Abstract
Background
Omega‐3 polyunsaturated fatty acids (omega‐3 PUFAs) from fish and plant sources are commonly considered as a promising non‐medical alternative to improve brain functions and slow down the progression of dementia. This assumption is mostly based on findings of preclinical studies and epidemiological research. Resulting explanatory models aim at the role omega‐3 PUFAs play in the development and integrity of the brain's neurons, their protective antioxidative effect on cell membranes and potential neurochemical mechanisms directly related to Alzheimer‐specific pathology. Epidemiological research also found evidence of malnutrition in people with dementia. Considering this and the fact that omega‐3 PUFA cannot be synthesised by humans, omega‐3 PUFAs might be a promising treatment option for dementia.
Objectives
To assess the efficacy and safety of omega‐3 polyunsaturated fatty acid (PUFA) supplementation for the treatment of people with dementia.
Search methods
We searched the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (ALOIS), MEDLINE, EMBASE, PsycINFO, CINAHL, ClinicalTrials.gov and the World Health Organization (WHO) portal/ICTRP on 10 December 2015. We contacted manufacturers of omega‐3 supplements and scanned reference lists of landmark papers and included articles.
Selection criteria
We included randomised controlled trials (RCTs) in which omega‐3 PUFA in the form of supplements or enriched diets were administered to people with Alzheimer's disease (AD), vascular dementia (VaD), dementia with Lewy bodies (DLB), Parkinson's disease dementia (PDD) or frontotemporal dementia (FTD).
Data collection and analysis
The primary outcome measures of interest were changes in global and specific cognitive functions, functional performance, dementia severity and adverse effects. Two review authors independently selected studies, extracted data and assessed the quality of trials according to the Cochrane Handbook for Systematic Reviews of Interventions. We rated the quality of the evidence using the GRADE approach. We received unpublished data from the trial authors and collected adverse effects information from the published articles. We conducted meta‐analyses for available outcome measures at six months.
Main results
We included three comparable randomised, placebo‐controlled trials investigating omega‐3 PUFA supplements in 632 participants with mild to moderate AD over six, 12 and 18 months. We found no studies investigating other types of dementia. All trials were of high methodological quality. The overall quality of evidence for most of the outcomes was high.
There was no evidence of a benefit from omega‐3 PUFAs on cognitive function when measured at six months with the Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (standardised mean difference (SMD) ‐0.02, 95% confidence interval (CI) ‐0.19 to 0.15; 566 participants; 3 studies; high quality evidence) or Mini‐Mental State Examination (mean difference (MD) 0.18, 95% CI ‐1.05 to 1.41; 202 participants; 2 studies; high quality evidence) or on activities of daily living (SMD ‐0.02, 95% CI ‐0.19 to 0.16; 544 participants; 2 studies; high quality evidence). There was also no effect at six months of treatment on severity of dementia measured with the Clinical Dementia Rating ‐ Sum of Boxes (MD ‐0.00, 95% CI ‐0.58 to 0.57; 542 participants; 2 studies; high quality evidence) or on quality of life measured with the Quality of Life Alzheimer's Disease scale (MD ‐0.10, 95% CI ‐1.28 to 1.08; 322 participants; 1 study; high quality evidence). There was no difference at six months on mental health measured with the Montgomery‐Åsberg Depression Rating Scale (MD ‐0.10, 95% CI ‐0.74 to 0.54; 178 participants: 1 study; high quality of evidence) or the Neuropsychiatric Inventory (SMD 0.10, 95% CI ‐0.07 to 0.27; 543 participants; 2 studies; high quality of evidence). One very small study showed a benefit for omega‐3 PUFAs in instrumental activities of daily living after 12 months of treatment (MD ‐3.50, 95% CI ‐4.30 to ‐2.70; 22 participants; moderate quality evidence). The included studies did not measure specific cognitive function. The studies did not report adverse events well. Two studies stated that all adverse events were mild and that they did not differ in overall frequency between omega‐3 PUFA and placebo groups. Data from one study showed no difference between groups in frequency of any adverse event (risk ratio (RR) 1.02, 95% CI 0.95 to 1.10; 402 participants; 1 study; moderate quality evidence) or any serious adverse event (RR 1.05, 95% CI 0.78 to 1.41; 402 participants; 1 study; high quality evidence) at 18 months of treatment.
Authors' conclusions
We found no convincing evidence for the efficacy of omega‐3 PUFA supplements in the treatment of mild to moderate AD. This result was consistent for all outcomes relevant for people with dementia. Adverse effects of omega‐3 PUFAs seemed to be low, but based on the evidence synthesised in this review, we cannot make a final statement on tolerability. The effects on other populations remain unclear.
Plain language summary
Omega‐3 fatty acids for the treatment of dementia
Background
Omega‐3 polyunsaturated fatty acids (omega‐3 PUFAs) are assumed to have a beneficial effect on the function of the brain. It has been suggested that they might improve or delay decline in memory and ability to carry out everyday tasks in people with dementia. In this review, we investigated randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) comparing omega‐3 PUFAs, given in the form of supplements or enriched diets, with placebo (a pretend treatment) in people with the most common types of dementia.
Included trials
We included three trials that investigated 632 people with Alzheimer's disease of mild to moderate severity. We found no trials on other types of dementia. In all trials participants took either placebo or omega‐3 PUFA supplements. The quality of the trials was good. The participants were allocated to the groups randomly. The participants and most of the investigators did not know which treatment was given.
Results
When we combined the results of the trials, we found that taking omega‐3 PUFA supplements for six months had no effect on cognition (learning and understanding), everyday functioning, quality of life or mental health. One very small study observed that omega‐3 PUFAs improved cognitively complex daily activities, such as shopping, when taken for a longer period of time. However, the quality of the evidence was only moderate, so this should be confirmed in further trials. Omega‐3 PUFAs also had no effect on ratings of the overall severity of the illness. The trials did not report side effects very well, but none of the studies reported significant harmful effects on health.
Conclusion
Altogether, the quality of the evidence was moderate or high for most of the effects that we measured, but we found no evidence for either benefit or harm from omega‐3 PUFA supplements in people with mild to moderate Alzheimer's disease. The effects on people with other types of dementia remain unclear.
Summary of findings
Summary of findings for the main comparison. Omega‐3 PUFA supplements compared to placebo for people with mild to moderate Alzheimer's disease.
| Omega‐3 PUFA supplements compared to placebo for people with mild to moderate Alzheimer's disease | ||||||
| Patient or population: people with mild to moderate Alzheimer's disease Setting: any setting Intervention: omega‐3 PUFA supplements Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo for mild to moderate Alzheimer's disease | Risk with omega‐3 PUFAs for mild to moderate Alzheimer's disease | |||||
| Any adverse event (combined: diarrhoea, urinary tract infection, falls, dizziness, agitations) Assessed with: unclear Follow‐up: mean 18 months | Study population | RR 1.02 (0.95 to 1.10) | 402 (1 RCT) | ⊕⊕⊕⊝ Moderate 1 | ‐ | |
| 878 per 1000 | 896 per 1000 (834 to 966) | |||||
| Moderate | ||||||
| 878 per 1000 | 896 per 1000 (834 to 966) | |||||
| Serious adverse events "Defined as events that result in death, hospitalization, prolongation of hospitalization, or are life threatening (based on the judgment of the study physician)" (Quinn 2010) | Study population | RR 1.05 (0.78 to 1.41) | 402 (1 RCT) | ⊕⊕⊕⊕ High | ‐ | |
| 305 per 1000 | 320 per 1000 (238 to 430) | |||||
| QoL Assessed with: QoL‐AD scale rated by participant Scale from 13 to 52 (higher = better) Follow‐up: mean 18 months |
The mean QoL was 40.02 scale points | The mean difference in QoL in the intervention group was 0.39 scale points fewer (1.79 fewer to 1.01 more) | ‐ | 269 (1 RCT) | ⊕⊕⊕⊝ Moderate 2 | ‐ |
| QoL Assessed with: QoL‐AD scale rated by participant Scale from 13 to 52 (higher = better) Follow‐up: mean 6 months | The mean QoL was 39.86 scale points | The mean difference in QoL in the intervention group was 0.1 scale points fewer (1.28 fewer to 1.08 more) | ‐ | 332 (1 RCT) | ⊕⊕⊕⊕ High | ‐ |
| Mental health (depression) Assessed with: MADRS Scale from 0 to 30 (lower = better) Follow‐up: mean 6 months | The mean depression (MADRS) score was 1.6 scale points | The mean difference in depression (MADRS) score in the intervention group was 0.1 scale points fewer (0.74 fewer to 0.54 more) | ‐ | 178 (1 RCT) | ⊕⊕⊕⊕ HIGH | ‐ |
| Mental health Assessed with: NPI Follow‐up: mean 6 months |
The mean difference in mental health (NPI) score in the intervention group was 0.1 standard deviations more (0.07 fewer to 0.27 more) 7 | ‐ | 543 (2 RCTs) | ⊕⊕⊕⊕ High | ‐ | |
| Global cognitive function Assessed with: ADAS‐Cog (different versions) Follow‐up: mean 6 months | The mean difference in global cognitive function (ADAS‐Cog) in the intervention group was 0.02 standard deviations fewer (0.19 fewer to 0.15 more) 4 | ‐ | 566 (3 RCTs) | ⊕⊕⊕⊕ High | ‐ | |
| Global cognitive function assessed with: MMSE scale Scale from 0 to 30 (higher = better) Follow‐up: mean 6 months | The mean global cognitive function ranged from 20.4 to 22.4 scale points | The mean difference in global cognitive function (MMSE) in the intervention group was 0.18 scale points more (1.05 fewer to 1.41 more) | ‐ | 202 (2 RCTs) | ⊕⊕⊕⊕ High | ‐ |
| IADL Assessed with: OARS‐IADL Scale from 0 to 14 (lower = better) Follow‐up: mean 12 months | The mean change in score for IADL was 4.2 scale points | The mean difference in the change in score for IADL in the intervention group was 3.5 scale points lower (4.3 lower to 2.7 lower) | ‐ | 22 (1 RCT) | ⊕⊕⊕⊝ Moderate 3 | ‐ |
| ADL Assessed with: DAD and ADCS‐ADL Follow‐up: mean 6 months | The mean difference in ADL in the intervention group was 0.02 standard deviations fewer (0.19 fewer to 0.16 more)5 | ‐ | 544 (2 RCTs) | ⊕⊕⊕⊕ High | ‐ | |
| Overall dementia severity (cognition and function combined) Assessed with: CDR‐SOB Scale from 0 to 18 (lower = better) Follow‐up: mean 6 months | The mean overall dementia severity (CDR‐SOB score) ranged from 6.5 to 6.75 scale points | The mean difference in overall dementia severity (CDR‐SOB score) in the intervention group was 0 scale points (0.58 fewer to 0.57 more) | ‐ | 542 (2 RCTs) | ⊕⊕⊕⊕ High | ‐ |
| Memory ‐ not measured | See comment | See comment | Not estimable | ‐ | ‐ | Outcome was not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living; ADL: activities of daily living; CDR‐SOB: Clinical Dementia Rating ‐ Sum of Boxes; CI: confidence interval; DAD: Disability Assessment for Dementia; IADL: instrumental activities of daily living; NPI: Neuropsychiatric Inventory; MADRS: Montgomery‐Åsberg Depression Rating Scale; MMSE: Mini‐Mental State Examination; OARS‐IADL: Older Americans Resources and Services ‐ Instrumental Activities of Daily Living; OR: odds ratio; PUFA: polyunsaturated fatty acid; QoL: quality of life; QoL‐AD: Quality of Life Alzheimer's Disease; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to serious risk of bias: combined outcome (i.e. diarrhoea, falls, agitation) that includes outcomes of unclear measurement methods (i.e. dizziness).
2 Downgraded one level due to serious risk of bias: follow‐up differed between groups: 63.0% (omega‐3 PUFAs) and 72.6% (placebo).
3 Downgraded one level due to serious imprecision: wide CI; only 22 participants overall.
4 SMD presented in place of absolute values in the intervention and comparison groups as studies used the different scale versions.
5 SMD presented in place of absolute values in the interventions and comparison groups as studies used different scales to measure the same construct.
Background
Description of the condition
The number of people living with dementia is increasing due to the ageing world population (United Nations 2013), with higher age being the major risk factor for the disease. In 2012, 35 million people were estimated to be affected worldwide. This number will double by 2030 resulting in high costs and considerable burden to individuals and societies (WHO 2012).
The term 'dementia' refers to a group of diseases that share a syndrome of typically chronic and progressive nature. The dementia syndrome involves disturbances of multiple higher cortical functions, such as memory, thinking, orientation, perception and behaviour, which are severe enough to affect the ability to perform everyday activities. Cognitive decline is often accompanied by deterioration in emotional control, social behaviour or motivation. The most common forms of dementia are Alzheimer's disease (AD) (60% to 70% of cases), vascular dementia (VaD), dementia with Lewy bodies (DLB), dementia in Parkinson's disease (PDD) and frontotemporal dementia (FTD).
The early stages of the disease are typically characterised by forgetfulness, communication problems and difficulties in carrying out complex activities. In the middle stage, the symptoms become more obvious and people gradually lose the ability to care for themselves without considerable support. In the late or severe stages of dementia, people are dependent on others for all care, and psychiatric and behavioural symptoms are increasingly common (WHO 2012).
Medical treatments for dementia are limited. Licensed medications are available only for dementia due to AD and PDD and these have only modest benefits for symptoms. Many people are interested in non‐medical options to slow down cognitive decline. These include lifestyle modifications and the reduction of modifiable risk factors (WHO 2012). Data from Larson 2013 indicate that the incidence of dementia may be falling, which supports the theory that individual risk might be modifiable. Currently, regular physical exercise, sleep hygiene, mental training and a healthy diet are often recommended to maintain a good physical and cognitive condition (Barnard 2014). Furthermore, there is a growing body of research indicating that malnutrition, which is strongly associated with cognitive decline, is a common problem of people with dementia (Reuther 2013; Roque 2013; Vellas 2005). Dietary recommendations for people with AD aim at a healthy balanced diet containing vegetables, legumes, fruits and whole grains (Barnard 2014). It is hoped that nutritional interventions might be a reasonable approach to delay the progression of the disease.
Description of the intervention
Omega‐3 long‐chain polyunsaturated fatty acids (omega‐3 PUFAs) play a major role in human organs and their function. They are involved in inflammatory and immunological processes and hormonal regulation. Furthermore, they are a component of neuronal membranes and involved in the development and function of the brain (Su 2010).
The human body cannot synthesise omega‐3 PUFAs. Therefore, they are classified as essential fatty acids. The most common omega‐3 PUFAs are eicosapentaenoic acid (EPA, 20:5n‐3), docosahexaenoic acid (DHA, 22:6n‐3) and alpha‐linolenic acid (ALA, 18:3n‐3). Chemically, fatty acids chains consist of carbon atoms with a carboxylic end ('alpha') and methyl end ('omega'). The first number of the chemical name refers to the number of carbons in the carbon chain. It is followed by the number of double bonds and their position counting from the omega end of the chain (i.e. 'n‐3' refers to the C=C double bond at position three).
Natural sources of EPA and DHA are algae, oily fish (e.g. salmon, mackerel, herring or sardines) and fish oils. In plants, the most common ALA is found in vegetable oils (e.g. canola, flax seed oil, soybean oil) and nuts (e.g. walnuts). Humans cannot synthesise ALA, but it can be partially metabolised into EPA and DHA (FAO 2010). Nutritional supplements containing oils rich in omega‐3 PUFAs are also available. There is broad scientific consensus about the importance of food sources rich in omega‐3 PUFAs to maintain healthy body function. However, the evidence on the supportive role of additional supplements is still insufficient (Campbell 2013; EFSA 2010; Hooper 2004). This applies in particular to the prevention of dementia (Sydenham 2012).
The human body has a limited storage capacity of PUFAs in adipose tissue, which implies their regular consumption (Arterburn 2006). Most guidelines recommend a daily intake of 250 to 1000 mg of EPA plus DHA to meet the requirements of a healthy diet in adults. An adequate intake of ALA is generally expressed as 'percentage of total dietary energy (E%)' (EFSA 2010) and usually defined to be 0.5E% to 1.0E% (Aranceta 2012; EFSA 2010). However dietary reference values and guideline recommendations vary across the world (Aranceta 2012; EFSA 2012). The optimal amounts for the prevention and treatment of chronic diseases are not well established (Micha 2014). Experts state that the recommended amounts of omega‐3 PUFAs can be consumed as part of a balanced diet with a regular intake of fish (EFSA 2010). For example, an intake of 500 mg of EPA plus DHA can be achieved by consuming two portions (90 g) of oily fish per week (FAO 2010). Nevertheless, omega‐3 PUFA supplements are among the most consumed of dietary supplements intended to improve or maintain overall health (Bailey 2013; Dickinson 2014). Even though current data show an overall increase of the consumption of polyunsaturated fats, people in most countries consume less than the recommended amount (EFSA 2012; Micha 2014). Supplements with combined doses of DHA and EPA up to 5 g/day, EPA alone up to 1.8 g/day or DHA up to 1 g/day for adults do not raise safety concerns of the European Food Safety Authority (EFSA 2012).
How the intervention might work
Omega‐3 PUFAs are involved in the structure and function of cell membrane phospholipid fractions in the brain (Cansev 2008), and are assumed to play an important role in cognitive processes. Several hypotheses have been presented to explain how the dietary intake of omega‐3 PUFAs might influence the cognitive performance of people with dementia.
