Abstract
Background
Cardiac ischemia induces myocardial dysfunction and ventricular wall stretch, which causes the release of B-type natriuretic peptide (BNP) into the bloodstream. However, it is unclear whether inducible ischemia produces a significant change in BNP levels (“stress delta-BNP”). The objective of this study was to determine the utility of stress-delta BNP levels and its precursor NT-proBNP for detecting inducible myocardial ischemia during cardiac stress testing.
Methods
We conducted a systematic review and meta-analysis. We searched PubMed, EMBASE, Web of Science, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Ovid. Studies examining the changes in levels of BNP and its precursor, N-terminal pro-B-type natriuretic peptide (NT-proBNP), after an exercise cardiac stress test were included. Two reviewers independently analyzed titles and abstracts. Abstracts that did not provide enough information regarding eligibility criteria were kept for full-text evaluation. The same two reviewers also performed data extraction for analyses. Any disagreement was resolved by a consensus and, if it persisted, by a third reviewer adjudication. We report the median and mean values in studies in the order of sample size.
Results
A total of 15 studies met the inclusion criteria. Nine studies reported results in medians and six studies reported results in means. Of the nine studies, five assessed BNP alone, three assessed NT-proBNP, and one assessed both. Due to the non-normal distribution of results in these studies, they could not be meta-analyzed. Of the six studies that reported results in means, three assessed BNP and three assessed NT-proBNP. The standardized difference between normal and ischemic patients' stress-delta BNP values was -0.39 (95% confidence interval (CI): -0.61; -0.17) in a fixed-effects model and -0.73 (95% CI: -1.72; 0.28) in the random-effects model with high heterogeneity (I^2 = 94%, Q test P = 0.001). For NT-proBNP, the meta-analysis model showed no significant difference between the stress-delta test for ischemic and normal patients (standardized mean difference (SMD): -0.02, 95% CI: -0.31; 0.28). Patients without inducible ischemia appeared to have a lower baseline BNP and NT-proBNP compared to patients with inducible ischemia by stress testing. Although some studies report higher stress-delta BNP in the ischemic group, this pattern was not seen consistently across studies. There was high heterogeneity across studies which was not robust to sensitivity analysis. A random-effects model failed to find statistically significant differences in stress-delta BNP or NT-proBNP.
Conclusions
We failed to find a relationship between stress-delta BNP or NT-proBNP and the presence or absence of ischemia. This may be due to high heterogeneity in the underlying studies.
Keywords: delta bnp, delta nt-pro-bnp, biomarkers, acute coronary syndrome, inducible myocardial ischemia, n-terminal pro-b-type natriuretic peptide (nt-pro-bnp), b-type natriuretic peptide (bnp)
Introduction
Acute coronary syndrome (ACS) remains a deadly and costly condition. One of the most common ways it is assessed is through a cardiac stress test. In this test, the patient’s heart is stressed using either exercise or medications to increase its oxygen demand. The patient’s heart is then assessed for inducible myocardial ischemia (a precursor to ACS) using some form of diagnostic imaging [1-3]. Accordingly, cardiac stress tests require sophisticated equipment and trained personnel to perform and interpret. This limits the test’s availability and affordability, leading to frequent hospitalization for patients with possible ACS. A different paradigm is needed to diagnose ACS.
One potential solution would be to utilize bloodstream biomarkers in the place of imaging studies to detect inducible myocardial ischemia. Multiple studies have attempted to determine whether there are dynamic biomarker correlates of inducible myocardial ischemia. B-type natriuretic peptide (BNP) is released by the myocardium in response to ventricular wall stretch. It has been hypothesized that BNP would also be released in myocardial ischemia, given that induced myocardial ischemia is associated with abnormal ventricular wall motion in the affected area. Prior individual studies examining changes in BNP as a predictor of stress test-induced myocardial ischemia were underpowered to make definitive conclusions and have findings that contradict one another [4-12]. Others have also studied the BNP precursor, N-terminal pro-B-type natriuretic peptide (NT-proBNP), which has similar characteristics as a biomarker and similarly found contradictory results [10, 13-15].
If clinicians could reliably use changes in bloodstream biomarkers following stress to detect myocardial ischemia, the need for highly trained personnel and sophisticated equipment for an imaging study could be precluded. Using “stress-delta” biomarkers in such a manner could have multiple advantages, including increased cost-effectiveness, efficiency, availability, and reproducibility. The goal of this systematic review and meta-analysis is to determine the utility of stress-delta BNP levels and its precursor, NT-proBNP, for detecting inducible myocardial ischemia during cardiac stress testing.
Materials and methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statement [16].
Eligibility criteria
Studies were eligible if they examined the changes in BNP or its precursor, NT-proBNP, before and after a stress test to assess myocardial ischemia in patients with known or suspected to have coronary artery disease. We excluded studies that examined these biomarkers in patients with systolic heart failure and in studies using them as prognostic rather than diagnostic markers. We also excluded studies that failed to exclude patients with congestive heart failure and studies that used pharmacological stress testing.
