Abstract
OBJECTIVE:
Differential diagnosis of mycosis fungoides (MF) in the early stages can be challenging. Dermoscopy has been reported to be useful in the evaluation of early MF. However, to our knowledge, there is no study that specifies these early stages as stage IA, IB or IIA. The present study aims to evaluate the dermoscopic findings of stage IIA MF in comparison with plaque psoriasis (PP).
METHODS:
Thirty-four patients aged between 16-70 years with stage IIA MF (n=17) and PP (n=17) were evaluated in this prospective study. Dermoscopic examinations were performed by manual dermatoscopy (Dermlite DL4). χ2 test was used.
RESULTS:
In patients with stage IIA MF, orange-yellow patches (88.2%), short, fine and linear vessels (82.3%), geometric white scales (70.5%), perifollicular white scales (47%) and white patches (35.2%) were common, while dotted vessels (94.1%), diffuse lamellar white scales (88.2%) and dotted and globular vessels (70.5%) were common in patients with PP. Although spermatozoa-like structures, purpuric dots, collarette white scales and Y-shaped arborizing vessels were common in patients with MF, this was not statistically significant. Geometric white scales (clinically; cigarette paper-like wrinkly scales) correlated with alternating parakeratosis and orthokeratosis in the stratum corneum histopathologically.
CONCLUSION:
A unique aspect of our study is that this study provides insights about the importance of scales in differentiating MF from PP. Orange-yellow and white patches, short, fine and linear vessels, geometric and perifollicular white scales may be useful in distinguishing stage IIA MF from PP by hand-held dermoscopy.
Keywords: Dermatoscopy, dermoscopy, mycosis fungoides
Mycosis fungoides (MF) is the most common primary cutaneous lymphoma that derives from T lymphocytes. MF may present with various clinical presentations, ranging from erythematous patches to plaque, nodules and tumors in different disease stages [1, 2]. Staging of MF is based on a “tumor node metastasis” (TNM) classification system originally described in 1979 [3]. A revision and expansion that also include blood involvement (TNMB) was published in 2007 [4]. Differential diagnosis of MF in these early stages (IA, IB, and IIA) can be challenging since lesions of MF may mimic chronic dermatitis, contact dermatitis, psoriasis, parapsoriasis, or tinea corporis. In addition to this, other forms of lymphoreticular malignancies, sarcoidosis, deep fungal infections, atypical mycobacterial infections, leishmaniasis, leprosy and metastases should be taken under consideration for the differential diagnosis of MF plaques [2]. The presence of clinically evident lymphadenopathy without pathologic nodal infiltration represents stage IIA MF [4, 5]. Plaque psoriasis (PP) with chronic recurrent erythematous squamous patches is the most important skin disease in the differential diagnosis. Therefore, in the latest years, some dermoscopic studies have been performed [6–12], in particular for early-stage MF [7, 10, 11] and some variants of MF, such as folliculotropic MF [8], poikilodermatous MF [9, 10], pagetoid reticulosis [12], granulomatous slack skin, and hypopigmented MF [1, 2]. However, early diagnosis of MF can be extremely difficult owing to its various clinical manifestations. Dermoscopy has been reported to be useful in the evaluation of early MF in Chinese [7], Egyptian [10] and Caucasian [11] populations. However, to our knowledge, there is no study that evaluates the dermoscopic findings of MF with specifying these early stages into stage IA, IB or IIA, probably due to the low prevalence of this skin lymphoma. The present study aims to evaluate the dermoscopic findings of stage IIA MF and to compare these with the dermoscopic findings of PP.
