Abstract
Acute heat shock has previously been shown to improve subsequent low O2 (hypoxia) tolerance in an intertidal fish species, a process known as cross-tolerance, but it is not known whether this is a widespread phenomenon. This study examined whether a rock pool specialist, the triplefin fish Bellapiscis medius, exhibits heat shock induced cross-tolerance to hypoxia, i.e., longer time to loss of equilibrium (LOE) and lower critical O2 saturation (Scrit) after recovering from an acute heat challenge. Non-heat shock controls had a median time to loss of equilibrium (LOE50) of 54.4 min under severe hypoxia (7% of air saturation) and a Scrit of 15.8% air saturation. Contrary to expectations, however, treatments that received an 8 or 10°C heat shock showed a significantly shorter LOE50 in hypoxia (+8°C = 41.5 min; +10°C = 28.7 min) and no significant change in Scrit (+8°C = 17.0% air saturation; +10°C = 18.3% of air saturation). Thus, there was no evidence of heat shock induced cross-tolerance to hypoxia in B. medius because exposure to acute heat shock impaired hypoxia tolerance.
Introduction
Marine organisms commonly have to contend with fluctuating environmental conditions (e.g. temperature, salinity, and oxygen) severe enough to elicit a physiological stress response. Moreover, environmental parameters rarely occur in isolation, so organisms must endure multiple environmental conditions that fluctuate either simultaneously or sequentially [1]. The response of organisms to multiple stressors can be complex and is largely dependent on the temporal pattern of exposure between each stressor. For example, when organisms experience multiple stressors simultaneously or in rapid succession, the combined negative effect is sometimes amplified (synergistic) relative to the additive effects of the individual stressors acting in isolation [1–3]. However, if there is a sufficient period of recovery between exposures, the combined negative effect of the stressors can be equal to the sum of the individual stressor effects (additive) or even reduced (antagonistic) [1,2]. In the case of an antagonistic response, exposure to one stressor can sometimes increase the tolerance of an organism to a second stressor; this phenomenon is known as cross-tolerance [4].
Due to the interaction between semi-diurnal tidal patterns and diel changes in air temperature and solar irradiance, certain physico-chemical parameters in intertidal rock pools tend to fluctuate out of phase and sequentially. Thus, for rock pool organisms such as intertidal fish, cross-tolerance could offer protection against different environmental stressors across consecutive tidal cycles. For example, temperature and oxygen availability in rock pools fluctuate widely, but when low tides roughly align with the middle of the day and night, peak daytime high temperatures occur out of phase with nocturnal hypoxia [1,5–9]. This is because the conditions associated with high temperature in isolated rock pools in the middle of the day (i.e. strong solar radiation) also promotes algal photosynthesis, which buffers against O2 depletion, and can even cause hyperoxia (O2 super-saturation). On the other hand, severe hypoxia can develop in isolated pools at night (i.e. only 8–11 h later) when respiring organisms draw down O2 at a time of comparatively stable temperatures [8]. Previous studies have demonstrated that high temperature exposure can induce cross-tolerance to hypoxia in fish. This includes examples where long-term (4–6 weeks) acclimation to a constant warm temperature results in an improved ability to tolerate hypoxia [10,11], and also examples where a one-off acute high temperature exposure (hours) has induced hypoxia cross-tolerance [12].
Rock pool habitats are prone to bouts of extreme high temperature followed by severe hypoxia, but only one study has addressed the tolerance of intertidal fish to this set of conditions [12]. Todgham et al., (2005) [12] showed that an intertidal species of sculpin (Oligocottus maculosus) had elevated tolerance (cross-tolerance) to both high salinity and hypoxia after recovering from an acute heat shock (2 h duration). With respect to measuring hypoxia cross-tolerance, these authors showed that fish exposed to an initial bout of acute heat shock had greater survival under subsequent exposure to severe hypoxia. It also appeared that cross-tolerance to high salinity could be maintained for quite a long period of time after heat shock (i.e. 8–48 h), and that the magnitude of heat shock was also important because high salinity cross-tolerance only developed with a +12°C heat shock and not a +15°C heat shock. Todgham et al. (2005) also examined the role of heat shock protein (Hsp) induction in conferring cross-tolerance. While these authors concluded that elevated Hsp were not required at the time of exposure to a second stressor to confer cross-tolerance, there was evidence that an initial heat shock primed the gills to respond to high salinity and the liver to hypoxia with an altered Hsp response. As 8 h was the minimum recovery time for cross-tolerance to develop in O. maculosus, and this period matched the approximate time between successive periods of low tide emersion in the intertidal zone, it was suggested that a protective effect of cross-tolerance might be relevant for fish under real life conditions [12].