Information sources
We searched the following electronic databases: PubMed, Excerpta Medica database (EMBASE), Web of Science, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and Ovid. In addition, we searched the references of the included articles manually for further studies that would meet the eligibility criteria. When necessary, we also contacted authors of eligible studies for data not reported.
Search
The initial search comprised the Medical Subject Headings (MeSH) terms “myocardial ischemia,” “brain natriuretic peptide,” “BNP,” “diagnostic test,” and related entry terms. The complete search strategy used for the PubMed and EMBASE databases is shown in Table 1. We did not use limits for language and date when conducting the searches.
Table 1. PubMed Search Strategy.
| Search | Query |
| #4 | Search (#1 AND #2 AND #3) |
| #3 | Search ("Natriuretic Peptide, Brain"[Mesh] OR "Peptide, Brain Natriuretic"[All Fields] OR "Brain Natriuretic Peptide"[All Fields] OR "BNP-32"[All Fields] OR "BNP 32"[All Fields] OR "Nesiritide"[All Fields] OR "B-Type Natriuretic Peptide"[All Fields] OR "Natriuretic Peptide, OR B-Type"[All Fields] OR "BNP Gene Product"[All Fields] OR "Type-B Natriuretic Peptide"[All Fields] OR "Natriuretic Peptide, Type-B"[All Fields] OR "Type B Natriuretic Peptide"[All Fields] OR "Natriuretic Peptide Type-B"[All Fields] OR "Natriuretic Peptide Type B"[All Fields] OR "Natriuretic Factor-32"[All Fields] OR "Natriuretic Factor 32"[All Fields] OR "Brain Natriuretic Peptide-32"[All Fields] OR "Brain Natriuretic Peptide 32"[All Fields] OR "Natriuretic Peptide-32, Brain"[All Fields] OR "Peptide-32, Brain Natriuretic"[All Fields] OR "Ventricular Natriuretic Peptide, B-type"[All Fields] OR "Ventricular Natriuretic Peptide, B type"[All Fields] OR "Natrecor"[All Fields] OR "Basic natriuretic peptide"[All Fields] OR "B-type Natriuretic Peptide (BNP) blood test"[All Fields] OR "B-type Natriuretic Peptide blood test"[All Fields] OR "B-type Natriuretic Peptide (BNP)"[All Fields] OR "BNP"[All Fields] OR "BNP blood test"[All Fields]) |
| #2 | Search ("Diagnostic Test Accuracy"[All Fields] OR "Diagnostic Study"[All Fields] OR "Sensitivity and Specificity"[Mesh] OR "Specificity and Sensitivity"[All Fields] OR "Sensitivity"[All Fields] OR "Specificity"[All Fields]) |
| #1 | Search ("Myocardial Ischemia"[Mesh] OR "Ischemia, Myocardial"[All Fields] OR "Ischemias, Myocardial"[All Fields] OR "Myocardial Ischemias"[All Fields] OR "Ischemic Heart Disease"[All Fields] OR "Heart Disease, Ischemic"[All Fields] OR "Disease, Ischemic Heart"[All Fields] OR "Diseases, Ischemic Heart"[All Fields] OR "Heart Diseases, Ischemic"[All Fields] OR "Ischemic Heart Diseases"[All Fields] OR "Coronary Artery Disease"[Mesh] OR "Artery Disease, Coronary"[All Fields] OR "Artery Diseases, Coronary"[All Fields] OR "Coronary Artery Diseases"[All Fields] OR "Disease, Coronary Artery"[All Fields] OR "Diseases, Coronary Artery"[All Fields] OR "Coronary Arteriosclerosis"[All Fields] OR "Arterioscleroses, Coronary"[All Fields] OR "Coronary Arterioscleroses"[All Fields] OR "Atherosclerosis, Coronary"[All Fields] OR "Atheroscleroses, Coronary"[All Fields] OR "Coronary Atheroscleroses"[All Fields] OR "Coronary Atherosclerosis"[All Fields] OR "Arteriosclerosis, Coronary"[All Fields]) |
Study selection
Two reviewers (SK and AH) independently evaluated titles and abstracts of the retrieved articles. Abstracts that did not provide enough information regarding the eligibility criteria were kept for full-text evaluation. Reviewers independently evaluated full-text articles and determined study eligibility. Disagreements were resolved by consensus, and if any disagreement persisted, a third reviewer’s opinion (ATL) was sought.
Risk of bias
The risk of bias was evaluated by ranking each study according to standard factors using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool. Bias assessment considered each study’s representativeness, reference standard acceptability, the delay between the index and reference standard, avoidance of partial or differential verification, avoidance of incorporation of index test into reference standard, blinding, the relevance of results, reporting of intermediate/indeterminate results, and explanation of withdrawals.
