Abstract
Background
Mycoplasma pneumoniae (M. pneumoniae) is a significant cause of community‐acquired pneumonia in children and adolescents. Treatment with macrolide antibiotics is recommended. However, M. pneumoniae is difficult to diagnose based on clinical symptoms and signs. Diagnostic uncertainty can lead to inappropriate antibiotic prescribing, which may worsen clinical prognosis and increase antibiotic resistance.
Objectives
The objectives of this review are (i) to assess the diagnostic accuracy of symptoms and signs in the clinical recognition of M. pneumoniae in children and adolescents with community‐acquired pneumonia; and (ii) to assess the influence of potential sources of heterogeneity on the diagnostic accuracy of symptoms and signs in the clinical recognition of M. pneumoniae.
Search methods
We searched MEDLINE (January 1950 to 26 June 2012) and EMBASE (January 1980 to 26 June 2012). We identified additional references by handsearching the reference lists of included articles and snowballing. We searched the reference lists of relevant systematic reviews identified by searching the Medion database, Database of Reviews of Effects 2012, Issue 6 (25 June 2012) and the Cochrane Register of Diagnostic Test Accuracy studies (2 July 2012). Experts in the field reviewed our list of included studies for any obvious omissions.
Selection criteria
We included peer‐reviewed published studies which prospectively and consecutively recruited children with community‐acquired pneumonia from any healthcare setting, confirmed the presence of M. pneumoniae using serology with or without other laboratory methods and reported data on clinical symptoms and signs in sufficient detail to construct 2 x 2 tables.
Data collection and analysis
One review author scanned titles to exclude obviously irrelevant articles. Two review authors independently scanned the remaining titles and abstracts, reviewed full‐text versions of potentially relevant articles, assessed the quality of included articles and extracted data on study characteristics and the following clinical features: cough, wheeze, coryza, crepitations, fever, rhonchi, shortness of breath, chest pain, diarrhea, myalgia and headache.
We calculated study‐specific values for sensitivity, specificity and positive and negative likelihood ratios with 95% confidence intervals (CIs). We estimated the post‐test probability of M. pneumoniae based on the absence or presence of symptoms and signs.
We calculated pooled sensitivities, specificities, positive and negative likelihood ratios with 95% CIs for symptoms and signs where data were reported by at least four included studies by fitting a bivariate normal model for the logit transforms of sensitivity and specificity. We explored potential sources of heterogeneity by fitting bivariate models with covariates using multi‐level mixed‐effects logistic regression. We performed sensitivity analyses excluding data from studies for which we were concerned about the representativeness of the study population and/or the acceptability of the reference standard.
Main results
Our search identified 8299 articles (excluding duplicates). We examined the titles and abstracts of 1125 articles and the full‐text versions of 97 articles. We included seven studies in our review, which reported data from 1491 children; all were conducted in hospital settings. Overall, study quality was moderate. In two studies the presence of chest pain more than doubled the probability of M. pneumoniae. Wheeze was 12% more likely to be absent in children with M. pneumoniae (pooled positive likelihood ratio (LR+) 0.76, 95% CI 0.60 to 0.97; pooled negative likelihood ratio (LR‐) 1.12, 95% CI 1.02 to 1.23). Our sensitivity analysis showed that the presence of crepitations was associated with M. pneumoniae, but this finding was of borderline statistical significance (pooled LR+ 1.10, 95% CI 0.99 to 1.23; pooled LR‐ 0.66, 95% CI 0.46 to 0.96).
Authors' conclusions
M. pneumoniae cannot be reliably diagnosed in children and adolescents with community‐acquired pneumonia based on clinical symptoms and signs. Although the absence of wheeze is a statistically significant diagnostic indicator, it does not have sufficient diagnostic value to guide empirical macrolide treatment. Data from two studies suggest that the presence of chest pain more than doubles the probability of M. pneumoniae. However, further research is needed to substantiate this finding. More high quality large‐scale studies in primary care settings are needed to help develop prediction rules based on epidemiological data as well as clinical and baseline patient characteristics.
Keywords: Adolescent; Child; Humans; Mycoplasma pneumoniae; Community‐Acquired Infections; Community‐Acquired Infections/diagnosis; Community‐Acquired Infections/microbiology; Pneumonia, Mycoplasma; Pneumonia, Mycoplasma/diagnosis; Randomized Controlled Trials as Topic; Respiratory Sounds; Symptom Assessment; Symptom Assessment/methods
Plain language summary
Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community‐acquired pneumonia
Chest infections are among the commonest reasons why children and young people go to see their doctor or nurse. At the moment, it is difficult for doctors and nurses to tell patients what type of infection they have based on their symptoms and signs. This can result in antibiotics being prescribed or withheld inappropriately. Mycoplasma pneumoniae (M. pneumoniae) is an important bacterial cause of chest infections in children and adolescents. This review assesses the value of clinical symptoms and signs in helping doctors and nurses decide whether a child or young person might have a chest infection caused by M. pneumoniae. We analysed data from seven studies including a total of 1491 children, all of which were conducted in hospital settings. We found that the presence of wheeze makes M. pneumoniae slightly less likely and the presence of crepitations (i.e. crackles heard on listening to the chest) makes M. pneumoniae slightly more likely. However, these clinical features are not sufficiently helpful to guide decisions about prescribing antibiotics for possible M. pneumoniae infections. Based on the results of two studies, the presence of chest pain doubles the likelihood of M. pneumoniae. However, further research in this area is needed, particularly in general practice and outpatient populations.
Summary of findings
Summary of findings 1. Overview of studies and population characteristics.
| Patients/populations | Children and adolescents aged 18 years or younger diagnosed with community‐acquired pneumonia based on clinical +/‐ radiological criteria. No evidence of serious underlying co‐morbidity or immunocompromise |
| Settings | All included studies were conducted in hospital settings |
| Index tests | Clinical symptoms and signs: cough, wheeze, coryza, crepitations, fever, rhonchi, shortness of breath, headache, chest pain, diarrhea and myalgia |
| Reference standard | M. pneumoniae serology (i.e. high antibody titre on a single serum sample or a significant rise in antibody titre between paired acute and convalescent sera) with or without the use of additional laboratory tests such as culture or polymerase chain reaction (PCR) analysis |
| Importance | M. pneumoniae cannot be reliably diagnosed in children and adolescents with community‐acquired pneumonia based on the absence or presence of individual clinical symptoms and signs. Absence of wheeze is a statistically significant diagnostic indicator, but does not have sufficient diagnostic value to guide empirical macrolide antibiotic treatment. Absence of wheeze is only 12% more likely in M. pneumoniae‐positive versus M. pneumoniae‐negative children. If empirical antibiotic treatment was given to children with community‐acquired pneumonia in whom wheeze was not reported, 61% to 89% of children receiving antibiotics would be M. pneumoniae‐negative (based on M. pneumoniae prevalence of 10% to 36%) and antibiotics would be withheld from 25% of M. pneumoniae‐positive children |
| Studies | Published peer‐reviewed studies (any design) including prospectively and consecutively recruited cohorts of children with community‐acquired pneumonia from any healthcare setting. This review included 7 studies which reported data on 1491 children |
| Quality concerns | The most common concern was unclear reporting of baseline study population characteristics. We had concerns about the spectrum of patients recruited and the validity of the reference standard in one study (Agarwal 2009) |
Summary of findings 2. Diagnostic value of clinical symptoms and signs ‐ pooled results with 95% confidence intervals.
|
Clinical feature (n = number of studies) |
Sensitivity | Specificity | Positive likelihood ratio | Negative likelihood ratio |
| Cough (n = 5) | 0.89 (0.67 to 0.97) | 0.15 (0.05 to 0.37) | 1.04 (0.95 to 1.13) | 0.78 (0.44 to 1.39) |
| Wheeze (n = 6)* | 0.25 (0.17 to 0.36) | 0.67 (0.56 to 0.76) | 0.76 (0.60 to 0.97) | 1.12 (1.02 to 1.23) |
| Coryza (n = 4) | 0.32 (0.08 to 0.72) | 0.66 (0.28 to 0.91) | 0.95 (0.71 to 1.26) | 1.03 (0.90 to 1.17) |
| Crepitations (n = 5)** | 0.84 (0.78 to 0.88) | 0.22 (0.14 to 0.32) | 1.06 (0.96 to 1.18) | 0.77 (0.52 to 1.12) |
*The absence of wheeze remained a statistically significant diagnostic indicator when M. pneumoniae was diagnosed based on serology only (pooled LR+ 0.68, 95% CI 0.50 to 0.92; pooled LR‐ 1.24, 95% 1.03 to 1.51) (Chan 2001; Somer 2006). However, the absence of wheeze was no longer a statistically significant diagnostic indicator based on data from studies which used other laboratory tests alongside serology to diagnose M. pneumoniae (pooled LR+ 0.84, 95% CI 0.63 to 1.12; pooled LR‐ 1.06, 95% CI 0.96 to 1.18).
**Our sensitivity analysis excluding data from Agarwal 2009 found that the presence of crepitations was a weak diagnostic indicator of borderline statistical significance (pooled LR+ 1.10, 95% CI 0.99 to 1.23; pooled LR‐ 0.66, 95% CI 0.46 to 0.96).
Background
Target condition being diagnosed
Mycoplasma pneumoniae (M. pneumoniae) is a significant and treatable cause of respiratory tract infections in children and adolescents. Data from previous studies suggest that M. pneumoniae is responsible for up to 40% of community‐acquired pneumonia in children over five years of age (Don 2005; Heiskanen‐Kosma 1998; Korppi 2004) and its highest incidence is found in the five to nine‐year age group (4 per 1000 children per year) (Hammerschlag 2001). M. pneumoniae tends to occur in epidemics lasting 12 to 15 months, which happen at approximately four‐yearly intervals (Chalker 2011; Hammerschlag 2001). Estimates of the prevalence of M. pneumoniae are therefore extremely variable, ranging from 1% during endemic periods (Sopena 1999) to 50% during outbreaks within closed institutional settings (Broome 1980).
At the moment, M. pneumoniae is diagnosed retrospectively using laboratory methods. However, there is no single 'gold standard' for laboratory diagnosis of M. pneumoniae. Serology is currently the most widely available method. Serological assays may be based on a single high antibody titre or on paired acute and convalescent serum samples taken two to four weeks apart (Loens 2010). Other laboratory methods include culture and polymerase chain reaction (PCR). Culture can take several weeks and has poor sensitivity (Loens 2010). PCR techniques are more rapid and more sensitive than serology at detecting acute M. pneumoniae infections but are less widely available (Nilsson 2008).
Index test(s)
In this review, our index tests were clinical symptoms and signs: cough, wheeze, coryza (nasal symptoms, including runny nose, nasal congestion and sneezing), crepitations (crackles audible on chest examination), fever (reported as a symptom or according to temperature threshold defined in study), rhonchi (wheeze audible on chest examination), shortness of breath, chest pain, diarrhea, myalgia (muscle aches) and headache. These clinical features have been reported in several case series of children with laboratory‐confirmed M. pneumoniae (Hsieh 2007; Othman 2005; Stevens 1978). This review formally assesses the diagnostic value of these symptoms and signs individually in the clinical recognition of M. pneumoniae in children and adolescents with community‐acquired pneumonia.
Alternative test(s)
There are no alternative tests applicable to this review, since a range of clinical symptoms and signs are being studied.
Rationale
Acute respiratory tract infections represent one of the commonest reasons for medical consultations and prescription of antibiotics. Despite the recommendations of recent guidelines (NICE 2008), almost two‐thirds of consultations for respiratory tract infections still result in antibiotic prescribing (Gulliford 2009). M. pneumoniae is an important cause of respiratory tract infections in children and adolescents. Macrolide antibiotics are recommended for the treatment of suspected M. pneumoniae infections (BTS 2011). However, it is difficult for clinicians to give patients accurate prognostic information or decide on appropriate antibiotic treatment in the absence of a microbiological diagnosis (Butler 2004). Diagnostic uncertainty can lead to inappropriate antibiotic prescribing, which may worsen clinical prognosis and increase antibiotic resistance within both communities (Goossens 2005) and individuals (Chung 2007). Macrolide‐resistant strains of M. pneumoniae have recently been reported in France (Pereyre 2007), Japan (Morozumi 2008), Germany (Dumke 2010), Israel (Averbuch 2011) and China (Zhao 2012).
This review will help us assess whether the absence or presence of symptoms and signs might help clinicians decide which children with clinically suspected community‐acquired pneumonia are most (and least) likely to benefit from empirical macrolide treatment at the time of initial presentation, when the results of laboratory tests for M. pneumoniae are not available.
Objectives
To assess the diagnostic accuracy of symptoms and signs in the clinical recognition of M. pneumoniae in children and adolescents with community‐acquired pneumonia.
Secondary objectives
To assess the influence of potential sources of heterogeneity on the diagnostic accuracy of symptoms and signs in the clinical recognition of M. pneumoniae in children and adolescents with community‐acquired pneumonia.
Investigation of sources of heterogeneity
We investigated the use of other laboratory investigations (such as culture and PCR) alongside serology to diagnose M. pneumoniae.
Methods
Criteria for considering studies for this review
Types of studies
Published peer‐reviewed studies (any design) including prospectively and consecutively recruited cohorts of children with community‐acquired pneumonia in any healthcare setting.
Participants
Participants aged 18 years or younger with no evidence of serious underlying comorbidity (e.g. cystic fibrosis, bronchiectasis, neoplasia) or immunocompromise (HIV‐positive or on immunosuppressant medication), who have been diagnosed with community‐acquired pneumonia based on clinical +/‐ radiological criteria.
Index tests
Clinical symptoms and signs reported in children and adolescents diagnosed with community‐acquired pneumonia: cough, wheeze, coryza (nasal symptoms, including runny nose, nasal congestion and sneezing), crepitations (crackles audible on chest examination), fever (reported as a symptom or according to temperature threshold defined in study), rhonchi (wheeze audible on chest examination), shortness of breath, chest pain, diarrhea, myalgia (muscle aches) and headache.
Comparator tests
None.
Target conditions
M. pneumoniae infection in children and adolescents with community‐acquired pneumonia.
Reference standards
Our reference standard was M. pneumoniae serology with or without the use of additional laboratory tests such as culture or PCR. M. pneumoniae serology is currently the most widely available diagnostic method. We defined a positive M. pneumoniae serology result as either a high antibody titre on a single serum sample or a significant rise in antibody titre between paired acute and convalescent sera, as defined by the manufacturers of the serology assay(s) being used in different studies.
Search methods for identification of studies
Electronic searches
We searched MEDLINE (January 1950 to 26 June 2012) and EMBASE (January 1980 to 26 June 2012) for suitable articles. Appendix 1 and Appendix 2 contain details of our electronic search strategies. We did not apply any language or publication restrictions to our search.
Searching other resources
We supplemented our electronic search by handsearching the reference lists of included studies and snowballing (i.e. reviewing full texts and reference lists of articles cited by previously identified publications). We also searched the Medion database (http://www.mediondatabase.nl) (25 June 2012), Database of Reviews of Effects 2012, Issue 6 (part of T he Cochrane Library, www.thecochranelibrary.com (accessed 25 June 2012) and the Cochrane Register of Diagnostic Test Accuracy studies (2 July 2012) to identify systematic reviews whose reference lists might provide additional references. We asked experts in the field to review our list of included studies for any obvious omissions.
Data collection and analysis
Selection of studies
We selected peer‐reviewed published studies (any design) which included prospectively and consecutively recruited cohorts of children with community‐acquired pneumonia in any healthcare setting and which reported data on clinical symptoms and signs in these children in sufficient detail for us to construct 2 x 2 tables. We excluded case reports, case series, systematic reviews and narrative reviews. We also excluded studies with unsuitable comparison groups (i.e. non‐consecutively recruited M. pneumoniae‐negative controls or participants with a different laboratory‐confirmed microbial diagnosis) because assessments of the diagnostic value of symptoms and signs are likely to be distorted in these types of study populations.
We included studies which confirmed the diagnosis of M. pneumoniae using serology based on single serum samples or paired acute and convalescent sera with or without the use of additional laboratory methods such as culture or PCR (see Reference standards).
One review author (KW) scanned the titles of studies identified by our search to exclude any obviously irrelevant articles. Two review authors (KW, PG) independently scanned the titles and abstracts of the remaining studies and reviewed full‐text versions of potentially relevant articles. We resolved any disagreements by discussion, if necessary with a third review author (DM or AH).
Data extraction and management
Two review authors (KW, PG) independently extracted data on study characteristics (study design, age and sex of participants, study inclusion and exclusion criteria, number of participants recruited, recruitment period, country(ies) where recruitment took place, healthcare setting, criteria for diagnosing community‐acquired pneumonia, laboratory methods used to diagnose M. pneumoniae, number of participants diagnosed with M. pneumoniae) and clinical symptoms and signs. We constructed 2 x 2 tables for clinical symptoms and signs (see Statistical analysis and data synthesis section for further details). We resolved any discrepancies by discussion, if necessary with a third review author (RP).
