Abstract
OBJECTIVE:
This study aims to evaluate the efficacy and safety of the addition of 10 or 25 mg of empagliflozin to patients with type 2 diabetes mellitus using a maximum tolerable dose of metformin and gliclazide.
METHODS:
A total of 60 patients who had been receiving a maximum tolerable dose of metformin plus gliclazide. was divided into two groups in this study. In the first group (Group 1, n=32), 10 mg empagliflozin was added to the current treatment once a day, and in the second group (Group 2, n=28) 25 mg empagliflozin was added to the same treatment once a day. Biochemical results, weight and blood pressure changes of the patients in both groups were evaluated before and after 12 weeks of empagliflozin addition. Patients who developed urinary tract and genital infections after treatment were recorded.
RESULTS:
There was a statistically significant decrease in HbA1c in both groups after empagliflozin treatment (Group 1, p<0.001 and Group2, p=0.001). When the lipid profile was evaluated, no significant difference was found between basal and post-treatment parameters (p>0.05). Patients in Group 1 and Group 2 lost 2.6±1.2 and 3.8±2.0 kg of body weight, respectively (p<0.0001 for each). There were also significant reductions in systolic and diastolic blood pressure for groups 1 and 2 (p<0.0001 for each). Although there was a numerical increase in the urinary tract and genital infections in both groups after empagliflozin treatment, there was no statistically significant difference compared to the pre-treatment period (p>0.05).
CONCLUSION:
Two doses of empagliflozin added to the present treatments showed a dose-independent improvement in glycemic control and a neutral effect on lipid metabolism.
Keywords: Empagliflozin, HbA1c, SGLT2
M etformin is the classical first-line treatment to provide glycemic control in patients with type 2 diabetes mellitus (T2DM). However, metformin alone may usually not sustain glycemic control for a long time [1], and additional treatments are needed in most patients [2]. Although they seem to be effective at first, sulfonylureas and oral hypoglycemic agents are difficult to tolerate due to the side effects [3]. Therefore, patients with T2DM need effective and well-tolerated new antidiabetic agents that can be used in combination with available treatments to improve glycemic control, especially without an additional risk of hypoglycemia and weight gain.
Sodium-glucose cotransporter 2 inhibitors (SGLT2-I) are a new class of drugs for the treatment of diabetes. SGLT2-I reduces hyperglycemia rates in patients with T2DM by diminishing renal glucose reabsorption and thus increasing urinary glucose excretion [4]. SGLT2-I can be used in combination with metformin, sulfonylurea (SU), dipeptidyl peptidase 4 inhibitors (DPP-4), thiazolidinediones (pioglitazone) or other antidiabetic agents, including insulin [5–7]. Since they act independently of insulin, the treatment can be used in appropriate patients at any stage of diabetes. The American Diabetes Association (ADA) and the European Association of Diabetes Research (EASD) T2DM guidelines also recommend SGLT2-I as one of the second-line treatment options [8]. Empagliflozin is a selective, potent sodium-glucose cotransporter -2 inhibitor used in the treatment of T2DM and has a >2500-fold affinity for SGLT-2 over SGLT-1 [9].
We, in this dose-comparison study, aimed to evaluate the efficacy and safety of two doses of empagliflozin (10 and 25 mg) when added to ongoing oral antidiabetic (metformin plus gliclazide) agents in patients with insufficient glycaemic control.
MATERIALS AND METHODS
Patient population
The ethics committee of Kartal Dr. Lutfi Kirdar Training and Research Hospital (date: 27.03.2019, number: 2019/514/150/24) approved the study protocol and the trial was directed in accordance with the Declaration of Helsinki.
In this study conducted between January 2018 and December 2018, medical records of 60 patients aged ≥18 years old with T2DM receiving gliclazide (60 mg/day) and metformin (2000 mg/day) and all of whom were added empagliflozin to their current treatment, were retrospectively evaluated. Study population were not on any kind of antihyperlipidemic treatment and during the 12-week period of the study, no dose or drug changes were made in their present antihypertensive drugs.