In order to build upon the findings of Todgham et al., (2005) [12], the present study examined cross-tolerance in another specialist intertidal fish (Bellapiscis medius, Günther, 1861) to assess whether heat shock induced cross-tolerance is a common response in rock pool fishes. To assess the presence of cross-tolerance in B. medius, the hypoxia tolerance of this species was assessed after a ~19 h period of recovery from either a +8°C (21–29°C) or +10°C (21–31°C) heat shock treatment over a 5 h ramping period. Todgham et al. (2005) [12] observed a peak in cross-tolerance 12–24 h following exposure to heat shock in O. maculosus; thus, as cross-tolerance was only assessed at one time point in the current study, we chose a recovery time (19 h) within the timeframe of peak cross-tolerance in their study. The +8°C heat shock (21–29°C) was used because peak temperatures of this magnitude have been observed in rock pools inhabited by B. medius on hot summer days [8]. The more extreme +10°C (21–31°C) heat shock was used to gauge how fish would cope with a future climate change scenario, where peak daytime temperatures may become higher in rock pools. Two measures of hypoxia tolerance were also employed. Firstly, the time to loss of equilibrium (LOE) under severe hypoxia (O2 = 7% air saturation) was measured. The hypoxia exposure of 7% air saturation was used as it is below previously reported critical oxygen tension (11% air saturation at 15°C; 25% air saturation at 25°C) for B. medius [13]. Thus, 7% air saturation was expected to be approximately 50% of critical oxygen tension at the temperature (21°C) used in the in the current study and represent a severe hypoxia challenge. As time to LOE is a standard proxy measure of hypoxia survival in fish [14,15], this allowed us to make comparisons to the findings of Todgham et al., (2005) [12] who demonstrated cross-tolerance as lower morbidity under hypoxia. It was therefore predicted that time to LOE under severe hypoxia would increase in B. medius after heat shock. Secondly, this study also measured the critical oxygen saturation (Scrit) of B. medius after heat shock because, although it is a commonly used indicator of hypoxia tolerance in fish [16,17], Scrit was not measured by Todgham et al. (2005) [12]. Scrit represents the change in O2 consumption as fish transition from being an O2 regulator to an O2 conformer under progressive hypoxia exposure [16,17]. Among sculpins, species with a lower Scrit showed LOE after longer periods under exposure to severe hypoxia [14]. Therefore, under the expectation that B. medius would show evidence of cross-tolerance (longer time to LOE), it was predicted that Scrit would decline after heat shock. This would indicate that heat shock treated fish could maintain resting O2 consumption to lower O2 levels, and also provide insight as to whether cross-tolerance to hypoxia operates via mechanisms associated with modifications to the cardio-respiratory cascade. Despite the widespread use of Scrit as a measure of hypoxia tolerance in fish [16], to our knowledge, the effect of prior acute heat shock on Scrit following a period of recovery (~19 h) at ambient temperature has never been addressed.