First, maintaining adequate levels of omega‐3 PUFAs may support the development and integrity of the brain's neurons and enhance synaptic plasticity (Cansev 2008; Su 2010). Research shows a risk of malnutrition in people with dementia (Reuther 2013; Roque 2013; Vellas 2005), which indicates that vulnerable people in particular can benefit from additional administration of omega‐3 PUFAs. Findings of decreased fatty acids in plasma within this population might support this idea (Lin 2012; Lopes da Silva 2013).
Second, omega‐3 PUFAs have anti‐oxidative and anti‐inflammatory effects (Molfino 2014; Vedin 2012). Especially in the ageing brain, this characteristic may contribute to the protection of neurons and prevent cellular death.
Third, Morris and Tangne have argued in their review that the fatty acid composition of the diet is an important determinant of blood cholesterol, which in turn seems to play a role in the pathology of AD (Morris 2014). For example, apolipoprotein‐E (ApoE) is involved in the transport of cholesterol and the ApoE‐ϵ4 allele is an important risk factor for AD (Morris 2014). Furthermore, there is growing evidence that serum cholesterol is strongly associated with the deposition of β‐amyloid in the human brain (Reed 2014).
Finally, it has also been suggested that omega‐3 PUFAs may be directly related to the decrease of AD‐specific pathology (e.g. Aβ levels) (Cole 2009; Su 2010). This hypothesis is supported to some extent by preclinical studies, and a wide range of models describing potential neurochemical mechanisms have been outlined (Murphy 2014; Su 2010).
Why it is important to do this review
Omega‐3 PUFAs have become increasingly important in several dietary recommendations. It is widely theorised that they slow cognitive decline in people with dementia. Considering the enormous impact of dementia on quality of life (QoL) and the limited treatment possibilities, a safe and effective dietary intervention would be of great interest to people with dementia. With this review, we aimed to assist them in their decision regarding dietary supplementation with omega‐3 PUFAs.
Objectives
To assess the efficacy and safety of omega‐3 polyunsaturated fatty acid (PUFA) supplementation for the treatment of people with dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). Since dementia is a progressive disease, we included only the data of the first period of cross‐over randomised trials.
Types of participants
We included people diagnosed with AD, VaD, DLB, PDD and FTD. The diagnosis of dementia should have been made in accordance with accepted guidelines, such as the Diagnostic and Statistical Manual of Mental Disorders (APA 1987; APA 1994; APA 2013), the International Classification of Diseases (ICD; WHO 1992; WHO 2010), the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS)‐Alzheimer's Disease and Related Disorders Association (ADRDA) Alzheimer's Criteria (McKhann 2011) or the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINDS‐AIREN) Criteria for the Diagnosis of Vascular Dementia (Román 1993).
AD and VaD are the most common types of dementia (WHO 2012). Therefore, we intended to evaluate studies in which the participants were diagnosed with dementia, even if the types of dementia were not specified. However, we found only studies investigating omega‐3 PUFAs in people diagnosed with AD.
We considered any stages and severity of dementia. Participants may have been recruited from any setting.
Types of interventions
We evaluated the following interventions:
Omega‐3 PUFA capsules as a dietary supplement versus placebo. We considered a supplement as appropriate for inclusion if its main active ingredient was omega‐3 PUFA;
Diets enriched with omega‐3 PUFAs in specific portions versus usual diet.
We considered any dosage of administration if the study participants received it on a regular basis (at least weekly) for at least 26 weeks.
We excluded studies that only investigated dietary advice. We also excluded trials that did not precisely specify the intake of omega‐3 PUFA.
Types of outcome measures
Primary outcomes
-
Changes in global and specific cognitive function measured by validated tools such as:
Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (Rosen 1984);
Mini‐Mental State Examination (MMSE) (Folstein 1975);
Rey Auditory Verbal Learning Test (RAVLT) (Schmidt 1996);
Wechsler Memory Scale (Wechsler 2010).
-
Changes in functional outcomes (e.g. activities of daily living (ADL)) measured by validated tools such as:
Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living (ADCS‐ADL) (Galasko 1997);
Gottries‐Brane‐Steen‐Skala, ADL subscale (GBS‐ADL) (Bråne 2001).
-
Overall dementia severity measured by validated tools such as:
Clinical Dementia Rating ‐ Sum of Boxes (CDR‐SOB) (O'Bryant 2008),
Alzheimer's Disease Cooperative Study ‐ Clinical Global Impression of Change (CIBIC‐Plus) (Schneider 1997).
-
Adverse effects of the intervention such as:
gastrointestinal effects;
dermatological effects;
taste disturbance;
infection.
Secondary outcomes
Effect of omega‐3 PUFAs on QoL.
Compliance with intervention.
Symptoms associated with dementia (e.g. changes in mood, alterations in circadian rhythm).
Entry to institutional care.
Hospital admissions.
Mortality.
We did not consider biomarker outcomes.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group's (CDCIG) specialised register on 10 December 2015.
The Trials Search Co‐ordinator for the CDCIG maintains ALOIS, which contains studies that fall within the areas of dementia prevention, dementia treatment and management, and cognitive enhancement in healthy elderly populations. The studies are identified through:
monthly searches of several major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and Lilacs;
monthly searches of several trial registers: ISRCTN; UMIN (Japan's Trial Register); the World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, and others);
quarterly search of the Cochrane Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of several grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website (www.medicine.ox.ac.uk/alois).
The 'methods used in reviews' section within the editorial information about the CDCIG shows details of the search strategies run in healthcare bibliographic databases that we use for the retrieval of reports of dementia, cognitive improvement and cognitive enhancement trials.
We ran additional searches in MEDLINE, EMBASE, PsycINFO, CINAHL, ClinicalTrials.gov and the WHO portal/ICTRP to ensure that the search was as comprehensive and as up‐to‐date as possible. Appendix 1 shows the search strategy.
Searching other resources
We contacted the following manufacturers of omega‐3 PUFA products and organisations for overlooked, unpublished and ongoing trials:
Global Organization for EPA and DHA Omega‐3s, USA;
Arjuna Natural Extracts Limited, India;
FMC Health and Nutrition Epax International, Norway;
Nordic Naturals, USA;
DSM Nutritional Products, Netherlands;
WHC Health Consulting, Belgium;
Carlson Laboratories, USA;
OmegaVia, USA;
Ocean Blue Professional, USA;
Prevention Pharmaceuticals, USA;
NeuroBioPharm Inc, USA.
We reviewed reference lists of included studies, trial registries and conference abstracts, and contacted authors of landmark papers for overlooked, unpublished and ongoing trials.
Data collection and analysis
Selection of studies
We managed all references retrieved by the searches using EndNote (X5) (EndNote 2011). The Trials Search Co‐ordinator of the CDCIG removed duplications of the same references. Afterwards, two review authors (MB and MH or MB and AF) independently examined titles and abstracts to identify eligible studies. If it was not clear whether a study was relevant, we made the decision based on the full text. Two review authors (MB and MH) evaluated full texts of relevant articles independently according to the eligibility criteria. They were not blinded to study data. We resolved disagreements by involving a third review author. We listed final decisions for the exclusion of articles that we retrieved in full text in the Characteristics of excluded studies table.
We planned to translate full texts that were not in English or German, and if necessary employ translation services. However, as all eligible studies were already presented in English this was not necessary. We linked multiple reports and conference abstracts of the same study together.
Data extraction and management
Two review authors (MB and MH) independently read and extracted the data presented in the respective article. In case of discrepancies, we involved a third review author until we reached consensus.
We used an electronic data extraction form, including source, eligibility, methods, participants, interventions, comparators, outcomes, results and miscellaneous notes according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). In addition, we assessed details of funding source, declarations of interest of the primary investigators and methods used to control possible conflicts of interests. Two review authors pre‐tested the form using the first two studies and adapted afterwards.
For continuous data, we extracted the mean value of the outcome measurement in each group (or, if this was not available, the mean change from baseline), the standard deviation (SD) and the number of participants used to measure the outcome for each group.
For dichotomous outcomes, we extracted the number of participants in each outcome group. If the data provided were insufficient, we attempted to obtain the omitted information from the authors of the report (see the section Dealing with missing data).
One review author (MB) entered the data into Review Manager 5 (RevMan 2014). A second review author (MH) checked the data for accuracy. We also extracted data from ongoing studies including study name, methods, participants, interventions, outcomes, starting date, contact information and notes.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study, using the Cochrane tool for assessing risk of bias (Higgins 2011b). We resolved any disagreements by discussion.
We described the risk of bias of all included studies in the Characteristics of included studies table and narrative. In addition, we provided an overall judgement of included studies by a 'Risk of bias' summary (see Figure 1). To prevent undue industry influence during the clinical trial process, we explicitly considered the appropriateness of all methods used. Therefore, we assessed additional criteria, which are presented in detail in Table 2. An overall rating on how these findings might have influenced the presented study results were considered as 'other bias' in the 'Risk of bias' tables.
1.
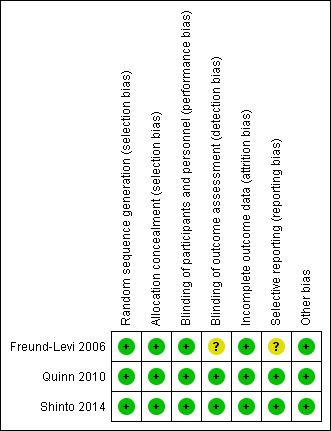
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
1. Methods used to control bias resulting from conflict of interest.
| Study | Beforehand published primary outcomes presented? | Planning phase and funding: role of industry | Conducting phase: role of industry | Analysing process: role of industry | Reporting process: role of industry | Overall judgement |
| Freund‐Levi 2006 | Yes | "The OmegAD study was funded in part by Pronova Biocare A/S, Lysaker, Norway. This company was represented in the trial steering committee for study design and the decision to submit for publication, and provided the EPAX1050TG and placebo preparations; however, the company was not involved in collection, analyses, or interpretation of the data" p. 1408 | The funding company provided the intervention and placebo preparations. "[...] the company was not involved in collection, analyses, or interpretation of the data" p. 1408 |
"[...] the company was not involved in collection, analyses, or interpretation of the data" p. 1408 | The funding company was in involved in the decision to submit for publication. It was not part of the author team. 1 author has received travel grants from Pronova Biocare A/S |
Low Rationale: data collection, analysis, presentation and interpretation seem not to be influenced by the manufacturer itself or other undue interests |
| Quinn 2010 | Yes | 2 employees of Martek Biosciences (manufacturers of DHA and inventor of a patent for DHA for treatment of AD) were involved in study concept and design | 2 employees of Martek Biosciences were involved in administrative, technical or material support | "Martek employees participated in design of the study and in revision of the manuscript ("Irish Endocrine Society 34th Annual Meeting,") The statistical analysis was conducted by the Alzheimer's Disease Cooperative Study Data Core. Martek employees did not participate in the statistical analysis and did not have access to the data prior to the completion of data analysis" p. 9 | 2 Martek employees were involved in the critical revision of the manuscript for important intellectual content. 2 other authors were (since 2010) co‐inventors on a patent for DHA for the treatment of AD, which was filed in 2009; both waived personal rights to royalties related to this patent. Both were involved in study design and concept, supervision and acquisition of data. 1 was additionally involved in administrative, technical or material support. 1 drafted the manuscript the other was involved in its critical revision No other authors reported disclosures. |
Low Rationale: some trial authors disclosed industry financial ties or employment in detail. However, we received all data that we had asked for and the results of the primary endpoints were reported as planned in the trials registration form. The study was otherwise accompanied by external experts and the statistical analysis seemed to be conducted independently from manufactures employees |
| The study design was "approved by an oversight committee of the National Institute on Aging. Representatives from the National Institute on Aging participated in meetings of the steering committee of the Alzheimer's Disease Cooperative Study during the course of the trial" p. 9 | ||||||
| Shinto 2014 | Yes | None described | Nordic Natural, Watsonville, CA, USA, supplied the fish oil and placebo oil, and Meda Pharma, Bad Homburg | None described | 2 from 11 authors disclosed fees for consultancy or lectures 1 author was also involved in the ADCS‐NIA trial. He stated in the related article (Quinn 2010) that he was co‐inventor on a patent for DHA for the treatment of AD but waived personal rights to royalties related to this patent |
Low Rationale: the study was well reported, we received all data requested and we judged the financial ties to the manufacturer as marginally |
AD: Alzheimer's disease; ADCS‐NIA: Alzheimer's Disease Cooperative Study ‐ National Institute on Aging; DHA: docosahexaenoic acid.
Measures of treatment effect
We used mean differences (MD) or standardised mean differences (SMD) with 95% confidence intervals (CI) for continuous outcomes, and risk ratios (RR) with 95% CIs for the analysis of dichotomous outcomes.
To date, no ranges for commonly accepted minimal clinically important differences (MCID) exist for most of the scales used to measure outcomes in people with dementia (IQWIG 2013; Molnar 2009; Schrag 2012; US Preventive Task Force 2014; Vellas 2008). We intended to present the proportion of participants with changes in the scale measures of the primary outcomes (i.e. more or less than 4 scale points for ADAS‐Cog) if data were available. However, considering the small insignificant effects, we did not request that data from the study authors.
Scales that are commonly used in dementia trials are often coded ordinally. We treated the data measured with scales comprising of more than 10 categories as continuous variables assuming a normal distribution.
Unit of analysis issues
The unit of analysis was the person with dementia. As defined in our protocol, we analysed only the first period of cross‐over trials considering the progressive nature of dementia. We intended to use comparable time points (± one week) for all meta‐analyses. Therefore, we conducted meta‐analyses from six‐month measurement data, which we were able to get from all trials.
Dealing with missing data
We contacted trial authors requesting missing information or to clarify any remaining ambiguity. All authors replied to our queries. We received unpublished data from two trials (Quinn 2010; Shinto 2014), and were able to clarify most questions with all trial authors. However, we were not able to obtain data from the OmegAD trial concerning data to adverse effects from each group (Freund‐Levi 2006). We considered this issue in the appraisal of the risk of bias.
None of the trials were able to assess the outcomes of all included participants. One trial used the last observation carried forward (LOCF) approach but did not publish the results, reasoning that the LOCF results did not differ from the per protocol analyses (Freund‐Levi 2006).
Two trials used logistic regression models to predict missing data over time (Quinn 2010; Shinto 2014). Quinn 2010 also presented some sensitivity analysis with multiple imputations. We described these results additionally as reported by the trial authors.
We also considered missing data by conducting sensitivity analysis (see Sensitivity analysis).
Assessment of heterogeneity
We evaluated clinical heterogeneity and statistical heterogeneity using Chi2 and I2 statistics.
Assessment of reporting biases
We tried to minimise reporting bias by inclusion of published and unpublished trials. Therefore, we compared conference abstracts and registered trials with published data. According to the trials registries, we found two studies that were completed but not published and contacted the responsible organisation or the researcher for more information (see Description of studies). We found no further indication of unpublished trials. It was not reasonable to perform a funnel plot and Egger's test for asymmetry (Egger 1997), since we included only three trials.
Data synthesis
We observed no considerable statistical heterogeneity and conducted fixed‐effect meta‐analyses to estimate an overall treatment effect. We performed all meta‐analyses by using Review Manager 5 (RevMan 2014). We combined outcomes measured with the same scales, by presenting MDs. When different or modified scales were used to measure the same construct, we used the SMD for the meta‐analysis. A precondition for this was that the same domains (i.e. global cognitive function) or subdomain (e.g. memory) were assessed.
Due to the progressive nature of dementia, we assumed that LOCF and per protocol analyses had a comparable distorting impact on the results. Therefore, we considered both in our meta‐analyses.
Subgroup analysis and investigation of heterogeneity
In the protocol for this review (Burckhardt 2015), we planned to conduct subgroup analyses of dementia subtype and stage, baseline nutritional status and dose of intervention. However, we included only three studies. All these included participants with mild to moderate AD. Analysing subgroups by the dosage was not reasonable either, because all study interventions were in a range of omega‐3 PUFAs 1.75 to 2.3 g in total. One study conducted subgroup analysis on MMSE and CDR‐SOB (Quinn 2010). However, they did not adjust their testing to multiple comparisons, which might bias the results. We presented the results briefly in the Effects of interventions section. None of the studies conducted subgroup analysis based on nutritional status. We investigated heterogeneity in terms of participants and omega‐3 PUFA dosage. We presented the main baseline characteristics and interventions in Table 3.
2. Baseline characteristics of participants and main interventions of included studies.
| Study | Number randomised | Diagnosis and severity of disease | Mean age (SD) (years) | Mean MMSE (SD) | Mean BMI (SD) | Use of AD medicine | Daily omega‐3 dose / treatment duration | Outcomes relevant to this review |
| Freund‐Levi 2006 | Total 204 IG 103 CG 101 |
AD mild to moderate |
73.47 (8.79) | 23.41 (3.8) (PP population) |
24.37 (3.04) | 100% cholinesterase inhibitors Memantine not reported |
DHA 1.7 g + EPA 0.6 g 26 weeks |
ADAS‐Cog MMSE CDR‐SOB NPI DAD MADRS Safety and tolerability |
| Quinn 2010 | 402 IG 238 CG 164 |
AD mild to moderate |
76 (8.71) | 20.66 (3.65) | 26 (4.0) | 85.8% cholinesterase inhibitors 60.4% memantine |
DHA 900‐1100 mg 18 months |
ADAS‐Cog CDR‐SOB MMSE ADCS‐ADL QoL NPI |
| Shinto 2014 | 26 IG 13 CG 13 |
AD mild to moderate |
75.55 (9.36) | 21.45 (2.95) | 25 (3.98) | 84.61% cholinesterase inhibitors or memantine | 675 mg DHA + 975 mg EPA 12 months |
ADAS‐Cog MMSE OARS‐ADL OARS‐IADL |
AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living; BMI: body mass index; CDR‐SOB: Clinical Dementia Rating ‐ Sum of Boxes; CG: control group; DAD: Disability Assessment for Dementia; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; IG: intervention group; MADRS: Montgomery‐Åsberg Depression rating scale; MMSE: Mini‐Mental State Examination; NPI: Neuropsychiatric Inventory; OARS‐ADL: Older Americans Resources and Services ‐ Activities of Daily Living; OARS‐IADL: Older Americans Resources and Services ‐ Instrumental Activities of Daily Living; PP: per protocol; QoL: quality of life; SD: standard deviation.