Exclusion Criteria
Patients with the acute coronary syndrome, cerebrovascular event, pregnancy, heart failure, chronic liver disease, renal function test abnormality, pregnancy and cancer,
Patients on treatments which may impair glucose metabolism, such as glucagon-like receptor agonist, antiobesity drugs and systemic or local steroid therapy,
Patients with known or suspected alcohol addiction and using narcotics or illegal drugs were excluded from this study.
Of the included 60 patients, 32 patients were treated with 10 mg/day of empagliflozin (group-1) and 28 subjects were administered 25 mg/day empagliflozin (group-2) in addition to metformin plus gliclazide. Patients in both groups continued their strict diet regimen and exercise programs. Fasting plasma glucose (FBG), urea, creatinine, HbA1c, total cholesterol, low-density lipoprotein cholesterol (LDL-cholesterol), high-density lipoprotein cholesterol (HDL-cholesterol), and triglyceride levels, estimated glomerular filtration rate (eGFR) (according to the Modification of Diet in Renal Disease), body weight, systolic (SBP) and diastolic blood pressure (DBP) measurements were recorded before and 12 weeks after empagliflozin treatment. Initial HbA1c level of ≥7% and ≤12% was allowed. Change in HbA1c and lipid profile from onset to 12th week, in and between empagliflozin treatment groups, was analyzed. This study also noted the frequency of urogenital tract infections during the course of empagliflozin treatment to detect safety issues.
Statistical Analysis
The characteristics of patients were evaluated with descriptive analysis. Descriptive analyses were presented using mean (±SD) or n (%), where appropriate. The categorical data of Group 1 and Group 2 were analyzed by Wilcoxon signed ranks test. Side effects, such as the presence of urinary system and genital infections, were evaluated with the use of McNemar test for Group 1 and Group 2. Between group comparisons were done using Mann-Whitney U test. The SPSS 23.0 version was used to perform the entire statistical analyses (IBM Corp., Armonk, NY, USA). A p-value under 0.05 was accepted as statistically significant.
RESULTS
Baseline data
Sixty patients with T2DM (32 female, 28 male) were included in this study. In Group 1, 13 patients were male and 19 were female, the mean age was 53.16±8.8 years and duration of diabetes was 15.4±7.1 years. In Group 2, 15 patients were male and 13 were female and the mean age was 52.5±7.5 years, the duration of diabetes was 14.6±6.8 years.
Efficacy
a. Glycaemic Control
In the first group, pre-empagliflozin treatment mean HbA1c was 10.3±2.5%, whereas in the second group, it was 9.6±1.7%. Both empagliflozin dose groups demonstrated statistically significant decreases in HbA1c from baseline to 12th week. The mean HbA1c decrease was 1.54±2.14% and 1.18±1.5% after treatment in the first and second group, respectively (p<0.001 and p=0.001, respectively) (Tables 1, 2). There was no statistically significant difference in HbA1c decreases among patients who used 10 and 25 mg of empagliflozin (p=0.935) (Table 3). The decrement in FPG at 12th week was significantly different from baseline treatments (Tables 1, 2). However, there was not a statistically significant difference in FPG among patients who used 10 and 25 mg of empagliflozin (p=0.739) (Table 3) (Fig. 1).
TABLE 1.