Assessment of methodological quality
Two review authors (KW, PG) independently assessed the quality of included articles. We resolved any disagreements by consensus. Table 3 outlines details of our quality assessment tool and coding criteria based on the QUADAS tool (Reitsma 2009).
1. Quality assessment tool and coding criteria.
| Item | Yes | No | Unclear |
| 1. Was the spectrum of patients representative of the patients who will receive the test in practice? |
|
|
|
| 2. Is the reference standard likely to classify the target condition correctly? |
|
|
|
| 3. Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? |
|
|
|
| 4. Did the whole sample or a random selection of the sample, receive verification using the intended reference standard? |
|
|
|
| 5. Did patients receive the same reference standard irrespective of the index test result? |
|
|
|
| 6. Was the reference standard independent of the index test (i.e. the index test did not form part of the reference standard)? |
|
|
|
| 7. Were the reference standard results interpreted without knowledge of the results of the index test? (index test results blinded) |
|
|
|
| 8. Were the index test results interpreted without knowledge of the results of the reference standard? |
|
|
|
| 9. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? |
|
|
|
| 10. Were uninterpretable/intermediate test results reported? |
|
|
|
| 11. Were withdrawals from the study explained? |
|
|
|
| 12. Did the study provide a clear definition of what was considered to be a positive result? |
|
|
|
Based on recommended quality criteria derived from the QUADAS tool (Reitsma 2009). PCR: polymerase chain reaction
Statistical analysis and data synthesis
We constructed 2 x 2 tables for each study we included in our review, cross‐classifying the absence or presence of M. pneumoniae with the absence or presence of clinical symptoms and signs. We used these tables to calculate study‐specific values for sensitivity, specificity and positive and negative likelihood ratios. We also examined how the prevalence of M. pneumoniae in the study population influences the post‐test probability of M. pneumoniae based on the absence or presence of different symptoms and signs (Van den Bruel 2010).
We calculated pooled sensitivities, specificities, positive and negative likelihood ratios with 95% confidence intervals (CIs) for clinical symptoms and signs where data were reported by at least four included studies, by fitting a bivariate normal model for the logit transforms of sensitivity and specificity (Leeflang 2008). We obtained estimates from these models using the command metandi (meta‐analysis of diagnostic accuracy) in Stata version 11. We obtained summary estimates for positive and negative likelihood ratios directly from the summary estimates of sensitivity and specificity.
Investigations of heterogeneity
We planned to explore the following potential sources of heterogeneity by fitting bivariate models with covariates using multi‐level mixed‐effects logistic regression (xtmelogit) in Stata version 11:
Participant age group (preschool (up to four years of age) versus school age (five to 12 years of age) versus adolescents (13 to 18 years of age))*.
Healthcare setting (community versus hospital).
Method of diagnosing community‐acquired pneumonia (based on clinical criteria only versus based on clinical and radiological criteria).
Serological method of diagnosing M. pneumoniae (high antibody titre on single serum sample versus significant rise in antibody titre between acute and convalescent sera).
Use of other laboratory investigations (such as culture or PCR) alongside serology to diagnose M. pneumoniae.
*We only planned to investigate heterogeneity if clinical symptoms and signs were reported in sufficient detail within studies to construct 2 x 2 tables in our specified age categories.
Sensitivity analyses
We used sensitivity analyses to explore the influence of negative classification of items 1 (representative spectrum) and 2 (acceptable reference standard) of our quality assessment tool (Table 3).
Assessment of reporting bias
We did not undertake any formal assessment of reporting bias in our review due to current uncertainty about how to assess reporting bias in diagnostic test accuracy reviews (Deeks 2005).
Results
Results of the search
Figure 1 summarises the numbers of articles that we identified, screened and selected for this review. We identified 8299 articles (excluding duplicates) in the search, of which we excluded 7174 based on title alone. We excluded a further 1028 articles after reviewing their abstracts. We reviewed full‐text versions of the remaining 97 articles. We included seven studies in our review. Agreement was very good based on title and abstract screening (% agreement = 97.2%; kappa = 0.84) and good based on full‐text screening (% agreement = 93.8%; kappa = 0.67).
1.
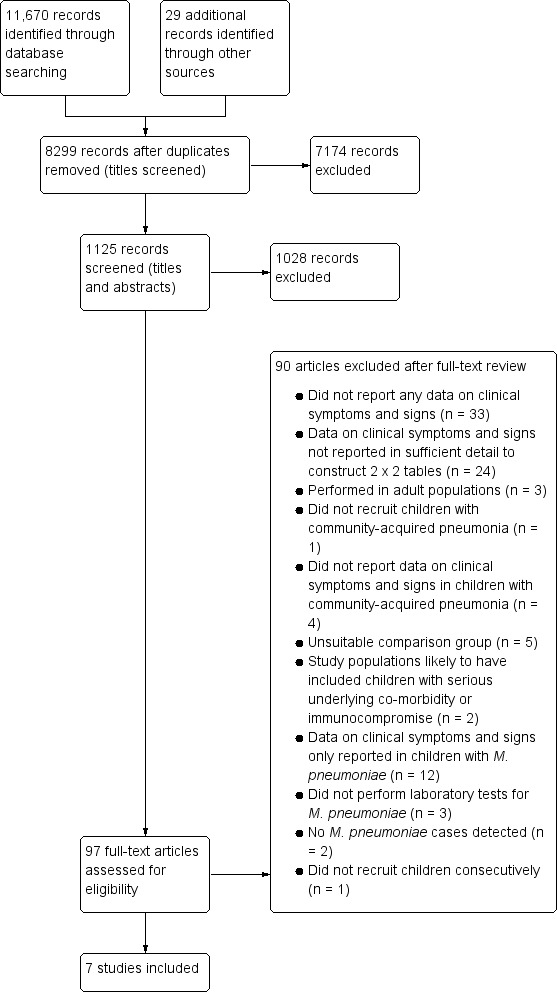
Study flow diagram.
Our Characteristics of excluded studies table lists the studies which we excluded after full‐text screening and the reasons for excluding these studies. We excluded studies for four main reasons: unsuitable population, inadequate reporting of clinical symptoms and signs, lack of suitable comparator group or lack of laboratory testing for M. pneumoniae.
Unsuitable population: We excluded three studies performed in adult populations (De Roux 2006; Javier Alvarez 2001; Marrie 2005) and two studies whose populations we felt were highly likely to include children with serious underlying comorbidity or immunocompromise (Samransamruajkit 2008; Vervloet 2010). One study did not recruit children with community‐acquired pneumonia (King 1991). Four studies recruited patients with different types of acute respiratory infections, including pneumonia, but did not report symptoms and signs in patients with community‐acquired pneumonia specifically (Almasri 2011; Peng 2009; Pocheville Guruceta 1998; Shenoy 2005). One study did not recruit children consecutively; only children with community‐acquired pneumonia in whom bacterial pathogens M. pneumoniae and C. pneumoniae were felt to be the causative organisms after clinical examination were included (Bamba 2006). In two studies, no M. pneumoniae cases were detected (Hortal 1994; Manfredi 1992).
Inadequate reporting of clinical symptoms and signs: We excluded 33 studies because they did not report any data on clinical symptoms or signs. We excluded a further 24 studies, which did report data on clinical symptoms and signs, but not in sufficient detail for us to be able to construct 2 x 2 tables.
Lack of suitable comparator group: Twelve studies were case series with no comparator group, which only reported data on clinical features in patients with M. pneumoniae (Broome 1980; Bunnag 2008; Defilippi 2008; Dular 1987; Gomez Campdera 2002; Guggenbichler 1977; Kurz 2011; Pereyre 2012; Putman 1975; Sakurai 1988; Touati 2010; Unay 2002). Five studies had unsuitable M. pneumoniae‐negative comparison groups consisting of patients with Chlamydia pneumoniae (C. pneumoniae) (Esposito 2001; Kicinski 2011; Ouchi 1999; Sidal 2007) or other microbial diagnoses (Nakayama 2007).
Lack of laboratory testing for M. pneumoniae: Three studies did not perform laboratory tests for M. pneumoniae (Gimenez Sanchez 2007; Holmes 2001; Rahman 1990).
Table 1 gives an overview of the characteristics of studies included in this review. We included seven studies in our review which included a total of 1491 children with community‐acquired pneumonia (Agarwal 2009; Chan 2001; Deerojanawong 2006; Kumar 2011; Maheshwari 2011; Principi 2001; Somer 2006). The prevalence of M. pneumoniae in these study populations ranged from 10% (Agarwal 2009) to 36% (Kumar 2011; Principi 2001). All of our included studies were prospective observational cohort studies conducted in hospital settings. Somer 2006 was nested within a larger prospective study evaluating the incidence of bacterial and atypical pathogens in hospitalised children with community‐acquired pneumonia.
Agarwal 2009 recruited children with severe community‐acquired pneumonia based on clinical features. Chan 2001 diagnosed children with community‐acquired pneumonia based on the presence of respiratory symptoms and respiratory signs or chest radiograph changes. All other studies diagnosed children with community‐acquired pneumonia based on both clinical and radiographic features. Two studies only used serology to diagnose M. pneumoniae (Chan 2001; Somer 2006). Four studies also used PCR of respiratory samples to detect M. pneumoniae. Maheshwari 2011 analyzed throat swabs, Kumar 2011 and Principi 2001 analyzed nasopharyngeal aspirates and Deerojanawong 2006 analyzed nasopharyngeal aspirates, sputum and throat swabs. Agarwal 2009 performed M. pneumoniae antigen detection in nasopharyngeal aspirate as well as serology. Only Agarwal 2009 diagnosed M. pneumoniae based on a single acute serum sample. All other included studies sought to obtain paired serum samples from participants.
Methodological quality of included studies
The methodological quality of included studies is summarised in Figure 2. Only two studies clearly reported that children with serious underlying co‐morbidity or who were immunocompromised were excluded from the study population (Principi 2001; Somer 2006). One study included 51/245 children with asthma (21%) but only nine children (4%) with serious co‐morbidities (seven children had congestive heart failure, one had hepatic disease and one had renal impairment) (Deerojanawong 2006). In three studies it was unclear whether or not a representative spectrum of patients had been recruited (Chan 2001; Kumar 2011; Maheshwari 2011). These studies did not state whether or not children with comorbid conditions were excluded, or report data on co‐morbidities or clinical outcomes. One study only recruited children with severe community‐acquired pneumonia based on World Health Organization (WHO) clinical criteria (Agarwal 2009); we therefore did not consider this study population to reflect a representative spectrum of children with community‐acquired pneumonia.
2.
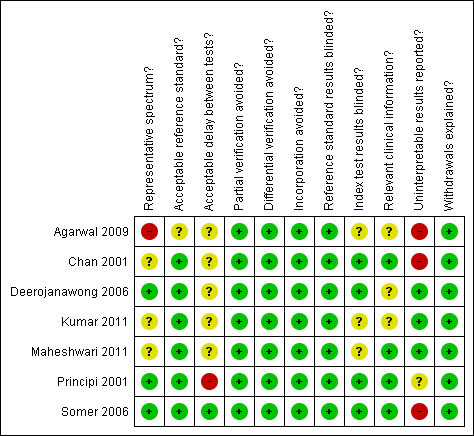
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
All except one of our included studies utilised acceptable reference standards for diagnosing M. pneumoniae. In two studies M. pneumoniae was diagnosed based on a single high antibody titre or a fourfold rise in antibody titre between acute and convalescent serum samples taken two to four weeks apart (Chan 2001; Somer 2006). Deerojanawong 2006 diagnosed M. pneumoniae based on a fourfold rise in antibody titre between acute and convalescent sera or a positive PCR result together with a persistently high antibody titre. Children with a positive PCR result in the absence of serological evidence of M. pneumoniae infection were considered to be carriers of M. pneumoniae and were therefore not categorised as having current M. pneumoniae infection.
In three studies children with positive PCR results were diagnosed with M. pneumoniae even in the absence of positive serology results (Kumar 2011; Maheshwari 2011; Principi 2001). However, the numbers of children who tested positive for M. pneumoniae on PCR but not on serology were low. In Kumar 2011 PCR was positive in 20 children of whom only three did not have serological evidence of M. pneumoniae. In Maheshwari 2011 PCR was positive in five children of whom only one did not have serological evidence of M. pneumoniae. Principi 2001 reported that 16 children with community‐acquired lower respiratory tract infections (acute bronchitis, wheezing or pneumonia) had positive PCR results without serological evidence of acute infection. The study did not report how many of these children were in the pneumonia subgroup, of whom 36% (150/418) were diagnosed with M. pneumoniae. However, even if all 16 children had been in this subgroup, they would only have accounted for 11% of M. pneumoniae diagnoses in children with community‐acquired pneumonia.
One study used antigen detection of M. pneumoniae in nasopharyngeal aspirate alongside serology as its reference standard (Agarwal 2009). However, it was unclear whether or not this reference standard was acceptable, as no children tested positive for M. pneumoniae using both laboratory methods. Of the 24 children who were diagnosed with M. pneumoniae, 14 were positive by serology and 10 by antigen detection in nasopharyngeal aspirate.
All of our included studies avoided partial and differential verification. Deerojanawong 2006 obtained paired serum samples from 245/257 children; the 12 children from whom convalescent serum samples could not be obtained were excluded from the study population. Somer 2006 obtained paired serum samples from 140/159 children; the 19 children from whom convalescent serum samples could not be obtained were excluded from the study population. Maheshwari 2011 sought to obtain convalescent serum samples from all 75 children who entered the study, but only managed this in 45 children. Children from whom convalescent serum samples were not obtained were still included in the study population. Only 2/23 children in whom M. pneumoniae was detected were diagnosed on the basis of a fourfold rise in antibody titre alone.
Only one study clearly reported that acute serum samples were obtained within 24 hours of hospital admission, when clinical symptoms and signs were assessed (Somer 2006). We were unable to assess whether or not the delay between assessment of clinical symptoms and signs and obtaining samples for M. pneumoniae detection was acceptable in five studies, as these did not report the time interval between clinical assessment and sample‐taking (Agarwal 2009; Chan 2001; Deerojanawong 2006; Kumar 2011; Maheshwari 2011). Principi 2001 reported that symptoms and signs were recorded at the time of hospital admission, whereas laboratory samples were taken at the time of enrolment into the study. The mean duration of hospitalisation ranged from 5.68 days in children with neither M. pneumoniae nor C. pneumoniae infection, to 6.63 days in children with both infections. We therefore considered that the delay between clinical assessment and laboratory sample taking was unacceptable in this study.
All of our included studies avoided incorporation bias, as M. pneumoniae was diagnosed based on laboratory test results and not on the absence or presence of clinical symptoms or signs. We also considered that blinding of the reference standard took place in our included studies. Somer 2006 reported that clinical assessment was performed at the time of hospital admission, when the results of convalescent serum samples would not have been available. Five other studies also sought convalescent serum samples from children, the results of which would not have been available during the acute community‐acquired pneumonia illness episode, when clinical symptoms and signs were recorded (Chan 2001; Deerojanawong 2006; Kumar 2011; Maheshwari 2011; Principi 2001). Agarwal 2009 only obtained acute laboratory samples but the results of these would not have been available on admission, when clinical symptoms and signs were recorded.
No studies explicitly reported that interpretation of laboratory tests was performed blinded to knowledge about clinical symptoms and signs. However, in four studies, we felt that this is unlikely to have occurred, since clear laboratory criteria and antibody titre thresholds were specified as being diagnostic of M. pneumoniae (Chan 2001; Deerojanawong 2006; Principi 2001; Somer 2006). Blinding to clinical symptoms and signs was unclear in three studies, which did not report diagnostic antibody titre thresholds (Agarwal 2009; Kumar 2011; Maheshwari 2011). None of our included studies reported borderline or uninterpretable serology results. The five studies which used additional laboratory methods alongside serology to diagnose M. pneumoniae all reported data on participants with discrepant results on different tests (Agarwal 2009; Deerojanawong 2006; Kumar 2011; Maheshwari 2011; Principi 2001). However, Principi 2001 did not report the number of discrepant test results within the pneumonia subgroup.
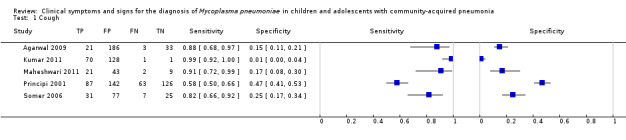
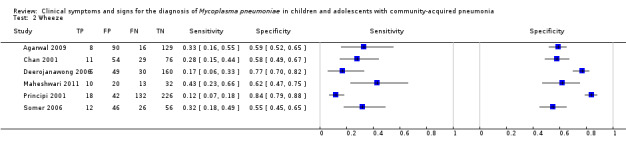
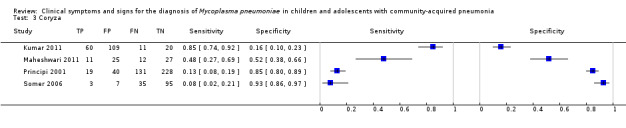
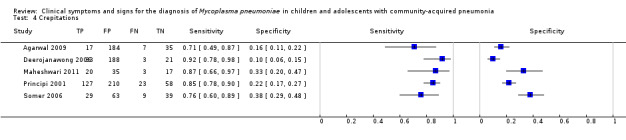
Findings
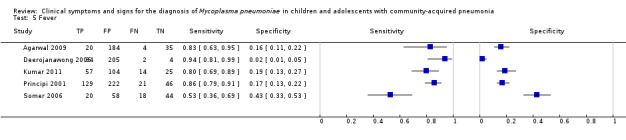
Figure 3 summarises study‐specific values for the sensitivities and specificities of cough, wheeze, coryza, crepitations, fever, rhonchi, shortness of breath, chest pain, diarrhea and myalgia with 95% CIs. For cough, coryza, fever and rhonchi, sensitivity and specificity varied widely between different studies. In particular, the variation in study‐specific specificity values was 20‐fold for fever (0.02 to 0.43) and 50‐fold for cough (0.01 to 0.47). There was a 10‐fold variation in study‐specific sensitivity for coryza (0.08 to 0.85).