Sensitivity analysis
We were only able to conduct our meta‐analyses by using means (or mean changes), which were observed (per protocol) or partially summarised over time (LOCF). Since dementia is a progressive disease, this might overestimate the effect in favour of omega‐3 PUFAs. As pre‐defined in our protocol, we conducted a sensitivity analysis using single imputation methods. We assumed that the mean and SD of the missing observations from both groups corresponded to those of the observed cases in the control group. For the ADAS‐Cog, we combined the assumed group results with the observed data with R statistics by using the formula for combining groups presented in Chapter 7.7. of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Due to the clear results and the similarity of the trials, we did not consider it necessary to perform further sensitivity analyses including imputed data.
We further decided to perform sensitivity analyses pooling MMSE and ADAS‐Cog results at the end of the treatment from all three trials, irrespective of study duration.
Presentation of results ‐ 'Summary of findings' tables
We used the GRADE approach to interpret the findings (Guyatt 2011), and presented them in 'Summary of findings' tables as recommended by Cochrane (Schünemann 2011). Together with our consumer group, we prioritised the above defined outcomes.
For that purpose, we conducted a small study involving people with early dementia, their relatives, nurses, and physicians of a geriatric ward. Data collection took place from May until November 2015 in the Department of Psychiatry, Psychotherapy and Psychosomatics of the University Hospital Halle (UKH). All participants were asked to take part in this survey anonymously. A simple questionnaire presented treatment outcomes in an understandable way. We asked the recipients to mark their subjective importance of each outcome on a 9‐point Likert scale ranging from 1 (unimportant) to 9 (important).
We collected 37 questionnaires from 14 people with dementia, 12 relatives and 11 staff members. However, in most cases the treatment goals were rated high and, therefore, resulted in a reduced variance in item scores. People with dementia, relatives and staff did not differ significantly in their evaluation, which is surely caused by the low sample size and the small variance within the ratings. In the total sample, low adverse effects of medication were rated most important, followed by enhancement of QoL, balanced state of mind, enhancement of general cognition, enhancement of memory and enhancement of instrumental activities of daily living (IADL). Enhancement of self care (ADL) was rated least important in the total sample as well as with people with dementia and relatives (see Table 4).
3. Prioritisation of outcomes.
| Outcome (measurement in trials) | People with AD (n = 14) | Relatives (n = 12) | Staff members (n = 11) | Total (n = 37) | Importance for decision‐making | ||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | ||
| Adverse effects of medication (number of adverse events; number of serious adverse events) | 8.71 (8.71) | 1 | 7.75 (1.42) | 4 | 7.91 (2.43) | 4 | 8.16 (1.59) | 1 | Critical |
| Quality of life (QoL‐AD) | 7.57 (2.07) | 5 | 8.33 (1.23) | 1 | 8.09 (1.14) | 1 | 7.97 (1.57) | 2 | Critical |
| Mental health (MADRS; NPI) | 7.79 (1.63) | 4 | 8.00 (0.63) | 3 | 8.00 (1.10) | 3 | 7.92 (1.20) | 3 | Critical |
| General cognition (ADAS‐Cog; MMSE) | 8.21 (1.37) | 2 | 8.08 (1.00) | 2 | 7.36 (2.42) | 6 | 7.92 (1.66) | 4 | Critical |
| Memory (not measured) | 7.86 (1.61) | 3 | 7.08 (1.62) | 5 | 7.00 (2.53) | 7 | 7.35 (1.92) | 5 | Critical |
| Complex activities of daily living (i.e. shopping) (OARS‐IADL) | 7.14 (1.96) | 6 | 6.82 (1.40) | 6 | 8.09 (1.45) | 2 | 7.33 (1.69) | 6 | Critical |
| Simple activities of daily living (i.e. dressing) (ADCS‐ADL; DAD) | 6.71 (3.17) | 7 | 6.00 (2.73) | 7 | 7.82 (1.60) | 5 | 6.81 (2.68) | 7 | Critical |
| Combined cognition and function (CDR‐SOB) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Critical |
ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living; CDR‐SOB: Clinical Dementia Rating ‐ Sum of Boxes; DAD: Disability Assessment for Dementia; MADRS: Montgomery‐Åsberg Depression rating scale; MMSE: Mini‐Mental State Examination; n: number of participants; NPI: Neuropsychiatric Inventory; OARS‐IADL: Older Americans Resources and Services ‐ Instrumental Activities of Daily Living; QoL‐AD: Quality of Life Alzheimer's Disease; SD: standard deviation.
We imported data of the meta‐analyses by using the GRADEpro GDT to create 'Summary of findings' tables. These included for each outcome: the estimate of the treatment effect, the quantity of supporting evidence and the quality of that evidence assessed using the GRADE approach (Guyatt 2011). Two review authors (MB and GL) used the recommended approach to downgrade the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
We included the outcomes in the 'Summary of findings' tables in the order that our consumer group prioritised them (Table 4). We did not ask for global measurements that included cognition and function. However, cognition and function were both rated as critical treatment goals. Therefore, we included results of the CDR‐SOB as well.
Results
Description of studies
Results of the search
The electronic searches from March and December 2015 retrieved 3064 results. After de‐duplication by Anna Noel‐Storr, Trials Search Coordinator of the CDCIG, two review authors (MB and MH or MB and AF) independently assessed the remaining 2331 references for relevance. We identified one further reference by scanning the reference lists of landmark papers and included studies. We received no information for further published or unpublished studies by experts or manufacturers. We discarded 2299 references that were not relevant. Two review authors (MB and MH) independently assessed 31 articles and conference abstracts for eligibility. Seven articles and two registered trials did not meet our inclusion criteria (see Characteristics of excluded studies table). We included 24 articles referring to three trials (Freund‐Levi 2006; Quinn 2010; Shinto 2014). The selection process is presented in the PRISMA statement (Liberati 2009) (see Figure 2).
2.
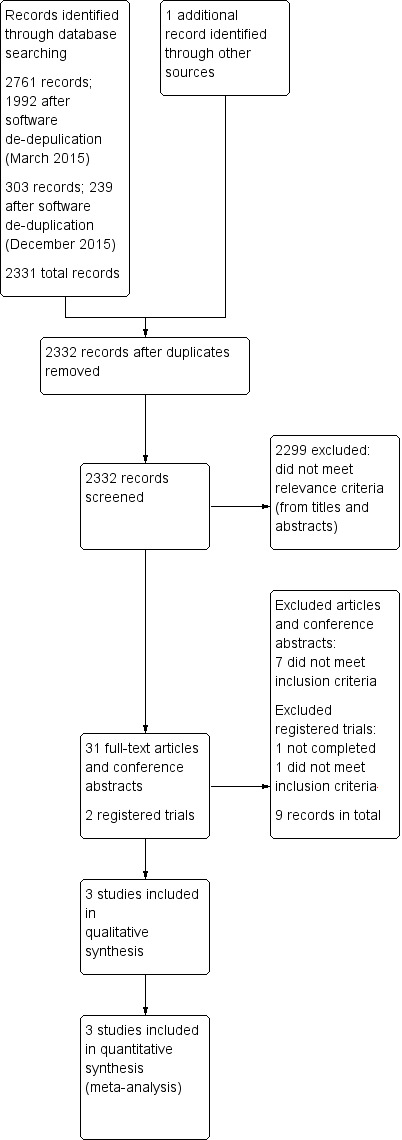
Study flow diagram.
Included studies
Three trials met the inclusion criteria for this review (Freund‐Levi 2006; Quinn 2010; Shinto 2014), and 632 participants were randomised in total. Clinically, the included studies were comparable with respect to the participants (mild to moderate AD) and dosage of the intervention (EPA plus DHA between 1750 and 2300 mg/day). The mean values of nutritional parameters presented in the studies, indicated no malnutrition, lack of DHA or other relevant baseline characteristics (Table 3). The trials had a considerable variation in duration. For most of the primary and secondary outcomes, the trial authors sent us the results from six months' follow‐up, which we combined in meta‐analyses. Statistically, we observed no relevant heterogeneity by using Chi2 and I2 statistics.
The largest trial investigated omega‐3 PUFAs in a parallel‐group design over a study period of 18 months with the primary aim of cognitive and functional outcomes (Quinn 2010). It was sponsored by the Alzheimer's Disease Cooperative Study (ADCS) in co‐operation with the National Institute of Aging (NIA) and DSM Nutritional Products. DSM Nutritional Products is a leading supplier of nutritional supplements. The trial is also referred to as the ADCS‐NIA trial. Dr. Joseph Quinn provided some unpublished data (Table 5).
4. Unpublished data from the ADCS trials (total scores, provided via personal communication).
| Measurement | Baseline | 6 months' follow‐up | 18 months' follow‐up | Linear mixed‐effects model at 18 months | |||
| Placebo mean (SD) | Omega‐3 PUFA mean (SD) |
Placebo mean (SD) |
Omega‐3 PUFA mean (SD) |
Placebo mean (SD) |
Omega‐3 PUFA mean (SD) |
||
| ADAS‐Coga | 23.96 (9.21) n = 162 |
23.77 (8.87) n = 236 |
26.73
(10.7) n = 148 |
26.53
(11.07) n = 217 |
31.53 (14.57) n = 128 | 31.17
(14.76) n = 175 |
‐ |
| ADCS‐ADL | 59.68 (12.9) n= 164 |
60.12 (12.32) n = 238 |
56.8
(15.43) n = 147 |
55.55
(14.94) n = 219 |
‐ | ‐ | ‐ |
| CDR‐SOB | 5.77
(2.61) n = 164 |
5.61 (2.62) n = 238 |
6.75
(3.16) n = 148 |
6.86
(3.3) n = 216 |
‐ | ‐ | ‐ |
| NPI | 9.15 (10.83) n = 164 |
8.92 (10.37) n = 238 |
9.58 (10.8) n = 146 |
11.17 (12.47) n = 219 |
‐ | ‐ | ‐ |
| QoL‐AD informant rated | 36.96 (6.13) n = 151 |
36.45 (5.78) n = 220 |
36.31 (5.82) n = 136 |
34.55 (5.84) n = 195 |
34.91 (6.3) n = 120 |
33.42 (5.95) n = 162 |
P value = 0.41 |
| QoL‐AD participant rated | 40.43 (5.38) n = 150 |
40.0 (4.84) n = 222 |
39.86 (5.41) n = 133 |
39.76 (5.33) n = 199 |
40.02 (6.09) n = 119 |
39.63 (5.45) n = 150 |
P value = 0.66 |
ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living; CDR‐SOB: Clinical Dementia Rating ‐ Sum of Boxes; n: number of participants; NPI: Neuropsychiatric Inventory; PUFA: polyunsaturated fatty acid; QoL‐AD: Quality of Life Alzheimer's Disease; SD: standard deviation.
aFor ADAS‐Cog missing items imputed with last observation carried forward; missing total scores not imputed.
The second largest trial, named the OmegAD study, was a cross‐over design trial of 12 months' duration sponsored by the Karolinska University Hospital in Sweden (Freund‐Levi 2006). The primary aim was to test efficacy of omega‐3 PUFAs on cognition. We included the results of the first period after a follow‐up of six months.
The third trial was a small pilot study with a three‐arm parallel design (Shinto 2014). Its primary aim was to evaluate the effects of omega‐3 PUFAs alone or in combination with alpha lipoic acid on oxidative stress parameters. We included the study's secondary, but patient‐relevant, outcomes on the comparison of omega‐3 PUFA versus placebo. Dr. Lynne Shinto provided unpublished six months data (Table 6), which we used for the meta‐analyses. The trial lasted 12 months and was sponsored by the Oregon Health and Science University in the USA and conducted in collaboration with NIA and the National Center for Complementary and Integrative Health (NCCIH).
5. Unpublished data as provided via personal communication by Dr. Shinto.
| Measurement | 6 months' follow‐up | |
| Placebo mean (SD) n = 11 | Omega mean (SD) n = 12 | |
| ADAS‐Cog | 32.10 (6.4) | 33.8 (9.6) |
| MMSE | 20.4 (4.6) | 18.9 (4.4) |
ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; MMSE: Mini‐Mental State Examination; n: number of participants; SD: standard deviation.
In addition to the Characteristics of included studies table, we presented an overview of the main baseline characteristics, interventions and outcomes of all three studies in Table 3.
We did not identify any trials investigating omega‐3 PUFAs in people with other types of dementia. We also found no trials investigating diets enriched with omega‐3 PUFAs.
Participants
The number of randomised men and women ranged from 26 to 402 with a range of mean age from 73.5 to 76 years. All trials were restricted to people with AD diagnosed with established criteria according to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV: Freund‐Levi 2006) or NINCDS‐ADRDA criteria (Quinn 2010; Shinto 2014). The severity of the disease was mild to moderate ranging from an MMSE of 23.6 (Freund‐Levi 2006) down to 20.66 (Quinn 2010), and the majority of the participants received a stable dose of cholinesterase inhibitors or memantine. All trials defined pre‐study intake of omega‐3 supplements as an exclusion criterion. The ADCS‐NIA trial also excluded participants who consumed on average DHA more than 200 mg/day in the form of food (Quinn 2010).
Two of the trials took place in the USA (Quinn 2010; Shinto 2014), and one in Sweden (Freund‐Levi 2006). All three trial were conducted in outpatient care. The baseline data showed no indication of poor nutrition. The mean body mass index ranged from 24 (SD 3) (Freund‐Levi 2006) to 26 (SD 4) (Quinn 2010). The baseline data from the blood samples indicated a sufficient intake of omega‐3 PUFAs. Table 3 presents the most relevant baseline characteristics of all three trials in detail.
Interventions
All participants received omega‐3 PUFAs as 1 g capsules containing various amounts of omega‐3 PUFAs versus placebo. In the OmegAD trial, participants consumed the highest dose of omega‐3 PUFAs with a combination of DHA 1.7 g and EPA 0.6 g (derived from fish oil) provided in four capsules per day (Freund‐Levi 2006). The capsules further contained vitamin E (tocopherol) 4 mg as a preservative. In the ADCS‐NIA trial, two capsules of an algal‐derived DHA were provided daily (Quinn 2010). This vegetarian source of omega‐3 PUFAs contained no EPA but approximately 45% to 55% of DHA by weight. This means that the participants received a daily dose of around DHA 900 to 1100 mg daily. In the trial of Shinto 2014, participants were recommended to ingest three lemon‐flavoured fish oil concentrate capsules with food. The daily dose contained DHA 675 mg and EPA 975 mg. All trials analysed blood samples for serum fatty acid levels, which increased significantly compared to unchanged levels in placebo groups; this can be interpreted as good compliance for the intervention.
Outcome measures
The trials used the following outcome measures. Table 3 summarises their use in the included studies. For a better interpretation, we presented related estimates of clinical important changes as identified in the literature. Appropriate methods for defining valid estimates of MCIDs are not yet fully developed and for scales, covering different constructs (i.e. global severity scales), almost impossible to determine (Molnar 2009). Furthermore, what is estimated to be a clinically important difference depends on the population (i.e. severity of dementia) and contextual characteristics (i.e. ratio of adverse effects and efficacy) and might vary from different points of view (i.e. researcher or participant) (Revicki 2008). This also applies to the following presented estimates of clinical important changes. They were developed with varying methods and address different circumstances and disease severity. Therefore, they should be considered with caution.
Global and specific cognitive function cognitive function measures
Mini‐Mental State Examination (MMSE) evaluates severity and progression of cognitive impairment in the five areas of orientation, immediate recall, attention and calculation, delayed recall, and language (Folstein 1975). The test score ranges from 0 to 30 with higher scores representing better cognitive function. The severity of cognitive impairment is usually classified by MMSE score points such as 20/21 to 26/27 as mild, 10 to 19/20 as moderate, and less than 10 as severe impaired (Hulstaert 2009). MCIDs of 1.4 to 3.7 score points are commonly estimated (corresponds to the estimates of Burback 1999; Hensel 2007; Qaseem 2008).
The Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) comprises spoken language ability, comprehension of spoken language, recall of test instructions, word finding difficulty, following commands, naming objects, construction drawing, ideational praxis, orientation, word recall and word recognition. The score ranges from 0 to 70, with a higher score indicating a greater impairment (Rosen 1984). MCID is mainly estimated between 2 and 4 score points (Huntley 2015; Molnar 2009; Schrag 2012; Vellas 2008). The OmegAD trial used an extended version of the ADAS‐Cog (scale range 0 to 85) (Mohs 1997).
None of the studies presented specific cognitive function measures (i.e. memory).
Functional outcome measures (e.g. activities of daily living)
The Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living (ADCS‐ADL) was specifically designed as part of a comprehensive test battery to assess ADL living in people with AD in clinical trials (Galasko 1997). It consists of 23 criteria comprising simple everyday skills and complex activities, which are rated based on an interview with an informant who knows the affected study participant well. The range is from 0 to 78 with a higher score indicating a lower interference. Data on MCID for ADCS‐ADL are limited. One study group defined a threshold of a 2 point score change as meaningful in an RCT investigating vitamin E and memantine in mild to moderate AD (Dysken 2014).