Clinical and demographic data in patients receiving empagliflozin therapy in Group 1
| Characteristic | Baseline treatment | Group 1 | p |
|---|---|---|---|
| Mean age, years (SD) | 53.16±8.8 | ||
| Sex, male/female, (%) | 13 (40.6%)/19 (59.4%) | ||
| Duration of diabetes, years (SD) | 15.4±7.1 | ||
| HbA1c, % | 10.3±2.5 | 8.7±1.9 | 0.000 |
| FPG, mg/dl (SD) | 206.5±91.3 | 167.5±58.5 | 0.013 |
| Creatinin, mg/dl (SD) | 0.75±0.14 | 0.73±0.14 | 0.428 |
| eGFR, mL/minper 1.73 m2 (SD) | 104.2±24.8 | 101.8±23.7 | 0.433 |
| Total cholesterol, mg/dL (SD) | 223.0±54.3 | 199.8±51.6 | 0.082 |
| LDL-cholesterol, mg/dL (SD) | 126.0±46.1 | 118.3±32.9 | 0.311 |
| HDL-cholesterol, mg/dL (SD) | 40.5±11.2 | 41.0±9.2 | 0.254 |
| Triglyceride, mg/dL (SD) | 295.0±206.4 | 236.8±125.5 | 0.150 |
| Weight, kg (SD) | 81.6±9.8 | 79.0±9.7 | 0.000 |
| SBP, mmHg (SD) | 136.2±8.2 | 129.3±4.9 | 0.000 |
| DBP, mmHg (SD) | 85.5±7.5 | 80.9±6.1 | 0.000 |
| Urinary tract infection (%) | 1/32 (%3.1) | 2/32 (6.2) | 0.985 |
| Genital infection (%) | 1/32 (%3.1) | 3/32 (9.3) | 0.625 |
Baseline treatment: Metformin (2000 mg/day) plus glyclazide (60 mg/day); SD: Standard deviation; FPG: Fasting plasma glucose; eGFR: Estimated glomerular filtration rate [using the Modification of Diet in Renal Disease (MDRD) equation]; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
TABLE 2.
Clinical and demographic data in patients receiving empagliflozin therapy in Group 2
| Compared parameters | Baseline treatment | Group 2 | p |
|---|---|---|---|
| Mean age, years (SD) | 52.5±7.5 | ||
| Sex male/female, (%) | 13 (%59)/9 (41) | ||
| Duration of diabetes, years (SD) | 14.6±6.8 | ||
| HbA1c, % (SD) | 9.6±1.7 | 8.4±1.4 | 0.001 |
| FPG, mg/dl (SD) | 217.1±75.5 | 180.3±50.4 | 0.006 |
| Creatinin, mg/dl (SD) | 0.76±0.10 | 0.80±0.11 | 0.088 |
| eGFR, mL/minper 1.73 m2 (SD) | 102.4±16.2 | 97.3±20.5 | 0.185 |
| Total cholesterol, mg/dL (SD) | 211.9±60.5 | 202.2±58.3 | 0.067 |
| LDL-cholesterol, mg/dL (SD) | 127.3±44.1 | 115.3±39.9 | 0.071 |
| HDL-cholesterol, mg/dL (SD) | 41.6±10.9 | 45.9±11.7 | 0.724 |
| Triglyceride, mg/dL (SD) | 208.9±140.9 | 206.1±145.8 | 0.820 |
| Weight, kg (SD) | 82.8±13.1 | 79.0±12.4 | 0.000 |
| SBP, mmHg (SD) | 137.2±8.6 | 127.6±5.0 | 0.000 |
| DBP, mmHg (SD) | 86.3±7.7 | 79.6±6.0 | 0.000 |
| Urinary tract infection (%) | 1 (%3.5) | 2 (%7.1) | 0.778 |
| Genital infection (%) | 1 (%3.5) | 2 (%7.1) | 0.778 |
Baseline treatment: Metformin (2000 mg/day) plus glyclazide (60 mg/day); SD: Standard deviation; FPG: Fasting plasma glucose; eGFR: Estimated glomerular filtration rate [using the Modification of Diet in Renal Disease (MDRD) equation]; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
TABLE 3.