3.
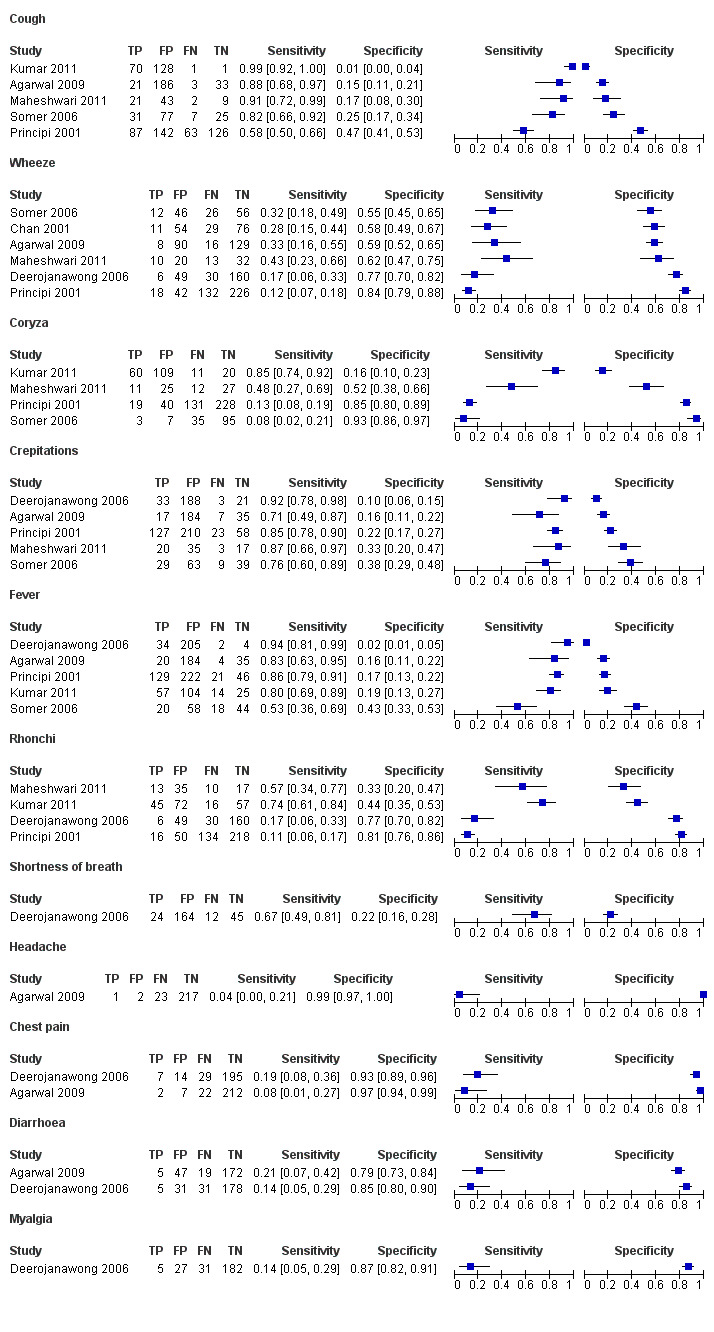
Forest plot of tests: 1 Cough, 2 Wheeze, 3 Coryza, 4 Crepitations, 5 Fever, 6 Rhonchi, 7 Shortness of breath, 8 Headache, 9 Chest pain, 10 Diarrhoea, 11 Myalgia.
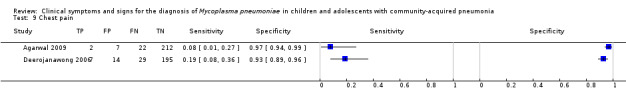
Table 4 shows that rhonchi were 32% more likely to be present in children with M. pneumoniae in one study (pooled positive likelihood ratio (LR+) 1.32, 95% confidence interval (CI) 1.07 to 1.64; pooled negative likelihood ratio (LR‐) 0.59, 95% CI 0.37 to 0.94) (Kumar 2011) but 10% more likely to be absent in children with M. pneumoniae in another study (LR+ 0.57, 95% CI 0.34 to 0.97; LR‐ 1.10, 95% CI 1.01 to 1.19) (Principi 2001). Table 4 also shows that in two studies the presence of chest pain more than doubled the probability of M. pneumoniae in children with community‐acquired pneumonia (Agarwal 2009, Deerojanawong 2006). The presence of chest pain increased the probability of M. pneumoniae from 10% to 22% in one study (Agarwal 2009) and from 15% to 33% in the other study (Deerojanawong 2006).
2. Pre‐/post‐test probabilities and likelihood ratios with 95% confidence intervals.
| Symptom/sign | Study | Pre‐test probability | Post‐test probability (symptom/sign positive) | Post‐test probability (symptom/sign negative) | Positive likelihood ratio | Negative likelihood ratio |
| Cough | Agarwal 2009 | 0.10 (0.06 to 0.14) | 0.10 (0.06 to 0.15) | 0.08 (0.02 to 0.22) | 1.03 (0.88 to 1.21) | 0.83 (0.28 to 2.50) |
| Kumar 2011 | 0.36 (0.29 to 0.43) | 0.35 (0.29 to 0.42) | 0.50 (0.01 to 0.99) | 0.99 (0.96 to 1.03) | 1.82 (0.12 to 28.6) | |
| Maheshwari 2011 | 0.31 (0.21 to 0.42) | 0.33 (0.22 to 0.46) | 0.18 (0.02 to 0.52) | 1.10 (0.93 to 1.32) | 0.50 (0.12 to 2.15) | |
| Principi 2001 | 0.36 (0.31 to 0.41) | 0.38 (0.32 to 0.45) | 0.33 (0.27 to 0.41) | 1.09 (0.92 to 1.31) | 0.89 (0.71 to 1.12) | |
| Somer 2006 | 0.27 (0.20 to 0.35) | 0.29 (0.20 to 0.38) | 0.22 (0.09 to 0.40) | 1.08 (0.90 to 1.30) | 0.75 (0.36 to 1.60) | |
| Wheeze | Agarwal 2009 | 0.10 (0.06 to 0.14) | 0.08 (0.04 to 0.15) | 0.11 (0.06 to 0.17) | 0.81 (0.45 to 1.46) | 1.13 (0.84 to 1.53) |
| Chan 2001 | 0.24 (0.17 to 0.31) | 0.17 (0.90 to 0.28) | 0.28 (0.16 to 0.55) | 0.66 (0.39 to 1.14) | 1.24 (0.98 to 1.58) | |
| Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.11 (0.04 to 0.22) | 0.16 (0.11 to 0.22) | 0.71 (0.33 to 1.54) | 1.09 (0.92 to 1.28) | |
| Maheshwari 2011 | 0.31 (0.21 to 0.42) | 0.33 (0.17 to 0.53) | 0.29 (0.16 to 0.44) | 1.13 (0.63 to 2.02) | 0.92 (0.61 to 1.40) | |
| Principi 2001 | 0.36 (0.31 to 0.41) | 0.30 (0.19 to 0.43) | 0.37 (0.32 to 0.42) | 0.77 (0.46 to 1.28) | 1.04 (0.97 to 1.13) | |
| Somer 2006 | 0.27 (0.20 to 0.35) | 0.21 (0.11 to 0.33) | 0.32 (0.22 to 0.43) | 0.70 (0.42 to 1.17) | 1.25 (0.94 to 1.65) | |
| Coryza | Kumar 2011 | 0.36 (0.29 to 0.43) | 0.36 (0.28 to 0.43) | 0.35 (0.19 to 0.55) | 1.00 (0.88 to 1.13) | 1.00 (0.51 to 1.97) |
| Maheshwari 2011 | 0.31 (0.21 to 0.42) | 0.31 (0.16 to 0.48) | 0.31 (0.17 to 0.48) | 0.99 (0.60 to 1.66) | 1.00 (0.51 to 1.97) | |
| Principi 2001 | 0.36 (0.31 to 0.41) | 0.32 (0.21 to 0.46) | 0.36 (0.32 to 0.42) | 0.85 (0.51 to 1.41) | 1.03 (0.95 to 1.11) | |
| Somer 2006 | 0.27 (0.20 to 0.35) | 0.30 (0.07 to 0.65) | 0.27 (0.20 to 0.35) | 1.15 (0.31 to 4.22) | 0.99 (0.89 to 1.10) | |
| Crepitations | Agarwal 2009 | 0.10 (0.06 to 0.14) | 0.08 (0.05 to 0.13) | 0.17 (0.07 to 0.31) | 0.84 (0.65 to 1.10) | 1.83 (0.91 to 3.65) |
| Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.15 (0.11 to 0.20) | 0.13 (0.03 to 0.32) | 1.02 (0.91 to 1.14) | 0.83 (0.26 to 2.64) | |
| Maheshwari 2011 | 0.31 (0.21 to 0.42) | 0.36 (0.24 to 0.50) | 0.15 (0.03 to 0.38) | 1.29 (1.01 to 1.65) | 0.40 (0.13 to 1.23) | |
| Principi 2001 | 0.36 (0.31 to 0.41) | 0.38 (0.32 to 0.43) | 0.28 (0.19 to 0.40) | 1.08 (0.99 to 1.19) | 0.71 (0.46 to 1.1) | |
| Somer 2006 | 0.27 (0.20 to 0.35) | 0.32 (0.22 to 0.42) | 0.19 (0.09 to 0.33) | 1.24 (0.98 to 1.56) | 0.62 (0.33 to 1.15) | |
| Fever | Agarwal 2009 | 0.10 (0.06 to 0.14) | 0.10 (0.06 to 0.15) | 0.10 (0.03 to 0.24) | 0.99 (0.82 to 1.20) | 1.04 (0.41 to 2.68) |
| Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.14 (0.10 to 0.19) | 0.33 (0.04 to 0.78) | 0.96 (0.89 to 1.05) | 2.90 (0.55 to 15.27) | |
| Kumar 2011 | 0.36 (0.29 to 0.43) | 0.35 (0.28 to 0.43) | 0.36 (0.21 to 0.53) | 1.00 (0.86 to 1.15) | 1.02 (0.57 to 1.83) | |
| Principi 2001 | 0.36 (0.31 to 0.41) | 0.37 (0.32 to 0.42) | 0.31 (0.21 to 0.44) | 1.04 (0.95 to 1.13) | 0.82 (0.51 to 1.31) | |
| Somer 2006 | 0.27 (0.20 to 0.35) | 0.26 (0.16 to 0.37) | 0.29 (0.18 to 0.42) | 0.93 (0.66 to 1.31) | 1.10 (0.73 to 1.64) | |
| Rhonchi | Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.11 (0.04 to 0.22) | 0.16 (0.11 to 0.22) | 0.71 (0.33 to 1.54) | 1.09 (0.92 to 1.28) |
| Kumar 2011 | 0.36 (0.29 to 0.43) | 0.38 (0.30 to 0.48) | 0.22 (0.13 to 0.33) | 1.32 (1.07 to 1.64) | 0.59 (0.37 to 0.94) | |
| Maheshwari 2011 | 0.31 (0.21 to 0.42) | 0.27 (0.15 to 0.42) | 0.37 (0.19 to 0.58) | 0.84 (0.56 to 1.26) | 1.33 (0.72 to 2.44) | |
| Principi 2001 | 0.36 (0.31 to 0.41) | 0.24 (0.15 to 0.36) | 0.38 (0.33 to 0.43) | 0.57 (0.34 to 0.97) | 1.10 (1.01 to 1.19) | |
| Shortness of breath | Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.13 (0.08 to 0.18) | 0.21 (0.11 to 0.34) | 0.85 (0.67 to 1.08) | 1.55 (0.91 to 2.63) |
| Headache | Agarwal 2009 | 0.10 (0.06 to 0.14) | 0.04 (0.00 to 0.21) | 0.01 (0.00 to 0.03) | 4.56 (0.43 to 48.48) | 0.97 (0.89 to 1.05) |
| Chest pain | Agarwal 2009 | 0.10 (0.06 to 0.14) | 0.22 (0.03 to 0.60) | 0.09 (0.06 to 0.14) | 2.61 (0.57 to 11.85) | 0.95 (0.84 to 1.07) |
| Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.33 (0.15 to 0.57) | 0.13 (0.09 to 0.18) | 2.90 (1.26 to 6.69) | 0.86 (0.73 to 1.02) | |
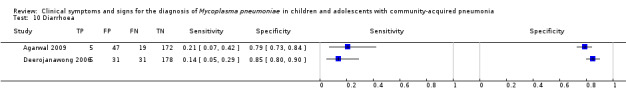
| Diarrhoea | Agarwal 2009 | 0.10 (0.06 to 0.14) | 0.10 (0.03 to 0.21) | 0.10 (0.06 to 0.15) | 0.97 (0.43 to 2.20) | 1.01 (0.81 to 1.25) |
| Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.14 (0.05 to 0.29) | 0.15 (0.10 to 0.20) | 0.94 (0.39 to 2.25) | 1.01 (0.88 to 1.17) | |
| Myalgia | Deerojanawong 2006 | 0.15 (0.11 to 0.20) | 0.16 (0.05 to 0.33) | 0.15 (0.10 to 0.20) | 1.08 (0.44 to 2.61) | 0.99 (0.86 to 1.14) |
We were able to obtain pooled estimates for cough, wheeze, coryza and crepitations (Table 2). Five studies reported data on cough (Agarwal 2009; Kumar 2011; Maheshwari 2011; Principi 2001; Somer 2006), six studies on wheeze (Agarwal 2009; Chan 2001; Deerojanawong 2006; Maheshwari 2011; Principi 2001; Somer 2006), four studies on coryza (Kumar 2011; Maheshwari 2011; Principi 2001; Somer 2006) and five studies on crepitations (Agarwal 2009; Deerojanawong 2006; Maheshwari 2011; Principi 2001; Somer 2006).
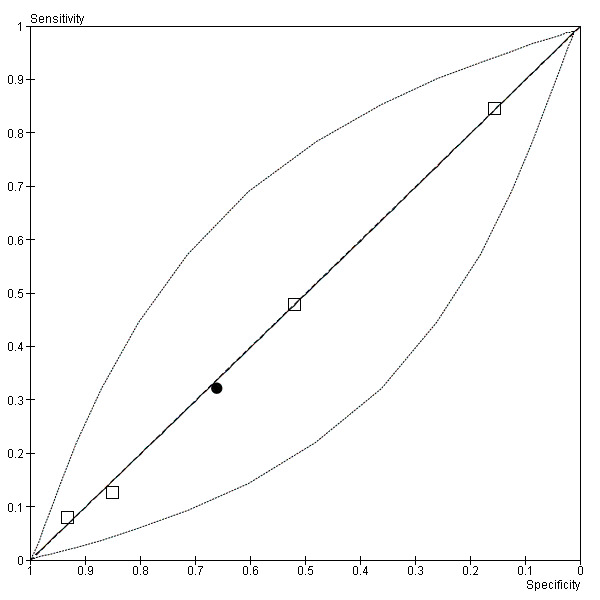
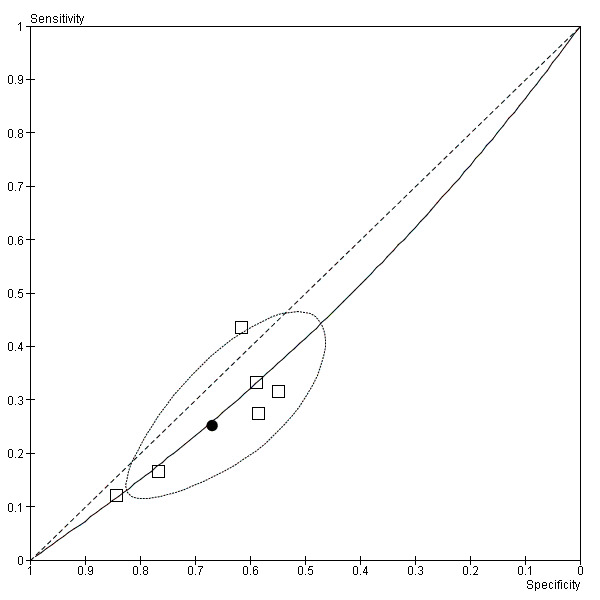
Cough (Figure 4) and crepitations (Figure 5) were sensitive but poorly specific indicators of M. pneumoniae (Cough: pooled sensitivity 0.89, 95% confidence interval (CI) 0.67 to 0.97; pooled specificity 0.15, 95% CI 0.05 to 0.37. Crepitations: pooled sensitivity 0.84, 95% CI 0.78 to 0.88; pooled specificity 0.22, 95% CI 0.14 to 0.32). The performance of coryza as a diagnostic indicator was no better than chance (Figure 6; pooled sensitivity 0.32, 95% CI 0.08 to 0.72; pooled specificity 0.66, 95% CI 0.28 to 0.91). Wheeze had poor sensitivity but moderate specificity (pooled sensitivity 0.25, 95% CI 0.17 to 0.36; pooled specificity 0.67, 95% CI 0.56 to 0.76). The summary curve for wheeze was below the diagonal, indicating that absence, rather than presence, of wheeze may indicate M. pneumoniae infection (Figure 7).