The Disability Assessment for Dementia (DAD) evaluates the performance of daily function in community‐dwelling people with dementia based on carer information (Gelinas 1999). The instrument evaluates initiation, planning and execution of simple and complex activities. A final score is formed by a percentage of all questions rated positive, indicating that the study participant is able to perform the respective task without help. Therefore, lower scores indicate more dysfunction. We found no estimates of a meaningful change.
The Older Americans Resources and Services ‐ Activities of Daily Living (OARS‐ADL) Questionnaire (Fillenbaum 1975; George 1985) is a part of a multidimensional functional assessment (Fillenbaum 1981). According to Dr. Shinto (personal communication), the pilot trial used a modified version with score ranges from 0 to 27 for ADL and 0 to 14 for IADL (Shinto 2014). A lower score indicates a better function. We found no estimates of a meaningful change.
Overall dementia severity measures
The Clinical Dementia Rating ‐ Sum of Boxes (CDR‐SOB) is a semi‐structured interview of people with dementia and informants for the assessment of cognition (memory, orientation, judgement/problem solving) and function (community affairs, home/hobbies, personal care) (O'Bryant 2008). The CDR‐SOB total score ranges from 0 to 18 with scores around 3 to 15.5 indicating mild to moderate dementia (O'Bryant 2008). A Clinical Dementia Rating ‐ Global score can be derived from the box scores. We found no estimates of a meaningful change.
Measures of symptoms associated with dementia
The Montgomery‐Åsberg Depression Rating Scale (MADRS) is a measure of mental health and was particularly developed to assess change secondary to treatment of depressive symptoms (Montgomery 1979). The scale encompasses 10 symptoms associated with depression (i.e. sadness or tension), the seriousness of which are rated by a clinician based on observation or reporting after an interview. The total score ranges from 0 to 60, higher scores indicating more severe symptoms. The cut‐off point for mild depression is usually at 13 points (Müller‐Thomsen 2005). MCID estimates range from 1 to 2 points in people with depressive symptoms (Duru 2008).
The 10‐item Neuropsychiatric Inventory (NPI) evaluates neuropsychiatric disturbance common in dementia and associated with mental health: delusions, hallucinations, dysphoria, anxiety, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy and aberrant motor activity (Cummings 1994). Scores range from 0 (normal) to 120 (severely disturbed). The 12‐item extension also assesses night‐time behavioural disturbances, appetite and eating abnormalities (score range 0 to 144) (Cummings 1997). The information is obtained from a person familiar with the patient's behaviour. A change of 4 to 8 points is suggested to be clinically meaningful (Cummings 2015; Howard 2011).
Measures of quality of life
The Quality of Life Alzheimer's Disease scale (QoL‐AD) exists in two versions both for people with AD (self reported) and their informal carers (proxy reported). QoL is assessed in 13 items, covering physical health, energy, mood, memory, living and financial situation, relationships to life partner, family and friends, the ability to perform household and leisure activities, and judgements of one's self and life as a whole. The total score ranges from 13 to 52 points with a higher score reflecting a better QoL (Logsdon 2002). We found no estimates of an MCID.
Adverse events
Safety and tolerability was a secondary outcome in Freund‐Levi 2006 but it was not reported in detail which parameters were assessed and how they were measured. The two other trials did not name adverse events explicitly as an outcome but presented the most reported adverse events. They were either reported by the study participants or partners (Shinto 2014), or it was not clear how they were assessed (Freund‐Levi 2006; Quinn 2010). Serious adverse events, as normally assessed by data and safety monitoring, were in the ADCS‐NIA trial defined as "...events that result in death, hospitalization, prolongation of hospitalization, or are life threatening (based on the judgment of the study physician)" (Quinn 2010).
Some secondary outcomes as defined in the protocol of this review (Burckhardt 2015), such as compliance with intervention, entry to institutional care, hospital admissions and mortality, were not assessed explicitly as outcomes in any of the trials. We considered the themes in the adverse events and tolerability section.
Quinn 2010 published score changes from baseline adjusted for baseline MMSE. If five or fewer items were missing on ADAS‐Cog, those items where imputed based on LOCF methods, on a per item, per participant basis. Missing score measures over time were predicted by linear mixed‐effects (LME) regression models. In a sensitivity analysis, they used analysis of covariance (ANCOVA) methods using multiple imputation methods. Dr. Quinn provided us with unpublished total mean values and QoL data, which were used in the meta‐analyses.
Shinto 2014 also published score changes analysed with LME model. They adjusted for age and education. Dr. Shinto sent us total mean values from six months' follow‐up, which we used for the meta‐analyses.
Freund‐Levi 2006 presented data as observed by presenting MDs. They also performed an LOCF analysis and stated that the results were comparable.
Excluded studies
We excluded eight publications and two registered trials and presented the reasons in the Characteristics of excluded studies table. The main reasons for exclusion were a duration of intervention of less than 26 weeks (Chiu 2008), inclusion of participants other than people with dementia (Hashimoto 2012; Mahmoudi 2014), or different study design (Terano 1999). According to the trials registries, we found two studies that were completed but not published. We then contacted the responsible organisation or the researcher. The North East London NHS Foundation Trust (UK) wrote to us, that their trial was not completed due to non‐significant results and low numbers recruited (Carter 2006). The sponsor (NeuroBioPharm Inc) of the other registered trial informed us that the company was "unable to share any information" with us at this time (NCT00867828). However, with a planned duration of treatment of 24 weeks, the trial does most appear to fulfil our inclusion criteria.
Risk of bias in included studies
Overall, we judged the quality of the trials as high (Figure 1). There were only a few uncertainties (see Characteristics of included studies table), which we do not think have an important influence on the overall results.
Allocation
All trial authors provided details of adequate sequence generation describing computer‐generated schemes (Freund‐Levi 2006 (reported in Faxen‐Irving 2009); Quinn 2010; Shinto 2014).
Blinding
All three trials used adequate blinding methods for participants by using placebo capsules with an identical appearance. Capsules were usually swallowed whole, therefore, we did not judge it as bias that only one trial team made efforts to match the fish‐like smell of omega‐3 PUFAs in their placebo capsules (Shinto 2014).
The reports of Quinn 2010 and Shinto 2014 indicated that outcome assessors were blinded during the whole study duration. However, it was not clear in the OmegAD study (Freund‐Levi 2006) if blinding was maintained long enough to blind the outcome assessor.
Incomplete outcome data
Quinn 2010 and Shinto 2014 presented numbers and reasons for participants who withdrew or dropped out. Numbers and reasons were similar in intervention and control groups. They included missing data by LMEs models.
In the OmegAD trial, an intention‐to‐treat (ITT) analysis on the basis of LOCF was carried out but not published, reasoning that there were no differences to the per‐protocol analysis (Freund‐Levi 2006).
Selective reporting
Overall, we judged the bias for selective reporting as low. The trials analysed and presented all primary outcomes as described in the published trial protocols. We received data to further outcomes as requested. Secondary outcomes and subgroup analysis of Freund‐Levi 2006 and Quinn 2010 were not congruent in detail with the published trial protocols, but there was no indication of favourable reporting of outcomes relevant to this review. However, there were some inconsistencies with reporting of adverse events. Freund‐Levi 2006 presented the reasons for participants leaving the study but not in regard to their group affiliation. We were not able to get this information by mail contact with Dr. Freund‐Levy. Even though these incidents were low in numbers, it cannot be excluded that this might favour omega‐3 PUFAs.
Other potential sources of bias
Omega‐3 suppliers provided all study drugs. Shinto 2014 reported having no further conflicts of interest.
A company producing omega‐3 PUFAs partly funded the OmegAD trial (Freund‐Levi 2006). An omega‐3 supplier was also involved as a collaborator in the ADCS‐NIA trial (Quinn 2010). Both trial authors reported that industry was involved in study design and the submission of the publication. Apart from that, they reported only minor conflicts of interest. However, authors of both trials explicitly state that industry was not involved in collection and analysis of study data, which, in our view, is the most vulnerable part of a trial (Freund‐Levi 2006; Quinn 2010). Considering this and the transparent reporting of all pre‐defined results, we do not judge the reported co‐operation with industry as a relevant source of bias. However, a possible influence of industry in presenting results cannot be ruled out with certainty.
We do not regard any imbalance of baseline data as relevant for the outcomes of this review. Furthermore, none of the studies stopped earlier than planned in the published protocol.
Effects of interventions
See: Table 1
There was no therapeutic benefit for all outcomes in people with mild to moderate AD. This result was irrespective of the omega‐3 PUFAs dose, which was between 1.75 and 2.3 g/day. For the meta‐analyses that we conducted for this review, we used published per‐protocol data from the OmegAD trial (Freund‐Levi 2006), and unpublished data that we received from Dr. Quinn (Quinn 2010) and Dr. Shinto (Shinto 2014).
None of the trials observed any significant effect on any of the outcomes relevant for this review. Therefore, we have largely refrained from presenting all of the effect measures and CIs separately. This especially applies to the pilot trial of Shinto 2014, which did not have enough power to detect a difference in any outcomes relevant to people with AD. We presented results of all outcomes separately.
Changes in global and specific cognitive function (primary outcomes)
Freund‐Levi 2006, Quinn 2010, and Shinto 2014 assessed the cognitive function with MMSE and ADAS‐Cog.
There was no evidence of a benefit for omega‐3 PUFAs compared to placebo in any of the studies. A meta‐analysis based on Freund‐Levi 2006 and Shinto 2014 showed no effect on cognition when measured with MMSE at six months (MD 0.18, 95% CI ‐1.05 to 1.41; 202 participants; 2 studies; I2 = 0%). We graded the quality of evidence across the studies as high (Table 1). Figure 3 shows the meta‐analysis (Analysis 1.1).
3.
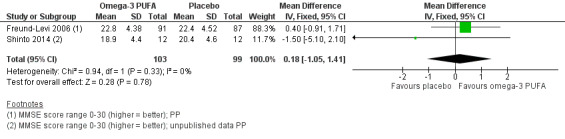
Forest plot of comparison: Omega‐3 PUFAs versus placebo for mild to moderate Alzheimer's disease. Analysis 1.1 Mini‐Mental State Examination (MMSE; 6 months' follow‐up, PP analysis).
1.1. Analysis.
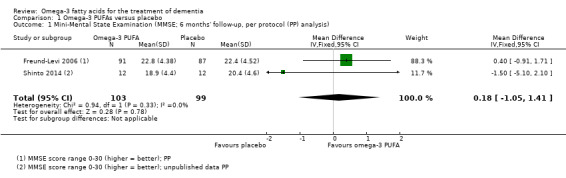
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 1 Mini‐Mental State Examination (MMSE; 6 months' follow‐up, per protocol (PP) analysis).
Quinn 2010 assessed cognition with MMSE at a follow‐up of 18 months in an ANCOVA analysis that showed no difference between groups (P value = 0.88). This was consistent with Shinto 2014 where there was no difference (P value = 0.80) at 12 months in an LME model when adjusted for age and education level.
This result also applied for cognition measured with ADAS‐Cog. We performed a meta‐analysis of six months' data of all trials, which revealed no significant benefit for omega‐3 PUFAs (SMD ‐0.02, 95% CI ‐0.19 to 0.15; 566 participants; 3 studies; I2 = 0%) (Analysis 1.2). We judged the quality of evidence across the studies as high (Table 1).
1.2. Analysis.
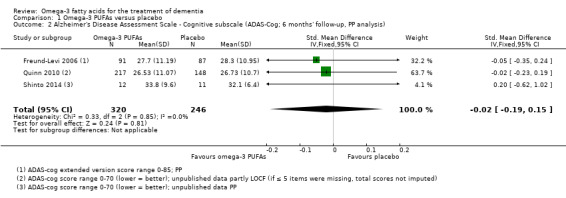
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 2 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog; 6 months' follow‐up, PP analysis).
In the ADCS trial, there was no significant difference observed at 18 months' follow‐up when missing data were considered with an LME model (P value = 0.41) adjusted for baseline MMSE or in an ANCOVA with data after multiple imputation (P value = 0.99; unpublished data) (Quinn 2010).
None of the included trials assessed specific cognitive functions.
Changes in functional outcome measures (e.g. activities of daily living (primary outcome))
A meta‐analysis with functional measures on DAD (Freund‐Levi 2006) and ADCS‐ADL (Quinn 2010) showed no difference at six months (SMD ‐0.02, 95% CI ‐0.19 to 0.16; 544 participants; 2 studies; I2 = 23%) (Analysis 1.3; Figure 4). We rated the quality of evidence across the studies as high (Table 1).
1.3. Analysis.
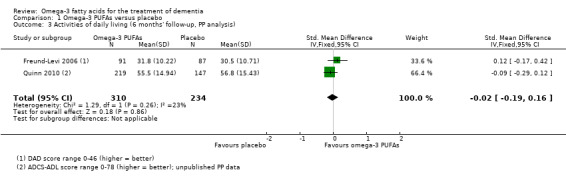
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 3 Activities of daily living (6 months' follow‐up, PP analysis).
4.
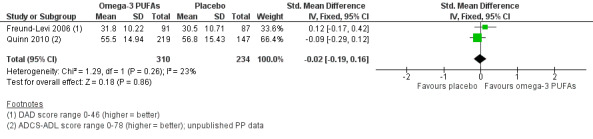
Forest plot of comparison: Omega‐3 PUFAs versus placebo for mild to moderate Alzheimer's disease. Published and unpublished. Analysis 1.3 Activities of daily living (6 months' follow‐up, PP analysis). ADCS‐ADL: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living; DAD: Disability Assessment for Dementia.
Considering missing data in LME models, this result was consistent when ADL was measured on a modified version of the OARS at 12 months (P value = 0.82) (Shinto 2014), or on the ADCS‐ADL at 18 months (P value = 0.38) (Quinn 2010).
Shinto 2014 observed a significant difference for IADL measured on the OARS‐IADL subscale in favour for omega‐3 PUFAs at 12 months (MD ‐3.50, 95% CI ‐4.30 to ‐2.70; 22 participants) (Analysis 1.4). When missing data were considered in an LME model adjusting for age and education at 12 months, the result remained positive in favour for omega‐3 PUFAs (P value < 0.01) (Shinto 2014). Although the difference was significant, the outcome was only presented by one very small study. We downgraded the quality of evidence to moderate because of a wide CI and a very low number of participants (Table 1).
1.4. Analysis.
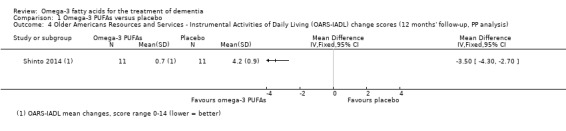
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 4 Older Americans Resources and Services ‐ Instrumental Activities of Daily Living (OARS‐IADL) change scores (12 months' follow‐up, PP analysis).
Overall dementia severity (primary outcome)
A meta‐analysis including measures of CDR‐SOB from two studies revealed no significant difference between omega‐3 PUFAs and placebo at six months (MD ‐0.00, 95% CI ‐0.58 to 0.57; 542 participants; 2 studies; I2 = 0%) (Analysis 1.5) (Freund‐Levi 2006; Quinn 2010). We graded the quality of evidence across the studies as high. The result was consistent in an LME model at 18 months (P value = 0.68) (Quinn 2010).
1.5. Analysis.
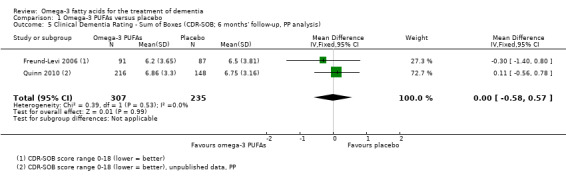
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 5 Clinical Dementia Rating ‐ Sum of Boxes (CDR‐SOB; 6 months' follow‐up, PP analysis).
Adverse effects (primary outcome)
The European Medicines Agency (EMA) recommends an on‐treatment follow‐up of at least 12 months to demonstrate long‐term safety (EMA 2014). Shinto 2014 and Quinn 2010 fulfilled these requirements by implementing a treatment duration of 12 (Shinto 2014) and 18 (Quinn 2010) months. Freund‐Levi 2006 did not report adverse events in detail.
Two of the three included studies described the intervention as well tolerated and with only mild adverse events (Freund‐Levi 2006; Shinto 2014). In the study of Shinto 2014, adverse events such as cold or influenza (omega‐3 PUFAs: 2/13; placebo: 2/13), loose stools (omega‐3 PUFAs: 2/13; placebo: 3/13), dizziness (omega‐3 PUFAs: 1/13; placebo: 2/13) or falls (omega‐3 PUFAs: 1/13: placebo: 2/13) were similar between treatment and placebo group. Serious adverse events (omega‐3 PUFAs: 1/13 (cardiac arrest); placebo: 1/13 (complications after a urinary tract infection)) were not considered to be related to omega‐3 PUFAs (Shinto 2014).
Freund‐Levi 2006 did not report adverse events or serious adverse events for each group. They described only the drop‐out rate as evenly distributed between the groups without unbundling the reasons. Reasons for overall group drop‐outs related to adverse events were diarrhoea (nine drop‐outs), dysphagia owing to the size of the capsules (nine drop‐outs) and new serious somatic disease (10 drop‐outs). We obtained no further detailed information regarding the distribution of these events to the groups by contacting Dr. Freund‐Levy by mail.