Materials and methods
All experiments were carried out at the Leigh Marine Laboratory, and the fish used were collected from rock pools (Hatfields Beach, Auckland, New Zealand, 36°34’S, 174°41’E) using hand nets. No access permit was required for the collection site as it is open to the public. Following capture, fish were housed in 30 L flow-through seawater tanks (air saturated, 200 μm filtered, 35 ppt salinity) and acclimated to constant temperature (~21°C), and a 12 h light-dark photoperiod for at least four weeks prior to experiments. Fish were fed daily on a mixture of crushed aquaculture feed (Skretting, Cambridge, TAS, Australia) and pilchard, and food was withheld for a period of 48 h prior to the start of experiments. All statistical analysis was performed using the Sigma Plot 13.0 software package and significance was accepted at P<0.05. The experiments were carried out under the approval of the University of Auckland Animal Ethics Committee (AEC approval number 001801) and all experiments were performed in accordance with relevant guidelines and regulations. Experiment 1 used LOE (i.e., the point when a fish can no longer maintain an upright position) as a proxy measure of survival. The fish used in this study maintained ventilation at LOE, and recovered rapidly (i.e. returned to an upright position) once returned to fully oxygenated conditions. As such, there was no mortality associated with LOE, and all animals were subsequently released to the wild. Nevertheless, fish were monitored daily for humane endpoints for 5 days following experimentation. The criteria for humane endpoints following a manipulation included: continued loss of equilibrium up to 24 h; loss of appetite and visible weight loss; prolonged erratic swimming, disorientation and tank collisions; and continued signs of infection, sores, or bleeding for up to 24 h. The planned method of euthanasia for any animal reaching humane endpoints and deemed irrecoverable was an overdose of anaesthetic (Aqui S 200mg/L). All authors have completed compulsory animal welfare training modules provided by the University of Auckland Animal Ethics Committee.
Experiment 1: Cross-tolerance for survival of severe hypoxia following heat shock
Experiment 1 aimed to determine if a prior heat shock exposure influences the ability of the intertidal triplefin B. medius to survive severe hypoxia. The time to loss of equilibrium (LOE) under a constant severe hypoxia exposure (~7% of air saturation) was therefore measured in three experimental groups that either did or did not receive an initial heat shock. This included: (1) an ambient temperature (21°C) control group not exposed to heat shock (body mass = 2.13 g ± 0.14, N = 12), (2) a group exposed to a +8°C (~21–29°C) heat shock (body mass = 1.95 g ± 0.16, N = 12) and (3) a group exposed to a +10°C (~21–31°C) heat shock (body mass = 1.92 g ± 0.15, N = 12). There was no difference in body mass of fish in each experimental group (ANOVA, DF = 2, F = 0.56, P = 0.58). The heat shock exposure was carried out in a 50 L aquarium in which seawater was heated with aquarium heaters. At ~09:00, fish were transferred from holding tanks to the thermal ramping tank where they were held at 21°C for an hour. The water temperature was then gradually heated from 21°C, to either 29 or 31°C, over a 5 h period (see S1 Table for the exact rate of temperature change in each experimental run). The control group was handled identically to the +8°C and +10°C treatments, but instead of facing acute heating in the heat shock exposure aquarium, they were held at ambient temperature (21°C) for 5 h. After heat shock, fish were transferred to a 50 L tank supplied with ambient temperature (~21°C) flow through seawater. The fish were then allowed to recover overnight for ~19 h before being subjected to a hypoxic challenge (~7% of air saturation, see S1 Table). Hypoxia was achieved by bubbling seawater with N2 until ~7% of air saturation was reached (7–10 min of continuous bubbling). N2 was then bubbled in short bursts to maintain O2 at 7%. Fish were immediately removed from hypoxia once LOE occurred and placed in air saturated seawater to recover. Two groups of 6 fish were run separately for each of the three experimental treatments (i.e. a total of 6 experimental runs; see S1 Table for the details of each run).