4.
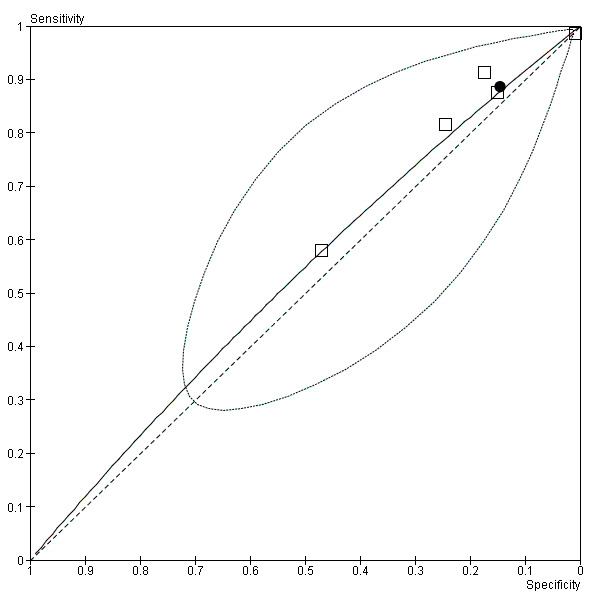
Summary ROC plot of cough (black dot ‐ summary point; black dotted line ‐ 95% confidence region for summary point; black solid line ‐ summary ROC curve)
5.
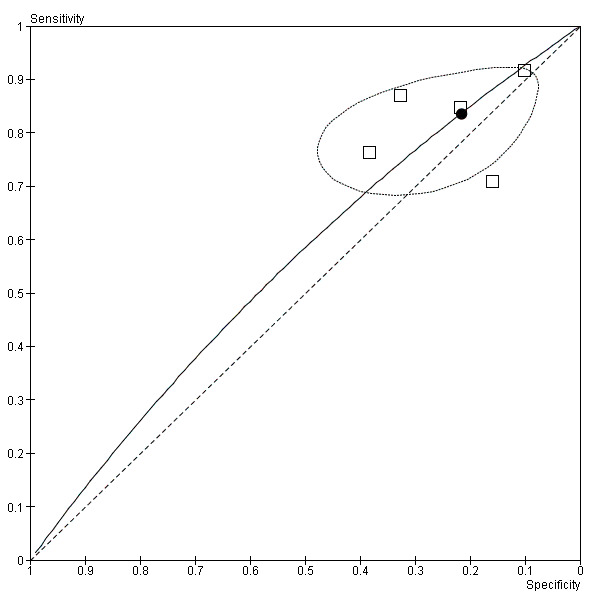
Summary ROC plot of crepitations (black dot ‐ summary point; black dotted line ‐ 95% confidence region for summary point; black solid line ‐ summary ROC curve)
6.
Summary ROC plot of coryza (black dot ‐ summary point; black dotted line ‐ 95% confidence region for summary point; black solid line ‐ summary ROC curve)
7.
Summary ROC Plot of Wheeze (black dot ‐ summary point; black dotted line ‐ 95% confidence region for summary point; black solid line ‐ summary ROC curve)
Although four studies reported data on rhonchi (Deerojanawong 2006; Kumar 2011; Maheshwari 2011; Principi 2001), the model failed to converge to a summary estimate. This failure to converge could have been due to the small number of studies combined with the data reported for the individual studies and is a well‐known problem in these models (Macaskill 2010).
Wheeze was 12% more likely to be absent in children with M. pneumoniae (pooled LR+ 0.76, 95% CI 0.60 to 0.97; pooled LR‐ 1.12, 95% CI 1.02 to 1.23). We performed a sensitivity analysis excluding Agarwal 2009 because we did not feel that this study recruited a representative spectrum of patients (only children with severe community‐acquired pneumonia included) and had concerns about the acceptability of the reference standard (no children tested positive for M. pneumoniae on both IgM serology and antigen detection in nasopharyngeal aspirate). However, this sensitivity analysis did not change our findings (pooled LR+ 0.75, 95% CI 0.58 to 0.98; pooled LR‐ 1.11, 95% CI 1.01 to 1.23). Our findings also did not change when we analyzed data from studies in which M. pneumoniae was diagnosed using serology only (pooled LR+ 0.68, 95% CI 0.50 to 0.92; pooled LR‐ 1.24, 95% 1.03 to 1.51) (Chan 2001; Somer 2006). However, wheeze was not a statistically significant diagnostic indicator based on data from studies which used other laboratory tests alongside serology to diagnose M. pneumoniae (pooled LR+ 0.84, 95% CI 0.63 to 1.12; pooled LR‐ 1.06, 95% CI 0.96 to 1.18) (Agarwal 2009; Deerojanawong 2006; Maheshwari 2011; Principi 2001). Figure 8 summarises individual and pooled sensitivity and specificity values for wheeze based on data from studies which diagnosed M. pneumoniae using serology only versus other laboratory tests alongside serology.
8.
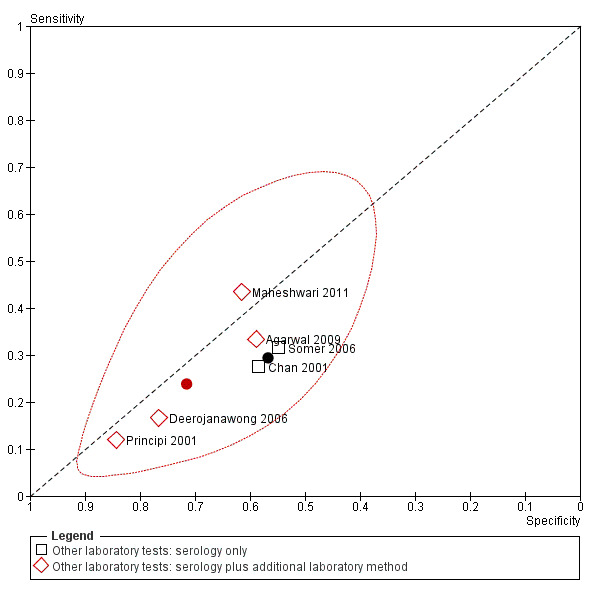
Summary ROC plot of wheeze including summary points (black dot ‐ summary point for studies which diagnosed M. pneumoniae using serology only; red dot ‐ summary point for studies which diagnosed M. pneumoniae using serology plus additional laboratory methods; red dotted line ‐ 95% confidence region for summary point calculated using data from studies which diagnosed M. pneumoniae using serology plus additional laboratory methods).
We did not investigate the influence of methods of diagnosing community‐acquired pneumonia or serological methods of diagnosing M. pneumoniae since Agarwal 2009 was the only study reporting data on wheeze which diagnosed community‐acquired pneumonia based on clinical criteria only and diagnosed M. pneumoniae using a single high antibody titre; we had already excluded data from Agarwal 2009 during our sensitivity analysis. Data were not sufficient to perform investigations of heterogeneity for any other clinical symptoms or signs apart from wheeze. It was not possible to explore the influence of healthcare setting or participant age group because all the studies included in this review were conducted in hospital settings and did not report data stratified according to our age groups of interest.
The presence of crepitations was not a statistically significant indicator of M. pneumoniae (pooled LR+ 1.06, 95% CI 0.96 to 1.18; pooled LR‐ 0.77, 95% CI 0.52 to 1.12). However, when we performed a sensitivity analysis excluding data from Agarwal 2009 we found that the presence of crepitations was a diagnostic indicator of M. pneumoniae, although this finding was only of borderline statistical significance (pooled LR+ 1.10, 95% CI 0.99 to 1.23; pooled LR‐ 0.66, 95% CI 0.46 to 0.96).
Five studies reported data on fever (Agarwal 2009; Deerojanawong 2006; Kumar 2011; Principi 2001; Somer 2006). However, we were only able fit a bivariate model when we performed a sensitivity analysis excluding data from Agarwal 2009 as the model did not converge when all five studies were included. Fever had high sensitivity (pooled sensitivity 0.85, 95% CI 0.63 to 0.95) but poor specificity (pooled specificity 0.15, 95% CI 0.05 to 0.38). Overall, fever was not a useful diagnostic indicator (pooled LR+ 1.00, 95% CI 0.94 to 1.07; pooled LR‐ 1.00, 95% CI 0.70 to 1.44).
Coryza and cough were not useful diagnostic indicators of M. pneumoniae (coryza: pooled LR+ 0.95, 95% CI 0.71 to 1.26; pooled LR‐ 1.03, 95% CI 0.90 to 1.17. Cough: pooled LR+ 1.04, 95% CI 0.95 to 1.13; pooled LR‐ 0.78, 95% CI 0.44 to 1.39). We attempted to perform a sensitivity analysis of data on cough excluding Agarwal 2009. However, we were unable to obtain a summary measure from the four remaining studies using the bivariate model as the algorithm failed to converge.
Discussion
Summary of main results
There is a paucity of high quality data relating to the diagnostic value of symptoms and signs in the clinical recognition of M. pneumoniae in children and adolescents with community‐acquired pneumonia. Based on currently available data, the absence or presence of individual clinical symptoms or signs cannot be used to help clinicians accurately diagnose M. pneumoniae. The absence of wheeze is a statistically significant diagnostic indicator of M. pneumoniae. However, its clinical utility is limited, since absence of wheeze is only 12% more likely in children with M. pneumoniae versus children without M. pneumoniae. However, this review did find preliminary evidence from two studies to suggest that the presence of chest pain approximately doubles the probability of M. pneumoniae in children with community‐acquired pneumonia. Chest pain may therefore be a useful clinical indicator of M. pneumoniae, whose diagnostic value should be evaluated further in future studies.
Strengths and weaknesses of the review
We used a systematic and comprehensive search strategy to identify articles for our review. We analyzed full‐text versions of any articles felt to be potentially relevant, including studies relating to community‐acquired pneumonia or respiratory tract infections generally, even if M. pneumoniae was not specifically mentioned in the title or abstract. We did not apply any language restrictions to our search. Two review authors independently screened abstracts and full‐text articles as well as extracted data from and assessed methodological quality of included studies. In order to assess the validity and robustness of our findings, we performed sensitivity analyses excluding data from one study (Agarwal 2009) because we had concerns that this study had recruited an unrepresentative spectrum of patients and were unclear about the reliability of the reference standard used to diagnose M. pneumoniae.
Since there is currently no 'gold standard' for the laboratory diagnosis of M. pneumoniae, we also assessed the impact on our findings of using other laboratory methods in addition to serology to detect M. pneumoniae. In this review, we selected serology as our reference standard because it is currently the most widely available test for M. pneumoniae. However, a combination of serological and polymerase chain reaction (PCR) methods is considered to be optimal for detecting M. pneumoniae in patients with community‐acquired pneumonia (Thurman 2009). PCR is a more sensitive method of detecting M. pneumoniae than serology during the first two weeks after symptom onset (Nilsson 2008). Although Nilsson 2008 found that M. pneumoniae DNA could persist in the oropharynx for up to seven months, a more recent study has demonstrated that M. pneumoniae carriage among asymptomatic individuals is rare (1/428 subjects, 0.2%) (Chalker 2011). Similarly, M. pneumoniae immunoglobulin (IgM) antibodies are detected in only 0.3% of healthy patients and patients with positive PCR results (sputum or other respiratory secretions) have similar clinical and demographic characteristics to patients with positive IgM serology (Von Baum 2009).
Our main limitations in this review were paucity of data and considerable heterogeneity in the findings of our included studies, particularly in relation to cough, coryza, fever and rhonchi. We only had sufficient data to obtain pooled estimates for cough, wheeze, coryza and crepitations. We were only able to obtain pooled estimates for fever after excluding data from Agarwal 2009 during our sensitivity analysis. We were also only able to investigate the use of additional laboratory tests alongside serology as a potential source of heterogeneity for one symptom (wheeze). We did not have sufficient data to investigate several other potential sources of heterogeneity, including age and healthcare setting (primary versus secondary care). No studies reported data on combinations of clinical symptoms and signs in children with and without M. pneumoniae.
There were also inconsistencies in the reporting of clinical symptoms and signs across different studies. Coryzal symptoms were described as coryza (Kumar 2011), rhinorrhoea (Maheshwari 2011), rhinitis (Principi 2001) or runny nose (Somer 2006). Rales rather than crepitations were described in four studies (Deerojanawong 2006; Maheshwari 2011; Principi 2001; Somer 2006). Although two studies reported data on chest pain (Agarwal 2009; Deerojanawong 2006) no further details were given about its character. In one study, data on the clinical features of two participants with concurrent M. pneumoniae and C. pneumoniae infections were not reported separately (Somer 2006). We therefore analyzed the data conservatively, assuming that the clinical features being studied were absent in both participants.
Applicability of findings to the review question
Based on currently published data, the absence or presence of individual clinical symptoms and signs should not be used to guide clinical decisions about empirical macrolide antibiotic prescribing for suspected M. pneumoniae infections. In two studies (Agarwal 2009; Deerojanawong 2006) the presence of chest pain more than doubled the probability of M. pneumoniae, but these findings were only based on small numbers of participants. The absence of wheeze is the only statistically significant diagnostic indicator, but has only limited clinical utility, since it is only 12% more likely in M. pneumoniae‐positive children with community‐acquired pneumonia.
Based on our pooled sensitivity estimate for wheeze, 25% of children with M. pneumoniae have wheeze as a clinical symptom. Therefore, a policy of empirical macrolide antibiotic treatment in children without wheeze would result in antibiotics being withheld from 25% of children with M. pneumoniae. More importantly, a high percentage of children receiving empirical antibiotic treatment would be M. pneumoniae‐negative and therefore be receiving antibiotics unnecessarily. Assuming that the specificity of wheeze is 67% (based on our pooled specificity estimate), the percentage of children receiving antibiotics unnecessarily would range from 61% if M. pneumoniae prevalence was 36% (the highest prevalence estimate among our included studies) to 89% if M. pneumoniae prevalence was 10% (the lowest prevalence estimate among our included studies).
The diagnostic value of clinical symptoms and signs may vary between children of different ages. Two case series found that coryza, tachypnoea, diarrhea and vomiting were more common in preschool than in older children with M. pneumoniae (Defilippi 2008; Othman 2005). In addition, our findings may not be applicable to children presenting in primary care with community‐acquired pneumonia because all of our included studies were conducted in hospital settings. Children admitted to hospital with community‐acquired pneumonia are likely to represent a narrower and more severe spectrum of illness than children who present in primary care. At the moment, the relationship between illness severity and clinical presentation of M. pneumoniae is uncertain. Greater variation in presenting symptoms and a higher prevalence of certain clinical features (including rhinorrhoea, headache and chest pain) during epidemic outbreaks have previously been observed (Gomez Campdera 2006) and may reflect a wider spectrum of disease severity when M. pneumoniae prevalence is high. Our findings may also have limited applicability in developed countries, since all our included studies except one (Principi 2001) were conducted in developing countries.
Population‐level data on M. pneumoniae incidence are likely to play an important role in helping clinicians to interpret the diagnostic value of clinical symptoms and signs. The performance of a clinical decision model for pertussis in infants has been shown to improve with the incorporation of local disease incidence data (Fine 2010). Clinicians should also take previous antibiotic use into account when considering possible microbial aetiology in children with community‐acquired pneumonia. Treatment with beta‐lactam antibiotics up to 14 days before admission to hospital with community‐acquired pneumonia is reported to be associated with a threefold increased chance of infection with atypical pathogens (including M. pneumoniae) and a threefold decreased probability of pneumococcal infection (Van de Garde 2008). Somer 2006 excluded children who had received antibiotics up to 10 days before admission and Principi 2001 excluded children who had received antibiotics within the last 48 hours.
The diagnostic value of wheeze may vary according to the prevalence of other respiratory pathogens, particularly respiratory viruses. Evidence of viral infection has previously been detected in 33% of acute asthma exacerbations in children, whereas evidence of M. pneumoniae infection was only detected in 2% (Freymuth 1999). The high prevalence of viral infections in children presenting with acute wheezing episodes is well established (Heymann 2004; Jartti 2004). One study found that M. pneumoniae was only present in 5% of children with acute wheezing episodes of proven viral aetiology (Lehtinen 2006). We may therefore have found that wheeze is less likely in children with M. pneumoniae because of the relatively infrequent association between M. pneumoniae and viral infections.