Quinn 2010 described adverse events at 18 months' follow‐up as diarrhoea (omega‐3 PUFAs: 7.6%; placebo: 6.1%), urinary tract infections (omega‐3 PUFAs: 9.7%; placebo: 7.3%), falls (omega‐3 PUFAs: 17.6%; placebo: 20.1%), dizziness (omega‐3 PUFAs: 5.0%; placebo: 5.5%) and agitation (omega‐3 PUFAs: 10.1%; placebo: 7.3%). Almost every participant had an adverse event when these outcomes were combined (omega‐3 PUFAs: 89.9%; placebo: 87.8%) (RR 1.02, 95% CI 0.95 to 1.10; 402 participants; 1 study). The distribution of "any adverse events" was similar between the treatment and the placebo group (Analysis 1.8).
1.8. Analysis.
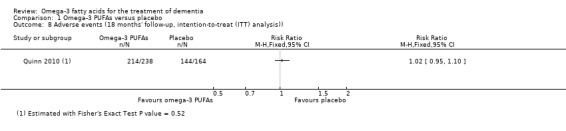
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 8 Adverse events (18 months' follow‐up, intention‐to‐treat (ITT) analysis)).
Serious adverse events were infrequent and the differences between the groups did not reach statistical significance (at the 5% level). Participants in the omega‐3 PUFAs group were more than twice as likely to die (omega‐3 PUFAs: 4.6%; placebo: 2.4%) or to develop a deep venous thrombosis or pulmonary embolus (omega‐3 PUFAs: 3.4%; placebo: 1.2%). Hospitalisation was a further reported serious adverse event (omega‐3 PUFAs: 28.2%; placebo: 26.2%). Considering all serious adverse events (death, hospitalisation, prolongation of hospitalisation and life‐threatening incidents) together, there was no difference between groups (RR 1.05, 95% CI 0.78 to 1.41; 402 participants; 1 study) (Analysis 1.9). We graded the quality of evidence for serious adverse events as high as we do not assume measurement errors for the included outcomes as likely. We downgraded the quality of evidence for the outcome 'any adverse events' for measurement uncertainties because the outcome was an accumulation of partial subjective outcomes (i.e. dizziness) and it was not clear how they were measured (Table 1).
1.9. Analysis.
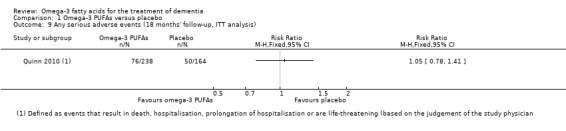
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 9 Any serious adverse events (18 months' follow‐up, ITT analysis).
Compliance was not explicitly reported in any of the trials and can be merely assumed by DHA levels presented in all three trials showing significant increases in the interventions groups but not in the placebo groups (Freund‐Levi 2006; Quinn 2010; Shinto 2014).
Symptoms associated with dementia (secondary outcome)
Mental health was depicted within the trials as depressive symptoms (MADRS) and neuropsychiatric disturbances (NPI). The ADCS trial used a 12‐item version of the NPI (Quinn 2010), and the OmegAD trial used an extended version (Freund‐Levi 2006). The meta‐analysis of both trials results revealed no difference at six months (SMD 0.10, 95% CI ‐0.07 to 0.27; 543 participants; 2 studies; I2 = 0%) (Analysis 1.6). We judged the quality of evidence across the studies as high (Table 1). Considering missing data in an LME model, there was no difference observed in the ADCS trial at 18 months (P value = 0.11) (Quinn 2010).
1.6. Analysis.
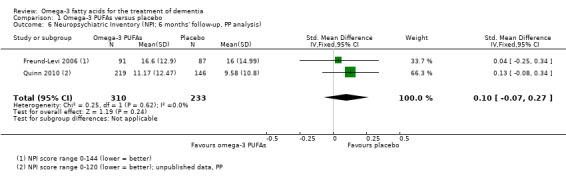
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 6 Neuropsychiatric Inventory (NPI; 6 months' follow‐up, PP analysis).
Only Freund‐Levi 2006 measured the severity of depressive episodes using the MADRS. However, the means of both groups were very low indicating no relevant depressive symptoms. There was no significant difference between groups (MD ‐0.10, 95% CI ‐0.74 to 0.54; 178 participants) (Analysis 1.7). The quality of evidence was high (Table 1).
1.7. Analysis.
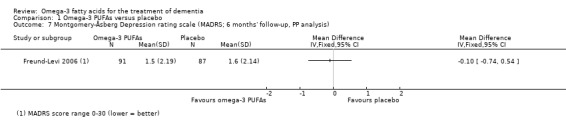
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 7 Montgomery‐Åsberg Depression rating scale (MADRS; 6 months' follow‐up, PP analysis).
Quality of life (secondary outcome)
The ADCS trial assessed QoL using participant‐reported and proxy‐reported by partners or carers QoL‐AD. Dr. Quinn provided us with unpublished data from both. In this trial, there was no difference when QoL was assessed by participants at six months (MD ‐0.10, 95% CI ‐1.28 to 1.08; 332 participants) (Analysis 1.10) (Quinn 2010). We judged the quality of the evidence at six months' follow‐up as high. There was a difference in favour for placebo when QoL was assessed using informant‐rated scores at six months (MD ‐1.76, 95% CI ‐3.04 to ‐0.48; 331 participants) (Analysis 1.11). Both results remained similar at 18 months (see Analysis 1.12 and Analysis 1.13). We downgraded the quality of evidence for QoL rated by participants at 18 months because of substantial group differences in the follow‐up (Table 1). We judged the rating of QoL by the participants themselves as more trustworthy than a proxy measurement. Therefore, we do not present the proxy measure of QoL in Table 1.
1.10. Analysis.
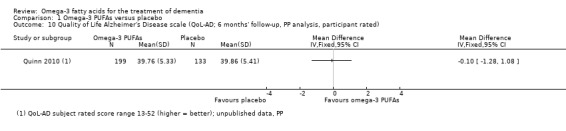
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 10 Quality of Life Alzheimer's Disease scale (QoL‐AD; 6 months' follow‐up, PP analysis, participant rated).
1.11. Analysis.
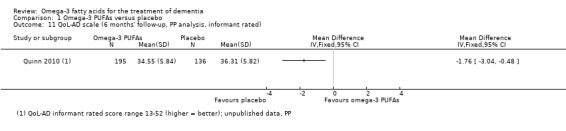
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 11 QoL‐AD scale (6 months' follow‐up, PP analysis, informant rated).
1.12. Analysis.
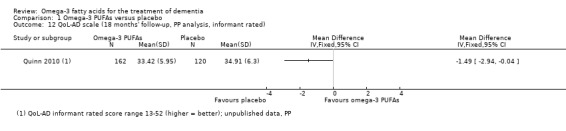
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 12 QoL‐AD scale (18 months' follow‐up, PP analysis, informant rated).
1.13. Analysis.
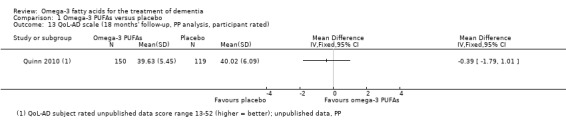
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 13 QoL‐AD scale (18 months' follow‐up, PP analysis, participant rated).
Considering missing data in LME models, there was no difference at 18 months, whether QoL was participant‐rated (P value = 0.66) or informant‐rated (P value = 0.41) (Quinn 2010).
Effects on subgroups
The data were not sufficient to perform our pre‐defined subgroup analyses by dementia stage and nutrition status.
Quinn 2010 conducted several subgroup ITT analyses (LME) of a more exploratory character (no adjustment for multiple testing and reduced power to detect a difference). There were no differences for any outcomes in subgroups based on higher and lower baseline MMSE scores (cut‐off: 21 score points) and CDR (cut‐off: 0.5, 1.0 and 2.0 score points). Further subgroup analyses reported in Quinn 2010 and Freund‐Levi 2006 were not pre‐defined in our protocol, therefore we have not included them in our review.
Sensitivity analysis
We assumed that the means and SDs of the outcomes for missing participants in both groups corresponded with the values for observed cases in the control group. We imputed missing six‐month values for ADAS‐Cog in all three trials (Freund‐Levi 2006; Quinn 2010; Shinto 2014). The meta‐analysis based on this assumption showed no difference between the groups on ADAS‐Cog (SMD ‐0.02, 95% CI ‐0.18 to 0.13; 632 participants; 3 studies; I2 = 0%) (Analysis 1.14) (see Figure 5).
1.14. Analysis.
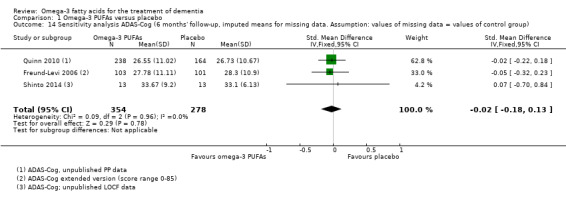
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 14 Sensitivity analysis ADAS‐Cog (6 months' follow‐up, imputed means for missing data. Assumption: values of missing data = values of control group).
5.
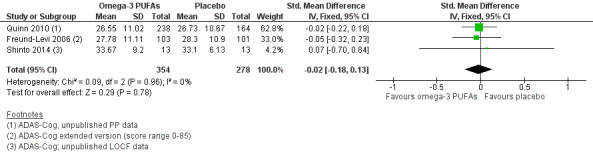
Forest plot of comparison: Omega‐3 PUFA versus placebo for mild to moderate Alzheimer's disease. Published and unpublished. Sensitivity analysis 1.15 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog; 6 months' follow‐up, imputed means for missing data. Assumption: values of missing data = values of control group). LOCF: last observation carried forward; PP: per protocol.
We also combined MMSE and ADAS‐Cog results at endpoint from all three trials, irrespective of study duration. In these analyses, there was no significant difference between groups for MMSE (MD 0.55, 95% CI ‐0.31 to 1.40; 464 participants; 3 studies; I2 = 0%) (Analysis 1.15) or ADAS‐Cog (SMD ‐0.03, 95% CI ‐0.20 to 0.15; 504 participants; 3 studies; I2 = 0%) (Analysis 1.16). Figure 6 shows the sensitivity analysis for MMSE.
1.15. Analysis.
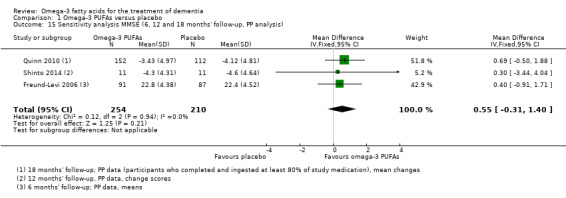
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 15 Sensitivity analysis MMSE (6, 12 and 18 months' follow‐up, PP analysis).
1.16. Analysis.
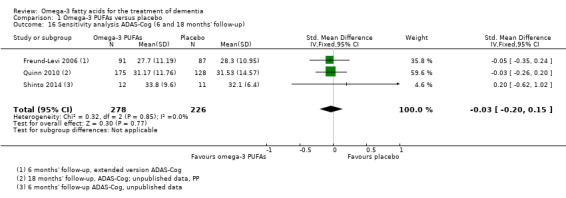
Comparison 1 Omega‐3 PUFAs versus placebo, Outcome 16 Sensitivity analysis ADAS‐Cog (6 and 18 months' follow‐up).
6.
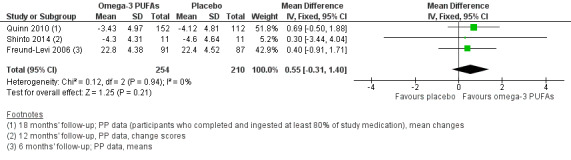
Forest plot of comparison: Omega‐3 versus placebo for mild to moderate Alzheimer's disease. Published and unpublished. Sensitivity analysis 1.19 Mini‐Mental State Examination (MMSE; 6, 12 and 18 months' follow‐up, per protocol (PP) analysis).
Discussion
Summary of main results
The review included three studies involving 632 participants with AD.
There was no convincing benefit of omega‐3 PUFAs for our pre‐defined primary outcomes of cognition, function or dementia severity, or for any other outcomes within the scope of this review, regardless of the dose of omega‐3 PUFAs or the duration of intake. There was a numerical advantage of omega‐3 PUFAs for cognition but even the upper boundaries of the related CIs were nearly always below published estimates of an MCID. Our results on the safety and adverse effects of dietary omega‐3 PUFAs were consistent with previous findings and assumptions (EFSA 2012; Eritsland 2000; FAO 2010; Sydenham 2012). There were adverse events in the study population, but these occurred equally in the treatment and the placebo groups.
Overall completeness and applicability of evidence
All trials assessed relevant endpoints to evaluate therapeutic efficacy in people with dementia. The larger studies addressed cognitive function, ADL and global severity of dementia together (Freund‐Levi 2006; Quinn 2010), as recommended by an expert group of the European Medicines Agency (EMA 2014). However, the pilot study of Shinto 2014 addressed a surrogate parameter as the primary endpoint and the study was not designed to test efficacy in the secondary endpoints relevant for this review.
All trials included participants with diagnoses of AD of mild to moderate severity and tested an appropriate dose (according to EFSA 2010; EFSA 2012). We found no trial investigating omega‐3 PUFAs in other dosages, or type or stage of dementia. Therefore, we cannot draw conclusions on people with VaD, DLB, PDD or FTD, or more severe forms of AD. Mean values of nutritional parameters presented in the studies indicated no malnutrition or lack of DHA at baseline and none of the trials investigated relevant subgroups. Therefore, we cannot rule out that trial participants with poorer baseline nutritional status may benefit more from the intervention.
Quality of the evidence
All three studies were RCTs. By using the GRADE approach, we rated the overall quality of evidence for most outcomes as high. By the GRADE definition, this means, "we are very confident that the true effect lies close to that of the estimate of the effect" (Schünemann 2013). However, Freund‐Levi 2006 did not report if blinding was maintained long enough to blind the outcome assessor but we assumed that this was a reporting issue and judged that the possible impact on the pooled outcomes was small when combined with the larger ADCS trial (Quinn 2010). All uncertainties regarding data that arose during the review process were resolved when we contacted the study authors.
Potential biases in the review process
More than one‐quarter of all randomised participants discontinued study participation. To date, there is no optimal method to address missing data in trials on dementia. Due to the progressive course of the disease, both ITT based on the LOCF data and per‐protocol analysis based on data assessed on completers of the trials are questionable, if not inappropriate, analysis methods (EMA 2014). We addressed this potential bias in a six‐month sensitivity analysis, imputing missing data on the assumption of similarity to the data of the control group and the results remained similar. However, it is conceivable that this assumption is violated by the possibility that participants who discontinued the studies could have had even worse results than the control groups.
Agreements and disagreements with other studies or reviews
This review is in line with several other Cochrane systematic reviews investigating the effect of omega‐3 PUFAs in the prevention or therapy of neurological diseases (Dennis 2013; Irving 2006; Montgomery 2008, Sydenham 2012), where there was no evidence from RCTs for the effectiveness of omega‐3 PUFAs. This applies also for a range of other diseases affecting people of advanced age (Campbell 2013; Hartweg 2008; Hooper 2004; Lawrenson 2015).
Authors' conclusions
Implications for practice.
We found no convincing evidence for efficacy of omega‐3 polyunsaturated fatty acids (omega‐3 PUFA) supplements in the treatment of mild to moderate Alzheimer's disease (AD). This result was based on high quality evidence and was consistent across all of the outcomes relevant for people with AD. It is possible that omega‐3 PUFAs improve instrumental activities of daily living, such as more complex activities (i.e. shopping), when taken for a longer period of time, but this has to be confirmed in further trials. Adverse effects of omega‐3 PUFAs seem to be uncommon, but based on the evidence synthesised in this review, we cannot make a definite statement on the tolerability of omega‐3 PUFA supplements.
The effects on other populations of people with dementia remain unclear.
Implications for research.
Based on consistent results from high quality evidence, we do not believe any further studies investigating the same treatment regimen in people with mild to moderate AD would yield any other results related to cognition and basic function. However, it remains unclear if people with other types of dementia or differing levels of severity of dementia would benefit from omega‐3 PUFAs. This applies in particular for people with a docosahexaenoic acid (DHA) deficit. Therefore, future trials should provide pre‐specified subgroup analyses for people with malnutrition or low DHA levels.
Based on current discussions (EMA 2014; Vellas 2008), it may prove favourable to assess cognition with outcome measures more sensitive to change versus the regular scales (e.g. Harrison 2007). It can also be hypothesised that changes in instrumental activities of daily living (i.e. doing finances) are more likely to be detected in early stages of dementia. Therefore, future trials should also consider using measures for instrumental activities of daily living.
More emphasis should be placed on statistical issues because the proportion of missing data in trials investigating dementia can be high. Simple methods such as last observation carried forward are seemingly attractive for longitudinal designs, but often cause bias due to several shortcomings. Possibly the most obvious and severe being that it ignores the progressive course of dementia disease (Molnar 2008). Mixed models for repeated measures and slope‐based analyses can also overestimate the effect (EMA 2014). Both models do not account for the possibility of a less favourable course for people discontinuing the study. In a European Medicines Agency (EMA) discussion paper, several alternative choices of analyses and sensitivity analyses were suggested to accompany the primary analysis (EMA 2014). Such additional calculations can be useful to interpret the data, provided that the assumptions and methods for imputed data are described and the assumed effect and variability measures are presented. Following the suggestion of Molnar 2009, it might further support interpretability and decision making, if minimal clinically important differences of outcome measures are determined as a complementary part in randomised controlled trials investigating omega‐3 PUFAs for dementia. It can be reasonably assumed that for many people affected by cognitive decline, the trade‐off between effectiveness, adverse effects and costs of nutritional supplements differs from that of drugs prescribed for dementia.