Experiment 2: Cross-tolerance for hypoxia following heat shock (critical oxygen saturation-Scrit)
The purpose of experiment 2 was to determine if a prior heat shock exposure induced changes in the critical oxygen saturation (Scrit) point of the intertidal triplefin B. medius. Scrit was assessed in three experimental groups: (1) an ambient temperature (21°C) control group not exposed to heat shock (body mass = 1.78 g ± 0.08, N = 10), (2) a group exposed to a +8°C (~21–29°C) heat shock challenge (body mass = 2.24 g ± 0.24, N = 10), and (3) a group exposed to a +10°C (~21–31°C) heat shock challenge (body mass = 2.06 ± 0.22, N = 8). There were no differences in body mass of fish in each experimental group (ANOVA, DF = 2, F = 1.65, P = 0.21), and the heat shock exposures were implemented following the same protocol as experiment 1. After heat shock, fish were transferred to respirometry chambers to assess Scrit using automated intermittent-flow respirometry (see McArley et al., (2018) [8] for detailed respirometry methods). Briefly, respirometers consisted of a cylindrical acrylic chamber fitted with an adjustable stopper, which allowed the chamber volume (60–110 ml) to be adjusted to match fish size. The inlet of each chamber was connected to an automated Eheim compact 3000 submersible flush pump (Eheim GmbH & Co. KG, Deizisau, Germany) controlled by custom-coded software (Leigh Marine Laboratory). An inline pump (modified Eheim compact 3000) was connected to the respirometry chamber in a closed loop to ensure adequate water mixing, and the oxygen concentration of water within the chamber was continuously measured using contactless sensor spots and FireSting O2 meters (PyroScience, Aachen, Germany). The R2 for the decline in O2 within the respirometer during the closed phase was >0.99 for the majority of measurement cycles and never <0.95.
The fish were first allowed to recover in the respirometers overnight at ~21°C (i.e., for 19 h after heat shock). During this recovery phase, mass specific oxygen consumption () was measured over repeated 8 min measurement cycles interspersed with 1 min flushing and wait periods (i.e. intermittent stop-flow respirometry). The standard metabolic rate (SMR) was estimated as the mean of the lowest 10% of values recorded in the overnight recovery period [18–20]. Following overnight recovery, a run of progressive hypoxia was initiated (~75, 55, 40, 30, 25, 20, 15, 10, 6% of air saturation) to resolve Scrit by identifying the oxygen saturation at which fish could no longer maintain above SMR [17]. Progressive hypoxia exposure began between 18–19.5 h following the conclusion of heat shock and was completed in ~4.5 h (see S2 Table). Temperature was maintained at ~21°C and N2 was bubbled into the water reservoir supplying respirometers to achieve each level of hypoxia. Three measurements were made at 75–20% of air saturation and one measurement was made at 15, 10 and 6% of air saturation. To determine Scrit, SMR and at each level of hypoxia were plotted against water oxygen air saturation, and a linear regression forced through zero was performed between values which fell below SMR. The point where the regression line intersected with SMR was taken as the Scrit for an individual fish [21–24]. Scrit in each of the treatment groups was assessed over 3–4 separate experimental runs (see S2 Table for the details of each experimental run).
As background oxygen consumption was evident within the respirometry chambers by the end of experimental runs, background respiration for each measurement cycle was back calculated using linear regression and subtracted from . The mean level of background respiration was equivalent to 4.7, 5.0, and 6.3% of in the control, +8°C heat shock and +10°C heat shock treatment groups respectively. There was no difference in the amount of background respiration among treatment groups (ANOVA, DF = 2, F = 2.18, P = 0.13).
Statistics
In experiment 1, there were no differences in the time to LOE between experimental runs of the same treatment group, and data were pooled for subsequent analysis (N = 12 per treatment). Median loss of equilibrium time (LOE50) among treatments was assessed using Kaplan-Meier log-rank survival analysis and one-way ANOVA with Holm-Sidak post-hoc comparisons [15]. In experiment 2, there was a significant correlation between SMR and Scrit in all treatment groups (P = 0.004). Thus, analysis of covariance (ANCOVA) with Holm-Sidak post-hoc comparisons was used to determine if there were differences in Scrit among treatments. SMR was set as the covariate, and Holm-Sidak comparisons were used for post-hoc tests. SMR was compared among the control and heat shock treatments using one-way analysis of variance (ANOVA) with Holm-Sidak post-hoc comparisons. Statistical analysis was carried out using the SigmaPlot 14 and IBM SPSS Statistics 26 software packages, and significance was accepted at P<0.05.