MATERIALS AND METHODS
Thirty-four patients aged between 16–70 years with stage IIA MF (n=17) and PP (n=17) were included in this prospective study. The protocol of this study was conducted according to the principles of the Declaration of Helsinki. This study was approved and conducted in accordance with the guidelines of the institutional review board at our hospital and all patients signed informed consent forms before enrollment in this study (IRB#5270/2017). Diagnosis of MF was confirmed histopathologically and immunohistochemically in all cases, while the diagnosis of PP was only confirmed histopathologically. Staging of MF is based on a “tumor node metastasis” (TNM) classification system originally published in 1979 [3]. A revision and expansion that also includes blood involvement (TNMB) were published in 2007 [4], and this revised version was used in this study as suggested in European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome in 2017 [5]. Determining stage IIA MF criteria was as follows: T1-2, N1-2, M0, B0-1. T1 means limited patches, papules, and/or plaques covering less than 10% of the skin surface, in details; T1a (patch only) vs. T1b (plaque +/- patch), T2 means patches, papules, or plaques covering 10% or more of the skin surface, in details; T2a (patch only) vs. T2b (plaque +/- patch). The other defines were as follows; N1-2: Clinically abnormal peripheral lymph nodes, M0: No visceral organ involvement, B0: Absence of significant blood involvement, 5% or less of peripheral blood lymphocytes were atypical (Sezary cells), B1: Low blood tumor burden: more than 5% of peripheral blood lymphocytes were atypical (Sezary cells) but did not meet the criteria of B2.
Exclusion criteria were the administration of topical or systemic treatment <1 or <6 months before the dermoscopic examination, respectively. Patients in whom the diagnosis was uncertain after recurrent histopathological and immunohistochemical examinations were excluded. Dermoscopic observation was performed by two independent dermoscopists after obtaining informed consent from the patients. Two observers were not blinded to patient diagnosis. Selection of the dermoscopic features included in the dermoscopic evaluation was based on the previous literature data [7, 11, 13–15]. The dermoscopic features included in this study were as follows: (i) vascular morphology (fine, short and linear (all vessels with linear morphology, curved or not), dotted (a dotted morphologic appearance, irrespectively of the diameter of the dots), globular, purpuric, spermatozoa-like structures (dotted and a short curved linear vessel, giving an overall shape similar to a spermatozoon); (ii) scales (white scales, yellow scales, perifollicular white scales, collarette white scales, diffuse lamellar white scales); (iii) other dermoscopic features (orange-yellowish patches). Demographic data, such as age and gender, were recorded. Dermoscopic examinations were performed by manual dermatoscopy (Dermlite DL4 PigmentBoost Plus X10; 3 Gen, San Juan Capistrano, CA, USA) with x10 magnification.
Statistical Analyses
Differences in the frequency of dermoscopic findings among the two groups were evaluated using the χ2 test with a statistical significance set at p<0.05.
RESULTS
The rate of female-to-male ratio was 10/7 in patients with MF in our study, and this rate was 8/9 in patients with PP. The average duration of disease was 8±2.3 years in patients with MF and 6±3.8 years in patients with PP. The mean age was 62±12.4 years in patients with MF, and it was 45±7.8 years in patients with PP.
In patients with MF, orange-yellow patches (p<0.00001, Fig. 1), fine, short, and linear vessels (p=0.00003, Fig. 1–3), geometric (rhomboid, triangular, parallel-shaped), fine, linear and white scales (p=0.0001, Fig. 1, 3), perifollicular white scales (p=0.02, Fig. 1) and white patches (p=0.033, Fig. 2) were common, while dotted vessels (p=0.0001), diffuse lamellar white scales (p<0.00001) and dotted and globular vessels (p=0.0001) were common in patients with PP (Table 1). Although spermatozoa-like structures (p=0.07, Fig. 1), purpuric dots (p=0.10, Fig. 1), collarette white scales (p=0.24) and Y-shaped arborizing vessels (p=0.07, Fig. 2) were common in patients with MF, their frequency was not statistically significant in this study.
FIGURE 1.