Data extraction
Two reviewers (SK and AH) independently conducted the data extraction and disagreements were resolved by the third reviewer (ATL). General characteristics of the studies were collected, such as study design, study settings, population description, age, and data collection time range. Most importantly, the required data for this study, including baseline and ending BNP or NT-Pro-BNP, disease prevalence, type of stress, and times that outcome was measured, were also collected.
Data analysis
Due to a wide skew in BNP and NT-Pro-BNP distributions in the studies that met the eligibility criteria, as well as the wide variation in methods among the studies, a meta-analysis was only performed on the studies reporting mean and a variability measure for both outcomes. Thus, to summarize all studies, we also qualitatively report studies’ median and mean values ordered by sample sizes.
Meta-analysis models were built for BNP and NT-Pro-BNP delta values (difference) between pre- and post-stress testing comparing normal and ischemic patients. Heterogeneity across the studies was examined through the Cochrane's Q (considering p-values lower than 0.1 to indicators of heterogeneity), H and I2statistics [17]. High I2 values indicate high heterogeneity with a proposed categorization of 25% (low), 45% (moderate), and 75% (high) [17]. In our study, we adopted the interpretation of the fixed-effect model because our main goal is to obtain the true-effect of BNP and NT-Pro-BNP along the different population sizes reported. Sensitivity analyses were conducted by including different subsets of articles based on their quality rank, which allowed us to test the robustness of our results. A forest plot was constructed to depict these results. All analyses were performed through the R language software (R-Project, version 3.1.0, 2016), specifically through the meta package and metafor package [18].
Results
Study selection
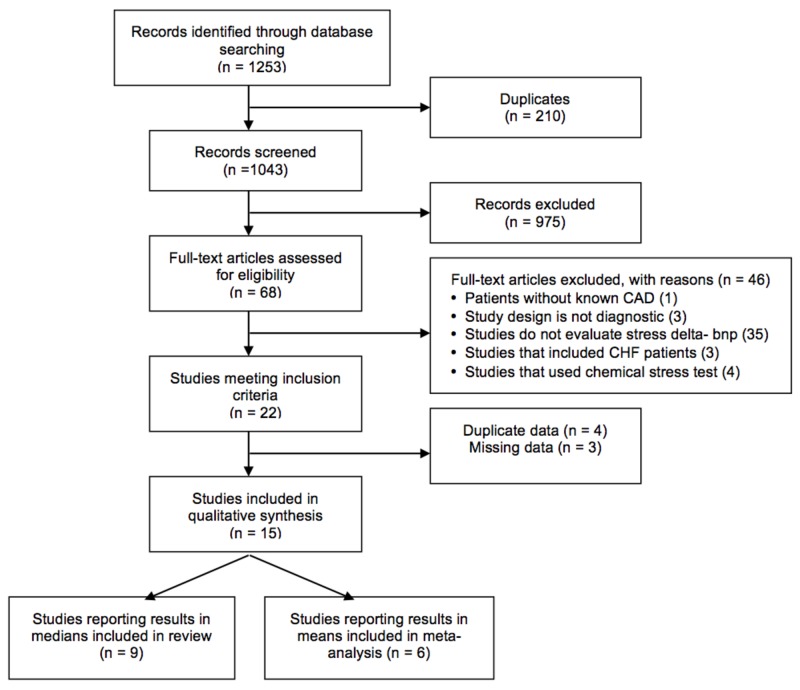
A total of 1,253 records were identified after searching through the various databases. Sixty-eight full-text articles were assessed for eligibility and 46 were excluded, leaving 22 articles that met the inclusion criteria. After further evaluation, only 15 studies contained unique data and were included in our synthesis. Of the 15 studies, six studies reported results in means and nine studies reported results in medians and were included in this systematic review (Figure 1).
Figure 1. Study selection flowchart.
BNP: B-type natriuretic peptide; CAD: coronary artery disease; CHF: congestive heart failure
Study characteristics
The population characteristics of these studies can be seen in Table 2. The mean age of participants ranged from 57 to 66 years old. The prevalence of ischemia in the studies ranged between 16.7% and 78%. The number of patients in each study ranged between 44 and 274. Only studies with exercise stress testing were included.
Table 2. Characteristics of Included Studies .