Acknowledgements
We acknowledge the work of Anna‐Noel Storr, Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) who designed the search strategy and conducted the search. We also acknowledge Jenny McCleery (Co‐ordinating Editor of the CDCIG) for her helpful editing and her valuable methodological comments. We also thank Alessio Molfino and Philip Calder for their thorough peer reviews, Sue Marcus (Managing Editor of CDCIG) for her excellent support and Jacquelene Lyda for her proof reading.
We thank Joseph Quinn (Quinn 2010), Yvonne Freund‐Levi (Freund‐Levi 2006), and Lynne Shinto (Shinto 2014) for providing the original data as requested and for answering every question. We are also grateful to all participants of the consumer group, which helped to prioritise the outcomes for the GRADE approach.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) but searched via the offline CRS (last searched 10 December 2015) |
[Omega OR "fatty acid*" OR PUFA OR EPA OR DHA OR ALA "alpha linolenic acid*" OR "docosahexaenoic acid*" OR "docosapentanoic acid*" OR "eicosapentaenoic acid*"] AND Study Aim: Treatment Dementia AND Study design: RCT OR CCT | March 2015: 70 December 2015: 2 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946‐present (OvidSP) (last searched 10 December 2015) | 1. dement*.mp. 2. alzheimer*.mp. 3. ((cognit* adj3 impair*) or mci).mp. 4. (memory adj3 (impair* or insufficien* or episode or complain*)).mp. 5. ("functional impair*" or MFI).ab. 6. cognit* declin*.mp. 7. ("cognitive impairment no dementia" or CIND).mp. 8. exp Dementia, Vascular/ 9. "vascular dementia".mp. 10. exp Lewy Bodies/ 11. ("lewy* bod*" or DLB).mp. 12. (AAMI or AACD).mp. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp "fatty acids"/ 15. "fatty acids, omega 3"/ 16. ("fatty acid*" or fats or omega‐3).mp. 17. (PUFA* or polyunsaturated).mp. 18. (EPA or "eicosapentaenoic acid*").mp. 19. (ALA or "alpha linolenic acid*").mp. 20. (DHA or "docosahexaenoic acid*").mp. 21. (DPA or "docosapentanoic acid*").mp. 22. n‐3‐fatty‐acid*.mp. 23. ("flaxseed oil" or "linseed oil" or "fish oil*" or "salmon oil" or "cod liver oil" or "mackerel oil" or "tuna* oil" or "tuna fish oil" or "blackcurrant oil" or "canola oil" or "rapeseed oil" or "mustard oil*" or "walnut oil" or "wheat germ oil" or "dental oil*").mp. 24. 21 or 17 or 20 or 15 or 14 or 22 or 18 or 23 or 16 or 19 25. 24 and 13 26. randomized controlled trial.pt. 27. Controlled clinical trial.pt. 28. randomi?ed.ti. 29. randomi?ed.ab. 30. placebo.ab. 31. drug therapy.fs. 32. randomly.ab. 33. trial.ab. 34. groups.ab. 35. "meta analys*".ab. 36. 35 or 27 or 33 or 32 or 28 or 26 or 34 or 30 or 29 or 31 37. (animals not (humans and animals)).sh. 38. 36 not 37 39. 38 and 25 |
March 2015: 863 December 2015: 76 |
| 3. EMBASE 1974‐2015 December 09 (OvidSP) (last searched 10 December 2015) |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. CADASIL.mp. 19. "cognit* impair*".mp. 20. exp mild cognitive impairment/ 21. MCI.ti,ab. 22. ACMI.ti,ab. 23. ARCD.ti,ab. 24. SMC.ti,ab. 25. CIND.ti,ab. 26. BSF.ti,ab. 27. AAMI.ti,ab. 28. MD.ti,ab. 29. LCD.ti,ab. 30. QD.ti,ab. 31. AACD.ti,ab. 32. MNCD.ti,ab. 33. MCD.ti,ab. 34. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 35. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 36. "preclinical AD".mp. 37. "pre‐clinical AD".mp. 38. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 39. (aMCI or MCIa).ti,ab. 40. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 41. ("GDS 3" or "stage 3 GDS").ti,ab. 42. ("global deterioration scale" and "stage 3").mp. 43. "Benign senescent forgetfulness".ti,ab. 44. "mild neurocognit* disorder*".ti,ab. 45. (prodrom* adj2 dement*).ti,ab. 46. "age‐related symptom*".mp. 47. (episodic adj2 memory).mp. 48. ("pre‐clinical dementia" or "preclinical dementia").mp. 49. or/1‐48 50. ("omega 3" or "fatty acid*" or "n‐3‐fatty‐acid*").ti,ab. 51. (PUFA* or polyunsaturated).ti,ab. 52. (EPA or "eicosapentaenoic acid*").ti,ab. 53. (ALA or "alpha linolenic acid*").ti,ab. 54. (DHA or "docosahexaenoic acid*").ti,ab. 55. (DPA or "docosapentanoic acid*").ti,ab. 56. ("flaxseed oil" or "linseed oil" or "fish oil*" or "salmon oil" or "cod liver oil" or "mackerel oil" or "tuna* oil" or "tuna fish oil" or "blackcurrant oil" or "canola oil" or "rapeseed oil" or "mustard oil*" or "walnut oil" or "wheat germ oil" or "dental oil*").ti,ab. 57. exp *fatty acid/ 58. or/50‐57 59. 49 and 58 60. randomized controlled trial/ 61. controlled clinical trial/ 62. randomly.ab. 63. placebo.ab. 64. groups.ab. 65. randomi?ed.ti,ab. 66. trial.ab. 67. "double‐blind*".ti,ab. 68. or/60‐67 69. 59 and 68 |
March 2015: 889 December 2015: 128 |
| 4. PsycINFO 1806‐December week 2 2015 (OvidSP) (last searched 10 December 2015) |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. "cognit* impair*".mp. 25. MCI.ti,ab. 26. ACMI.ti,ab. 27. ARCD.ti,ab. 28. SMC.ti,ab. 29. CIND.ti,ab. 30. BSF.ti,ab. 31. AAMI.ti,ab. 32. MD.ti,ab. 33. LCD.ti,ab. 34. QD.ti,ab. 35. AACD.ti,ab. 36. MNCD.ti,ab. 37. MCD.ti,ab. 38. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 39. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 40. "preclinical AD".mp. 41. "pre‐clinical AD".mp. 42. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 43. (aMCI or MCIa).ti,ab. 44. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 45. ("GDS 3" or "stage 3 GDS").ti,ab. 46. ("global deterioration scale" and "stage 3").mp. 47. "Benign senescent forgetfulness".ti,ab. 48. "mild neurocognit* disorder*".ti,ab. 49. (prodrom* adj2 dement*).ti,ab. 50. "age‐related symptom*".mp. 51. (episodic adj2 memory).mp. 52. ("pre‐clinical dementia" or "preclinical dementia").mp. 53. or/1‐52 54. exp Fatty Acids/ 55. ("fatty acid*" or fats or omega‐3).mp. 56. ("flaxseed oil" or "linseed oil" or "fish oil*" or "salmon oil" or "cod liver oil" or "mackerel oil" or "tuna* oil" or "tuna fish oil" or "blackcurrant oil" or "canola oil" or "rapeseed oil" or "mustard oil*" or "walnut oil" or "wheat germ oil" or "dental oil*").mp. 57. n‐3‐fatty‐acid*.mp. 58. (PUFA* or polyunsaturated).mp. 59. (EPA or "eicosapentaenoic acid*").mp. 60. (ALA or "alpha linolenic acid*").mp. 61. (DHA or "docosahexaenoic acid*").mp. 62. (DPA or "docosapentanoic acid*").mp. 63. or/54‐62 64. 53 and 63 65. random*.ti,ab. 66. placebo.ti,ab. 67. trial.mp. 68. ("double‐blind*" or "single‐blind*").ti,ab. 69. groups.ab. 70. crossover.ti,ab. 71. "cross‐over".ti,ab. 72. or/65‐71 73. 64 and 72 |
March 2015: 175 December 2015: 19 |
| 5. CINAHL (EBSCOhost) (last searched 10 December 2015) |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 TX MCI OR CIND OR AAMI OR AACD S20 TX "cognit* impair*" S21 (MH "Cognition Disorders") S22 TX "pre‐clinical alzheimer*" OR "pre‐clinical AD" S23 TX "N‐MCI" OR "A‐MCI" OR "M‐MCI" S24 TX aMCI OR nMCI OR mMCI S25 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S26 (MH "Fatty Acids, Omega 3") OR (MM "alpha‐Linolenic Acid") OR (MM "Docosahexaenoic Acids") OR (MM "Eicosapentaenoic Acid") S27 (MH "Linseed Oil") OR (MH "Margarine") OR (MH "Olive Oil") OR (MH "Peanut Oil") OR (MH "Rapeseed Oil") OR (MH "Safflower Oil") OR (MH "Sesame Oil") OR (MH "Soybean Oil") S28 TX "fatty acid*" OR fats OR "omega‐3" S29 TX "flaxseed oil" OR "linseed oil" OR "fish oil*" OR "salmon oil" OR "cod liver oil" OR "mackerel oil" OR "tuna* oil" OR "tuna fish oil" OR "blackcurrant oil" OR "canola oil" OR "rapeseed oil" OR "mustard oil*" OR "walnut oil" OR "wheat germ oil" OR "dental oil*" S30 TX n‐3‐fatty‐acid* S31 TX PUFA* OR polyunsaturated S32 TX EPA OR "eicosapentaenoic acid*" S33 TX ALA OR "alpha linolenic acid*" S34 TX DHA OR "docosahexaenoic acid*" S35 TX DPA OR "docosapentanoic acid*" S36 S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 S37 S25 and S36 S38 AB random* S39 TI random* S40 TI placebo* S41 AB placebo* S42 AB trial S43 (MH "Clinical Trials") OR (MH "Randomized Controlled Trials") S44 AB groups S45 AB "double‐blind*" S46 S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 S47 S37 and S46 |
March 2015: 68 December 2015: 14 |
| 6. ISI Web of Knowledge ‐ all databases (includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports) (last searched 10 December 2015) |
(dement* OR alzheimer* OR "lewy bod*" OR DLB OR "vascular cognitive impairment*" OR FTD OF FTLD OR "cerebrovascular insufficienc*") AND TOPIC: (omega OR "fatty acid*" OR ALA OR "alpha linolenic acid*" OR PUFA* or polyunsaturated OR EPA or "eicosapentaenoic acid*" OR "flaxseed oil" OR "linseed oil" OR "fish oil*" OR "salmon oil" OR "cod liver oil" OR "mackerel oil" OR "tuna* oil" OR "tuna fish oil" OR "blackcurrant oil" OR "canola oil" OR "rapeseed oil" OR "mustard oil*" OR "walnut oil" OR "wheat germ oil" OR "dental oil*") AND TOPIC: (randomly OR randomised OR randomized OR placebo OR "double‐blind*" OR trial OR RCT OR CCT) Timespan: All years Search language=Auto |
March 2015: 513 December 2015: 116 |
| 7. LILACS (BIREME) (last searched 10 December 2015) |
Omega‐3 OR PUFA OR polyunsaturated OR EPA OR DHA OR "poli‐insaturados" OR "ômega‐3" [Words] and dementia OR demencia OR alzheimer OR alzheimers [Words] | March 2015: 7 December 2015: 0 |
| 8. The Cochrane Central Register of Controlled Trials (CENTRAL) (2015 Issue 11 of 12) (last searched 10 December 2015) |
#1 MeSH descriptor: [Dementia] explode all trees #2 MeSH descriptor: [Delirium] this term only #3 MeSH descriptor: [Wernicke Encephalopathy] this term only #4 MeSH descriptor: [Delirium, Dementia, Amnestic, Cognitive Disorders] this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 MCI or "cognit* impair*" or AAMI or "memory impair*" or "cognit* declin*" or AACD #22 #20 or #21 in Trials #23 "omega 3" or "fatty acid*" or PUFA or EPA or ALA or DHA or DPA #24 "eicosapentaenoic acid*" or "alpha linolenic acid*" or "docosahexaenoic acid*" or "docosapentanoic acid*" #25 "n‐3‐fatty‐acid*" or polyunsaturated or "flaxseed oil" or "linseed oil" or "fish oil*" or "salmon oil" or "cod liver oil" or "mackerel oil" or "tuna* oil" or "tuna fish oil" or "blackcurrant oil" or "canola oil" or "rapeseed oil" or "mustard oil*" or "walnut oil" or "wheat germ oil" or "dental oil*" #26 MeSH descriptor: [Fatty Acids] this term only #27 #23 or #24 or #25 or #26 in Trials #28 #22 and #27 in Trials |
March 2015: 159 December 2015: 18 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) (last searched 10 December 2015) |
(dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy) AND (omega OR PUFA OR EPA OR DHA OR "fatty acid" OR "fatty acids" OR polyunsaturated) | March 2015: 14 December 2015: 5 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry ‐ India; Clinical Research Information Service ‐ Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] (last searched 10 December 2015) |
(dementia OR alzheimer OR alzheimers OR alzheimer's OR lewy) AND (omega OR PUFA OR EPA OR DHA OR "fatty acid" OR "fatty acids" OR polyunsaturated) | March 2015: 3 December 2015: 0 |
| TOTAL before de‐duplication | March 2015: 2761 December 2015: 303 |
|
| TOTAL after software de‐duplication | March 2015: 1992 December 2015: 239 |
|
Data and analyses
Comparison 1. Omega‐3 PUFAs versus placebo.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Freund‐Levi 2006.
| Methods | Randomised, double‐blind, placebo‐controlled trial; cross‐over design (second sequence not included in this review); trial duration from December 2000 to March 2004) | |
| Participants | Country: Sweden Diagnosis: AD Follow‐up (first sequence): 6 months Inclusion criteria: AD according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria; MMSE‐15 score 15‐30 points, person living in his or her own home; treatment with a stable dose of acetylcholinesterase inhibitors for ≥ 3 months before the start of the study; and plan to continue acetylcholine esterase inhibitors for the duration of the study Exclusion criteria: people were excluded if treated with non‐steroidal anti‐inflammatory drugs (low‐dose aspirin (acetylsalicylic acid) was accepted), omega‐3 preparations or anticoagulant agents; alcohol abuse; had a concomitant serious disease or did not have a carer Total number of participants: 204 (103 in omega‐3 group, 101 in placebo group) Per‐protocol population: 178 (91 in omega‐3 group, 87 in placebo group) Baseline characteristics:
*relative amount in percentage of all fatty acids analysed in total plasma |
|
| Interventions | Intervention 1: omega‐3 PUFA capsules 1 g containing DHA 430 mg and EPA 150 mg and vitamin E 4 mg, 4 capsules/day, total daily dose of DHA 1.7 g and EPA 0.6 g Intervention 2: placebo containing isocaloric placebo oil (corn oil 1 g, including linoleic acid 0.6 g) and vitamin E 4 mg Treatment duration (first part of cross‐over trial): 6 months |
|
| Outcomes | Primary:
Secondary:
Data of second part of cross‐over trial, carer burden, anthropometry and biochemical outcomes, and blood pressure not included in this review |
|
| Notes | Authors stated: "In the intention‐to‐treat analyses, the last observation was carried forward to the subsequent registration. Since no differences in outcomes between the two methods were found, we have chosen to show these data using the per‐protocol mode" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Faxen Irving, 2009: "Patients were randomized in blocks of four, using sealed envelopes and according to a computerized table of random numbers, to receive four 1 g capsules daily, each containing 430 mg DHA and 150mg EPA [...] or an isocaloric placebo oil (containing 1 g of corn oil, including 0.6 g of linoleic acid)..." p. 12 |
| Allocation concealment (selection bias) | Low risk | Faxen Irving, 2009: "Patients were randomized in blocks of four, using sealed envelopes and according to a computerized table of random numbers, to receive four 1 g capsules daily, each containing 430 mg DHA and 150mg EPA [...] or an isocaloric placebo oil (containing 1 g of corn oil, including 0.6 g of linoleic acid)..." p. 12 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules filled with either verum or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as 'double blind' but authors did not describe how long blinding was maintained or if outcome assessors were blinded too. Overestimation of effects possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs equal in both groups Both ITT using LOCF and per‐protocol analyses performed, no significant differences detected when analysed with the 2 methods |
| Selective reporting (reporting bias) | Unclear risk | Data to both primary outcomes described as in study protocol planned. Relevant adverse effects mentioned but not described which group. This might favour omega. However, authors reported, "the Omega 3 fatty acid preparation was well tolerated and safe" and drop‐outs are equally distributed |
| Other bias | Low risk | The OmegAD study was initially partly funded by Pronova Biocare A/S, Lysaker, Norway. Industry was involved in planning phase and the decision of submitting the publication, not in collection, analysis or interpretation of data |
Quinn 2010.