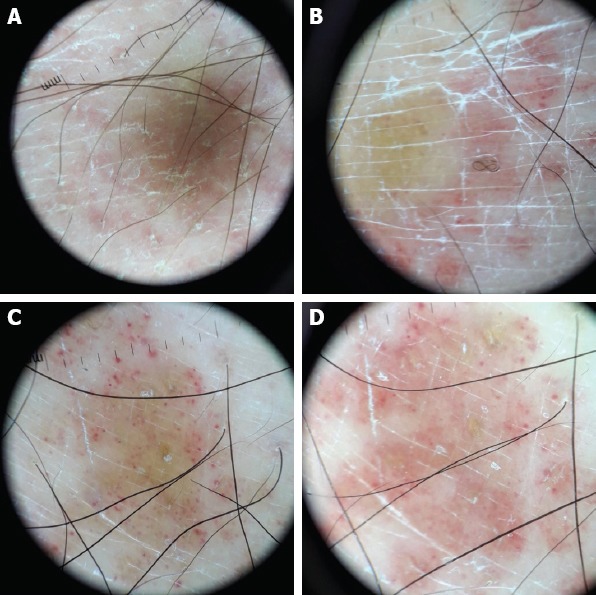
Orange-yellow patches, fine, short and linear vessels, geometric, fine, linear and scattered perifollicular white scales, purpuric dots and spermatozoa-like structures.
FIGURE 3.
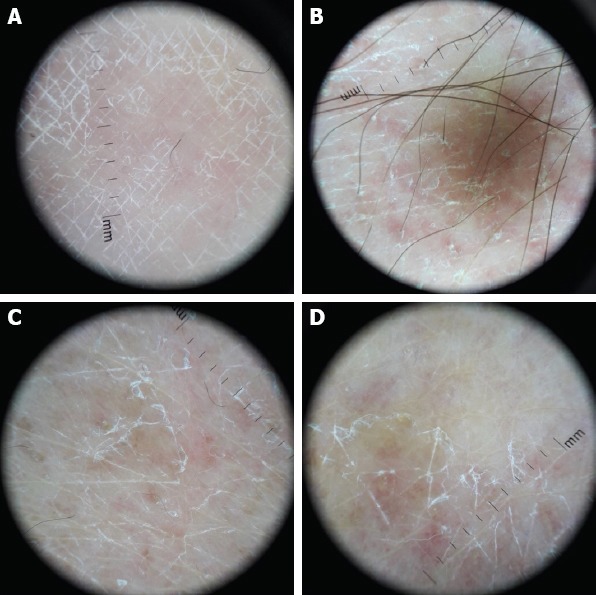
Geometric shaped, fine, linear and white scales and fine, short and linear vessels.
FIGURE 2.
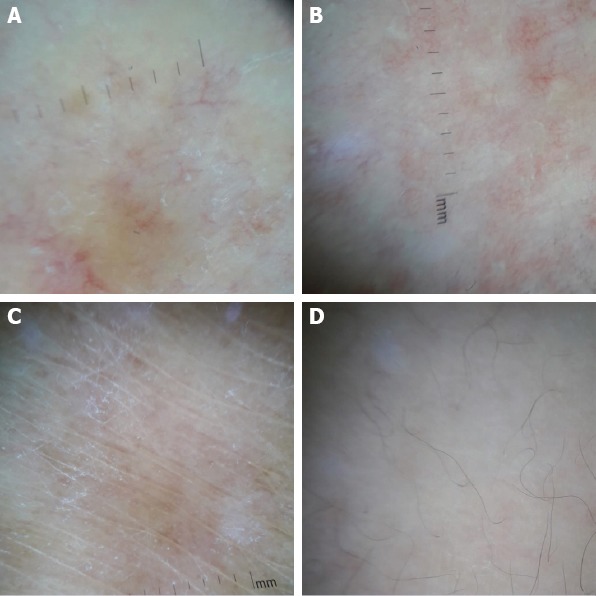
Y-shaped arborizing vessels (A), white patches and fine, short and linear vessels (B–D).
TABLE 1.