Based on scarce preliminary data, chest pain may be a useful indicator of M. pneumoniae in children who present with community‐acquired pneumonia in outpatient settings. Among adults with community‐acquired pneumonia, 41.8% of outpatients report pleuritic chest pain compared to 29.3% of inpatients (Wattanathum 2003). Wattanathum 2003 also reported a significantly higher prevalence of M. pneumoniae in outpatients (29.6%) than in inpatients (6.8%; P < 0.001). However, establishing the absence or presence of subjective symptoms, such as chest pain, may be difficult in preschool children (Hay 2002). Moreover, further research is needed to substantiate the diagnostic value of chest pain in the clinical recognition of M. pneumoniae.
Authors' conclusions
Implications for practice.
This review has found that M. pneumoniae cannot be reliably diagnosed in children and adolescents with community‐acquired pneumonia based on the absence or presence of individual clinical symptoms and signs. Although the absence of wheeze is a statistically significant diagnostic indicator, it does not have sufficient diagnostic value to guide empirical macrolide antibiotic treatment in children with community‐acquired pneumonia. Data from two studies suggest that the presence of chest pain more than doubles the probability of M. pneumoniae. However, further research is needed to substantiate this finding. No data on the diagnostic value of combinations of clinical symptoms and signs has yet been published. Clinicians should therefore consider other factors, including previous antibiotic use and population‐based data on M. pneumoniae incidence, to help them estimate the likelihood of M. pneumoniae infection in the context of a clinical consultation.
Implications for research.
More high quality, large‐scale studies are needed to help develop prediction rules based on epidemiological data as well as clinical and baseline patient characteristics, which clinicians can use to help them diagnose M. pneumoniae during clinical consultations. These studies should also consider a wider range of extra‐pulmonary clinical features, such as headache, diarrhea and myalgia. In particular, more studies conducted in primary care settings are needed. Given that there is no gold standard for M. pneumoniae diagnosis, greater consistency in the laboratory methods used across different studies is needed to ensure that acute M. pneumoniae infections are reliably detected. The development of rapid point of care tests for the diagnosis of M. pneumoniae is also an important area for further research. The ability to diagnose M. pneumoniae more accurately will not only help inform more appropriate clinical decisions, but will also facilitate the design and conduct of more definitive research to determine the efficacy of antibiotics in the treatment of community‐acquired pneumonia and other respiratory tract infections caused by M. pneumoniae (Mulholland 2010).
Acknowledgements
We would like to thank Nia Roberts and Sarah Thorning for assisting us with preparing our search strategy, performing our searches and retrieving articles for this review. We thank Yemisi Takwoingi for her advice on statistical analysis. We wish to thank Paul Glasziou and Inge Axelsson for commenting on drafts of the protocol and full review. We also wish to thank Chanpen Choprapawon, Anne Lyddiatt and Amita Jain for commenting on the draft protocol and Rakesh Lodha, Elizabeth Ayres and Conor Teljeur for commenting on the draft of the full review. Kay Wang's post is funded by the National Institute for Health Research and this review is part of a wider programme of research.
Appendices
Appendix 1. MEDLINE search strategy
Pneumonia/
Pneumonia, Bacterial/
Pneumonia, Mycoplasma/
mycoplasma pneumon*.tw.
"m. pneumoniae".tw.
(community‐acquired pneumon* or community acquired pneumon*).tw.
or/1‐6
Cough/
cough*.tw.
wheez*.tw.
"shortness of breath".tw.
sore throat*.tw.
coryza.tw.
"chest pain".tw.
crepitation*.tw.
Fever/
fever*.tw.
Exanthema/
(rash or rashes).tw.
exp Diarrhea/
(diarrhoea or diarrhea).tw.
myalgia.tw.
Headache/
headache*.tw.
clinical assessment*.tw.
clinical feature*.tw.
(symptom* or sign* or characteristic* or manifestation*).tw.
or/8‐27
7 and 28
exp Infant/
(infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur*).tw.
exp Child/
(child* or schoolchild* or school age* or preschool* or kid or kids or toddler*).tw.
Adolescent/
(adoles* or teen* or boy* or girl*).tw.
Minors/
Puberty/
(minor* or pubert* or pubescen*).tw.
exp Pediatrics/
(pediatric* or paediatric*).tw.
exp Schools/
(nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*).tw.
or/30‐42
29 and 43
Pneumonia, Mycoplasma/di [Diagnosis]
Pneumonia, Bacterial/di [Diagnosis]
45 or 46
43 and 47
44 or 48
Appendix 2. EMBASE search strategy
1. *PNEUMONIA/ 2. bacterial pneumonia/ or infectious pneumonia/ 3. Mycoplasma pneumonia/ 4. COMMUNITY ACQUIRED PNEUMONIA/ 5. mycoplasma pneumon*.tw. 6. "m. pneumoniae".tw. 7. (community‐acquired pneumon* or community acquired pneumon*).tw. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. coughing/ or wheezing/ 10. cough*.tw. 11. wheez*.tw. 12. "short of breath*".tw. 13. "shortness of breath".tw. 14. sore throat*.tw. 15. coryza.tw. 16. "chest pain".tw. 17. crepitation*.tw. 18. fever/ 19. (fever* or febrile).tw. 20. exp rash/ 21. (rash or rashes).tw. 22. diarrhea/ 23. (diarrhoea* or diarrhea*).tw. 24. myalgia.tw. 25. HEADACHE/ 26. (headache* or head ache*).tw. 27. clinical feature/ 28. clinical assessment*.tw. 29. clinical feature*.tw. 30. (symptom* or sign* or characteristic* or manifestation*).tw. 31. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. 8 and 31 33. exp infant/ 34. (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur*).tw. 35. child/ or boy/ or girl/ or preschool child/ or school child/ or toddler/ 36. (child* or schoolchild* or school age* or preschool* or kid or kids or toddler*).tw. 37. adolescent/ 38. (adoles* or teen* or boy* or girl*).tw. 39. juvenile/ 40. Puberty/ 41. (minor* or pubert* or pubescen*).tw. 42. pediatrics/ 43. (pediatric* or paediatric*).tw. 44. school/ or high school/ or kindergarten/ or middle school/ or nursery school/ or primary school/ 45. (nursery school* or kindergar* or primary school* or secondary school* or elementary school* or high school* or highschool*).tw. 46. 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 47. 32 and 46
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Cough | 5 | 1076 |
| 2 Wheeze | 6 | 1291 |
| 3 Coryza | 4 | 833 |
| 4 Crepitations | 5 | 1121 |
| 5 Fever | 5 | 1246 |
| 6 Rhonchi | 4 | 928 |
| 7 Shortness of breath | 1 | 245 |
| 8 Headache | 1 | 243 |
| 9 Chest pain | 2 | 488 |
| 10 Diarrhoea | 2 | 488 |
| 11 Myalgia | 1 | 245 |
1. Test.
Cough.
2. Test.
Wheeze.
3. Test.
Coryza.
4. Test.
Crepitations.
5. Test.
Fever.
6. Test.

Rhonchi.
7. Test.

Shortness of breath.
8. Test.

Headache.
9. Test.
Chest pain.
10. Test.
Diarrhoea.
11. Test.

Myalgia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwal 2009.
| Clinical features and settings | Hospital setting (Chhatrapati Shahuji Mahraj Medical University, India) Clinical features for study inclusion: cough or difficulty in breathing (an increased respiratory rate or chest indrawing) |
|
| Participants | Children aged 1 to 59 months admitted with clinical diagnosis of WHO‐defined severe pneumonia Number of participants: 243 Male participants: 160, (65.8%) Number of participants with M. pneumoniae: 24, (9.9%) |
|
| Study design | Prospective observational cohort study | |
| Target condition and reference standard(s) |
M. pneumoniae detected using:
Presence of M. pneumoniae infection was defined as M. pneumoniae detected using either laboratory method |
|
| Index and comparator tests | Symptoms: fever, cough, headache, chills, vomiting, chest pain, diarrhea, wheeze, sore throat, sinus pain Signs: temperature > 100 °F, central cyanosis, nasal flaring, altered sensorium, crepitations, respiratory rate > 40 breaths per minute, inability to feed |
|
| Follow‐up | Symptoms and signs recorded at the time of admission. Blood and nasopharyngeal aspirate samples obtained at the same time, but timing in relation to admission not stated | |
| Notes | Mean duration of hospital stay in children with M. pneumoniae was 8.74 days (standard deviation 6.52), which was not significantly different from that in children with other infections. No children tested positive for M. pneumoniae on both serology and antigen detection in nasopharyngeal aspirate | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | No | Participants were children with severe community‐acquired pneumonia based on WHO clinical criteria. The study population therefore only represented a limited part of the spectrum of disease severity among children admitted with community‐acquired pneumonia. Co‐morbidities in study population were not reported |
| Acceptable reference standard? All tests | Unclear | No children were positive for M. pneumoniae on both types of laboratory testing; 14 children had M. pneumoniae based on IgM serology and 10 based on antigen detection in nasopharyngeal aspirate. Timing of sample taking in relation to symptom onset was not reported |
| Acceptable delay between tests? All tests | Unclear | Symptoms and signs recorded on admission, but timing of sample taking in relation to admission was not reported |
| Partial verification avoided? All tests | Yes | All study participants were subjected to the same laboratory tests |
| Differential verification avoided? All tests | Yes | All study participants were subjected to the same laboratory tests |
| Incorporation avoided? All tests | Yes | The diagnosis of M. pneumoniae was based on laboratory test results only |
| Reference standard results blinded? All tests | Yes | Clinical symptoms and signs likely to have been recorded on study entry, since only children meeting WHO clinical criteria for severe community‐acquired pneumonia were eligible to take part in this study. Laboratory test results would therefore not have been available at the time that clinical assessment took place |
| Index test results blinded? All tests | Unclear | Diagnostic antibody titre for M. pneumoniae detection not reported |
| Relevant clinical information? All tests | Unclear | Baseline data on participant age, sex, weight and height were recorded. Baseline data were also collected on antibiotic intake, previous hospitalisation and oxygen administration during the preceding 6 days. Unclear whether data on duration of illness were collected |
| Uninterpretable results reported? All tests | No | No intermediate or borderline test results reported. Study did not report definitions of intermediate or borderline results for either type of ELISA test |
| Withdrawals explained? All tests | Yes | Data reported for all 243 participants, no withdrawals |
Chan 2001.
| Clinical features and settings | Hospital setting (University of Malaya Medical Center, Malaysia) Clinical features for study inclusion: fever > 37.5 °C with respiratory symptoms and chest signs or chest radiograph changes compatible with a diagnosis of pneumonia |
|
| Participants | Children aged 1 month to 15 years with community‐acquired pneumonia Number of participants: 170 Male participants: 112, (65.9%) Number of participants with M. pneumoniae: 40 (23.5%) |
|
| Study design | Prospective observational cohort study | |
| Target condition and reference standard(s) | M. pneumoniae detected based on serological testing of acute and convalescent blood samples taken 2 to 4 weeks apart (passive particle agglutination test). Presence of M. pneumoniae infection defined as single antibody titre of more than 1:160 or a fourfold or greater rise in antibody titre between acute and convalescent samples. The antibody titre threshold used to diagnose M. pneumoniae on a single serum sample was adjusted from 1:40 (manufacturer's recommendation) to 1:160. A titre of 1:40 was considered too low, as this was found in 45% of healthy blood donors. However, a titre of 1:160 was only found in 10% of the healthy population | |
| Index and comparator tests | Wheeze | |
| Follow‐up | Children followed up during admission. Duration between admission and recording of clinical features/initial sample taking was not reported | |
| Notes | Extra‐pulmonary complications were encountered in 3 children with M. pneumoniae. Two children had elevated liver enzymes which normalised after a week and one died from multi‐organ failure on day 15 of illness | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Co‐morbidities in study population were not reported. Did not state whether or not children with co‐morbidities were excluded |
| Acceptable reference standard? All tests | Yes | M. pneumoniae detected based on serological testing of acute and convalescent blood samples taken 2 to 4 weeks apart |
| Acceptable delay between tests? All tests | Unclear | Timing of collection of acute blood sample in relation to recording of clinical features was not reported |
| Partial verification avoided? All tests | Yes | All study participants were subjected to the same laboratory tests |
| Differential verification avoided? All tests | Yes | All study participants were subjected to the same laboratory tests |
| Incorporation avoided? All tests | Yes | Clear thresholds for positive serological diagnosis of M. pneumoniae reported |
| Reference standard results blinded? All tests | Yes | Clinical symptoms and signs were recorded during the acute community‐acquired pneumonia illness episode, when the results of convalescent serum samples would not have been available |
| Index test results blinded? All tests | Yes | Clear thresholds for positive serological diagnosis of M. pneumoniae reported |
| Relevant clinical information? All tests | Yes | Baseline data were collected from participants on age, sex, ethnicity and duration of illness |
| Uninterpretable results reported? All tests | No | No intermediate or borderline serology results reported. Study did not provide definition of intermediate or borderline serology result |
| Withdrawals explained? All tests | Yes | Data reported for all 170 participants; no withdrawals |
Deerojanawong 2006.
| Clinical features and settings | Multicentre study performed at 3 hospitals in Bangkok, Thailand (Queen Sirkit National Insititute of Health, King Chulalongkorn Memorial Hospital, Ramathibodi Hospital) Clinical features for study inclusion: clinical and radiological diagnosis of community‐acquired pneumonia, defined as new infiltrates or consolidation on chest X‐ray that could not be attributed to other aetiology and the presence of 3 or more of: cough, acute change in quality of sputum, fever or hypothermia (> 38 °C or < 36.1 °C) within the preceding 24 hours, rales or evidence of pulmonary consolidation, leukocytosis, malaise/myalgia or gastrointestinal symptoms |
|
| Participants | Children aged 2 to 15 years with community‐acquired pneumonia Children were excluded if they had evidence or history of tuberculosis, nosocomial pneumonia, aspiration pneumonia or bronchiectasis. Children were also excluded if they were HIV‐positive or had been hospitalised within 2 weeks prior to consultation Number of participants: 257 Number of participants who underwent testing for M. pneumoniae: 245 Male participants: 135, (55.1%) Number of participants with M. pneumoniae: 36, (14.7%) |
|
| Study design | Prospective observational cohort study | |
| Target condition and reference standard(s) |
M. pneumoniae detected using:
Results of single serum samples were excluded from the analysis. The presence of positive PCR for M. pneumoniae in the absence of a positive serologic response was interpreted as possible carriage |
|
| Index and comparator tests | Symptoms: cough, fever, chill, chest pain, dyspnoea, malaise, myalgia, diarrhea, wheezing Signs: rales, rhonchi, bronchial breath sounds |
|
| Follow‐up | Children followed up during admission. Duration between admission and recording of clinical features/initial sample taking was not reported | |
| Notes | In total, 199 children (81%) were treated in hospitals and 3 children (1%) required treatment in the intensive care unit | |
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Inclusion criteria not based on indicators of disease severity. Co‐morbidities in study population: asthma (n = 51), congestive heart failure (n = 7), hepatic disease (n = 1), renal impairment (n = 1) |
| Acceptable reference standard? All tests | Yes | In total 36 children met laboratory diagnostic criteria for current M. pneumoniae infection; 24 children were diagnosed by a fourfold or greater increase in antibody titre between acute and convalescent sera and 12 were diagnosed by positive PCR with persistent high antibody titre. 16 children with a 4‐fold or greater increase in antibody titre were also positive by PCR |
| Acceptable delay between tests? All tests | Unclear | Timing of nasopharyngeal aspirate and acute blood sample collection in relation to recording of clinical features was not reported |
| Partial verification avoided? All tests | Yes | Of the 257 children enrolled in the study with a diagnosis of community‐acquired pneumonia, paired sera could only be obtained from 245 children |
| Differential verification avoided? All tests | Yes | All study participants were subjected to the same laboratory tests |
| Incorporation avoided? All tests | Yes | The diagnosis of M. pneumoniae was based on laboratory test results only |
| Reference standard results blinded? All tests | Yes | Clinical symptoms and signs were recorded during the acute community‐acquired pneumonia illness episode, when the results of convalescent serum samples would not have been available |
| Index test results blinded? All tests | Yes | Clear laboratory criteria for laboratory diagnosis of M. pneumoniae were reported |
| Relevant clinical information? All tests | Unclear | Baseline data on participant age, sex and co‐morbidity were recorded. However, unclear whether data on duration of illness were collected at the time of study entry |
| Uninterpretable results reported? All tests | Yes | Six children had positive PCR results without serological evidence of M. pneumoniae infection. These children were considered to be carriers of M. pneumoniae and were hence not classified as having current M. pneumoniae infection |
| Withdrawals explained? All tests | Yes | Explained that they were only able to obtain paired sera from 245/257 children. The 12 children from whom convalescent serum samples could not be obtained were excluded from the study population |
Kumar 2011.