*studies examining both BNP and NT-proBNP
BNP: B-type natriuretic peptide; CAD: coronary artery disease; MPI: myocardial perfusion imaging; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SPECT: single-photon emission computed tomography
| Study | Year | Setting | Study Population | Mean Age | Men (%) | Type of Stress | N | Prevalence of ischemia |
| BNP | ||||||||
| Lee et al. [7] | 2014 | University Hospital of Basel | Consecutive patients with suspected CAD referred for stress testing | 62 | 68% | Bicycle exercise with SPECT | 274 | 37.6% |
| Staub et al. [8] | 2006 | University Hospital of Basel | Consecutive patients referred for CAD evaluation by bicycle ergometry MPI SPECT | 63 | 70% | Bicycle exercise MPI SPECT | 256 | 49.6% |
| Zaid et al. [4] | 2006 | Bnai-Zion Medical Center | Consecutive patients referred for chest pain evaluation | 59 | 67% | Exercise with nuclear perfusion imaging | 203 | 52% |
| Moller et al. [9] | 2008 | Mayo Clinic, Rochester | Patients referred for evaluation of angina or exertional dyspnea | 65 | 55% | Bicycle exercise echo | 140 | 46% |
| Paraskevaidis et al. [5] | 2011 | Outpatient clinic | Consecutive patients with chest pain | 58.7 | 80% | Treadmill exercise | 100 | 78% |
| *Foote et al. [10] | 2004 | Dartmouth Hitchcock Medical Center | Consecutive patients with CAD referred for MPI SPECT | 58.8 | 84% | Exercise with MPI SPECT | 74 | 54% |
| Marumoto et al. [6] | 1995 | - | 35 patients with angiographically proven angina and 35 angiographically normal patients | 61.1 | 67% | Exercise stress test with SPECT | 70 | 50% |
| Bergeron et al. [11] | 2006 | - | Patients referred for clinically indicated exercise echo | 66 | 82% | Treadmill exercise with echo | 60 | 31.7% |
| Win et al. [12] | 2005 | - | Patients undergoing treadmill exercise for evaluation of chest pain or screening for ischemia | 57.2 | 68% | Treadmill exercise with SPECT | 60 | 16.7% |
| NT-Pro BNP | ||||||||
| Staub et al. [13] | 2005 | University Hospital Basel | Consecutive patients referred for CAD evaluation | 63 | 70% | Bicycle exercise with SPECT | 260 | 49.6% |
| Vanzetto et al. [14] | 2007 | University Hospital of Grenoble | Patients with known stable CAD referred for exercise MPI | 61 | 88% | Bicycle exercise MPI | 102 | 55.9% |
| Van der Zee et al. [15] | 2009 | - | Consecutive patients referred for evaluation of inducible ischemia | 61 | 64% | Bicycle exercise with SPECT | 101 | 36.6% |
| Başkurt et al. [19] | 2011 | Outpatient clinic | Patients with a history of exercise-induced angina or atypical angina | 57 | 63% | Exercise stress test | 96 | 50% |
| *Foote et al. [12] | 2004 | Dartmouth Hitchcock Medical Center | Consecutive patients with CAD referred for MPI SPECT | 58.8 | 84% | Exercise with MPI SPECT | 74 | 54% |
| Chatha et al. [20] | 2006 | Rapid access chest pain clinic | All patients with chest pain from rapid access chest pain clinic | 58 | 46% | Exercise stress test | 59 | 23.7% |
| Zhu et al. [21] | 2010 | Zhongshan Hospital | Patients with chest pain and suspected CAD | - | - | Treadmill exercise | 44 | 56.8% |
Out of the total 15 studies included, eight studies examined BNP, six examined NT-proBNP, and one examined both. Table 3 depicts the data in order of sample size for studies examining BNP with median and mean stress-deltas, respectively. Eight of the nine BNP studies demonstrated a higher stress-delta value in patients with ischemic stress tests than in patients with normal stress tests. These studies represent 1,177 of the 1,237 patients (95%) with stress-delta BNP.
Table 3. Stress-delta BNP/NT-proBNP Studies (ng/L) .
“Delta-delta” refers to the stress-delta BNP for patients with ischemia on stress imaging, minus the stress-delta BNP for patients with normal stress imaging.