Dermoscopic findings of 17 patients with stage IIA mycosis fungoides and 17 patients with plaque psoriasis and comparison of these findings
| Dermoscopic features | Mycosis fungoides | Psoriasis | *p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Orange- yellow patches | 15 | 88.2 | 2 | 11.7 | <0.00001 |
| Fine, short, linear vessels | 14 | 82.3 | 2 | 11.7 | 0.00003 |
| Geometric white scales | 12 | 70.5 | 1 | 5.8 | 0.0001 |
| Perifollicular white scales | 8 | 47 | 2 | 11.7 | 0.02 |
| Purpuric dots | 6 | 35.2 | 2 | 11.7 | 0.10 |
| Collarette white scales | 6 | 35.2 | 3 | 17.6 | 0.24 |
| White patches | 6 | 35.2 | 1 | 5.8 | 0.033 |
| Spermatozoa like structures | 5 | 29.4 | 1 | 5.8 | 0.07 |
| Y shaped arborizing vessels | 5 | 29.4 | 1 | 5.8 | 0.07 |
| Dotted vessels | 5 | 29.4 | 16 | 94.1 | 0.0001 |
| Diffuse lamellar white scales | 1 | 5.8 | 15 | 88.2 | <0.00001 |
| Dotted and globular vessels | 1 | 5.8 | 12 | 70.5 | 0.0001 |
P<0.05 was considered statistically significant. The findings that are statistically significant are bolded.
DISCUSSION
In patients with stage IIA MF, orange-yellow patches (88.2%), short, fine and linear vessels (82.3%), geometric (rhomboid, triangular, parallel-shaped), fine, linear and white scales (70.5%), perifollicular white scales (47%) and white patches (35.2%) were common in dermoscopic evaluation in comparison with PP. In the literature, dermoscopy has been reported to be useful in the evaluation of early MF (stage IA, IB, IIA) in Chinese [7], Egyptian [10] and Caucasian [11] populations. In all these studies, dermoscopic evaluation was performed by two independent dermoscopists, similar to this study; yet unlike other studies, this study was performed in a prospective manner, with long term follow up and with additional skin biopsies if necessary, to rule out transformation to another clinical type and to rule out folliculotropism and anaplastic large cell transformation histopathologically.
Lallas et al., in their study [11], investigated the dermoscopic features of 32 patients with early MF without specifying the disease stage and compared with 35 patients with chronic dermatoses (CD) in Caucasian populations. In their study, fine and linear vessels (93.8%), orange-yellow patches (90.6%) and spermatozoa-like vessels (50.0%) were common and concluded fine short linear vessels and orange-yellow patchy areas as specific dermoscopic features of early MF in comparison with CD. On the other hand, Xu et al. [7] examined 31 patients with early MF without specifying disease stage similar to the above study, 36 patients with CD and 34 patients with PP dermoscopically, histopathologically and immunohistochemically in the Chinese population. They reported the most common dermoscopic features as linear vessels (90.3%) and spermatozoa-like structures (74.2%) in their study and concluded linear vessels, spermatozoa-like structures and orange-yellowish patchy areas as highly predictive features for the diagnosis of eMF in comparison with CD and PP. Interestingly, Bosseila et al. [10] evaluated angiogenesis in 25 patients with early MF (16 of them were classic, seven of them were hypopigmented and two of them were poikilodermatous MF) both dermoscopically and immunohistochemically, and reported the dotted vessels as the most frequent vascular pattern followed by the linear pattern in the early cases of MF lesions. Our results were parallel with the previous two studies that concluded linear vessels and orange-yellowish patchy areas as being the most common dermoscopic features. Xu et al., in their study [7], observed dotted/globular vessels in 67.7% of patients with MF, in 97.2% of patients with CD and in 97.1% of patients with PP. The difference was not significant, similar to our study that the frequencies of dotted and globular vessels (70.5%) and isolated dotted vessels (94.1%) were statistically higher in PP patients than MF patients. In addition to this, in patients with PP, dotted vessels (94.1%), diffuse lamellar white scales (88.2%) and dotted and globular vessels (70.5%) were common and this finding was consistent with the literature [15]. We believe that varying frequency rates of vessels in different studies may be related to the insufficiency of dermatoscope with x10 magnification in observing detailed shapes of vessels. Similarly, spermatozoa like structures was seen in 5/17 (29.4%) of patients with MF in this study, which was not statistically significant, while the frequency rate was higher in the other study [7] that used x20 magnification dermoscope that provides better visualization of the dermoscopic features. This vascular pattern could not be identified exactly in our study, as with a magnification of x10 by the dermatoscope. We could not well differentiate a spermatozoa-like structure from a dot/globule or linear vascular structure similar to findings of the study of Bosseila et al. [10].