Results
Experiment 1: Loss of equilibrium under a hypoxic challenge following acute heat shock
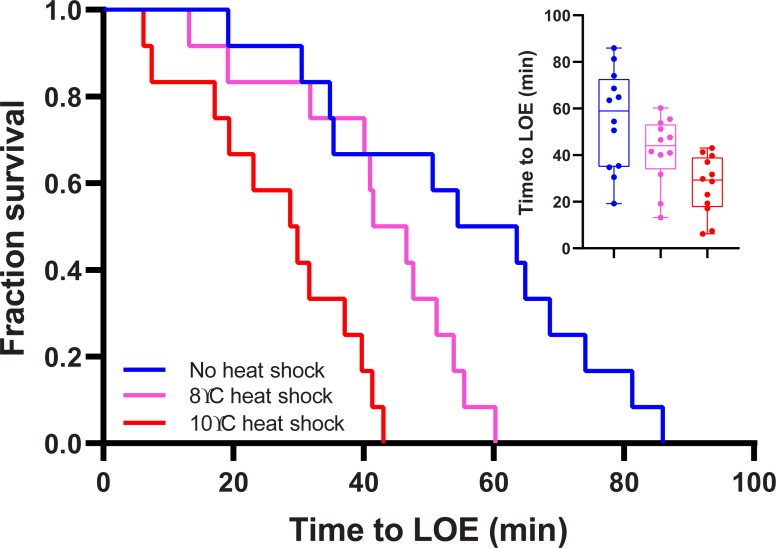
During severe hypoxia exposure (~7% of air saturation) LOE occurred after 19–85 min in the control group, after 13–60 min in the +8°C heat shock group, and after 6–43 min in the +10°C heat shock group (Fig 1). The survival curves for time to LOE were significantly different among treatment groups (Log-Rank test, DF = 2, Statistic = 19.52, P<0.001). Post-hoc comparisons showed the median time to loss of equilibrium was significantly shorter in the +8°C heat shock group (LOE50 = 41.5 min) compared to the control group ((LOE50 = 54.4 min), and significantly shorter again in the +10°C heat shock group (LOE50 = 28.7 min) compared to both the control and +8°C heat shock group (Fig 1).
Fig 1. Survivorship curves and time to loss of equilibrium (LOE) in Bellapiscis medius exposed to severe hypoxia (7% air saturation) after 19 h recovery from acute heat shock.
Blue line = control group (no heat shock), pink line = +8°C heat shock (21–29°C), red line = +10°C heat shock (21–31°C). Inset shows median time to loss of equilibrium and individual data points (N = 12 per treatment group). Different lower case letters in the inset indicate significant differences (P<0.05) in the median time to LOE among treatment groups.
Experiment 2: Critical oxygen saturation (Scrit) following acute heat shock
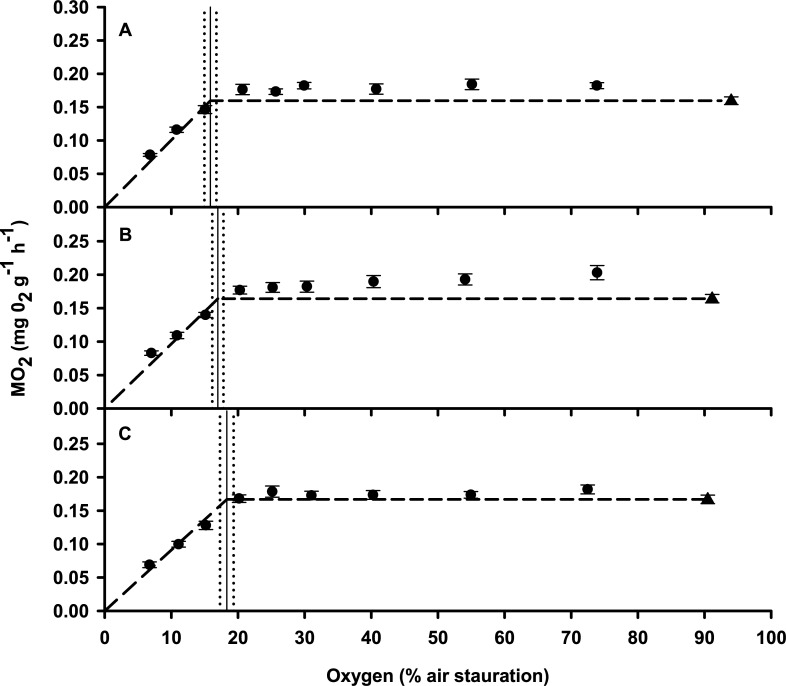
The of fish was elevated in all treatment groups following entry to respirometers but had fallen to within 1 standard deviation of SMR after 8–11 h recovery. After overnight recovery in the respirometers, there was no difference in SMR between the control fish (0.16 mg O2 g-1 h-1 ± 0.005) and the fish exposed to an +8°C (0.16 mg O2 g-1 h-1 ± 0.006) or +10°C heat shock (0.16 mg O2 g-1 h-1 ± 0.005) (ANOVA, DF = 2, F = 0.184, P = 0.83, Fig 2). remained stable in all treatment groups during progressive hypoxia before declining abruptly below SMR between 15 and 20% of air saturation (Fig 2). The Scrit of the control fish was 15.8% of air saturation (± 0.9), but was slightly higher in fish exposed to an 8°C (Scrit = 17% of air saturation ±0.85) or 10°C (Scrit = 18.3% of air saturation ± 1.03) heat shock (Fig 2). There was, however, no significant difference in Scrit between any of the treatments (ANCOVA, DF = 2, F = 2.36, P = 0.12).