Comparison of pre and post added empagliflozin therapy differences in parameters between Group 1 and Group 2
| Compared parameters | Change from baseline treatment | p | |
|---|---|---|---|
| Group 1 | Group 2 | ||
| Δ HbA1c, % | -1.54±2.14 | -1.18±1.5 | 0.935 |
| Δ FPG, mg/dl | -39.0±94.1 | -36.7±81.1 | 0.739 |
| Δ Creatinine, mg/dl | 0.01±0.10 | 0.03±011 | 0.067 |
| Δ eGFR, mL/min per 1.73 m2 | -2.4±14.5 | -5.1±17.6 | 0.068 |
| Δ Total cholesterol, mg/dL | -23.1±38.5 | -9.6±19.9 | 0.313 |
| Δ LDL-cholesterol, mg/dL | -7.6±36.0 | -12.0±21.2 | 0.299 |
| Δ HDL-cholesterol, mg/dL | 0.5±7.6 | 4.2±7.9 | 0.267 |
| Δ Triglyceride, mg/dL | -58.2±181.2 | -2.7±47.4 | 0.224 |
| Δ Weight, kg | -2.6±1.2 | -3.8±2,0 | 0.021 |
| Δ SBP, mmHg | -6.8±5.1 | -9.6±7.5 | 0.032 |
| Δ DBP, mmHg | -4.6±4.0 | -6.7±4.6 | 0.005 |
FPG: Fasting plasma glucose; HbA1c: Glycosylated haemoglobin; eGFR: Estimated glomerular filtration rate [using the Modification of Diet in Renal Disease (MDRD) equation]; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
FIGURE 1.
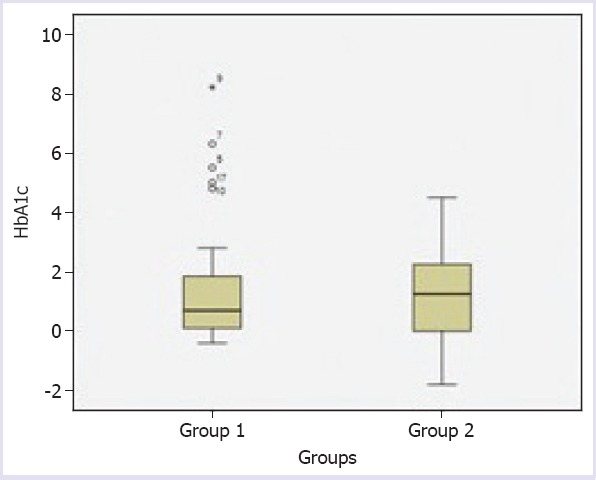
Comparison of pre- and post empagliflozin in mean HbA1c between Group 1 and Group 2.
b. Serum Lipids
Despite numerical differences from baseline in mean plasma lipid levels, serum levels of total, LDL-cholesterol, HDL-cholesterol and triglyceride did not show any significant changes for both groups after 12-weeks of empagliflozin treatment (p>0.05) (Tables 1, 2). There was no statistically significant difference relevant to the change in lipid parameters between patients who used 10 and 25 mg of empaglifosin, as well (p>0.05) (Table 3).
c. Blood Pressure
We observed statistically significant difference in systolic and diastolic blood pressure measurements between the beginning and 12th week of the empagliflozin treatment (p<0.05) (Tables 1, 2). When both empagliflozin groups were compared, there was a propensity of dose-dependent decline in blood pressure (p<0.05). The decrease in blood pressure in the 25 mg group of empagliflozin was −9.6±7.5 mmHg (systolic) and − 6.7±4.6 mmHg (diastolic), while the decrease in the group-1 was -6.8±5.1 mmHg (systolic) and -4.6±4.0 mmHg (diastolic) (for SBP p=0.032 and for DBP p<0.005) (Table 3, Fig. 2, 3).
FIGURE 2.

Comparison of pre- and post empagliflozin in mean systolic blood pressure (SBP) between Group 1 and Group 2.
FIGURE 3.

Comparison of pre- and post empagliflozin in mean diastolic blood pressure (DBP) between Group 1 and Group 2.
d. Body Weight
In the twelfth week, we noted a loss in body weight from baseline, with both low and high dose empagliflozin groups (p<0.001, for each) (Tables 1, 2).