As another dermoscopic vessel structure, we saw Y shaped arborizing vessels in 5/17 patients with MF, but this was not statistically significant. Arborizing blood vessels were detected only in the poikilodermatous variant of MF in a study [10]. In addition, purpuric dots were identified in six of 17 cases (35.2%) in our study, but this was not also significant. In Lallas et al.’s study [11], they saw purpuric dots in three of 32 (9.4%) early MF cases similar to this study, while purpuric dots were not observed in other dermoscopic studies with classical MF.
In this study, we observed geometric (rhomboid, triangular, parallel-shaped), fine, linear and white scales (70.5%) and perifollicular white scales (47%) in patients with MF while diffuse lamellar white scales (88.2%) were common in patients with PP. In contrast with our findings, Lallas et al. [11] observed white scales (65.7%) and yellow scales (57.1%) more frequently in CD, while fine white scales were present in a few patients with MF (18.8%, 6/32) in their study, although the difference was not statistically significant. Xu et al., [7] in their study, observed white scales only in one patient (3.2%) with MF, while it was statistically more frequent in patients with PP (79.6%). On the other hand, similar to our results, Ghahramani et al. [8] observed white scales in 4/7 (57.1%) of their patients with patch/plaque MF. We believe that scales are important dermoscopic findings in differentiating between these two dermatoses. In MF lesions, in the patch stage with well-established lesions, the epidermis may show acanthosis and hyperkeratosis [16]. Hence, it is rational to expect scales in the dermoscopy of MF. The early patch stage of MF presents with single or multiple erythematous, scaly (cigarette paper-like wrinkly scales) macules and patches that vary from orange to red that are located mostly on sun-protected areas of skin clinically [2]. In our study, the linear white scales that form geometric shapes were correlated with alternating parakeratosis and orthokeratosis in the stratum corneum histopathologically (Fig. 4).We hypothesize that these geometric (rhomboid, triangular, parallel-shaped), fine, linear and white scales (70.5%) may be the dermoscopic appearance of cigarette paper-like wrinkly scales. Geometric scales are a novel finding that has been described in this study.
FIGURE 4.
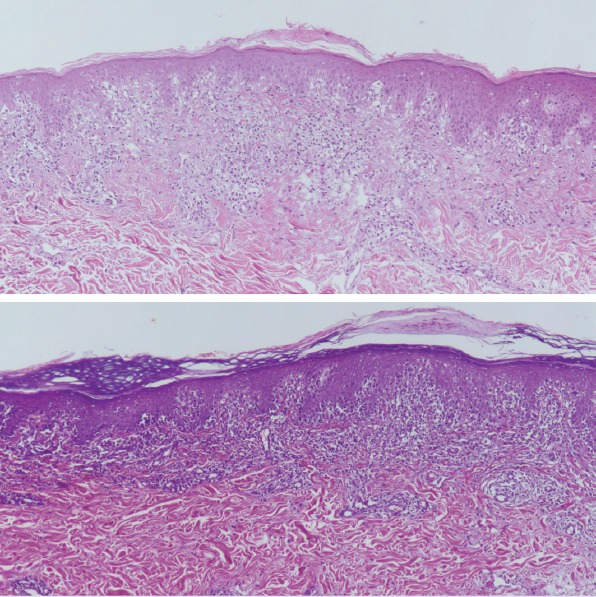
Dermatoscopic geometric white scales (clinically; cigarette paper-like wrinkly scales) correlated with alternating parakeratosis and orthokeratosis in the stratum corneum histopathologically (haematoxylin and eosin, original magnification x 100).