Fig 2. Critical oxygen saturation (Scrit) and standard metabolic rate (SMR) of Bellapiscis medius following recovery from acute heat shock.
All values represent mean ± S.E.M. A = control (N = 10), B = +8°C heat shock (N = 10), C = +10°C heat shock (N = 8). Triangles show SMR under normoxia, circles show routine under progressive hypoxia exposure, vertical line shows Scrit and vertical dotted line shows Scrit S.E.M. The dashed horizontal line shows the break point between oxygen regulation and oxygen conformation and is for illustrative purposes only.
Discussion
Knowledge relating to the impacts of multiple stressors is crucial to understanding the tolerance of marine organisms to real life environmental conditions [1–3]. The majority of studies have applied stressors simultaneously to assess organismal tolerance to multiple stressors, but this does not always represent ecologically relevant conditions because in many habitats different stressor types occur asynchronously [1]. Todgham et al., (2005) [12] sequentially exposed rock pool sculpins to high temperature then hypoxia, and provided evidence of cross-tolerance as fish showed improved hypoxia tolerance 8 h after recovering from the initial heat shock. Sequential stressor exposures are highly relevant for intertidal fish because, in rock pools, critically high temperatures can occur with intense solar radiation during daytime low tides, but then during the next low tide (~8–11 h later) severe hypoxia can develop under cooler night-time conditions [7–9]. However when examining the response of B. medius, a rock pool specialist within the family of New Zealand triplefin fishes, to sequential high temperature then hypoxia exposure we found no evidence of heat shock induced cross-tolerance.
Based on evidence of cross-tolerance from the study of Todgham et al., (2005) [12], our predictions were that the LOE50 of B. medius under severe hypoxia would increase, and that the Scrit would decrease after recovery from acute heat shock (i.e. B. medius would be preconditioned). However, neither prediction was supported because the LOE50 of B. medius decreased and Scrit showed no significant change. These findings therefore reveal no evidence for cross-tolerance in B. medius after acute exposure to peak temperatures observed in rock pools. In fact, the direction of change in LOE50 suggested that the hypoxia tolerance of B. medius was impaired by an initial heat shock. We cannot, however, discount the possibility that cross-tolerance is induced following exposure to less extreme heat shock (i.e. less than +8°C). Indeed, Todgham et al. (2005) [12] found the tolerance of sculpins to high salinity improved after a +12°C heat shock but not when a +15°C shock was applied. Thus, there may be a heat shock intensity threshold which, if surpassed, prevents the development of cross-tolerance. In the current study, the magnitude of heat shock exposures was based on field observations of temperature extremes in rock pools occupied by B. medius [8]. Moreover, fish were subjected to a thermal ramping protocol (i.e. increased temperature over 5 h) in order to replicate environmentally realistic heat shock in rock pools, where temperature gradually increases to a peak throughout the duration of low tide emersion. Thus, the concern raised by the current study is that B. medius showed a reduced tolerance to hypoxia after heat shock representative of current day scenarios (+8°C heat shock treatment) and hypoxia tolerance was further impaired in near-future warming scenario (+10°C heat shock treatment). This is a cause-for-concern as climate change is predicted to increase the frequency and intensity of heat wave events [25], which may result in more extreme rock pool temperatures in the near future and render B. medius less capable of coping with multiple stressors across successive low tides. On top of more extreme heat wave events, climate change involves chronic increases in mean environmental temperature occurring over prolonged time periods (i.e., decades). Physiological plasticity and/or adaptive capacity in the face of chronic warming may allow species to acclimate or adapt and gain additional resilience to climate change [26]. Thus, to better understand the vulnerability of B. medius to global warming, future studies should assess the influence of chronic warming on tolerance to hypoxia and acute high temperatures.