BNP: B-type natriuretic peptide; IQR: interquartile range; N: number; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SD: standard deviation
| Authors | Ischemic Patients | Normal Patients | Delta-Delta | Total N | ||||||||
| N | Baseline | Post | Delta | N | Baseline | Post | Delta | |||||
| Median BNP (IQR) | ||||||||||||
| Lee et al. [7] | 103 | 105.7 (57.1 - 176.9) | 130.5 (74.3 - 260.9) | 24.8 | 171 | 56.6 (30.4 - 94.3) | 72.4 (44.4 - 116.3) | 15.8 | 9 | 274 | ||
| Staub et al. [8] | 127 | 70.8 (34.8 - 146.5) | 87.5 (42.2 - 173.6) | 16.7 | 129 | 38.1 (19.6 - 81.4) | 52.2 (24.7 - 102.3) | 14.1 | 2.6 | 256 | ||
| Moller et al. [9] | 65 | 19 (8.8 - 34.6) | 29.6 (17.6 - 46.5) | 10.6 | 75 | 12.1 (4.6 - 26.9) | 16.3 (7.1 - 34.1) | 4.2 | 6.4 | 140 | ||
| Foote et al. [10] | 40 | 40.5 (24 - 54) | 77 - | 36.5 (15 - 49.5) | 34 | 16.5 (9.5 - 30.5) | 24 - | 7.5 (3.5 - 17.5) | 29 | 74 | ||
| Win et al. [12] | 10 | 13.4 (9.5 - 30.6) | 26.7 (19.3 - 61.5) | 13.2 | 50 | 15.05 (7 - 37.7) | 34.7 (14.9 - 67.6) | 19.65 | -6.45 | 60 | ||
| Bergeron et al. [11] | 19 | 24 (10 - 69) | 50 (30 - 94) | 26 | 41 | 15 (8.1 - 24) | 29 (13 - 40) | 14 | 12 | 60 | ||
| Mean BNP (SD) | ||||||||||||
| Zaid et al. [4] | 106 | 117 (+/- 292) | 149 (+/- 356) | 32 | 97 | 50 (+/- 66) | 67 (+/- 88) | 17 | 15 | 203 | ||
| Paraskevaidis et al. [5] | 78 | 21.8 (+/- 15.3) | 69.9 (+/- 63.2) | 48.1 | 22 | 14.2 (+/- 17.0) | 38.2 (+/- 51.1) | 24 | 24.1 | 100 | ||
| Marumoto et al. [6] | 35 | 2.8 (+/- 0.8) | 6.9 (+/- 2.6) | 4.1 | 35 | 2.7 (+/- 0.7) | 2.9 (+/- 1.0) | 0.2 | 3.9 | 70 | ||
| Median Pro-BNP (IQR) | ||||||||||||
| Staub et al. [13] | 129 | 155 (84 - 360) | 169 (90 - 391) | 14 | 131 | 91 (43 - 228) | 100 (50 - 244) | 9 | 5 | 260 | ||
| Vanzetto et al. [14] | 57 | 182 (97 - 265) | 212 (104 - 315) | 30 | 45 | 85 (44 - 164) | 99 (50 - 179) | 14 | 16 | 102 | ||
| Van der Zee et al. [15] | 37 | 184 (57 - 386) | 214 - | 30 (7 - 45) | 64 | 74 (21 - 255) | 89 - | 15 (4 - 46) | 15 | 101 | ||
| Foote et al. [10] | 40 | 120.5 (76 - 158) | 135 - | 14.5 (10.5 - 19.5) | 34 | 53.5 (28 - 74) | 57.5 - | 4 (0.5 - 9.5) | 10.5 | 74 | ||
| Mean Pro-BNP (SD) | ||||||||||||
| Başkurt et al. [19] | 48 | 175.1 (+/- 392.3) | 201.5 (+/- 461.6) | 26.4 | 48 | 92.2 (+/- 130.5) | 102.5 (+/- 139.2) | 10.3 | 16.1 | 96 | ||
| Chatha et al. [20] | 14 | 71.4 (+/- 41.2) | 76.8 (+/- 44.0) | 5.4 | 45 | 54 (+/- 61.2) | 60.1 (+/- 69.0) | 6.1 | -0.7 | 59 | ||
| Zhu et al. [21] | 25 | 187.97 (+/- 166.29) | 197 (+/- 178.82) | 9.03 | 19 | 107.27 (+/- 105.15) | 117.911 (+/- 93.34) | 10.64 | -1.61 | 44 | ||
Table 3 also demonstrates the data from studies examining NT-proBNP with median and mean stress-deltas, respectively. Five of the seven studies demonstrated that patients with ischemic stress tests had higher stress-delta values compared to normal patients. These studies represent 633 of the 736 patients (86%) with stress-delta NT-proBNP calculated.
Van der Zee et al. [15] assessed the change of NT-proBNP at time-points greater than two hours post-stress. The study appears to show similar results at the time-points greater than two hours. In fact, this study shows higher stress-delta values at time-points greater than two hours compared to immediately post-stress.