| Methods | Randomised, double‐blind, placebo‐controlled trial; trial duration from February 2007 to May 2009 | |
| Participants | Country: USA Diagnosis: probable AD Follow‐up: 18 months Inclusion criteria: probable AD (according to trial protocol mild to moderate AD, aged ≥ 50 years and neuroimaging consistent with the diagnosis of AD at some time after the onset of the memory decline), MMSE 14‐26, medically stable, mean consumption of DHA ≤ 200 mg/day (assessed by a brief 7‐item food frequency questionnaire), no consumption of DHA or omega‐3 fatty acid supplements Exclusion criteria: intake of central anticholinergic effects or sedatives or people who received investigational treatment for AD Stable use (≥ 3 months) of cholinesterase inhibitors or memantine was permitted Total number of participants: 402 ITT population: 402 (238 in omega‐3 PUFA group, 164 in placebo group) Baseline characteristics:
*relative amount in percentage of all fatty acids analysed in total plasma |
|
| Interventions | Intervention 1: algal‐derived DHA capsules, 1 g twice per day, total daily dose DHA approximately 900‐1100 mg. Martek Biosciences, Columbia, Maryland, Algal DHA contains approximately 45‐55% of DHA by weight and does not contain EPA Intervention 2: placebo capsules (made up of corn or soy oil), identical in appearance Treatment duration: 18 months |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Disproportionate enrolment in groups (60% omega, 40% placebo) was intended to enhance recruitment Statistical analysis by linear mixed‐effects model with baseline MMSE score as covariate. Unpublished 6‐month results provided by personal communication by Dr. Quinn (Table 5) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was achieved with a centralized interactive voice response system, using a block design with a block size of 5 (3 in the DHA group and 2 in the placebo group)" p. 3 |
| Allocation concealment (selection bias) | Low risk | "Randomization was achieved with a centralized interactive voice response system, using a block design with a block size of 5 (3 in the DHA group and 2 in the placebo group)" p. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo identical in appearance. "When asked to guess treatment assignment for each participant at the final study visit, the majority of study partners (48.5%), study coordinators (50%), and site physicians (59.2%) responded "do not know" p. 7 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo identical in appearance. "When asked to guess treatment assignment for each participant at the final study visit, the majority of study partners (48.5%), study coordinators (50%), and site physicians (59.2%) responded "do not know" p. 7 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After 6 months approximately 10% drop‐outs in both groups. Reasons for drop‐outs at 18 months described. Missing data additionally considered by mixed‐effects models Comment: higher drop‐outs and unequal distribution at 18 months considered in GRADE as limitations for quality of life outcomes. Distribution of drop‐outs similar for all other outcomes used in this review |
| Selective reporting (reporting bias) | Low risk | Primary outcomes and secondary outcomes that were defined in study protocol were assessed and reported. Quality of life measures not published but provided by Dr. Quinn (personal communication) |
| Other bias | Low risk | 2 employees of a DHA manufacturer were involved in the planning, conducting and reporting of the trial, 1 of them also in analysis and interpretation of data. Authors explicitly stated that, "Martek employees did not participate in the statistical analysis and did not have access to the data prior to the completion of data." 2 authors named as co‐inventors on a patent for DHA for the treatment of AD in apolipoprotein E ϵ4‐negative people but have waived personal rights to royalties related to this patent The study was otherwise supervised: "The National Institute of aging (NIA) approved the study design, its representatives participated in meetings of the steering committee of the Alzheimer's Disease Cooperative Study [...]" p. 9 No relevant baseline imbalance and free of early stopping |
Shinto 2014.
| Methods | 3‐arm (omega‐3; placebo, parallel group, alpha lipoic acid) placebo‐controlled, double‐blind, randomised controlled trial The arm with alpha lipoic acid was not included in this review; trial duration from April 2004 to December 2009. |
|
| Participants | Country: USA Diagnosis: probable AD (NINCDS‐ADRDA criteria) Follow‐up: 12 months Inclusion criteria: probable AD; aged ≥ 55 years, MMSE score 15‐26, Clinical Dementia Rating Scale 0.5‐1.0, not depressed (Center for Epidemiological Studies of Depression Score < 4.0) Exclusion criteria: non‐AD dementia; residence at long‐term care facility at screening visit; history of clinically significant stroke; health conditions such as cancer (prostate cancer Gleason grade < 3 and non‐metastatic cancers were acceptable), liver disease, history of ventricular fibrillation or ventricular tachycardia, major psychiatric disorder, major central nervous system diseases (e.g. brain tumour, seizure disorder); taking lipid‐lowering medication; hyperlipidaemia (triglycerides > 500 mg/dL, low‐density lipoprotein > 160 mg/dL, total cholesterol > 240 mg/dL); fish oil or cod liver oil supplementation within 30 days of enrolment; > 1 x 6 ounce (150 g) serving per week of fish or seafood within 30 days of enrolment; lipoic acid supplementation within 30 days of enrolment; taking systemic corticosteroids, neuroleptics, antiparkinsonian agents or narcotic analgesics Acetylcholinesterase inhibitors, memantine, vitamin E and ginkgo biloba were allowed if stable for 4 months prior to study enrolment Total number of participants: 39 (13 in omega‐3 PUFA group, 13 in alpha lipoic acid group, 13 in placebo group) Per‐protocol population: 34 (11 in omega‐3 PUFA group, 12 in alpha lipoic acid group, 11 in placebo group) Baseline characteristics:
|
|
| Interventions | Intervention 1: 1 placebo tablet (replacing alpha lipoic acid) in the morning, 2 placebo capsules (replacing omega‐3 PUFA) in the morning and 1 in the afternoon with food. Placebo for omega‐3 contained soybean oil with 5% fish oil and lemon flavour Intervention 2: omega‐3 PUFA capsules (fish oil concentrate in the triglyceride form at 3 g/day, daily dose of DHA 675 mg and EPA 975 mg, flavoured with lemon), 2 capsules in the morning with food, 1 capsule in the evening with food. 1 placebo tablet (replacing alpha lipoic acid) was additionally given in the morning Intervention 3: alpha lipoic acid 600 mg/day in 1 tablet and 2 omega‐3 capsules in the morning with food, 1 omega‐3 capsule in the afternoon with food (daily dose of DHA 675 mg and EPA 975 mg) Treatment duration: 12 months Only data of intervention 2 (omega‐3 PUFA) and intervention 1 (placebo) was included in this review |
|
| Outcomes | Primary:
Secondary:
The primary outcome of the study was not included in this review (surrogate outcome). Adverse effects reported, but not assessed as outcome |
|
| Notes | Study registration number on ClincalTrials.gov, NCT00090402 The research was supported by the National Institutes of Health/National Institute of Aging (NIH/NIA) R21AG023805, NIH/NIA AG08017 and NIH General Clinical Research Grant M01RR00334. Nordic Natural, Watsonville, CA, USA, supplied the fish oil and placebo oil. There was no visible influence by industry in the planning phase, conducting phase or analysing process Statistical analysis by linear mixed‐effects model adjusted for age and education |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised by a computer generated scheme that was stratified by smoking status (current smoker versus nonsmoker) [...]" p. 3 |
| Allocation concealment (selection bias) | Low risk | "Participants were randomized by a computer generated scheme that was stratified by smoking status (current smoker versus nonsmoker) as this would have the greatest impact on the primary outcome" p. 3 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The study assessed the maintenance of blinding over 12 months by asking the participant's study partner, the participant, and all research staff involved in administering outcome measures about knowledge of group assignment at 12 months" p. 4 "When asked about treatment assignment at the end of the study, the majority reported no knowledge of treatment assignment: research staff (100%), AD participant (84%), participant study partner (81%)" p. 5 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study assessed the maintenance of blinding over 12 months by "asking […] all research staff involved in administering outcome measures about knowledge of group assignment at 12 months" p. 4 "When asked about treatment assignment at the end of the study, the majority reported no knowledge of treatment assignment: research staff (100%), AD participant (84%), participant study partner (81%)" p. 5 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs equally distributed in omega‐3 (1 death, 1 moved) and placebo group (1 death, 1 discarded). Missing data considered by mixed‐effects models |
| Selective reporting (reporting bias) | Low risk | Outcomes were congruent with trials protocol |
| Other bias | Low risk | Small baseline imbalance but we did not judge it relevant for this review Second author was named in Quinn 2010 as co‐inventor on a patent for DHA for the treatment of AD but waived rights to royalties related to this patent |
AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living; ADL: activities of daily living; ADRDA: Alzheimer's Disease and Related Disorders Association; CDR‐SOB: Clinical Dementia Rating ‐ Sum of Boxes; DAD: Disability Assessment for Dementia; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; IADL: instrumental activities of daily living; ITT: intention to treat; LOCF: last observation carried forward; MADRS: Montgomery‐Åsberg Depression rating scale; MMSE: Mini‐Mental State Examination; NINCDS: National Institute of Neurological and Communicative Disorders and Stroke; NPI: Neuropsychiatric Inventory; OARS‐ADL: Older Americans Resources and Services ‐ Activities of Daily Living; OARS‐IADL: Older Americans Resources and Services ‐ Instrumental Activities of Daily Living; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carter 2006 | According to information of the North East London NHS Foundation Trust (UK), the trial was not completed due to non‐significant results and low numbers recruited |
| Chiu 2008 | RCT investigated omega‐3 fatty acids on people with mild cognitive impairment and AD but duration of intervention was only 24 weeks |
| Corrigan 1991 | RCT investigated omega‐6 fatty acids |
| Hashimoto 2011 | Conference abstract. Refers to the trial published in Hashimoto 2012 |
| Hashimoto 2012 | RCT investigating omega‐3 PUFAs in healthy participants. Excluded "[...] neurological disorder that could produce cognitive deterioration, including AD [...]" |
| Mahmoudi 2014 | Included "normal cognitive elderly accompanied by mild to moderate cognitive impaired participants." No diagnose of dementia |
| NCT00867828 | According to CinicalTrials.gov registry, the treatment duration was planned for 24 weeks. The study was completed at 1 January 2011. The responsible company NeuroBioPharm Inc. announced that it was not possible to share any information on the trial |
| Terano 1999 | Not an RCT |
AD: Alzheimer's disease; PUFA: polyunsaturated fatty acid; RCT: randomised controlled trial.
Differences between protocol and review
For a better interpretation of the results, we intended to present the proportion of people with changes in the scale measures of the primary outcomes. However, considering the small insignificant effects, we did not request that data from the study authors. Instead, we provided minimal clinically important differences extracted from the literature.
Contributions of authors
MB: correspondence; project management, drafting review versions; selection of randomised controlled trials (RCTs); extraction of data; assessing risk of bias; data entry, data analysis; GRADE; interpretation of data/analyses.
MH: selection of RCTs; extraction of data; assessing risk of bias data entry, data analysis; interpretation of data/analyses.
AF: selection of RCTs (update), adverse events section, interpretation of data/analyses.
GL: GRADE; interpretation of data/analyses.
TW: description of condition chapter; prioritisation of outcomes study.
SW: description of condition chapter; prioritisation of outcomes study.
TW and SW provided the description of the condition, which MB, MH, AF and GL complemented and commented on.
MB wrote the remaining sections of the review, which were complemented and commented by all authors.
Sources of support
Internal sources
-
Roux Program of Martin Luther University Halle‐Wittenberg, medical faculty, Germany.
University grant
External sources
-
NIHR, UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
None known.
New
References
References to studies included in this review
Freund‐Levi 2006 {published data only (unpublished sought but not used)}
- Eriksdotter M, Falahati F, Vedin I, Freund‐Levi Y, Hjorth E, Faxen‐Irving G, et al. Effects of omega‐3 fatty acid supplementation on plasma fatty acids, gender effects, and effects on cognition in Alzheimer's patients: the OmegAD study. Alzheimer's & Dementia 2014;40(4 Suppl):P455. [Google Scholar]
- Eriksdotter M, Vedin I, Falahati F, Freund‐Levi Y, Hjorth E, Faxen‐Irving G, et al. Plasma fatty acid profiles in relation to cognition and gender in Alzheimer's disease patients during oral omega‐3 fatty acid supplementation: the OmegAD study. Journal of Alzheimer's Disease 2015;48(3):805‐12. [DOI] [PubMed] [Google Scholar]
- Faxen‐Irving G, Freund‐Levi Y, Eriksdotter‐Jonhagen M, Basun H, Brismar K, Hjorth E, et al. Omega‐3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer's disease: the Omega‐3 Alzheimer's Disease study. Journal of the American Geriatrics Society 2009;57:11‐7. [DOI] [PubMed] [Google Scholar]
- Faxen‐Irving G, Freund‐Levi Y, Eriksdotter‐Jonhagen M, Basun H, Hjorth E, Palmblad J, et al. Effects on transthyretin in plasma and cerebrospinal fluid by DHA‐rich n ‐ 3 fatty acid supplementation in patients with Alzheimer's disease: the OmegAD study. Journal of Alzheimer's disease 2013;36:1‐6. [DOI] [PubMed] [Google Scholar]
- Freund‐Levi Y, Basun H, Cederholm T, Faxen‐Irving G, Garlind A, Grut M, et al. Omega‐3 supplementation in mild to moderate Alzheimer's disease: effects on neuropsychiatric symptoms. International Journal of Geriatric Psychiatry 2008;23:161‐9. [DOI] [PubMed] [Google Scholar]
- Freund‐Levi Y, Cederholm T, Basu S. Oxidative stress and cox‐mediated inflammation in patients with Alzheimer's disease and effects of N‐3 fatty acid supplementation the omegAD study. Clinical Nutrition 2011;Suppl:9. [Google Scholar]
- Freund‐Levi Y, Hjorth E, Lindberg C, Cederholm T, Faxen‐Irving G, Vedin I, et al. Effects of omega‐3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer's disease: the OmegAD study. Dementia and Geriatric Cognitive Disorders 2009;27:481‐90. [DOI] [PubMed] [Google Scholar]
- Freund‐Levi Y, Vedin I, Cederholm T, Basun H, Faxen Irving G, Eriksdotter Jonhagen M, et al. Effects of a DHA rich omega‐3 fatty acid supplementation for Alzheimer disease patients on fatty acid composition in cerebrospinal fluid, disease biomarkers and cognition: the OmegAD study. Journal of Molecular Neuroscience 2012;48:S36. [Google Scholar]
- Freund‐Levi Y, Vedin I, Cederholm T, Basun H, Faxen Irving G, Eriksdotter M, et al. Transfer of omega‐3 fatty acids across the blood‐brain barrier after dietary supplementation with a docosahexaenoic acid‐rich omega‐3 fatty acid preparation in patients with Alzheimer's disease: the OmegAD study. Journal of Internal Medicine 2014;275:428‐36. [DOI] [PubMed] [Google Scholar]
- Freund‐Levi Y, Vedin I, Hjorth E, Basun H, Irving GF, Schultzberg M, et al. Effects of supplementation with omega‐3 fatty acids on oxidative stress and inflammation in patients with Alzheimer's disease: the OmegAD study. Journal of Alzheimer's Disease 2014;42:823‐31. [DOI] [PubMed] [Google Scholar]
- Freund‐Levi, Y, Eriksdotter‐Jonhagen, M, Cederholm, T, Basun, H, Faxen‐Irving, G, Garlind, et al. Omega‐3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study ‐ a randomized double‐blind trial. Archives of Neurology 2006; Vol. 63, issue 10:1402‐8. [DOI] [PubMed]
- Irving GF, Freund‐Levi Y, Eriksdotter‐Jonhagen M, Basun H, Brismar K, Hjorth E, et al. Omega‐3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer's disease: the omega‐3 Alzheimer's disease study [erratum appears in Journal of the American Geriatrics Society 2009;57(3):579]. Journal of the American Geriatrics Society 2009;57:11‐7. [DOI] [PubMed] [Google Scholar]
- Irving GF, Freund‐Levi Y, Eriksdotter‐Jonhagen M, Basun H, Palmblad J, Vedin I, et al. N‐3 fatty acid treatment and plasma transthyretin in patients with Alzheimer's disease. FASEB Journal 2009;23:Suppl 543.10. [Google Scholar]
- Levi Freund Y, Vedin I, Cederholm T, Basun H, Irving Faxen G, Jönhagen Eriksdotter M, et al. Effects of a DHA rich omega‐3 fatty acid supplementation for Alzheimer disease patients on fatty acid composition in cerebrospinal fluid, disease biomarkers and cognition: the OmegAD study. Journal of Molecular Neuroscience 2012;48:S36. [Google Scholar]
- Palmblad J. Effects of a DHA rich omega‐3 fatty acid supplementation on fatty acid composition in cerebrospinal fluid and relation to Alzheimer disease. The OmegAD study. Clinical Nutrition 2011;Suppl:117‐8. [Google Scholar]
- Vedin I, Cederholm T, Freund Levi Y, Basun H, Garlind A, Irving GF, et al. Effects of docosahexaenoic acid‐rich n‐3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. American Journal of Clinical Nutrition 2008;87:1616‐22. [DOI] [PubMed] [Google Scholar]
- Vedin I, Cederholm T, Freund‐Levi Y, Basun H, Garlind A, Irving GF, et al. Effects of DHA‐rich n‐3 fatty acid supplementation on gene expression in blood mononuclear leukocytes: the OmegAD study. PLoS One 2012;7:e35425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedin I, Cederholm T, Freund‐Levi Y, Basun H, Hjorth E, Irving GF, et al. Reduced prostaglandin F‐2 alpha release from blood mononuclear leukocytes after oral supplementation of omega 3 fatty acids: the OmegAD study. Journal of Lipid Research 2010;51:1179‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedin I, Freund Levi Y, Cederholm T, Basun H, Hjorth E, Irving GF, et al. Effects of a DHA rich n‐3 fatty acid supplementation on fatty acid composition in cerebrospinal fluid in Alzheimer disease. The OmegAD study. FASEB Journal 2013;27:lb343. [DOI] [PubMed] [Google Scholar]
- Wang X, Hjorth E, Vedin I, Eriksdotter M, Freund‐Levi Y, Wahlund LO, et al. Effects of n‐3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: the OmegAD study. Journal of Lipid Research 2015;56(3):674‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 2010 {published and unpublished data}
- Quinn J. Alzheimer's Disease Cooperative Study DHA primary analysis. Individual patient data (as supplied 22 May 2015). Data on file.