Patients with the classic form of MF are known to have no follicular changes, and follicles are not usually observed in MF lesions dermoscopically [6]. Interestingly, scattered perifollicular white scales (47%) were common in this study that correlated with perifollicular hyperkeratosis histopathologically (Fig. 5). In a recently published report of a pilot study of primary cutaneous lymphomas, comedo-like openings were observed in folliculotropic MF, likely reflecting follicular plugging [8] and a white halo around the follicles (perifollicular accentuation) is hypothesized to be an early dermoscopic sign of folliculotropism with patch stage MF in a case report [6]. In addition to this, we observed collarette white scales in 6/17 of the patients in this study, but this was not statistically significant. To our knowledge, this is the first clinical investigation to report the novel finding, “perifollicular and collarette white scales” in the early stage MF.
FIGURE 5.
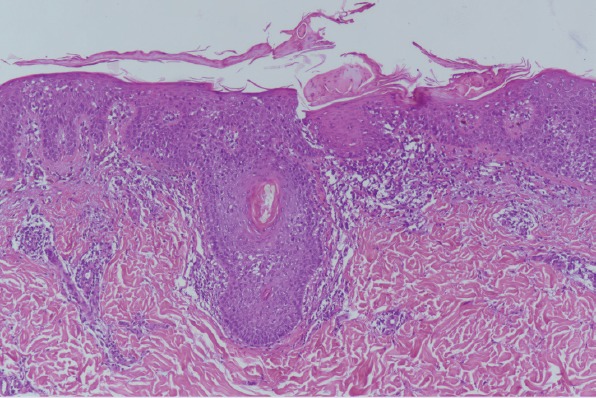
Dermatoscopic scattered perifollicular white scales correlated with perifollicular hyperkeratosis histopathologically (haematoxylin and eosin, original magnification x 100).
White patches (35.2%) were common in this study even though all of the cases included in this study were in the form of classical MF (not hypopigmented MF). In a report of a pilot study of primary cutaneous lymphomas, similar to above finding of this study, Ghahramani et al. [8] evaluated seven patients with patch stage MF and reported the most common dermoscopic feature in their study as a fenestrated to trabeculated the pattern of patchy white structureless areas (100%). They correlated their findings with epidermotropism, atypical pleomorphic cells, and lichenoid infiltrate histopathologically [8]. Similarly, Xu et al., in their study [9] evaluated dermoscopy of a 59-year-old man with poikilodermatous MF, and it showed multiple polygonal structures consisting of lobules of white storiform streaks and the skin biopsy specimen revealed vacuolar alterations of the basal layer, scattered melanophages, intermittent fibrosis of the collagen and lymphocytic infiltration in the papillary dermis histopathologically. Papillary dermal fibrosis may be present in the lesions of the patch stage of the classical MF [16]. Thus, we hypothesize that these dermoscopic white patches could correlate with this dermal fibrosis of the collagen with lichenoid alterations histopathologically.
Our study had several limitations. The two observers in this study were not blinded to the histopathologic diagnosis of the disease. The patient population was limited that potentially decreased the statistical power of this study. In addition, other chronic scaly erythematous dermatoses that could be part of the differential diagnosis of stage IIA MF are not included in this study. Further studies with larger samples in varying disease stages that assess the correlation of these dermoscopic structures with histological changes are necessary to elicit the usefulness of dermoscopy in the differential diagnosis of MF.
As a conclusion, to our knowledge, this is the first study that emphasizes the usefulness of dermoscopy in the differentiation stage IIA MF from PP. Orange-yellow and white patches, short, fine and linear vessels, geometric and perifollicular white scales may be important in distinguishing this dermatosis from PP by hand-held dermoscopy. A unique aspect of our study is that it provides insights about the importance of scales in differentiating MF from PP.
Footnotes
Ethics Committee Approval: University of Health Sciences, Istanbul Umraniye Training and Research Hospital, December 21, 2017; B.10.1.TKH.4.34. H.GP.0.01/130.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – MKO, IZ, EZ; Design – MKO, IZ; Supervision – MKO; Materials – MKO, IZ, EZ; Data collection and/or processing – MKO, IZ, EZ; Analysis and/or interpretation – MKO; Writing – MKO; Critical review – MKO.