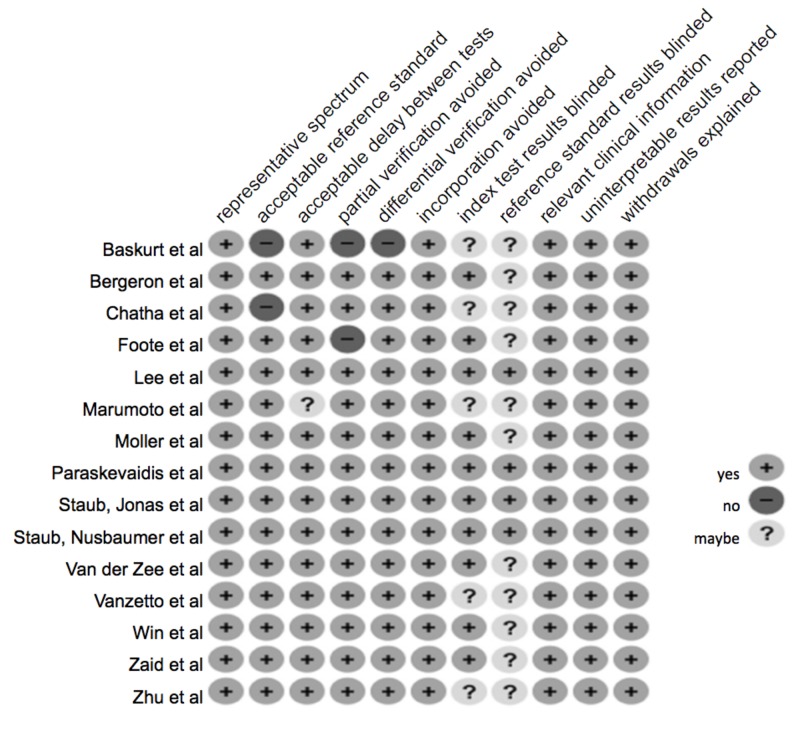
Risk of bias
Our bias assessment results are demonstrated in Figure 2. Four studies (Paraskevaidis et al., Lee et al., Staub et al., and Staub et al.) addressed all of the 11 characteristics evaluated [5, 7-8, 13]. The remaining 11 studies did not address whether the reference standard results were blinded and five of those studies also did not mention if the index test results were blinded. Studies by Başkurt et al. and Chatha et al. used an exercise treadmill test alone without imaging as a reference standard for the evaluation of coronary artery disease [19-20]. Foote et al. and Başkurt et al. were also at risk for partial verification [10, 19]. Başkurt et al. only evaluated with angiography if the exercise stress test was positive [19]. Foote et al. only used an exercise treadmill in healthy volunteers while using an exercise treadmill, as well as imaging, in the population with suspected coronary artery disease [10].
Figure 2. Assessment of risk.
Meta-analysis
The standardized difference between normal and ischemic patients’ stress-delta BNP values was -0.39 (95% confidence interval (CI): -0.61; -0.17) in a fixed-effect model and -0.73 (95% CI: -1.72; 0.28) in the random-effects model (Figure 3). The model revealed high heterogeneity (I2 = 94%, Q test P = 0.001). Sensitivity analysis showed that excluding the study by Marumoto et al. [6] would produce a significant improvement to the model, reaching almost no heterogeneity (I2 = 0%, Q test P = 0.001), and a decrease in the estimated pooled standardized mean difference to -0.13 (CI 95%: -0.37; 0.11). Overall, the results correspond to a homogeneous model and show that ischemic patients had a slightly higher BNP delta than normal patients, although the difference was not statistically significant.
Figure 3. Meta-analysis of studies reporting mean values.
BNP [4-6]; ProBNP [19, 21, 20]
BNP: B-type natriuretic peptide; ProBNP: pro-B-type natriuretic peptide
For NT-proBNP, the meta-analysis model showed no significant difference between the stress-delta test for ischemic and normal patients (standardized mean difference (SMD): -0.02, 95% confidence interval (CI): -0.31, 0.28). The NT-proBNP delta values for ischemic patients were similar to the normal patient group in a low heterogeneity fixed-effects model (Figure 11).
Discussion
While some have questioned the utility of routine stress testing, it remains the guideline-recommended care for patients presenting with symptoms of the potential acute coronary syndrome [22-24]. Routine stress testing has further come into question in light of findings that coronary interventions in stable patients with positive stress tests do not prevent future acute myocardial infarction or death [25]. The ability to utilize a bloodstream biomarker to reliably detect inducible myocardial ischemia could reduce the need for sophisticated equipment and highly trained personnel currently used for stress testing [1-3]. Since the imaging component of contemporary stress testing is not usually available 24 hours per day, the need for stress testing drives many of the hospitalizations for patients with symptoms of acute coronary syndrome. A biomarker-based stress test, therefore, could make ACS assessment more affordable and efficient since laboratory testing is routinely available continuously in most hospitals.
It has long been recognized that BNP is released by the myocardium in response to ventricular wall stretch [26]. This has been observed in patients with chronic and acute-on-chronic congestive heart failure [27]. It has, therefore, been hypothesized that BNP would also be released in the setting of myocardial ischemia, given the observation that induced myocardial ischemia is associated with abnormal ventricular wall motion activity [26, 28]. A prior meta-analysis examined whether a single, resting BNP, or NT-proBNP level can predict inducible myocardial ischemia on stress testing and found relatively high diagnostic test characteristics [29].
Accordingly, we attempted to systematically review and synthesize the existing literature exploring the relationship of dynamic BNP release from induced myocardial ischemia occurring during stress testing by examining the stress-delta BNP levels. In our analysis, patients without inducible ischemia appeared to have lower baseline BNP and NT-proBNP values compared to patients with inducible ischemia by stress testing. Stress-delta BNP values were slightly higher in patients with inducible ischemia. However, we were unable to definitively identify a relationship between inducible ischemia and statistically different stress-delta BNP or NT-proBNP values after sensitivity analysis.
This systematic review and analysis have several strengths. We conducted a comprehensive search of various databases in addition to going through all citations of the included studies. We used standardized definitions of inclusion and exclusion criteria and trained abstractors with excellent agreement.