The body weight losses of Group 1 and Group 2 were -2.6±1.2 and -3.8±2.0, respectively. When Group 1 and Group 2 were compared according to the amount of weight losses, there was a significant difference in support of the group using 25 mg of empagliflozin (p=0.021) (Fig. 4).
FIGURE 4.

Comparison of pre- and post empagliflozin in mean weight loss between Group 1 and Group 2.
Safety and Adverse Events
Mean creatinine and eGFR decreased in both Group 1 (p=0.428 and p=0.433, respectively) and Group 2 (p=0.088 and p=0.185, respectively) (Tables 1, 2) after empagliflozin treatment. There was not a significant difference in creatinine and eGFR decrease when the two groups were compared (p=0.067 and p=0.068, respectively). Although urogenital tract infections seem to be numerically increased in both groups, there was no statistically significant difference in incidence between the pre- and post-empagliflozin period (p>0.05) (Table 3).
DISCUSSION
The addition of both doses of empagliflozin as a third treatment agent to patients with poorly controlled T2DM receiving sulfonylureas plus metformin resulted in a significant HbAlc decline and no adverse effects on lipid parameters over 12 weeks. Our analysis revealed no significant difference between patients treated with two doses of empagliflozin.
In the study by Ferrannini et al. [10], a dose-dependent decrement in Hba1c was observed after 12 weeks of treatment duration with empagliflozin. Patients receiving 10 mg Empagliflozin showed a 0.5%, while the group on 25 mg empagliflozin showed a 0.6% of decrease. In our study, we found a statistically significant decrease in HbA1c levels in both dose groups compared to which was achieved by ongoing treatment of metformin plus gliclazide. Unexpectedly, HbA1c reduction was lower in the group that used 25 mg empagliflozin compared to the group of empagliflozin-10 mg. This might be attributed to the slightly higher level of baseline HbA1c in the empagliflozin-10 mg group than that of the group using 25 mg of empagliflozin.
Several trials have addressed the potential role of empagliflozin on lipid metabolism and most ended up with a negative effect [11–13]. Briand et al. [14] reported that empagliflozin moderately increased LDL cholesterol levels, ketone production and energy metabolism from carbohydrate to lipid use. Despite the existing evidence, in the present study, no significant change was documented in lipid profiles between the pre and post addition of the empagliflozin to the groups. A possible explanation could be the neutralization of the negative effect on the lipid profile via the positive effect in terms of Hba1c decrease which then, improved lipid metabolism given that the HbA1c decrease in our study is much more than the reported in many previous studies in the literature [10, 15].
Weight loss is an additional treatment target for most patients with T2DM and may increase adherence to treatment [16]. The potential loss of calories through urinary glucose excretion and the resulting decrease in body weight is a prominent characteristic of SGLT2 inhibitors [15]. Unexpectedly, Rosenstock et al., in their study comparing 10 and 25 mg of empagliflozin, stated that 10 mg of empagliflozin was more likely than the 25 mg of the drug to achieve weight loss [15]. It turns out that, in our study, although a significant loss in body weight was reached with both doses, high-dose empagliflozin seems to be a reasonable option, particularly for Type 2 DM patients in whom there appears to be a goal of more weight loss.
In our study, a statistically significant decrease in systolic and diastolic blood pressure at both doses is an indicator that empagliflozin acts as an antihypertensive agent with osmotic diuresis potential. The impact of SGLT2 inhibitors on the renin-angiotensin-aldosterone system is another possible way to take into account [15].
From the point of side effects, in the meta-analysis by Dai et al., the authors reported a significant increase in urogenital tract infections in patients with T2DM [17]. In our study, the number of the urinary tract and genital infections increased with the addition of both 10 mg and 25 mg of empaglifozin to the baseline treatment, but it was not found to be statistically significant.
The short follow-up duration of 12 weeks and a small sample size are among the potential limitations of this study. Selection bias might be another limitation of the study given the retrospective nature. Lastly, although no agent-dose change had been made in the antihypertensive drugs utilized by the patients, the usage of different agents is regarded as a limitation.