- Quinn J. Effects of docosahexaenoic acid (DHA) in slowing the progression of Alzheimer's disease. Neuropsychopharmacology 2006;31:S1‐69. [Google Scholar]
- Quinn J, Shinto L, Yurko‐Mauro K, Galasko D, Aisen P, Montine T. Biomarkers of fatty acid oxidation in a clinical trial of DHA for Alzheimer's disease. Alzheimer's & Dementia 2012;8(4):P585. [Google Scholar]
- Quinn JF, Raman R, Thomas RG, Yurko‐Mauro K, Nelson EB, Dyck C, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 2010;304(17):1903‐11. [DOI: 10.1001/jama.2010.1510] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinto 2014 {published and unpublished data}
- Shinto L. Unpublished six months results. Individual patient data (as supplied 16 July 2015). Data on file.
- Shinto L, Quinn J, Montine T, Dodge HH, Woodwarda W, Baldauf‐Wagnera S, et al. A randomized placebo‐controlled pilot trial of omega‐3 fatty acids and alpha lipoic acid in Alzheimer's disease. Journal of Alzheimers Disease 2014; Vol. 38, issue 1. [DOI] [PMC free article] [PubMed]
References to studies excluded from this review
Carter 2006 {published data only}
- Carter J. A randomised placebo‐controlled trial of polyunsaturated omega‐3 fatty acid (PFA), in the treatment of dementia; a pilot study, 2006. www.isrctn.com/ISRCTN27372325 (accessed 31 March 2016).
Chiu 2008 {published data only}
- Chiu C‐C, Su K‐P, Cheng T‐C, Liu H‐C, Chang C‐J, Dewey ME, et al. The effects of omega‐3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double‐blind placebo‐controlled study. Progress in Neuro‐psychopharmacology & Biological Psychiatry 2008;32:1538‐44. [DOI] [PubMed] [Google Scholar]
Corrigan 1991 {published data only}
- Corrigan FM, Rhijn A, Horrobin DF. Essential fatty acids in Alzheimer's disease. Annals of the New York Academy of Sciences 1991;640:250‐2. [DOI] [PubMed] [Google Scholar]
Hashimoto 2011 {published data only}
- Hashimoto M, Yamashita K, Kato S, Tamai T, Matsumoto I, Tanabe Y, et al. Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function in elderly people with very mild dementia in Japan. Alzheimer's & Dementia 2011;7(4 Suppl):S610‐1. [Google Scholar]
Hashimoto 2012 {published data only}
- Hashimoto M, Yamashita K, Kato S, Tamai T, Mitarai M, Matsumoto I, et al. Beneficial effects of daily dietary omega‐3 polyunsaturated fatty acid supplementation on age‐related cognitive decline in elderly Japanese with very mild dementia: a 2‐year randomized, double‐blind, placebo‐controlled trial. Journal of Aging Research & Clinical Practice 2012;1(3):193‐201. [Google Scholar]
Mahmoudi 2014 {published data only}
- Mahmoudi MJ, Hedayat M, Sharifi F, Mirarefin M, Nazari N, Mehrdad N, et al. Effect of low dose ‐3 poly unsaturated fatty acids on cognitive status among older people: a double‐blind randomized placebo‐controlled study. Journal of Diabetes & Metabolic Disorders 2014;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00867828 {published data only}
- NCT00867828. Multi‐center, double‐blind, placebo‐controlled, monotherapy study of Neptune Krill Oil (NKO™) in early stage Alzheimer's disease, 2009. clinicaltrials.gov/show/NCT00867828 (accessed 31 March 2016).
Terano 1999 {published data only}
- Terano T, Fujishiro S, Ban T, Yamamoto K, Tanaka T, Noguchi Y, et al. Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases. Lipids 1999;34 Suppl:S345‐6. [DOI] [PubMed] [Google Scholar]
Additional references
APA 1987
- American Psychiatric Association. Diagnostic and StatisticalManual of Mental Disorders [American Psychiatric Association, 1987.]. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington D.C.: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington D.C.: American Psychiatric Association, 1994. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
Aranceta 2012
- Aranceta J, Perez‐Rodrigo C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: a systematic review. British Journal of Nutrition 2012;107 Suppl 2:S8‐22. [DOI] [PubMed] [Google Scholar]
Arterburn 2006
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n‐3 fatty acids in humans. American Journal of Clinical Nutrition 2006;83:1467S‐76S. [DOI] [PubMed] [Google Scholar]
Bailey 2013
- Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA International Medicine 2013;173:355‐61. [DOI] [PubMed] [Google Scholar]
Barnard 2014
- Barnard ND, Bush AI, Ceccarelli A, Cooper J, Jager CA, Erickson KI, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiology of Aging 2014;35:S74‐8. [DOI] [PubMed] [Google Scholar]
Bråne 2001
- Bråne G, Gottfries CG, Winblad B. The Gottfries‐Bråne‐Steen scale: validity, reliability and application in anti‐dementia drug trials. Dementia and Geriatric Cognitive Disorders 2001;12:1‐14. [DOI] [PubMed] [Google Scholar]
Burback 1999
- Burback D, Molnar FJ, John P, Man‐Son‐Hing M. Key methodological features of randomized controlled trials of Alzheimer's disease therapy. Dementia and Geriatric Cognitive Disorders 1999;10:534‐40. [DOI] [PubMed] [Google Scholar]
Burckhardt 2015
- Burckhardt M, Herke M, Wustmann T, Watzke S, Langer G, Fink A. Omega 3 fatty acids for the treatment of dementia. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009002.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2013
- Campbell A, Price J, Hiatt WR. Omega‐3 fatty acids for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003833.pub4] [DOI] [PubMed] [Google Scholar]
Cansev 2008
- Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimer's & Dementia 2008;4:S153‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 2009
- Cole GM, Ma QL, Frautschy SA. Omega‐3 fatty acids and dementia. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2009;81:213‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi D A, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308‐14. [DOI] [PubMed] [Google Scholar]
Cummings 1997
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997;48(5 Suppl 6):S10‐6. [DOI] [PubMed] [Google Scholar]
Cummings 2015
- Cummings J. Neuropsychiatric Inventory (NPI) setting the standard for Alzheimer research: FAQs. npitest.net/faqs.html (accessed 31 March 2016).
Dennis 2013
- Dennis CL, Dowswell T. Interventions (other than pharmacological, psychosocial or psychological) for treating antenatal depression. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2013; Vol. 7. [DOI: 10.1002/14651858.CD006795.pub3] [DOI] [PMC free article] [PubMed]
Dickinson 2014
- Dickinson A, Blatman J, El‐Dash N, Franco JC. Consumer usage and reasons for using dietary supplements: report of a series of surveys. Journal of the American College of Nutrition 2014;33:176‐82. [DOI] [PubMed] [Google Scholar]
Duru 2008
- Duru G, Fantino B. The clinical relevance of changes in the Montgomery‐Åsberg Depression Rating Scale using the minimum clinically important difference approach. Current Medical Research and Opinion 2008;24(5):1329‐35. [DOI: 10.1185/030079908X291958] [DOI] [PubMed] [Google Scholar]
Dysken 2014
- Dysken MW, Sano M, Asthana S, Vertrees J E, Pallaki M, Llorente M, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM‐AD VA cooperative randomized trial. JAMA 2014;311(1):33‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
EFSA 2010
- EFSA Panel on Dietetic Products, Nutrition, Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal 2010;8(3):1461. [DOI: 10.2903/j.efsa.2010.1461] [DOI] [Google Scholar]
EFSA 2012
- European Food Safety Authority (EFSA). Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA Journal 2012;10(7):2815. [DOI: 10.2903/j.efsa.2012.2815] [DOI] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2014
- European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Discussion paper on the clinical investigation of medicines for the treatment of Alzheimer's disease and other dementias. EMA/CHMP/539931/2014 Corr. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176827.pdf (accessed 23 October 2014).
EndNote 2011 [Computer program]
- Thomson Reuters. EndNote X5. Thomson Reuters, 2011.
Eritsland 2000
- Eritsland J. Safety considerations of polyunsaturated fatty acids. American Journal of Clinical Nutrition 2000;71(Suppl):197S‐201S. [DOI] [PubMed] [Google Scholar]
FAO 2010
- Food, Agriculture Organization of the United Nations (FAO). Fats and fatty acids in human nutrition. Report of an expert consultation. 10‐14 November 2008, Geneva. FAO Food and Nutrition Paper 91. Rome, 2010:188. [ISBN 978‐92‐5‐106733‐8] [PubMed]
Fillenbaum 1975
- Fillenbaum GG. OARS multidimensional functional assessment questionnaire complete activities of daily living section (ADL, IADL, Summary Scale), 1975. www.dementia‐assessment.com.au/symptoms/oars_adl_iadl.pdf (accessed 31 March 2016).
Fillenbaum 1981
- Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journal of Gerontology 1981;36(4):428‐34. [PUBMED: 7252074] [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease & Associated Disorders 1997;11(Suppl 2):33‐9. [PubMed] [Google Scholar]
Gelinas 1999
- Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the Disability Assessment for Dementia. American Journal of Occupational Therapy 1999;53(5):471‐81. [DOI] [PubMed] [Google Scholar]
George 1985
- George LK, Fillenbaum GG. OARS methodology. A decade of experience in geriatric assessment. Journal of the American Geriatrics Society 1985;33(9):607‐15. [PUBMED: 4031339] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [DOI] [PubMed] [Google Scholar]
Harrison 2007
- Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Archives of Neurology 2007;64(9):1323‐9. [DOI] [PubMed] [Google Scholar]
Hartweg 2008
- Hartweg J, Perera R, Montori VM, Dinneen SF, Hawn AN, Farmer AJ. Omega‐3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003205.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hensel 2007
- Hensel A, Angermeyer MC, Riedel‐Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini‐Mental State Examination. Journal of Neurology, Neurosurgery and Psychiatry 2007;78(12):1298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hooper 2004
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2011
- Howard R, Phillips P, Johnson T, O'Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. International Journal of Geriatric Psychiatry 2011;26(8):812‐7. [DOI] [PubMed] [Google Scholar]
Hulstaert 2009
- Hulstaert F, Thiry N, Eyssen M, Vrijens F. Pharmaceutical and non‐pharmaceutical interventions for Alzheimer's Disease, a rapid assessment. Brussels: Belgian Health Care Knowledge Centre (KCE), 2009. [Google Scholar]
Huntley 2015
- Huntley JD, Gould RL, Liu K, Smith M, Howard RJ. Do cognitive interventions improve general cognition in dementia? A meta‐analysis and meta‐regression. BMJ Open 2015;5(4):e005247. [PUBMED: 25838501] [DOI] [PMC free article] [PubMed] [Google Scholar]
IQWIG 2013
- Institute for Quality and Efficiency in Health Care (IQWIG). Positronenemissionstomographie (PET) und PET/CT bei Alzheimer‐Demenz. Berichtsplan Version 1.0. Available from www.iqwig.de. Köln: Institute for Quality and Efficiency in Health Care, 2013. [Google Scholar]
Irving 2006
- Irving CB, Mumby‐Croft R, Joy LA. Polyunsaturated fatty acid supplementation for schizophrenia. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001257.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larson 2013
- Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. New England Journal of Medicine 2013;369:2275‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrenson 2015
- Lawrenson JG, Evans JR. Omega 3 fatty acids for preventing or slowing the progression of age‐related macular degeneration. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD010015.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. [DOI] [PubMed] [Google Scholar]
Lin 2012
- Lin PY, Chiu CC, Huang SY, Su KP. A meta‐analytic review of polyunsaturated fatty acid compositions in dementia. Journal of Clinical Psychiatry 2012;73(9):1245‐54. [DOI] [PubMed] [Google Scholar]
Logsdon 2002
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine 2002;64(6):510‐9. [DOI] [PubMed] [Google Scholar]
Lopes da Silva 2013
- Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, et al. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta‐analysis. Alzheimer's & Dementia 2013;10(4):485‐502. [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CRJ, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7:263‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Micha 2014
- Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country‐specific nutrition surveys. BMJ 2014;348:g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohs 1997
- Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997; Vol. 11, issue Suppl 2. [PubMed]
Molfino 2014
- Molfino A, Gioia G, Fanelli FR, Muscaritoli M. The role for dietary omega‐3 fatty acids supplementation in older adults. Nutrients 2014;6:4058‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molnar 2008
- Molnar FJ, Hutton B, Fergusson D. Does analysis using "last observation carried forward" introduce bias in dementia research?. Canadian Medical Association Journal 2008;179(8):751‐3. [PUBMED: 18838445] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molnar 2009
- Molnar FJ, Man‐Son‐Hing M, Fergusson D. Systematic review of measures of clinical significance employed in randomized controlled trials of drugs for dementia. Journal of the American Geriatrics Society 2009;57:536‐46. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Montgomery 2008
- Montgomery P, Richardson AJ. Omega‐3 fatty acids for bipolar disorder. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005169.pub2] [DOI] [PubMed] [Google Scholar]
Morris 2014
- Morris MC, Tangney CC. Dietary fat composition and dementia risk. Neurobiology of Aging 2014;35 Suppl 2:S59‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2014
- Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plasticity 2014;2014:563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller‐Thomsen 2005
- Müller‐Thomsen T, Arlt S, Mann U, Maß R, Ganzer S. Detecting depression in Alzheimer's disease: evaluation of four different scales. Archives of Clinical Neuropsychology 2005;20:271‐6. [DOI] [PubMed] [Google Scholar]
O'Bryant 2008
- O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's Research Consortium study. Archives of Neurology 2008;65(8):1091‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qaseem 2008
- Qaseem A, Snow V, Cross JT Jr, Forciea MA, Hopkins R Jr, Shekelle P, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Annals of Internal Medicine 2008;148(5):370‐8. [PUBMED: 18316755] [DOI] [PubMed] [Google Scholar]
Reed 2014
- Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurology 2014;71:195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reuther 2013
- Reuther S, Nie N, Meijers J, Halfens R, Bartholomeyczik S. Malnutrition and dementia in the elderly in German nursing homes. Results of a prevalence survey from the years 2008 and 2009 [Mangelernährung und Demenz bei Bewohnern in Einrichtungen der stationären Altenpflege in Deutschland]. Zeitschrift für Gerontologie und Geriatrie 2013;46:260‐7. [DOI] [PubMed] [Google Scholar]
Revicki 2008
- Revicki D, Hays R D, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Román 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. [DOI] [PubMed] [Google Scholar]
Roque 2013
- Roque M, Salva A, Vellas B. Malnutrition in community‐dwelling adults with dementia (NutriAlz Trial). Journal of Nutrition, Health and Aging 2013;17:295‐9. [DOI] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:1356‐64. [DOI] [PubMed] [Google Scholar]
Schmidt 1996
- Schmidt M. Rey Auditory Verbal Learning Test™ (RAVLT™). A handbook, 1996. www.wpspublish.com/app/ (accessed 31 March 2016).
Schneider 1997
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:S22‐32. [DOI] [PubMed] [Google Scholar]
Schrag 2012
- Schrag A, Schott JM, Alzheimer's Disease Neuroimaging Initiative. What is the clinically relevant change on the ADAS‐Cog?. Journal of Neurology, Neurosurgery and Psychiatry 2012;83:171‐3. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Chapter 5. Updated October 2013. gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (accessed 31 March 2016).
Su 2010
- Su HM. Mechanisms of n‐3 fatty acid‐mediated development and maintenance of learning memory performance. Journal of Nutritional Biochemistry 2010;21:364‐73. [DOI] [PubMed] [Google Scholar]
Sydenham 2012
- Sydenham E, Dangour AD, Lim W‐S. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005379.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
United Nations 2013
- United Nations Department of Economic and Social Affairs Population Division. World Population Ageing 2013. www.un.org/en/development/desa/population/publications/ (accessed 10 June 2014).
US Preventive Task Force 2014
- US Preventive Task Force. Screening for cognitive impairment in older adults: final recommendation statement. AHRQ Publication No. 14‐05198‐EF‐2, 2014. www.uspreventiveservicestaskforce.org/uspstf14/dementia/dementiafinalrs.htm (accessed 31 March 2016).
Vedin 2012
- Vedin I, Cederholm T, Freund‐Levi Y, Basun H, Garlind A, Irving GF, et al. Effects of DHA‐rich n‐3 fatty acid supplementation on gene expression in blood mononuclear leukocytes: the OmegAD study. PLoS One 2012;7:e35425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vellas 2005
- Vellas B, Lauque S, Gillette‐Guyonnet S, Andrieu S, Cortes F, Nourhashemi F, et al. Impact of nutritional status on the evolution of Alzheimer's disease and on response to acetylcholinesterase inhibitor treatment. Journal of Nutrition, Health and Aging 2005;9:75‐80. [PubMed] [Google Scholar]
Vellas 2008
- Vellas B, Andrieu S, Sampaio C, Coley N, Wilcock G. Endpoints for trials in Alzheimer's disease: a European task force consensus. Lancet Neurology 2008;7:436‐50. [DOI] [PubMed] [Google Scholar]
Wechsler 2010
- Wechsler D. Wechsler Memory Scale. 4th Edition. San Antonio, TX: Pearson, 2010. [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 classification of mental and behavioural disorders: clinical description and diagnostic guidelines. Geneva: World Health Organization, Division of Mental Health, 1992. [Google Scholar]
WHO 2010
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th revision. Vol. 2, Geneva: World Health Organization, 2010. [ISBN 978 92 4 154834 2] [Google Scholar]
WHO 2012
- World Health Organization, Alzheimer's Disease International. Dementia. A public health priority, 2012. Available from www.who.int/mental_health/publications/dementia_report_2012/en/ (accessed 31 March 2016). [ISBN 978 92 4 156445 8]