Qualitative analysis of the results suggests that there is not a strong relationship between median stress-delta BNP and inducible ischemia when the blood samples are drawn at peak exercise or immediately after stress testing. Thus, the timing of blood sampling may play a critical role in assessing the utility of any stress-delta biomarker paradigm.
We note some limitations to our systematic review. The study population of included papers appears to have a wide range of pretest probability of disease among subjects, and there was a wide range of prevalence of ischemia noted as well. There was a great deal of heterogeneity in the studies examined with regard to methods and results. There are many factors that can influence BNP levels, and although we eliminated studies that included patients with systolic heart failure, other causes of elevated baseline BNP that could skew results (such as valvular disorders, pulmonary hypertension, and left ventricular diastolic dysfunction) were not consistently controlled. There was variation in the BNP and NT-proBNP assays used. There was also no standardization of blood draw time points; some studies sampled during peak exercise while others were drawn within minutes after finishing. Further increase after stress may occur and thus requires further investigation. NT-proBNP has a longer half-life of two to three hours and has a higher baseline compared to BNP. Therefore, small absolute increases may be harder to detect [30]. The study by Van Der Zee et al. examining changes in NT-proBNP over time after stress showed a peak change immediately at peak exercise, at the second peak at four hours in patients with ischemia, and at five hours in patients without ischemia [15]. In addition, treadmill exercise might not be a significant enough stressor to induce myocardial ischemia or it might produce an ischemic state that is too short to create significant stress-delta changes. Finally, stress modalities for inducing myocardial ischemia may also affect the result. The pharmacologic stress test, such as dobutamine and dipyridamole, has not been widely studied and was excluded from our studies.
Conclusions
In conclusion, a systematic review failed to reach a consensus on whether stress-delta BNP or NT-proBNP reliably elevates in response to myocardial ischemia caused by a cardiac stress test. Our meta-analysis of the available data showed no statistical difference; however, the type of data reported in the literature added limitations to the meta-analysis model. Further studies with larger sample sizes and with longer post-stress time sampling will be necessary to better determine their utility in the assessment of potential ACS patients.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared financial relationships, which are detailed in the next section.
Alexander Limkakeng declare(s) a grant from Abbott Laboratories and Roche Diagnostics. Dr. Limkakeng has had funded research from Abbott Laboratories and Roche Diagnostics (both of whom make BNP and Pro-BNP assays, respectively).
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Stress echocardiography for the diagnosis of coronary artery disease: an evidence-based analysis. Medical Advisory Secretariat. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3377563/ Ont Health Technol Assess Ser. 2010;10:1–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Single photon emission computed tomography for the diagnosis of coronary artery disease: an evidence-based analysis. Medical Advisory Secretariat. http://www.ncbi.nlm.nih.gov/pubmed/23074411. Ont Health Technol Assess Ser. 2010;10:1–64. [PMC free article] [PubMed] [Google Scholar]
- 3.Cardiovascular nuclear imaging: balancing proven clinical value and potential radiation risk. Cardiovascular Council Board of Directors. J Nucl Med. 2011;52:1162–1164. doi: 10.2967/jnumed.111.090654. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic accuracy of serum B-type natriuretic peptide for myocardial ischemia detection during exercise testing with spect perfusion imaging. Zaid G, Tanchilevitch A, Rivlin E, Gropper R, Rosenschein U, Lanir A, Goldhammer E. Int J Cardiol. 2007;117:157–164. doi: 10.1016/j.ijcard.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Exercise-induced changes of B-type natriuretic peptide uncover the unknown coronary artery disease in patients with chest pain and normal left ventricular systolic function. Paraskevaidis IA, Tsougos E, Varounis C, Dagres N, Karatzas D, Parissis J, Kremastinos DT. Eur J Cardiovasc Prev Rehabil. 2011;18:72–78. doi: 10.1097/HJR.0b013e32833a4529. [DOI] [PubMed] [Google Scholar]
- 6.Increased secretion of atrial and brain natriuretic peptides during acute myocardial ischaemia induced by dynamic exercise in patients with angina pectoris. Marumoto K, Hamada M, Hiwada K. Clin Sci (Lond) 1995;88:551–556. doi: 10.1042/cs0880551. [DOI] [PubMed] [Google Scholar]