Conclusion
In this study investigating the two doses of empagliflozin added to metformin plus gliclazide therapy in patients with T2DM, we documented a dose-independent improvement on glycaemic control, the neutral effect on lipid metabolism and statistically insignificant side effect development. Empagliflozin was revealed to be a promising therapy concerning efficacy and tolerability in a daily practice population of T2DM.
Footnotes
Ethics Committee Approval: The ethics committee of Kartal Dr. Lutfi Kirdar Training and Research Hospital (date: 27.03.2019, number: 2019/514/150/24) approved the study protocol and the trial was directed in accordance with the Declaration of Helsinki.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – SO, MC, AV, BA; Design – SO, MC, AV, BA; Supervision – SO, MC, AV, BA; Fundings – SO, MC, AV, BA; Materials – SO, AV, BA; Data collection and/or processing – SO, AV, BA; Analysis and/or interpretation – SO, MC, BA; Literature review – SO, MC, AV, BA; Writing – SO, MC; Critical review – SO, MC, AV, BA.
REFERENCES
- 1.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes:a patient-centered approach Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–96. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 3.Morgan CL, Jenkins-Jones S, Evans M, Barnett AH, Poole CD, Currie CJ. Weight change in people with type 2 diabetes:secular trends and the impact of alternative antihyperglycaemic drugs. Diabetes Obes Metab. 2012;14:424–32. doi: 10.1111/j.1463-1326.2011.01552.x. [DOI] [PubMed] [Google Scholar]
- 4.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes:basic physiology and consequences. Diab Vasc Dis Res. 2015;12:78–89. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, et al. EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes:a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovacs CS, Seshiah V, Merker L, Christiansen AV, Roux F, Salsali A, et al. EMPA-REG EXTEND™PIO investigators. Empagliflozin as Add-on Therapy to Pioglitazone With or Without Metformin in Patients With Type 2 Diabetes Mellitus. Clin Ther. 2015;37:1773–88. doi: 10.1016/j.clinthera.2015.05.511. e1. [DOI] [PubMed] [Google Scholar]
- 7.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin:efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–36. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes 2015:a patient-centered approach:update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 9.Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor:characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:721–8. doi: 10.1111/dom.12081. [DOI] [PubMed] [Google Scholar]
- 11.Kohler S, Salsali A, Hantel S, Kaspers S, Woerle HJ, Kim G, et al. Safety and Tolerability of Empagliflozin in Patients with Type 2 Diabetes. Clin Ther. 2016;38:1299–313. doi: 10.1016/j.clinthera.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Halimi S, Vergès B. Adverse effects and safety of SGLT-2 inhibitors. Diabetes Metab. 2014;40:S28–34. doi: 10.1016/S1262-3636(14)72693-X. [DOI] [PubMed] [Google Scholar]
- 13.Lund SS, Sattar N, Salsali A, Crowe S, Broedl UC, Ginsberg HN. Potential relevance of changes in haematocrit to changes in lipid parameters with empagliflozin in patients with type 2 diabetes. Diabetologie und Stoffwechsel. 2016;11:135. [Google Scholar]
- 14.Briand F, Mayoux E, Brousseau E, Burr N, Urbain I, Costard C, et al. Empagliflozin, via Switching Metabolism Toward Lipid Utilization, Moderately Increases LDL Cholesterol Levels Through Reduced LDL Catabolism. Diabetes. 2016;65:2032–8. doi: 10.2337/db16-0049. [DOI] [PubMed] [Google Scholar]
- 15.Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–60. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 16.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, et al. EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes:a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–19. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 17.Dai X, Luo ZC, Zhai L, Zhao WP, Huang F. Adverse Drug Events Associated with Low-Dose (10 mg) Versus High-Dose (25 mg) Empagliflozin in Patients Treated for Type 2 Diabetes Mellitus:A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Ther. 2018;9:753–70. doi: 10.1007/s13300-018-0399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