| Clinical features and settings | Hospital setting (Lok Nayak Hospital, India) Clinical features for study inclusion: cough and fever with breathlessness of less than 30 days duration, increased respiratory rate on examination, signs of consolidation or bronchopneumonia with or without wheeze on auscultation, radiological findings suggestive of consolidation, bronchopneumonia or interstitial infiltrates with or without hyperinflation |
|
| Participants | Children aged 2 months to 12 years admitted to hospital with lower respiratory tract infections Number of participants: 200 Male participants: 127 (63.5%) Number of participants with M. pneumoniae: 71 (35.5%) |
|
| Study design | Prospective observational cohort study | |
| Target condition and reference standard(s) |
M. pneumoniae detected using:
Presence of M. pneumoniae infection was defined as M. pneumoniae detected using either or both laboratory methods |
|
| Index and comparator tests | Symptoms: cough, coryza, afebrile Signs: wheeze audible on auscultation |
|
| Follow‐up | Duration of follow‐up not reported | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Co‐morbidities in study population were not reported. Did not state whether or not children with co‐morbidities were excluded |
| Acceptable reference standard? All tests | Yes | Children with positive PCR results but negative serology results were still diagnosed with M. pneumoniae. However, there was good agreement between PCR and serology results; 20 children had positive PCR results of whom only 3 did not also have serological evidence of M. pneumoniae infection |
| Acceptable delay between tests? All tests | Unclear | Timing of nasopharyngeal aspirate and acute blood sample collection in relation to recording of clinical features was not reported |
| Partial verification avoided? All tests | Yes | All study participants were subjected to the same laboratory tests |
| Differential verification avoided? All tests | Yes | All study participants were subjected to the same laboratory tests |
| Incorporation avoided? All tests | Yes | The diagnosis of M. pneumoniae was based on laboratory test results only |
| Reference standard results blinded? All tests | Yes | Clinical symptoms and signs were recorded during the acute community‐acquired pneumonia illness episode, when the results of convalescent serum samples would not have been available |
| Index test results blinded? All tests | Unclear | Laboratory criteria/thresholds for serological diagnosis of M. pneumoniae detection were not reported |
| Relevant clinical information? All tests | Unclear | Baseline data on participant age and sex were collected. However, unclear whether data on duration of illness were collected at the time of study entry |
| Uninterpretable results reported? All tests | Yes | Three children had positive PCR results without serological evidence of M. pneumoniae infection |
| Withdrawals explained? All tests | Yes | Data reported for all 200 participants; no withdrawals reported |
Maheshwari 2011.
| Clinical features and settings | Hospital setting (Maulana Azad Medical College, India) Clinical features for study inclusion: presence of cough and fever with breathlessness of less than 30 days duration, tachypnoea and presence of radiological features of lower respiratory tract infections |
|
| Participants | Children aged 6 months to 12 years with clinically suspected lower respiratory tract infections Number of participants: 75 Male participants: 42, (56.0%) Number of participants with M. pneumoniae: 23, (30.7%) |
|
| Study design | Prospective observational cohort study | |
| Target condition and reference standard(s) |
M. pneumoniae detected using:
Presence of M. pneumoniae infection was defined as M. pneumoniae detected using either or both laboratory methods |
|
| Index and comparator tests | Symptoms: rhinorrhoea, wheezing, dry cough Signs: rales, rhonchi |
|
| Follow‐up | Duration of follow‐up not reported | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Unclear | Co‐morbidities in study population were not reported. Did not state whether or not children with co‐morbidities were excluded |
| Acceptable reference standard? All tests | Yes | Children with positive PCR results but negative serology results were still diagnosed with M. pneumoniae. However, there was good agreement between PCR and serology results; 5 children had positive PCR results of whom only 1 did not also have serological evidence of M. pneumoniae infection |
| Acceptable delay between tests? All tests | Unclear | Timing of throat swab and acute blood sample collection in relation to recording of clinical features was not reported |
| Partial verification avoided? All tests | Yes | Tried to obtain convalescent blood samples from all 75 children, but only managed to obtain samples in 45 children |
| Differential verification avoided? All tests | Yes | Tried to obtain convalescent blood samples from all 75 children, but only managed to obtain samples in 45 children |
| Incorporation avoided? All tests | Yes | The diagnosis of M. pneumoniae was based on laboratory test results only |
| Reference standard results blinded? All tests | Yes | Clinical symptoms and signs were recorded during the acute community‐acquired pneumonia illness episode, when the results of convalescent serum samples would not have been available |
| Index test results blinded? All tests | Unclear | Laboratory criteria/thresholds for serological diagnosis of M. pneumoniae detection were not reported |
| Relevant clinical information? All tests | Yes | A detailed history and clinical examination were performed for all participants |
| Uninterpretable results reported? All tests | Yes | One child had a positive PCR result without serological evidence of M. pneumoniae infection |
| Withdrawals explained? All tests | Yes | Data were presented for all 75 participants. Reported that only managed to obtain convalescent serum samples from 45/75 participants |
Principi 2001.
| Clinical features and settings | Multicentre study performed at 21 centres in Italy (hospital setting) Children were classified into 3 groups based on clinical and radiological findings:
|
|
| Participants | Previously healthy children aged 2 to 14 years who had been hospitalised for signs and/or symptoms of community‐acquired lower respiratory tract infection Exclusion criteria: severe concomitant diseases (neoplasia, kidney or liver disease, immunodepression, cardiovascular disease, malabsorption syndrome), nosocomial infections, use of antibiotics during the last 48 hours Total number of participants: 613 Male participants: 310 (50.6%) Number of participants with pneumonia: 418 Number of participants with M. pneumoniae: 150 (35.9%) |
|
| Study design | Prospective observational cohort study | |
| Target condition and reference standard(s) | Acute M. pneumoniae infection defined as significant antibody response on paired serum samples (IgM specific antibody >= 1:100; IgG specific antibody >= 1:400 or 4‐fold increase in IgG antibody titre) and/or nasopharyngeal aspirate positive for M. pneumoniae on PCR analysis Past M. pneumoniae infection defined as IgG antibody titre >= 1:100 but < 1:400 without a fourfold increase in paired serum samples and/or nasopharyngeal aspirate positive for M. pneumoniae on PCR analysis Convalescent serum samples were obtained 4 to 6 weeks after acute serum samples |
|
| Index and comparator tests | Symptoms: cough, rhinitis, tachypnoea, wheezing Signs: fever (temperature >= 37.8 °C), rales, rhonchi |
|
| Follow‐up | Clinical features (medical history and physical examination) were recorded for each child at the time of admission. Acute serum samples and nasopharyngeal aspirates were obtained at the time of study entry. Children were re‐evaluated 4 to 6 weeks after study entry, when convalescent serum samples were obtained | |
| Notes | ||
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Clearly reported that children with severe concomitant diseases, nosocomial infections and who had received antibiotics within the last 48 hours were excluded from the study |
| Acceptable reference standard? All tests | Yes | 16 children with community‐acquired lower respiratory tract infections (acute bronchitis, wheezing or pneumonia) had positive PCR results without serological evidence of acute M. pneumoniae infection. The study did not report how many of these children were in the pneumonia subgroup. However, even if all 16 children had been in this subgroup, they would only have represented 11% of the 150 children with pneumonia who were diagnosed with M. pneumoniae |
| Acceptable delay between tests? All tests | No | Clinical symptoms and signs were recorded on admission. Acute serum samples and nasopharyngeal aspirates were taken on enrolment into the study. Mean duration of hospitalisation at time of enrolment ranged from 5.68 days in children with neither M. pneumoniae nor C. pneumoniae infections to 6.63 days in children with both infections |
| Partial verification avoided? All tests | Yes | Acute and convalescent serum samples were obtained from all children |
| Differential verification avoided? All tests | Yes | The same laboratory methods were used to detect M. pneumoniae in all children |
| Incorporation avoided? All tests | Yes | The diagnosis of M. pneumoniae was based on laboratory test results only |
| Reference standard results blinded? All tests | Yes | Clinical symptoms and signs were recorded during the acute community‐acquired pneumonia illness episode, when the results of convalescent serum samples would not have been available |
| Index test results blinded? All tests | Yes | Clear laboratory criteria for diagnosis of acute and past M. pneumoniae infection were reported |
| Relevant clinical information? All tests | Yes | At the time of admission, systematic recordings were made of each patient's medical history, including the date of onset of illness, the underlying respiratory symptoms and the presence of fever (temperature >= 37.8 °C) |
| Uninterpretable results reported? All tests | Unclear | Sixteen children with community‐acquired lower respiratory tract infections had positive PCR results without serological evidence of acute M. pneumoniae infection. However, the study did not report how many of these were in the pneumonia subgroup |
| Withdrawals explained? All tests | Yes | Data were reported for all 418 children with pneumonia; no withdrawals were reported |
Somer 2006.
| Clinical features and settings | Hospital setting (Istanbul, Turkey) Clinical features for study inclusion: combination of acute respiratory symptoms (tachypnoea, dyspnoea, cough, difficulty in breathing, chest indrawing and nasal flaring or with non specific symptoms such as lethargy, fever and rigours) and chest radiographic findings compatible with pneumonia |
|
| Participants | Previously healthy children aged 2 months to 15 years hospitalised because of a diagnosis of community‐acquired pneumonia Exclusion criteria: active tuberculosis, malignancy, hospital‐acquired pneumonia, underlying pulmonary or immunological disease. This study was undertaken as part of a larger prospective study evaluating the incidence of bacterial and atypical pathogens in hospitalised children with community‐acquired pneumonia. As a result of the protocol of this larger study, children who had received antibiotics during the 10 days before admission were excluded Number of participants: 140 Male participants: 85 (60.7%) Number of participants with M. pneumoniae: 38 (27%) |
|
| Study design | Nested prospective observational cohort study | |
| Target condition and reference standard(s) | M. pneumoniae detected based on serological testing of acute and convalescent serum samples (ELISA). Evidence of infection was defined as either a single positive serum IgM titre (>= 1:10) at any visit or a fourfold increase in IgG titres at visit 2 | |
| Index and comparator tests | Symptoms: cough, chest indrawing, nasal flaring, fever, wheeze, sputum, runny nose Signs: cyanosis, crepitations, expiration prolonged |
|
| Follow‐up | A convalescent serum sample was obtained from each child 2 to 4 weeks after the acute serum sample | |
| Notes | A total of 206 children were admitted with community‐acquired pneumonia during the study period of whom 47 did not meet the study inclusion criteria (pulmonary tuberculosis, n = 12; congenital immunodeficiency, n = 10; chronic pulmonary disease, n = 9; malignancy, n = 8; antibiotic use in the 10 days before admission, n = 8) All patients were treated with antimicrobials, mostly ampicillin, clarithromycin or cefuroxime |
|
| Table of Methodological Quality | ||
| Item | Authors' judgement | Description |
| Representative spectrum? All tests | Yes | Previously healthy children admitted with a diagnosis of community‐acquired pneumonia based on clinical and radiological criteria |
| Acceptable reference standard? All tests | Yes | Serological testing of acute and convalescent serum samples |
| Acceptable delay between tests? All tests | Yes | Acute serum was obtained within 24 hours of admission and convalescent serum was obtained 2 to 4 weeks later |
| Partial verification avoided? All tests | Yes | Convalescent serum was not obtained from 19 patients who did not return for follow‐up appointments. Paired serum samples were obtained from all 140 participants |
| Differential verification avoided? All tests | Yes | Paired serum samples were obtained from all 140 participants |
| Incorporation avoided? All tests | Yes | The diagnosis of M. pneumoniae was based on laboratory test results only |
| Reference standard results blinded? All tests | Yes | Data on clinical symptoms and signs were recorded at the time of admission, when laboratory test results would not have been available |
| Index test results blinded? All tests | Yes | Clear laboratory criteria for diagnosis of M. pneumoniae infection were reported |
| Relevant clinical information? All tests | Yes | A thorough medical history was obtained at admission |
| Uninterpretable results reported? All tests | No | No intermediate or borderline serology results were reported. Study did not provide definition of intermediate or borderline serology result |
| Withdrawals explained? All tests | Yes | Convalescent serum was not obtained from 19 patients who did not return for follow‐up appointments. These patients were excluded from the study population |
ELISA: enzyme‐linked immunoadsorbent assay IgG: immunoglobulin G IgM: immunoglobulin M PCR: polymerase chain reaction WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Moyed 2003 | Study population consisted of participants aged 10 to 60 years with clinically and radiographically diagnosed lower respiratory tract infections. Reported data on clinical symptoms and signs in all participants according to whether or not M. pneumoniae was detected, but did not report these data in children specifically |
| Al‐Rashed 1998 | Did not report any data on clinical symptoms or signs |
| Almasri 2011 | Reported data on clinical symptoms and signs in children with community‐acquired respiratory tract infections (pneumonia, pharyngitis or tracheobronchitis) in relation to whether or not M. pneumoniae was detected. However, these data were not reported specifically in the subgroup of children with community‐acquired pneumonia |
| Angadi 1980 | Did not report any data on clinical symptoms or signs |
| Antonelli 2009 | Reported data on clinical symptoms and signs but not in relation to whether or not M. pneumoniae was detected |
| Baer 2003 | Did not report any data on clinical symptoms or signs |
| Bamba 2006 | Did not recruit children with community‐acquired pneumonia consecutively. Only recruited children in whom bacterial pathogens, M. pneumoniae and Chlamydia pneumoniae were felt to be the causative organisms after clinical examination |
| Bii 2002 | Did not report any data on clinical symptoms or signs |
| Block 1995 | Did not report any data on clinical symptoms or signs |
| Broome 1980 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Bunnag 2008 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Butun 2006 | Reported data on clinical symptoms and signs but not in relation to whether or not M. pneumoniae was detected |
| Chaudhry 1998 | Did not report any data on clinical symptoms or signs |
| Chkhaidze 2006 | Did not report any data on clinical symptoms or signs |
| De Roux 2006 | Study was performed in an adult population |
| Defilippi 2008 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Dowdle 1967 | Did not report any data on clinical symptoms or signs |
| Drummond 2000 | Did not report any data on clinical symptoms or signs |
| Dular 1987 | Only reported clinical symptoms and signs in children with M. pneumoniae |
| Elkholy 2009 | Did not report any data on clinical symptoms or signs |
| Esposito 2001 | Unsuitable comparison group (children with C. pneumoniae infection) |
| Ferwerda 2001 | Double‐blind, randomised, comparative trial comparing the efficacy of a 3‐day course of azithromycin with a 10‐day course of co‐amoxiclav in the treatment of lower respiratory tract infections in children. Provided data on clinical symptoms and signs according to treatment arm. Did not provide laboratory confirmation of M. pneumoniae |
| Fischer 2002 | Reported longer duration of fever as a significant predictor of M. pneumoniae, but did not report data on absence or presence or fever or any other clinical features |
| Forgie 1991 | Reported data on clinical symptoms and signs but not in relation to whether or not M. pneumoniae was detected |
| Gendrel 1997 | Did not report any data on clinical symptoms or signs |
| Gimenez Sanchez 2007 | Did not perform laboratory tests to detect M. pneumoniae |
| Gomez Campdera 2002 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Guggenbichler 1977 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Gutierrez 2005 | Did not report any data on clinical symptoms or signs |
| Harris 1998 | Double‐blind, randomised, comparative trial comparing the safety and efficacy of azithromycin with amoxicillin/clavulanate or erythromycin for the treatment of community‐acquired pneumonia, including atypical pneumonia caused by M. pneumoniae and C. pneumoniae. Data on clinical symptoms and signs were reported according to treatment arm but according to whether or not M. pneumoniae infection was detected |
| Heinz 1983 | Did not report any data on clinical symptoms or signs |
| Heiskanen‐Kosma 1998 | Did not report any data on clinical symptoms or signs |
| Holmes 2001 | Did not perform laboratory tests to detect M. pneumoniae |
| Hortal 1994 | No M. pneumoniae‐positive cases detected |
| Javier Alvarez 2001 | Study was performed in an adult population |
| Jensen 1967 | Did not report any data on clinical symptoms or signs |
| Jokinen 1993 | Did not report any data on clinical symptoms or signs |
| Juven 2000 | Did not report any data on clinical symptoms or signs |
| Kapellerova 2007 | Reported complications in children with M. pneumoniae. Did not report data on clinical symptoms or signs |