The Scrit of fish subjected to acute heat shock remained unchanged, clearly demonstrating there is no adjustment in the capacity of B. medius to meet basal O2 demands under low O2 following overnight recovery from heat shock. Scrit is determined by an interaction between the extractive capacity of a fish’s cardio-respiratory system for O2 and minimal resting metabolic demand for O2 (i.e. SMR) [27,28]. Since Scrit and SMR were similar across treatments, prior exposure to heat shock neither enhanced, nor hindered the extractive capacity of the cardio-respiratory system for O2. Yet, despite no differences in Scrit, the LOE50 of B. medius under exposure to severe hypoxia (~7% air saturation) was still reduced after heat shock. It is important to note, however, that severe hypoxia in the current study exposed fish to an O2 level approximately 50% of Scrit. The ability of an organism to survive hypoxia below Scrit partly relies on utilising anaerobic ATP production to maintain energy balance [28]. Thus, a reduced ability to survive levels of hypoxia below Scrit might reflect either faster energy expenditure and/or lower availability of endogenous fermentable fuel stores such as glycogen. Since there was no difference in SMR between treatment groups a faster rate of energy expenditure in heat shock exposed fish seems unlikely. As blood lactate is raised in fish exposed to acute high temperatures [29], it is possible heat shock treated fish lost equilibrium faster because tissue glycogen stores were diminished when they faced hypoxia ~19 h later. Further studies assessing tissue glycogen dynamics following acute heat shock, however, are required to substantiate this hypothesis.
The studies of Hilton et al., (2008; 2010) [13,30] demonstrate a 1.7–2.1 fold increase in the Scrit of B. medius with an acute increase in temperature from 15°C to 25°C over 2 h. However, in both these previous studies, hypoxia tolerance was assessed when the 25°C (i.e. +10°C) heat shock was applied, so the large increases in Scrit were likely due to elevated metabolic costs at high temperatures. As shown by McArley et al., (2018) [8], an acute +10°C increase in rock pool temperature is only likely to occur during daytime low tides associated with direct solar radiation and high air temperatures, conditions which also promote algal mediated hyperoxia due to photosynthesis. Thus, while the results of Hilton and colleagues demonstrate that B. medius suffers a substantial impairment of Scrit when hypoxia and acute heat shock co-occur, this is probably a circumstance rarely, if ever, faced under natural conditions.
Whilst cross-tolerance was not seen in the current study it is important to discuss two limitations in our experimental design that may have impacted our findings. B. medius in this study were allowed ~19 h to recover from heat shock before facing hypoxia but, in reality, fish undergoing a natural tidal cycle in the wild would experience a shorter time in which to recover from heat shock. For example, in one rock pool inhabited by B. medius, nocturnal O2 levels fell to 20% air saturation approximately 10 h after the peak in daytime temperature [8]. We therefore cannot discount the possibility that a shorter 10 h recovery period after acute heat shock might reveal cross-tolerance in B. medius where it was absent in the current study. In reality, it is more likely that a shorter recovery would lead to greater level of cross-sensitivity, so future studies should replicate the natural temporal pattern of multiple stressor exposures with greater precision if a complete, ecologically relevant result is to be formed. A second limitation is that rock pools occupied by B. medius also become hyperoxic during acute high temperature events [8]. This is important because, when facing an acute heat challenge combined with hyperoxia, the aerobic metabolic scope of B. medius is not constrained to the same degree as it is under normoxia [8]. It is thus possible that an expanded aerobic scope under hyperoxia during heat shock could dampen the requirement for anaerobic metabolism, thus preserving tissue glycogen stores and permitting fish to survive longer during a subsequent hypoxic exposure. We note that hyperoxia mitigated the rise in plasma and muscle lactate levels in two Antarctic notothenioid fishes exposed to acute heat shock [31], but clearly future research is required to resolve this issue fully.
