Highlights
-
•
Kobuviruses were found in cats with diarrhoea.
-
•
Kobuviruses were not detected in asymptomatic cats.
-
•
The complete genome sequence of one such strains was determined.
-
•
Kobuviruses resembling the newly described feline kobuviruses were identified.
-
•
First evidence on the circulation of feline kobuviruses outside the Asian continent.
Keywords: Kobuviruses, Cats, Diarrhoea, Genome, Aichivirus A
Abstract
Kobuviruses have been identified in the enteric tract of several mammalian species but their role as enteric pathogens is still not defined. In this study, feline kobuviruses were found in 13.5% of cats with diarrhoea, but not in asymptomatic animals. In the full-length genome, one such strains, TE/52/13/ITA, displayed the highest nucleotide identity (96.0%) to the prototype strain FK-13. These results provide firm evidence that kobuviruses are common constituents of feline enteric viroma and that they are not geographically restricted to the Asian continent, where they were first signalled.
1. Introduction
Kobuviruses are non-enveloped, polyadenylated, single-stranded, positive-sense RNA viruses and represent a distinct genus in the family Picornaviridae. Based on their genomic organization and sequence similarities, kobuviruses are currently classified into 3 species, Aichivirus A (formerly Aichi virus), Aichivirus B (formerly Bovine kobuvirus) and Aichivirus C (porcine kobuvirus) (Adams et al., 2013). Human Aichi virus (AiV) was first recognized in 1989 as the cause of oyster-associated nonbacterial gastroenteritis in humans in Aichi Prefecture, Japan (Yamashita et al., 1991). Several investigations have subsequently revealed that AiVs are involved in 0.9–4.1% of sporadic cases of pediatric gastroenteritis (Ambert-Balay et al., 2008, Reuter et al., 2009b, Kaikkonen et al., 2010, Jonsson et al., 2012). Novel kobuviruses genetically closely related to human AiVs have been recently found in domestic and wild carnivores (Kapoor et al., 2011, Li et al., 2011, Carmona-Vicente et al., 2013, Chung et al., 2013, Di Martino et al., 2014a, Di Martino et al., 2014b). Carnivore kobuviruses were firstly discovered in diarrhoeic and asymptomatic dogs in USA (Kapoor et al., 2011, Li et al., 2011). Upon genome sequencing, canine kobuvirus (CaKoV) was found to be genetically closely related to mouse kobuvirus (MuKoV) (84.0% amino acid [aa] identity) (Phan et al., 2011) and to human AiV (80.0% aa identity). Subsequently, CaKoV-like RNA was detected in diarrhoeic cats in South Korea (Chung et al., 2013). The aa identity between CaKoVs and feline kobuvirus (FeKoV) in the full-length genome ranged from 82.0% to 86.0%, while aa identity to human AiV was 79.0% (Cho et al., 2014). Molecular investigations worldwide have revealed that CaKoVs are common in both symptomatic and asymptomatic dogs (Kapoor et al., 2011, Li et al., 2011, Carmona-Vicente et al., 2013, Di Martino et al., 2013, Oem et al., 2014). By converse, there is no information on the epidemiology of the newly described FeKoVs after their initial description in Asia (Chung et al., 2013, Cho et al., 2014).
In order to start filling this gap, we screened faecal samples obtained from asymptomatic and diarrhoeic cats. Also, the full-length genome sequence of a FeKoV strain was determined.
2. Materials and methods
2.1. Collection of faecal samples and extraction of DNA and RNA
A total of 83 stool samples from domestic cats aged 2–12 months were collected from April to July 2013 in three distinct shelters located in different Italian regions. The faecal panel consisted of 37 samples from cats with signs of mild to severe gastroenteritis and 46 samples from asymptomatic animals.
The RNA was extracted from 200 μl of 10% (wt/vol) faecal suspension by using the TRIzol LS (Invitrogen, Ltd, Paisley, UK) procedure. The final RNA pellet was resuspended in 50 μl of RNase free water and used directly in RT-PCR assays or stored at −80 °C. Total DNA was extracted from the supernatants of faecal homogenates by boiling for 10 min and chilling on ice. To reduce residual inhibitors of DNA polymerase activity to ineffective concentrations, the DNA extracts were diluted 1:10 in distilled water as previously described (Decaro et al., 2005).