| Kicinski 2011 | Unsuitable comparison group (children with C. pneumoniae infection) |
| King 1991 | Did not recruit children with community‐acquired pneumonia (recruited children who had not been treated with antibiotics during the previous 7 days whether or not they had respiratory symptoms) |
| Kogan 2003 | Comparative randomised trial to determine the efficacy of azithromycin versus erythromycin and amoxicillin in the treatment of presumed bacterial community‐acquired pneumonia in ambulatory children. Children were tested for M. pneumoniae (serology and PCR) but data on clinical symptoms and signs were reported according to treatment arm and not according to whether or not M. pneumoniae was detected |
| Korppi 2004 | Did not report any data on clinical symptoms or signs |
| Kurz 2011 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Lagerstrom 2003 | Study population included children and adults (patients with community‐acquired pneumonia aged 10 years and over). Data on clinical symptoms and signs reported but not in relation to whether or not M. pneumoniae was detected. Data in children not reported separately |
| Lassmann 2008 | Reported data on clinical symptoms and signs in relation to whether or not atypical infections (Bordetella pertussis, Mycoplasma pneumoniae or Chlamydia pneumoniae) were detected |
| Lee 2008 | Reported data on clinical symptoms and signs but not in relation to whether or not M. pneumoniae was detected |
| Liam 2001 | Did not report any data on clinical symptoms or signs |
| Lochindarat 2007 | Reported data on clinical symptoms and signs in relation to whether or not atypical infections (M. pneumoniae or C. pneumoniae) were detected |
| Maltezou 2004 | Did not report any data on clinical symptoms or signs |
| Manfredi 1992 | No M. pneumoniae‐positive cases detected |
| Marrie 1996 | Reported data on clinical symptoms and signs in relation to whether or not atypical infections (M. pneumoniae, C. pneumoniae, Legionella species or respiratory viruses) were detected |
| Marrie 2005 | Study was performed in an adult population |
| Masia 2006 | Reported data on clinical symptoms and signs in relation to whether or not atypical infections (M. pneumoniae, Legionella pneumophila, Chlamydophila species or Coxiella burnetti) were detected |
| Matute 2006 | Reported data on clinical symptoms and signs in relation to whether or not pneumococcus was detected |
| Michelow 2004 | Reported data on clinical symptoms and signs in relation to whether or not atypical infections (M. pneumoniae or C. pneumoniae) were detected |
| Murphy 1981 | Did not report any data on clinical symptoms or signs |
| Nagalingam 2004 | Reported that several symptoms (abdominal pain, chest pain, chills, diarrhea, difficulty breathing, fever, ear pains, headache, hoarseness, muscle pains, productive cough, vomiting/nausea, wheezing and mental confusion) did not significantly affect the prevalence of M. pneumoniae in patients with pneumonia but did not report absence or presence of these symptoms in children with or without M. pneumoniae |
| Nagayama 1988 | Study population was divided into 3 groups (pneumonia, fever and wheezing). Prevalence of M. pneumoniae was estimated in each group but clinical symptoms and signs not reported in relation to whether or not M. pneumoniae was detected |
| Nagayama 1990 | Study reported clinical manifestations in children with pleuropneumonia versus children with pneumonia without pleural effusion |
| Nakayama 2007 | Unsuitable comparison group (children in whom other viral and/or bacterial infections were detected) |
| Ngeow 2005 | Reported data on clinical symptoms and signs but not in relation to whether or not M. pneumoniae was detected |
| Ouchi 1999 | Unsuitable comparison group (children with C. pneumoniae infection) |
| Pandey 2000 | Reported data on clinical symptoms and signs but not in relation to whether or not M. pneumoniae was detected |
| Peng 2009 | Recruited children hospitalised with acute respiratory infections but not community‐acquired pneumonia specifically. Also excluded children with signs of bacterial infections |
| Pereyre 2012 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Pocheville Guruceta 1998 | Recruited patients with wide range of presentations, including respiratory disease, arthritis or skin disease. Only performed M. pneumoniae serology on children with clinical features felt to be compatible with M. pneumoniae infection |
| Prapphal 2006 | Reported data on clinical symptoms and signs in relation to whether or not atypical infections (M. pneumoniae, C. pneumoniae or Legionella pneumoniae) were detected |
| Putman 1975 | Case series (children and adults). Only reported data on clinical features in patients with M. pneumoniae |
| Rahman 1990 | Did not perform laboratory tests to detect M. pneumoniae. Recruited patients with cough and diarrhea |
| Sakurai 1988 | Only reported data on clinical symptoms and signs in children with M. pneumoniae |
| Sakurai 1988a | Did not report any data on clinical symptoms or signs |
| Samransamruajkit 2008 | Clinical outcome data suggest that study population likely to include a high proportion of children with severe underlying comorbidity and/or immunocompromise (mean duration of hospitalisation was 18.8 days and 25% of children developed respiratory failure) |
| Shenoy 2005 | Recruited children with upper and lower acute respiratory tract infections, not community‐acquired pneumonia specifically. Age range of study population was not defined |
| Sidal 2007 | Unsuitable comparison group (children with C. pneumoniae infection) |
| Stawarski 2001 | Did not report any data on clinical symptoms or signs |
| Szabo 1977 | Did not report any data on clinical symptoms or signs |
| Tajima 2006 | Did not report any data on clinical symptoms or signs |
| Tinsa 2009 | Reported data on clinical symptoms and signs but not in relation to whether or not M. pneumoniae was detected |
| Touati 2010 | Only reported clinical symptoms and signs in children with M. pneumoniae |
| Tsolia 2004 | Did not report any data on clinical symptoms or signs |
| Unay 2002 | Only reported clinical symptoms and signs in children with M. pneumoniae |
| Van der Straeten 1976 | Did not report any data on clinical symptoms or signs |
| Vervloet 2010 | Clinical outcome data suggest that study population likely to include a high proportion of children with severe underlying comorbidity and/or immunocompromise (23% of children with M. pneumoniae and 17% without M. pneumoniae required mechanical ventilation) |
| Virkki 2002 | Did not report any data on clinical symptoms or signs |
| Woodhead 1987 | Did not report any data on clinical symptoms and signs |
| Wubbel 1999 | Did not report any data on clinical symptoms or signs |
| Yang 2001 | Reported data on clinical symptoms and signs in relation to whether or not atypical infections (M. pneumoniae or C. pneumoniae) were detected |
| Yin 2003 | Did not report any data on clinical symptoms or signs |
| Zoricic‐Letoja 1995 | Did not report any data on clinical symptoms or signs |
PCR: polymerase chain reaction
Differences between protocol and review
We had intended to assess the diagnostic value of both individual and combinations of clinical symptoms and signs. However, we were only able to analyze individual clinical features because none of our included studies reported data on combinations of clinical symptoms and signs. In our protocol we had proposed to extract and analyze data on rash and sore throat. However, data on rash were not reported in our included studies. One study presented data on sore throat, but this was not present in any children within the study population (Agarwal 2009). We summarised study‐specific sensitivity and specificity values with 95% CIs using forest plots. We also calculated 95% CIs for post‐test probabilities based on the absence or presence of each clinical feature studied.
In our protocol, we stated that we would add an item to our quality assessment tool (item 12) on whether a study provided a clear definition of what was considered to be a positive M. pneumoniae result. However, we decided not to include this item in our review because, having extracted data from our included studies, we felt that items 1 (representative spectrum) and 2 (acceptability of the reference standard) would be more appropriate factors on which to base our sensitivity analysis. We included an additional criterion to item 1 (representative spectrum) stating that we would not consider a study which only recruited participants from a limited spectrum of disease severity within that study's chosen healthcare setting to have included a representative spectrum of patients. We had planned to perform sensitivity analyses based on items 10 (reporting of uninterpretable or intermediate test results) and 11 (explanation of withdrawals) of our quality assessment tool but did not have sufficient data to do so.
We had also planned to explore several factors as potential sources of heterogeneity: participant age group (preschool (up to four years) versus school age (five to 12 years) versus adolescents (13 to 18 years)), healthcare setting (community versus hospital), method of diagnosing community‐acquired pneumonia (based on clinical criteria only or on clinical and radiological criteria), serological methods of diagnosing M. pneumoniae (high antibody titre on single serum sample versus significant rise in antibody titre) and use of other laboratory methods alongside serology to diagnose M. pneumoniae. However, in this review, we only had sufficient data to explore the use of other laboratory methods alongside serology as a potential source of heterogeneity in our analysis of wheeze. Among the six studies which reported data on wheeze, only one study (Agarwal 2009) diagnosed community‐acquired pneumonia based on clinical criteria only and diagnosed M. pneumoniae based on a single high antibody titre. We therefore did not investigate these factors as potential sources of heterogeneity, since we had already performed sensitivity analyses excluding data from Agarwal 2009. We did not have sufficient data to perform investigations of heterogeneity for any of the other clinical symptoms or signs studied in this review. We were unable to explore healthcare setting as a potential source of heterogeneity because all of our included studies were conducted in hospital settings. We were also unable to explore participant age group as a potential source of heterogeneity because our included studies did not report data stratified according to our age groups of interest.
Contributions of authors
KW, AH and DM developed the scope of the protocol. RP developed the data analysis plan. KW, PG, AT, DM and AH developed the search strategy and criteria for quality assessment of articles. All authors were involved in writing the protocol. KW and PG screened articles, assessed methodological quality and extracted data from included studies. KW and RP performed the data analysis. KW wrote the first draft of the review. All authors contributed towards the final manuscript.
Sources of support
Internal sources
Department of Primary Care Health Sciences, University of Oxford, UK.
External sources
National Institute for Health Research, UK.
Declarations of interest
None declared.
New
References
References to studies included in this review
Agarwal 2009 {published data only}
- Agarwal J, Awasthi S, Rajput A, Tiwari M. Atypical bacterial pathogens in community‐acquired pneumonia in children: a hospital based study. Tropical Doctor 2009;39:109‐11. [DOI] [PubMed] [Google Scholar]
Chan 2001 {published data only}
- Chan PWK, Lum LCS, Ngeow YF, Yasim MY. Mycoplasma pneumoniae infection in Malaysian children admitted with community acquired pneumonia. Southeast Asian Journal of Tropical Medicine and Public Health 2001;32:397‐401. [PubMed] [Google Scholar]
Deerojanawong 2006 {published data only}
- Deerojanawong J, Prapphal N, Suwanjutha S, Lochindarat S, Chantarojanasiri T, Kunakorn M, et al. Prevalence and clinical features of Mycoplasma pneumoniae in Thai children. Journal of the Medical Association of Thailand 2006;89:1641‐7. [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar S, Saigal SR, Sethi GR. Rapid diagnosis of Mycoplasma pneumoniae by polymerase chain reaction in community‐acquired lower respiratory tract infections. Tropical Doctor 2011;41:160‐2. [DOI] [PubMed] [Google Scholar]
Maheshwari 2011 {published data only}
- Maheshwari M, Kumar S, Sethi GR, Bhalla P. Detection of Mycoplasma pneumoniae in children with lower respiratory tract infections. Tropical Doctor 2011;41:40‐2. [DOI] [PubMed] [Google Scholar]
Principi 2001 {published data only}
- Principi N, Esposito S, Blasi F, Allegra L: Mowgli Study Group. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired lower respiratory tract infections. Clinical Infectious Diseases 2001;32:1281‐9. [DOI] [PubMed] [Google Scholar]
Somer 2006 {published data only}
- Somer A, Salman N, Yalcin I, Agacfidan A. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community‐acquired pneumonia in Istanbul, Turkey. Journal of Tropical Paediatrics 2006;52:173‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almasri 2011 {published data only}
- Almasri M, Diza E, Papa A, Eboriadou M, Souliou E. Mycoplasma pneumoniae respiratory tract infections among Greek children. Hippokratia 2011;15:147‐52. [PMC free article] [PubMed] [Google Scholar]
Al‐Moyed 2003 {published data only}
- Al‐Moyed KA, Al‐Shamahy HA. Mycoplasma pneumoniae infection in Yemen: incidence, presentation and antibiotic susceptibility. Eastern Mediterranean Health Journal 2003; Vol. 9, issue 3:279‐90. [PubMed]
Al‐Rashed 1998 {published data only}
Angadi 1980 {published data only}
- Angadi SA, Mukerji S, Nimbkar YS, Merchant SM. Serological study of acute respiratory tract infection in children with reference to Mycoplasma pneumoniae complement fixing and cold hemagglutinating antibodies. Indian Journal of Pathology and Microbiology 1980; Vol. 23, issue 1:65‐8. [PubMed]
Antonelli 2009 {published data only}
- Antonelli F, Brasi D, Siani P. Appropriateness of hospitalization for CAP‐affected pediatric patients: report from a Southern Italy General Hospital. Italian Journal of Pediatrics 2009; Vol. 35, issue 1:26. [DOI] [PMC free article] [PubMed]
Baer 2003 {published data only}
- Baer G, Engelcke G, Abele‐Horn M, Schaad UB, Heininger U. Role of Chlamydia pneumoniae and Mycoplasma pneumoniae as causative agents of community‐acquired pneumonia in hospitalised children and adolescents. European Journal of Clinical Microbiology and Infectious Diseases 2003; Vol. 22, issue 12:742‐5. [DOI] [PubMed]
Bamba 2006 {published data only}
Bii 2002 {published data only}
Block 1995 {published data only}
- Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community‐acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatric Infectious Disease Journal 1995; Vol. 14, issue 6:471‐7. [DOI] [PubMed]
Broome 1980 {published data only}
- Broome CV, LaVenture M, Kaye HS, Davis AT, White H, Plikaytis BD, et al. An explosive outbreak of Mycoplasma pneumoniae infection in a summer camp. Pediatrics 1980; Vol. 66, issue 6:884‐8. [PubMed]
Bunnag 2008 {published data only}
- Bunnag T, Lochindarat S, Srisan P, Jetanachai P. Mycoplasma pneumonia in young children, 2‐5 years of age. Journal of the Medical Association of Thailand 2008; Vol. 91 Suppl 3:124‐7. [PubMed]
Butun 2006 {published data only}
- Butun Y, Kose S, Babayigit A, Olmez D, Anal O, Uzuner N, et al. Chlamydia and Mycoplasma serology in respiratory tract infections of children. Tuberkuloz ve Toraks 2006; Vol. 54, issue 3:254‐8. [PubMed]
Chaudhry 1998 {published data only}
Chkhaidze 2006 {published data only}
Defilippi 2008 {published data only}
De Roux 2006 {published data only}
Dowdle 1967 {published data only}
Drummond 2000 {published data only}
- Drummond P, Clark J, Wheeler J, Galloway A, Freeman R, Cant A. Community acquired pneumonia ‐ a prospective UK study. Archives of Disease in Childhood 2000; Vol. 83, issue 5:408‐12. [DOI] [PMC free article] [PubMed]
Dular 1987 {published data only}
- Dular R, Lambert M, Bruce BW, Phipps PH, Rossier E, Kasatiya S. Mycoplasma pneumoniae infections in a rural setting in Canada. Canadian Medical Association Journal 1987; Vol. 136, issue 12:1271‐3. [PMC free article] [PubMed]
Elkholy 2009 {published data only}
Esposito 2001 {published data only}
Ferwerda 2001 {published data only}
- Ferwerda A, Moll HA, Hop WCJ, Kouwenberg JM, Tjon Pian CV, Robben SGF, et al. Efficacy, safety and tolerability of 3‐day azithromycin versus 10‐day co‐amoxiclav in the treatment of children with acute lower respiratory tract infections. Journal of Antimicrobial Chemotherapy 2001; Vol. 47:441‐6. [DOI] [PubMed]
Fischer 2002 {published data only}
Forgie 1991 {published data only}
- Forgie IM, O'Neill KP, Lloyd‐Evans N, Leinonen M, Campbell H, Whittle HC, et al. Etiology of acute lower respiratory tract infections in Gambian children: II. Acute lower respiratory tract infection in children ages one to nine years presenting at the hospital. Pediatric Infectious Disease Journal 1991; Vol. 10, issue 1:42‐7. [DOI] [PubMed]
Gendrel 1997 {published data only}
Gimenez Sanchez 2007 {published data only}
Gomez Campdera 2002 {published data only}
- Gomez Campdera JA, Rodriquez Fernandez R, Megias Montijano A, Gonzalez Sanchez MI, Navarro Gomez M, Ruiz Magro P. Mycoplasma pneumoniae in patients under three years of age. Acta Pediatrica Espanola 2002; Vol. 60, issue 7:343‐7.
Guggenbichler 1977 {published data only}
- Guggenbichler JP, Berger H. Mycoplasma pneumonias in childhood (author's transl). Padiatrie und Padologie 1977; Vol. 12, issue 1:10‐8. [PubMed]
Gutierrez 2005 {published data only}
Harris 1998 {published data only}
Heinz 1983 {published data only}
- Heinz F, Januska J, Raszka J, Cervenka P, Mlynkova H, Kupec V. Contribution to the problem of sporadic acute respiratory infections (ARI) caused by RS viruses and Mycoplasma pneumoniae in preschool‐age children and adults. Ceskoslovenska Epidemiologie, Mikrobiologie, Imunologie 1983; Vol. 32, issue 4:197‐203. [PubMed]
Heiskanen‐Kosma 1998 {published data only}
Holmes 2001 {published data only}
- Holmes WF, Macfarlane JT, Macfarlane RM, Hubbard R. Symptoms, signs, and prescribing for acute lower respiratory tract illness. British Journal of General Practice 2001; Vol. 51:177‐81. [PMC free article] [PubMed]
Hortal 1994 {published data only}
Javier Alvarez 2001 {published data only}
- Javier Alvarez Gutierrez F, Castillo Otero D, Garcia Fernandez A, Romero Romero B, Jose Del Rey Perez J, Soto Campos G, et al. Prospective study of 221 community acquired pneumonias followed up in an outpatient clinic. Etiology and clinical‐radiological progression. Medicina Clinica 2001; Vol. 116:161‐6. [DOI] [PubMed]
Jensen 1967 {published data only}
Jokinen 1993 {published data only}
Juven 2000 {published data only}
Kapellerova 2007 {published data only}
- Kapellerova A, Zlocha J, Kukova Z, Luptakova M, Tarhini A. Mycoplasmal pneumonia in childhood [Slovak]. Cesko‐Slovenska Pediatrie 2007; Vol. 62, issue 1:16‐24.