REFERENCES
- 1.Piccolo V, Russo T, Agozzino M, Vitiello P, Caccavale S, Alfano R, et al. Dermoscopy of Cutaneous Lymphoproliferative Disorders:Where Are We Now?Dermatology. 2018;234:131–6. doi: 10.1159/000490412. [DOI] [PubMed] [Google Scholar]
- 2.Bombonato C, Pampena R, Lallas A, Giovanni P, Longo C. Dermoscopy of Lymphomas and Pseudolymphomas. Dermatol Clin. 2018;36:377–88. doi: 10.1016/j.det.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Bunn PA, Jr, Lamberg SI. Report of the Committee on Staging and Classification of Cutaneous T-Cell Lymphomas. Cancer Treat Rep. 1979;63:725–8. [PubMed] [Google Scholar]
- 4.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. ISCL/EORTC. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome:a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–22. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 5.Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. Eur J Cancer. 2017;77:57–74. doi: 10.1016/j.ejca.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Toncic RJ, Drvar DL, Bradamante M, Rados J, Jerkovic-Gulin S, Caccavale S, et al. Early dermoscopic sign of folliculotropism in patients with mycosis fungoides. Dermatol Pract Concept. 2018;8:328–29. doi: 10.5826/dpc.0804a17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, Liu J, Wang T, Luo Y, Liu Y. Dermoscopic patterns of early-stage mycosis fungoides in a Chinese population. Clin Exp Dermatol. 2019;44:169–75. doi: 10.1111/ced.13680. [DOI] [PubMed] [Google Scholar]
- 8.Ghahramani GK, Goetz KE, Liu V. Dermoscopic characterization of cutaneous lymphomas:a pilot survey. Int J Dermatol. 2018;57:339–43. doi: 10.1111/ijd.13860. [DOI] [PubMed] [Google Scholar]
- 9.Xu P, Tan C. Dermoscopy of poikilodermatous mycosis fungoides (MF) J Am Acad Dermatol. 2016;74:e45–7. doi: 10.1016/j.jaad.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Bosseila M, Sayed Sayed K, El-Din Sayed SS, Abd El Monaem NA. Evaluation of Angiogenesis in Early Mycosis Fungoides Patients:Dermoscopic and Immunohistochemical Study. Dermatology. 2015;231:82–6. doi: 10.1159/000382124. [DOI] [PubMed] [Google Scholar]
- 11.Lallas A, Apalla Z, Lefaki I, Tzellos T, Karatolias A, Sotiriou E, et al. Dermoscopy of early stage mycosis fungoides. J Eur Acad Dermatol Venereol. 2013;27:617–21. doi: 10.1111/j.1468-3083.2012.04499.x. [DOI] [PubMed] [Google Scholar]
- 12.Morariu SH, Rotaru M, Vartolomei MD, Turcu M, Chiotoroiu AL, Suciu M, et al. Pagetoid reticulosis Woringer-Kolopp type, a particular variant of mycosis fungoides:a case report. Rom J Morphol Embryol. 2014;55:1469–72. [PubMed] [Google Scholar]
- 13.Lallas A, Kyrgidis A, Tzellos TG, Apalla Z, Karakyriou E, Karatolias A, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. 2012;166:1198–205. doi: 10.1111/j.1365-2133.2012.10868.x. [DOI] [PubMed] [Google Scholar]
- 14.Vázquez-López F, Kreusch J, Marghoob AA. Dermoscopic semiology:further insights into vascular features by screening a large spectrum of nontumoral skin lesions. Br J Dermatol. 2004;150:226–31. doi: 10.1111/j.1365-2133.2004.05753.x. [DOI] [PubMed] [Google Scholar]
- 15.Golińska J, Sar-Pomian M, Rudnicka L. Dermoscopic features of psoriasis of the skin, scalp and nails - a systematic review. J Eur Acad Dermatol Venereol. 2019;33:648–60. doi: 10.1111/jdv.15344. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita T, Abbade LP, Marques ME, Marques SA. Mycosis fungoides and Sézary syndrome:clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817–30. doi: 10.1590/S0365-05962012000600001. [DOI] [PMC free article] [PubMed] [Google Scholar]