- 7.B-type natriuretic peptide and clinical judgment in the detection of exercise-induced myocardial ischemia. Lee G, Sou SM, Twerenbold R, et al. Am J Med. 2014;127:427–435. doi: 10.1016/j.amjmed.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Use of B-type natriuretic peptide in the detection of myocardial ischemia. Staub D, Nusbaumer C, Zellweger MJ, et al. Am Heart J. 2006;151:1223–1230. doi: 10.1016/j.ahj.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Influence of left ventricular filling pattern on exercise-induced changes of natriuretic peptides in patients with suspected coronary artery disease. Møller JE, Bergeron S, Jaffe A, Pellikka PA. Int J Cardiol. 2008;124:204–210. doi: 10.1016/j.ijcard.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Detection of exercise-induced ischemia by changes in B-type natriuretic peptides. Foote RS, Pearlman JD, Siegel AH, Yeo KT. J Am Coll Cardiol. 2004;44:1980–1987. doi: 10.1016/j.jacc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Exertional changes in circulating cardiac natriuretic peptides in patients with suggested coronary artery disease. Bergeron S, Møller JE, Bailey KR, Chen HH, Burnett JC, Pellikka PA. J Am Soc Echocardiogr. 2006;19:772–776. doi: 10.1016/j.echo.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Percent change in B-type natriuretic peptide levels during treadmill exercise as a screening test for exercise-induced myocardial ischemia. Win HK, Chang SM, Raizner M, et al. Am Heart J. 2005;150:695–700. doi: 10.1016/j.ahj.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Use of N-terminal pro-B-type natriuretic peptide to detect myocardial ischemia. Staub D, Jonas N, Zellweger MJ, et al. Am J Med. 2005;118:1287–1280. doi: 10.1016/j.amjmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 14.N-terminal pro-brain natriuretic peptide predicts myocardial ischemia and is related to post-ischemic left-ventricular dysfunction in patients with subtle coronary artery disease. Vanzetto G, Jacon P, Calizzano A, Neuder Y, Faure P, Fagret D, Machecourt J. J Nucl Cardiol. 2007;14:835–842. doi: 10.1016/j.nuclcard.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Relation of N-terminal pro B-type natriuretic peptide levels after symptom-limited exercise to baseline and ischemia levels. Van der Zee PM, Verberne HJ, van Spijker RC, et al. Am J Cardiol. 2009;103:604–610. doi: 10.1016/j.amjcard.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. PLoS Med. 2009;6:0. [PMC free article] [PubMed] [Google Scholar]
- 17.Quantifying heterogeneity in a meta-analysis. Higgins JP, Thompson SG. https://onlinelibrary.wiley.com/doi/abs/10.1002/sim.1186. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2010. R: A Language and Environment for Statistical Computing: Reference Index. [Google Scholar]
- 19.Serum high-sensitivity C-reactive protein, amyloid associated protein and N-terminal proBNP levels do not predict reversible myocardial ischaemia. Başkurt M, Aktürk F, Keskin K, et al. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3721907/ Cardiovasc J Afr. 2011;22:85–89. doi: 10.5830/CVJA-2010-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.B-type natriuretic peptide in reversible myocardial ischaemia. Chatha K, Alsoud M, Griffiths MJ, et al. J Clin Pathol. 2006;59:1216–1217. doi: 10.1136/jcp.2005.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Analysis of serum cardiac biomarkers and treadmill exercise test-electrocardiogram for the diagnosis of coronary heart disease in suspected patients. Zhu Z, Yan Y, Wang Q, Qian J, Ge J. Acta Biochim Biophys Sin (Shanghai) 2010;42:39–44. doi: 10.1093/abbs/gmp103. [DOI] [PubMed] [Google Scholar]
- 22.Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. Foy AJ, Liu G, Davidson WR, Sciamanna C, Leslie DL. JAMA Intern Med. 2015;175:428–436. doi: 10.1001/jamainternmed.2014.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. Hermann LK, Newman DH, Pleasant WA, et al. JAMA Intern Med. 2013;173:1128–1133. doi: 10.1001/jamainternmed.2013.850. [DOI] [PubMed] [Google Scholar]
- 24.2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Fihn SD, Gardin JM, Abrams J, et al. J Am Coll Cardiol. 2012;60:0–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 25.International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. ISCHEMIA Trial Research Group, Maron DJ, Hochman JS, et al. Am Heart J. 2018;201:124–135. doi: 10.1016/j.ahj.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Weber M, Hamm C. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.B-type natriuretic peptide in heart failure: diagnostic, prognostic, and therapeutic use. Mehra R, Maisel A. Crit Pathw Cardiol. 2005;4:10–20. doi: 10.1097/01.hpc.0000155219.26524.19. [DOI] [PubMed] [Google Scholar]
- 28.The effects of coronary occlusion on myocardial contraction. Tennant R, Wiggers C. Am J Physiol. 1935;112:351–361. [Google Scholar]
- 29.Meta-analysis of B-type natriuretic peptide's ability to identify stress induced myocardial ischemia. Nadir MA, Witham MD, Szwejkowski BR, Struthers AD. Am J Cardiol. 2011;107:662–667. doi: 10.1016/j.amjcard.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Acute changes in circulating natriuretic peptide levels in relation of myocardial ischemia. Sabatine MS, Morrow DA, de Lemos JA, et al. J Am Coll Cardiol. 2004;44:1988–1995. doi: 10.1016/j.jacc.2004.07.057. [DOI] [PubMed] [Google Scholar]