.
Conclusion & future perspective
In conclusion, the present study revealed a higher resistance rate to fosfomycin and colistin in K. pneumoniae. Synergistic activity of both fosfomycin–meropenem and fosfomycin–colistin combinations was demonstrated against NDM, OXA-48-like producers and co-producers of NDM plus OXA-48-like. However, the synergistic activity was not found to be remarkable. Therefore, these combinations may not be promising for treating CR-Kp infections.
CR-Kp infections are difficult to manage because of the limited treatment options. Combination therapy is often preferred to treat CR-Kp. NDM and OXA-48-producing CR-Kp are expanding globally. A combination of fosfomycin with meropenem or colistin is not considerably promising in treating CR-Kp infections. A novel approach of ceftazidime-avibactam in combination with aztreonam would be a promising option. In the future, novel antibiotics including cefipime tazobactam, cefiderocol and ervacycline will be more beneficial in treating carbapenem-resistant infections.
Executive summary.
Carbapenem resistant carbapenem-resistant Klebsiella pneumoniae infections are difficult to treat and are associated with high mortality and morbidity.
Synergistic activity of fosfomycin–meropenem and fosfomycin–colistin combination was observed in 20 and 16% of carbapenem-resistant Klebsiella pneumoniae isolates, respectively.
Antagonism was not observed with these combinations.
Synergistic activity of these combinations were seen against NDM, OXA-48 and co-producers.
Footnotes
Author contributions
YD Bakthavatchalam and DPM Sethuvel analyzed the data and wrote the manuscript. A Shankar, K Asokan, K Kanthan performed analysis. B Veeraraghavan critically reviewed the manuscript.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 28th Informational Supplement. Clinical and Laboratory Standards Institute, PA, USA, CLSI document M100-S29 (2019). [Google Scholar]
- 2.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. (2019). www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf
- 3.Roussos N, Karageorgopoulos DE, Samonis G. et al. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents 34(6), 506–515 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Davies TA, Shang W, Bush K. et al. Affinity of doripenem and comparators to penicillin-binding proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52(4), 1510–1512 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakthavatchalam YD, Pragasam AK, Biswas I. et al. Polymyxin susceptibility testing, interpretative breakpoints and resistance mechanisms: an update. J. Glob. Antimicrob. Resist. 12, 124–136 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution of Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. 9th ed. Document M07-A9, 9th Edition, Clinical and Laboratory Standards Institute, PA, USA: (2012). [Google Scholar]
- 7.Poirel L, Walsh TR, Cuvillier V. et al. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70, 119–123 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Ellington MJ, Kistler J, Livermore DM. et al. Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J. Antimicrob. Chemother. 59, 321–322 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Yigit H, Queenan AM, Anderson GJ. et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45, 1151–1161 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isenberg H. Time-kill assay. : Clinical Microbiology Procedures Handbook. Garcia LS, Isenberg HD. (). ASM, DC, USA, 5.10.2.1–5.10.2.12 (2010). [Google Scholar]
- 11.Silva A, Sousa AM, Alves D. et al. Heteroresistance to colistin in Klebsiella pneumoniae is triggered by small colony variants sub-populations within biofilms. Pathog Dis. 74(5), ftw036 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Sorlí L, Luque S, Grau S. et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect. Dis. 13(1), 380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdellatif S, Trifi A, Daly F, Mahjoub K, Nasri R, Lakhal SB. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann. Intensive Care 6(1), 26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalopoulos AS, Falagas ME. Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann. Intensive Care 1(1), 30 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falagas ME, Vouloumanou EK, Samonis G. et al. Fosfomycin. Clin. Microbiol. Rev. 29(2), 321–347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matzi V, Lindenmann J, Porubsky C. et al. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J. Antimicrob. Chemother. 65(5), 995–998 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Sastry S, Clarke LG, Alrowais H. et al. Clinical appraisal of fosfomycin in the era of antimicrobial resistance. Antimicrob. Agents Chemother. 59(12), 7355–7361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaase M, Szabados F, Anders A. et al. Fosfomycin susceptibility in carbapenem-resistant Enterobacteriaceae from Germany. J. Clin. Microbiol. 52(6), 1893–1897 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veeraraghavan B, Perumalla SK, Ragupathi NK. et al. Coexistence of fosfomycin and colistin resistance in Klebsiella pneumoniae: whole-genome shotgun sequencing. Microbiol. Resour. Announc. 4(6), e01303–01316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flamm RK, Rhomberg PR, Lindley JM. et al. Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents when tested against gram-negative bacterial strains using time-kill curves. Antimicrob. Agents Chemother. 63(5), pii: e02549–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku YH, Chen CC, Lee MF. et al. Comparison of synergism between colistin, fosfomycin and tigecycline against extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates or with carbapenem resistance. J. Microbiol. Immunol. Infect. 50(6), 931–939 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Albiero J, Sy SK, Mazucheli J, Caparroz-Assef SM. et al. Pharmacodynamic evaluation of the potential clinical utility of fosfomycin and meropenem in combination therapy against KPC-2-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 60(7), 4128–4139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samonis G, Maraki S, Karageorgopoulos DE. et al. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 31(5), 695–701 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Tseng SP, Wang SF, Ma L. et al. The plasmid-mediated fosfomycin resistance determinants and synergy of fosfomycin and meropenem in carbapenem-resistant Klebsiella pneumoniae isolates in Taiwan. J. Microbiol. Immunol. Infect. 50(5), 653–661 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Zhao M, Bulman ZP, Lenhard JR. et al. Pharmacodynamics of colistin and fosfomycin: a ‘treasure trove’combination combats KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 72(7), 1985–1990 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the synergistic activity of colistin and fosfomycin against colistin and fosofmycin susceptible Klebsiella pneumoniae carbapenemase-producing K. pneumoniae.
- 26.Albur MS, Noel A, Bowker K. et al. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. Int. J. Antimicrob. Agents 46(5), 560–567 (2015). [DOI] [PubMed] [Google Scholar]; •• Highlights the antibacterial activity of colistin and fosfomycin against NDM-producing K. pneumoniae.
- 27.Evren E, Azap ÖK, Çolakoğlu Ş. et al. In vitro activity of fosfomycin in combination with imipenem, meropenem, colistin and tigecycline against OXA 48–positive Klebsiella pneumoniae strains. Diagn. Microbiol. Infect. Dis. 76(3), 335–338 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Benzerara Y, Gallah S, Hommeril B. et al. Emergence of plasmid-mediated fosfomycin-resistance genes among Escherichia coli isolates, France. Emerg. Infect. Dis. 23(9), 1564–1567 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castañeda-García A, Blázquez J, Rodríguez-Rojas A. Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics (Basel) 2(2), 217–236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albiero J, Mazucheli J, Barros JPDR. et al. Pharmacodynamic attainment of the synergism of meropenem and fosfomycin combination against Pseudomonas aeruginosa producing metallo-β-lactamase. Antimicrob. Agents Chemother. 63(6), pii: e00126–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perdigão Neto LV, Oliveira MS, Martins RC. et al. Fosfomycin in severe infections due to genetically distinct pan-drug-resistant Gram-negative microorganisms: synergy with meropenem. J. Antimicrob. Chemother. 74(1), 177–1781 (2018). [DOI] [PubMed] [Google Scholar]