2.2. Screening by RT-PCR and PCR and sequence analysis
Kobuvirus RNA was detected by RT-PCR using a broadly reactive primer pair, UNIV-kobu-F/UNIV-kobu-R (Reuter et al., 2009a), which amplifies a 217-bp fragment of the 3D region of all kobuvirus species. The samples were also tested by PCR or RT-PCR for feline parvovirus (FPV) (Buonavoglia et al., 2001) and feline enteric coronavirus (FECV) (Gunn-Moore et al., 1998).
The amplicons were excised from the gel and purified using a QIAquick gel extraction kit (Qiagen GmbH, Hilden, Germany). The fragment was then subjected to direct sequencing using BigDye Terminator Cycle chemistry and 3730 DNA Analyzer (Applied Biosystems, Foster, CA). Basic Local Alignment Search Tool (BLAST; http://www.ncbi.nlm.nih.gov) and FASTA (http://www.ebi.ac.uk/fasta33) with default values were used to find homologous hits.
2.3. Determination of the full-length genome of FeKoV TE/52/13/ITA
In order to determine the complete genome sequence of one FeKoV strain (sample TE/52/13/ITA), 5′ and 3′ RACE protocols and long RT-PCRs with consensus primers designed on the basis of sequence alignments were used. A ∼3.0 kb region at the 3′ end of the genome was amplified by a 3′ RACE protocol. The cDNA was synthesized by SuperScript III First-Strand cDNA synthesis kit (Invitrogen Ltd, Italy) with an oligo(dT). The PCR was performed with TaKaRa La Taq polymerase (Takara Bio Europe S.A.S. Saint-Germain-en-Laye, France) using consensus primers. Finally, the amplicon was purified and cloned by using TOPO® XL Cloning Kit (Invitrogen Ltd, Milan, Italy). The complete 3.0 kb sequence was determined by a primer walking strategy. To obtain the 5′ end sequence of the genome, a 5′ RACE kit was used (Invitrogen Ltd, Italy). Sequence gaps were filled by long RT-PCR with either specific or consensus primers. Sequence editing and multiple alignments were performed with Bioedit software package vers. 2.1 (Hall, 1999). Phylogenetic analysis (Neighbor-Joining) with bootstrap analysis (1000 replicates) and Poisson correction was conducted by using the MEGA software package v3.0 (Kumar et al., 2004).
3. Results and discussion
Out of 83 samples, 5 (6.0%) contained FeKoV RNA. All the positive samples were identified from diarrhoeic cats (5/37), with a prevalence rate of 13.5%. Three of the FeKoV-infected samples were also positive for FPV and FECV, while two samples tested positive only for FeKoV. Partial 3D sequences were determined from all the positive samples and compared with cognate sequences available in the databases. By FASTA and BLAST analyses all the sequences displayed the closest nucleotide (nt) identity (91.0–94.0%) to the FeKoVs recently discovered in South Korea (Chung et al., 2013).
The full-length genome sequence of one FeKoV strain (TE/52/13/ITA) was determined and deposited in Genbank under accession number KM091960. The TE/52/13/ITA genome was 8,201-nt in length excluding the poly(A) tail and shared the highest nt identity (96.0%) to the Asian prototype strain FK-13 (Cho et al., 2014), while identity to CaKoVs and AiVs was 80.0–83.0% and 75.0–77.0%, respectively.
The predicted polyprotein (2436 aa) encoded by a single open reading frame (7,467 nt), was flanked by a 646-nt-long 5′ untranslated region (5′ UTR) and a 244-nt-long 3′ UTR. The polyprotein comprised a leader protein (L), three structural proteins (VP0, VP3, and VP1), and seven nonstructural proteins (2A to 2 C and 3A to 3D). The positions of the cleavage sites on the polyprotein were predicted by sequence alignments and NetPicoRNA analysis (http://www.cbs.dtu.dk/services/NetPicoRNA). Likewise in other kobuviruses, the predicted cleavage sites were consistent with L/VP0, VP3/VP1, VP1/2A, 2A/2B, 2B/2 C, 2 C/3A, 3B/3 C and 3 C/3D sites, all which contained Q/G residues, but the VP0/VP3 and 3A/3B cleavage sites, which contained E/G and Q/A residues. The aa identity between strain TE/52/13/ITA and FK-13 in the P1 (853 aa), P2 (636 aa) and P3 (779 aa) regions was 98.0%, 100% and 98.0%, respectively. Based on sequence and phylogenetic analysis (Fig. 1 ), the two FeKoV strains formed a tight group (bootstrap value 100%) distinct from CaKoVs, MuKoV and AiVs, within the species Aichivirus A.