Kicinski 2011 {published data only}
King 1991 {published data only}
- King DE, Muncie HL. High prevalence of Mycoplasma pneumoniae in patients with respiratory tract symptoms: a rapid detection method. Journal of Family Practice 1991; Vol. 32, issue 5:529‐31. [PubMed]
Kogan 2003 {published data only}
Korppi 2004 {published data only}
Kurz 2011 {published data only}
- Kurz H, Göpfrich H. Epidemiology and morbidity of mycoplasma as causative agent for community‐acquired pneumonia in hospitalized children in a community hospital in Vienna. Wiener Medizinische Wochenschrift 2011;161:180‐3. [DOI] [PubMed] [Google Scholar]
Lagerstrom 2003 {published data only}
Lassmann 2008 {published data only}
- Lassmann B, Poetschke M, Ninteretse B, Issifou S, Winkler S, Kremsner PG, et al. Community‐acquired pneumonia in children in Lambarene, Gabon. American Journal of Tropical Medicine and Hygiene 2008; Vol. 79, issue 1:109‐14. [PubMed]
Lee 2008 {published data only}
- Lee P‐I, Wu M‐H, Huang L‐M, Chen J‐M, Lee C‐Y. An open, randomized, comparative study of clarithromycin and erythromycin in the treatment of children with community‐acquired pneumonia. Journal of Microbiology, Immunology and Infection 2008; Vol. 41, issue 1:54‐61. [PubMed]
Liam 2001 {published data only}
Lochindarat 2007 {published data only}
- Lochindarat S, Suwanjutha S, Prapphal N, Chantarojanasiri T, Bunnag T, Deerojanawong J, et al. Mycoplasma pneumoniae and Chlamydophila pneumoniae in children with community‐acquired pneumonia in Thailand. International Journal of Tuberculosis and Lung Disease 2007; Vol. 11, issue 7:814‐9. [PubMed]
Maltezou 2004 {published data only}
- Maltezou HC, La‐Scola B, Astra H, Constantopoulou I, Vlahou V, Kafetzis DA, et al. Mycoplasma pneumoniae and Legionella pneumophila in community‐acquired lower respiratory tract infections among hospitalized children: diagnosis by real time PCR. Scandinavian Journal of Infectious Diseases 2004; Vol. 36, issue 9:639‐42. [DOI] [PubMed]
Manfredi 1992 {published data only}
Marrie 1996 {published data only}
Marrie 2005 {published data only}
Masia 2006 {published data only}
Matute 2006 {published data only}
Michelow 2004 {published data only}
Murphy 1981 {published data only}
Nagalingam 2004 {published data only}
- Nagalingam NA, Adesiyun AA, Swanston WH, Bartholomew M. Prevalence of Mycoplasma pneumoniae and Chlamydia pneumoniae in pneumonia patients in four major hospitals in Trinidad. New Microbiologica 2004; Vol. 27, issue 4:345‐51. [PubMed]
Nagayama 1988 {published data only}
Nagayama 1990 {published data only}
Nakayama 2007 {published data only}
- Nakayama E, Hasegawa K, Morozumi M, Kobayashi R, Chiba N, Iitsuka T, et al. Rapid optimization of antimicrobial chemotherapy given to pediatric patients with community‐acquired pneumonia using PCR techniques with serology and standard culture. Journal of Infection and Chemotherapy 2007; Vol. 13, issue 5:305‐13. [DOI] [PMC free article] [PubMed]
Ngeow 2005 {published data only}
Ouchi 1999 {published data only}
Pandey 2000 {published data only}
Peng 2009 {published data only}
- Peng D, Zhao D, Liu J, Wang X, Yang K, Xicheng H, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virology Journal 2009; Vol. 6:155. [DOI] [PMC free article] [PubMed]
Pereyre 2012 {published data only}
- Pereyre S, Renaudin H, Charron A, Bebear C. Clonal spread of Mycoplasma pneumoniae in primary school, Bordeaux, France. Emerging Infectious Diseases 2012;18:343‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pocheville Guruceta 1998 {published data only}
- Pocheville Guruceta I, Angulo Barrena P, Ortiz Andres A, Fernandez Fernandez B, Vazquez Ronco MA, Garea Ibanez C, et al. Clinical and epidemiological spectrum of Mycoplasma pneumoniae infections at a pediatric hospital. Anales Espanoles de Pediatria 1998; Vol. 48, issue 2:127‐31. [PubMed]
Prapphal 2006 {published data only}
- Prapphal N, Suwanjutha S, Durongkaveroj P, Lochindarat S, Kunakorn M, Deerojanawong J, et al. Prevalence and clinical presentations of atypical pathogens infection in community acquired pneumonia in Thailand. Journal of the Medical Association of Thailand 2006; Vol. 89, issue 9:1412‐9. [PubMed]
Putman 1975 {published data only}
Rahman 1990 {published data only}
Sakurai 1988 {published data only}
- Sakurai N, Nagayama Y. The clinical observation of M. pneumoniae infections among children in Asahi General Hospital with special reference to mixed viral infections. Acta Paediatrica Japonica (Overseas Edition) 1988; Vol. 30, issue 3:267‐75. [PubMed]
Sakurai 1988a {published data only}
Samransamruajkit 2008 {published data only}
- Samransamruajkit R, Jitchaiwat S, Wachirapaes W, Deerojanawong J, Sritippayawan S, Prapphal N. Prevalence of Mycoplasma and Chlamydia pneumonia in severe community‐acquired pneumonia among hospitalized children in Thailand. Japanese Journal of Infectious Diseases 2008; Vol. 61, issue 1:36‐9. [PubMed]
Shenoy 2005 {published data only}
Sidal 2007 {published data only}
Stawarski 2001 {published data only}
- Stawarski A, Choroszy‐Krol I, Iwanczak B, Ruczkowska J, Iwanczak F. Epidemiology of atypical pneumonia caused by Mycoplasma pneumoniae and Chlamydia sp. in children. Advances in Clinical and Experimental Medicine 2001; Vol. 10, issue 1:37‐44.
Szabo 1977 {published data only}
- Szabo N, Loos T, Szabo I, Martha I. Illness due to Mycoplasma pneumoniae in childhood. Zeitschrift fur Erkrankungen der Atmungsorgane 1977; Vol. 148, issue 3:307‐11. [PubMed]
Tajima 2006 {published data only}
Tinsa 2009 {published data only}
- Tinsa F, Boussetta K, Gharbi A, Bousnina D, Abdelaziz R, Brini I, et al. Community acquired pneumonia in children. Tunisie Medicale 2009; Vol. 87, issue 12:851‐6. [PubMed]
Touati 2010 {published data only}
- Touati A, Pereyre S, Bouziri A, Achour W, Khaldi A, Ben Jaballah N, et al. Prevalence of Mycoplasma pneumoniae‐associated respiratory tract infections in hospitalized children: results of a 4‐year prospective study in Tunis. Diagnostic Microbiology and Infectious Disease 2010; Vol. 68, issue 2:103‐9. [DOI] [PubMed]
Tsolia 2004 {published data only}
- Tsolia MN, Psarras S, Bossios A, Audi H, Paldanius M, Gourgiotis D, et al. Etiology of community‐acquired pneumonia in hospitalized school‐age children: evidence for high prevalence of viral infections. Clinical Infectious Diseases 2004; Vol. 39, issue 5:681‐6. [DOI] [PMC free article] [PubMed]
Unay 2002 {published data only}
- Unay B, Kismet E, Guney C, Akin R, Ozcan O. The frequency and treatment of childhood pneumonia due to Mycoplasma pneumoniae [Turkish]. Gulhane Medical Journal 2002; Vol. 44, issue 2:125‐8.
Van der Straeten 1976 {published data only}
Vervloet 2010 {published data only}
Virkki 2002 {published data only}
- Virkki R, Juven T, Rikalainen H, Svedström E, Mertsola J, Ruuskanen O. Differentiation of bacterial and viral pneumonia in children. Thorax 2002; Vol. 57:438‐41. [DOI] [PMC free article] [PubMed]
Woodhead 1987 {published data only}
Wubbel 1999 {published data only}
Yang 2001 {published data only}
Yin 2003 {published data only}
Zoricic‐Letoja 1995 {published data only}
- Zoricic‐Letoja I, Basic‐Grbac M, Mikulic Z, Krstic‐Buric M. Pneumonia in school children and adolescents ‐ the clinical and etiological aspects [Serbian]. Paediatria Croatica 1995; Vol. 39, issue 3:175‐8.
Additional references
Averbuch 2011
- Averbuch D, Hidalgo‐Grass C, Moses AE, Engelhard D, Nir‐Paz R. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerging Infectious Diseases 2011;17:1079‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 2011
- British Thoracic Society Community Acquired Pneumonia in Children Guideline Group. Guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011;66:ii1‐ii23. [DOI] [PubMed] [Google Scholar]
Butler 2004
- Butler C, Rollnick S, Kinnersley P, Tapper‐Jones L, Houston H. Communicating about expected course and re‐consultation for respiratory tract infections in children: an exploratory study. British Journal of General Practice 2004;54:536‐8. [PMC free article] [PubMed] [Google Scholar]
Chalker 2011
- Chalker VJ, Stocki T, Mentasti M, Fleming D, Sadler C, Ellis J, et al. Mycoplasma pneumoniae infection in primary care investigated by real‐time PCR in England and Wales. European Journal of Clinical Microbiology and Infectious Diseases 2011;30:915‐21. [DOI] [PubMed] [Google Scholar]
Chung 2007
- Chung A, Perera R, Brueggemann AB, Elamin AE, Harnden A, Mayon‐White R, et al. Effect of antibiotic prescribing on antibiotic resistance in individual children in primary care: prospective cohort study. BMJ 2007;335:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58:882‐93. [DOI] [PubMed] [Google Scholar]
Don 2005
- Don M, Fasoli L, Paldanius M, Vainionpaa R, Kleemola M, Raty R, et al. Aetiology of community‐acquired pneumonia: serological results of a paediatric survey. Scandinavian Journal of Infectious Diseases 2005;37(11‐2):806‐12. [DOI] [PubMed] [Google Scholar]
Dumke 2010
- Dumke R, Baum H, Lück PC, Jacobs E. Occurrence of macrolide‐resistant Mycoplasma pneumoniae strains in Germany. Clinical Microbiology and Infection 2010;16:613‐6. [DOI] [PubMed] [Google Scholar]
Fine 2010
- Fine AM, Reis BY, Nigrovic LE, Goldmann DA, LaPorte TN, Olson KL, et al. Use of population health data to refine diagnostic decision‐making for pertussis. Journal of American Medical Informatics Association 2010;17(1):85‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freymuth 1999
- Freymuth F, Vabret A, Brouard J, Toutain F, Verdon R, Petitjean J, et al. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. Journal of Clinical Virology 1999;13(3):131‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez Campdera 2006
- Gomez Campdera JA, Rodriguez Fernandez R, Navarro Gomez ML, Gonzalez Sanchez MI, Megias Montijano A. Mycoplasma pneumoniae: endemic or epidemic disease. Acta Pediatrica Espanola 2006;64(11):536‐44. [Google Scholar]
Goossens 2005
- Goossens H, Ferech M, Stichele RV, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross‐national database study. Lancet 2005;365:579‐87. [DOI] [PubMed] [Google Scholar]
Gulliford 2009
- Gulliford M, Latinovic R, Charlton J, Little P, Staa T, Ashworth M. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. Journal of Public Health (Oxford) 2009;31:512‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammerschlag 2001
- Hammerschlag MR. Mycoplasma pneumoniae infections. Current Opinion in Infectious Diseases 2001;14:181‐6. [DOI] [PubMed] [Google Scholar]
Hay 2002
- Hay AD, Wilson AD. The natural history of acute cough in children aged 0 to 4 years in primary care: a systematic review. British Journal of General Practice 2002;52:401‐9. [PMC free article] [PubMed] [Google Scholar]
Heymann 2004
- Heymann PW, Carper HT, Murphy DM, Platts‐Mills TAE, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. Journal of Allergy and Clinical Immunology 2004;114:239‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsieh 2007
- Hsieh S‐C, Kuo Y‐T, Chern M‐S, Chen C‐Y, Chan W‐P, Yu C. Mycoplasma pneumonia: clinical and radiographic features in 39 children. Pediatrics International 2007;49:363‐7. [DOI] [PubMed] [Google Scholar]
Jartti 2004
- Jartti T, Lehtinen P, Vuorinen T, Osterback R, Hoogen B, Osterhaus AD, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerging Infectious Diseases 2004;10(6):1095‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeflang 2008
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM, Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Annals of Internal Medicine 2008;149:889‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehtinen 2006
- Lehtinen P, Jartti T, Virkki R, Vuorinen T, Leinonen M, Peltola V, et al. Bacterial coinfections in children with viral wheezing. European Journal of Clinical Microbiology and Infectious Diseases 2006;25(7):463‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loens 2010
- Loens K, Goossens H, Ieven M. Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. European Journal of Clinical Microbiology and Infectious Diseases 2010;29:1055‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. Chichester: Wiley‐Blackwell, 2010. [Google Scholar]
Morozumi 2008
- Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, et al. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community‐acquired pneumonia. Antimicrobial Agents and Chemotherapy 2008;52:348‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulholland 2010
- Mulholland S, Gavranich JB, Chang AB. Antibiotics for community‐acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD004875.pub3] [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Respiratory tract infections ‐ antibiotic prescribing. Clinical Guideline 69 2008. [PubMed]
Nilsson 2008
- Nilsson AC, Bjorkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiology 2008;8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Othman 2005
- Othman N, Isaacs D, Kesson A. Mycoplasma pneumoniae infections in Australian children. Journal of Paediatrics and Child Health 2005;41:671‐6. [DOI] [PubMed] [Google Scholar]
Pereyre 2007
- Pereyre S, Charron A, Renaudin H, Bebear C, Bebear CM. First report of macrolide‐resistant strains and description of a novel nucleotide sequence variation in the P1 adhesin gene in Mycoplasma pneumoniae clinical strains isolated in France over 12 years. Journal of Clinical Microbiology 2007;45:3534‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2009
- Reitsma JB, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ. Chapter 9: Assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration. Available from http://srdta.cochrane.org/. Chichester: Wiley‐Blackwell, 2011. [Google Scholar]
Sopena 1999
- Sopena N. Prospective study of community‐acquired pneumonia of bacterial etiology in adults. European Journal of Clinical Microbiology and Infectious Diseases 1999;18:852‐8. [DOI] [PubMed] [Google Scholar]
Stevens 1978
- Stevens D, Swift PGF, Johnston PGB, Kearney PJ, Corner BD, Burman D. Mycoplasma pneumoniae infections in children. Archives of Disease in Childhood 1978;53:38‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thurman 2009
- Thurman KA, Walter ND, Schwartz SB, Mitchell SL, Dillon MT, Baughman AL, et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clinical Infectious Diseases 2009;48:1244‐9. [DOI] [PubMed] [Google Scholar]
Van de Garde 2008
- Garde EMW, Endeman H, Hemert RN, Voorn GPR, Deneer VHM, Leufkens HGM, et al. Prior outpatient antibiotic use as predictor for microbial aetiology of community‐acquired pneumonia: hospital‐based study. European Journal of Clinical Pharmacology 2008;64(4):405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Bruel 2010
- Bruel A, Haj‐Hassan T, Thompson M, Buntinx F, Mant D, European Research Network on Recognising Serious Infection investigators. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet 2010;375:834‐45. [DOI] [PubMed] [Google Scholar]
Von Baum 2009
- Baum H, Welte T, Marre R, Suttorp N, Lück C, Ewig S. Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for Community‐acquired pneumonia (CAPNETZ). BMC Infectious Diseases 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wattanathum 2003
- Wattanathum A, Chaoprasong C, Nunthapisud P, Chantaratchada S, Limpairojn N, Jatakanon A, et al. Community‐acquired pneumonia in Southeast Asia. The microbial differences between ambulatory and hospitalized patients. Chest 2003;123:1512‐9. [DOI] [PubMed] [Google Scholar]
Zhao 2012
- Zhao F, Lv M, Tao X, Huang H, Zhang B, Zhang Z, et al. Antibiotic sensitivity of 40 Mycoplasma pneumoniae isolates and molecular analysis of macrolide‐resistant isolages from Beijing, China. Antimicrobial Agents and Chemotherapy 2012;56:1108‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]