Fig. 1.
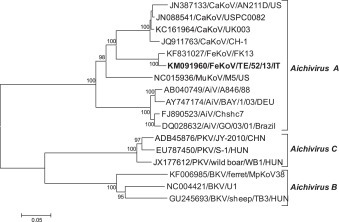
Phylogenetic tree based on the full-length amino acid sequence (2436 aa) of the FeKoV TE/52/13/ITA. Tree was generated using the neighbor joining method and Poisson correction, supplying a statistical support with bootstrapping of 1000 replicates. The scale bar indicates nucleotide substitutions per site. Abbreviations: CaKoV, canine kobuvirus; FeKoV, feline kobuvirus; AiV, aichi virus; PKV, porcine kobuvirus; BKV, bovine kobuvirus.
The findings of this study demonstrate that FeKoVs are not geographically restricted to South Korea, where they were first signalled (Chung et al., 2013, Cho et al., 2014). Our results also provide further evidence that a distinct group of kobuviruses (i.e. FeKoVs) within Aichivirus A species, but with peculiar genetic signatures, circulates in cats. Accordingly, FeKoVs should be considered as common constituents of feline enteric viroma. In this study, FeKoVs were detected only in diarrhoeic animals, with a prevalence rate similar to that reported in South Korea (Chung et al., 2013). Some of the FeKoV-positive cats were also infected by other feline pathogens such as FPV or FECV. These findings were not unexpected, as mixed infections are not infrequent, chiefly in animal communities such as shelters. Although these results demonstrated that FeKoVs are common viruses of cats, it is not clear whether they also play a role as enteric pathogens. Large structured epidemiological studies and comprehensive characterization of FeKoV strains identified from other geographic areas and experimental infections might help clarify their global distribution, genetic heterogeneity and possible association with enteric diseases in cats.
Several pieces of evidences indicate that enteric viruses of carnivores may have a zoonotic potential: (i) infection of young children by rotavirus strains of canine and feline origin has been documented repeatedly (De Grazia et al., 2007); (ii) having dogs in or near a home has been recognized as a risk factor for acquisition of IgA antibodies specific for noroviruses (NoVs) in infants in a seroepidemiologic study conducted in rural Mexico (Peasey et al., 2004); (iii) IgG antibodies specific for NoVs of carnivores (genotype GIV.2) have been detected in human serum samples (Di Martino et al., 2014a, Di Martino et al., 2014b) and dogs can be infected, in turn, with human NoVs of different genotypes (Summa et al., 2012); (iv) IgG antibodies specific for human AiV (strain A846/88) were detected in dog and cat sera in a serological study conducted in Europe (Carmona-Vicente et al., 2013). Accordingly, considering the close genetic relatedness of these novel kobuviruses with AiVs, and the strict social interactions between humans and cats, it will be important to address whether cross-species transmission of kobuviruses might occur between carnivores and humans.
Conflict of interest
All Authors declare that there are no financial or other relationships that might lead to a conflict of interest. All authors have seen and approved the manuscript and have contributed significantly to the work.
Acknowledgements
The work was financed by grants from the University of Teramo, Italy, and from the Italian Ministry of University and Research.
References
- Adams M.J., King A.M., Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2013) Arch. Virol. 2013;158:2023–2030. doi: 10.1007/s00705-013-1688-5. [DOI] [PubMed] [Google Scholar]
- Ambert-Balay K., Lorrot M., Bon F., Giraudon H., Kaplon J., Wolfer M., Lebon P., Gendrel D., Pothier P. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J. Clin. Microbiol. 2008;46:1252–1258. doi: 10.1128/JCM.02140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- Carmona-Vicente N., Buesa J., Brown P.A., Merga J.Y., Darby A.C., Stavisky J., Sadler L., Gaskell R.M., Dawson S., Radford A.D. Phylogeny and prevalence of kobuviruses in dogs and cats in the UK. Vet. Microbiol. 2013;164:246–252. doi: 10.1016/j.vetmic.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.Y., Lim S.I., Kim Y.K., Song J.Y., Lee J.B., An D.J. Molecular characterization of the full kobuvirus genome in a cat. Genome Announc. 2014 doi: 10.1128/genomeA.00420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.Y., Kim S.H., Kim Y.H., Lee M.H., Leea K.K., Oem J.K. Detection and genetic characterization of feline kobuviruses. Virus Genes. 2013;47:559–562. doi: 10.1007/s11262-013-0953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Desario C., Campolo M., Di Trani L., Tarsitano E., Tempesta M., Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs. Vet. Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- De Grazia S., Martella V., Giammanco G.M., Iturriza-Gomara M., Ramirez S., Cascio A., Colomba C., Serenella A. Canine-origin G3P[3] rotavirus strain in child with acute gastroenteritis. Emerg. Infect. Dis. 2007;13:1091–1093. doi: 10.3201/eid1307.070239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Felice E., Ceci C., Di Profio F., Marsilio F. Canine kobuviruses in diarrhoeic dogs in Italy. Vet. Microbiol. 2013;166:246–249. doi: 10.1016/j.vetmic.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Profio F., Ceci C., Di Felice E., Green K.Y., Bok K., Giammanco G.V., Massirio I., Lorusso E., Buonavoglia C., Marsilio F., Martella V. Seroprevalence of Norovirus genogroup IV antibodies among humans, Italy, 2010-2011. Emerg. Infect. Dis. 2014 doi: 10.3201/eid2011.131601. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Profio F., Melegari I., Robetto S., Di Felice E., Orusa R., Marsilio F. Molecular evidence of kobuvirus in free-ranging red foxes (Vulpes vulpes) Arch. Virol. 2014;159:1803–1806. doi: 10.1007/s00705-014-1975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn-Moore D.A., Gruffydd-Jones T.J., Harbour D.A. Detection of feline coronaviruses by culture and reverse transcriptase-polymerase chain reaction of blood samples from healthy cats and cats with clinical feline infectious peritonitis. Vet. Microbiol. 1998;62:193–205. doi: 10.1016/S0378-1135(98)00210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- Jonsson N., Wahlström K., Svensson L., Serrander L., Lindberg A.M. Aichi virus infection in elderly people in Sweden. Arch. Virol. 2012;157:1365–1369. doi: 10.1007/s00705-012-1296-9. [DOI] [PubMed] [Google Scholar]
- Kaikkonen S., Räsänen S., Rämet M., Vesikari T. Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol. Infect. 2010;138:1166–1171. doi: 10.1017/S0950268809991300. [DOI] [PubMed] [Google Scholar]
- Kapoor A., Simmonds P., Dubovi E.J., Qaisar N., Henriquez J.A., Medina J., Shields S., Lipkin W.I. Characterization of a canine homolog of human Aichi virus. J. Virol. 2011;85:11520–11525. doi: 10.1128/JVI.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004;5:153–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Li L., Pesavento P.A., Shan T., Leutenegger C.M., Wang C., Delwart E. Viruses in diarrhoeic dogs include novel kobuviruses and sapoviruses. J. Gen. Virol. 2011;92:2534–2541. doi: 10.1099/vir.0.034611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oem J.K., Choi J.W., Lee M.H., Lee K.K., Choi K.S. Canine kobuvirus infections in Korean dogs. Arch. Virol. 2014 doi: 10.1007/s00705-014-2136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peasey A.E., Guillermo M., Ruiz-Palacios G.M., Qugley M., Newsholme W., Martinez J., Rosales G., Jiang X., Blumenthal U.J. Seroepidemiology and risk factors for sporadic norovirus/Mexico strain. J. Infect. Dis. 2004;189:2027–2036. doi: 10.1086/386310. [DOI] [PubMed] [Google Scholar]
- Phan T.G., Kapusinszky B., Wang C., Rose R.K., Lipton H.L., Delwart E.L. The fecal viral flora of wild rodents. PLoS Pathog. 2011 doi: 10.1371/journal.ppat.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Boldizsár A., Pankovics P. Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae. Arch. Virol. 2009;154:101–108. doi: 10.1007/s00705-008-0288-2. [DOI] [PubMed] [Google Scholar]
- Reuter G., Boldizsár A., Papp G., Pankovics P. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch. Virol. 2009;154:1529–1532. doi: 10.1007/s00705-009-0473-y. [DOI] [PubMed] [Google Scholar]
- Summa M., von Bonsdorff C.H., Maunula L. Pet dogs--a transmission route for human noroviruses? J. Clin. Virol. 2012;53:244–247. doi: 10.1016/j.jcv.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Kobayashi S., Sakae K., Nakata S., Chiba S., Ishihara Y., Isomura S. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J. Infect. Dis. 1991;164:954–957. doi: 10.1093/infdis/164.5.954. [DOI] [PubMed] [Google Scholar]