The present study shows that prior exposure to heat shock impairs the subsequent hypoxia tolerance of the intertidal triplefin B. medius, thus providing no evidence of heat shock induced cross-tolerance to hypoxia in this species. Instead, B. medius appears to be mildly cross-sensitive to hypoxia after acute heat shock exposure (+8°C) representative of a current day situation. Perhaps more concerning, a +10°C heat shock representing a future climate change scenario results in a more significant sensitisation to hypoxia. Overall, B. medius is likely to retain relatively normal levels of hypoxia tolerance under current day conditions, but if heat shock events intensify with climate change as expected, this might substantially reduce the ability of this species to tolerate multiple ecologically relevant stressors.
Supporting information
(XLSX)
(DOCX)
(DOCX)
Acknowledgments
Technical staff Peter Browne and Errol Murray are acknowledged for their assistance with design and fabricating the respirometry setups and John Atkins is acknowledged for design of the respirometry software.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
T.J.M. would like to acknowledge support from the University of Auckland Scholarship Office for doctoral funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gunderson AR, Armstrong EJ, Stillman JH. Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Annu Rev Mar Sci. 2016;8: 357–378. [DOI] [PubMed] [Google Scholar]
- 2.McBryan T, Anttila K, Healy T, Schulte P. Responses to temperature and hypoxia as interacting stressors in fish: implications for adaptation to environmental change. Integr Comp Biol. 2013;53: 648–659. 10.1093/icb/ict066 [DOI] [PubMed] [Google Scholar]
- 3.Crain CM, Kroeker K, Halpern BS. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett. 2008;11: 1304–1315. 10.1111/j.1461-0248.2008.01253.x [DOI] [PubMed] [Google Scholar]
- 4.Todgham AE, Stillman JH. Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr Comp Biol. 2013;53: 539–544. 10.1093/icb/ict086 [DOI] [PubMed] [Google Scholar]
- 5.Huggett J, Griffiths C. Some relationships between elevation, physico-chemical variables and biota of intertidal rock pools. Mar Ecol Prog Ser. 1986;29: 189–197. [Google Scholar]
- 6.Legrand E, Riera P, Pouliquen L, Bohner O, Cariou T, Martin S. Ecological characterization of intertidal rockpools: Seasonal and diurnal monitoring of physico-chemical parameters. Reg Stud Mar Sci. 2018;17: 1–10. [Google Scholar]
- 7.Morris S, Taylor AC. Diurnal and seasonal variation in physico-chemical conditions within intertidal rock pools. Estuar Coast Shelf Sci. 1983;17: 339–355. [Google Scholar]
- 8.McArley TJ, Hickey AJR, Herbert NA. Hyperoxia increases maximum oxygen consumption and aerobic scope of intertidal fish facing acutely high temperatures. J Exp Biol. 2018;221. [DOI] [PubMed] [Google Scholar]
- 9.Truchot J, Duhamel-Jouve A. Oxygen and carbon dioxide in the marine intertidal environment: diurnal and tidal changes in rockpools. Respir Physiol. 1980;39: 241–254. 10.1016/0034-5687(80)90056-0 [DOI] [PubMed] [Google Scholar]
- 10.Anttila K, Lewis M, Prokkola JM, Kanerva M, Seppanen E, Kolari I, et al. Warm acclimation and oxygen depletion induce species-specific responses in salmonids. J Exp Biol. 2015;218: 1471–1477. 10.1242/jeb.119115 [DOI] [PubMed] [Google Scholar]
- 11.McBryan TL, Healy TM, Haakons KL, Schulte PM. Warm acclimation improves hypoxia tolerance in Fundulus heteroclitus. J Exp Biol. 2016;219: 474–484. 10.1242/jeb.133413 [DOI] [PubMed] [Google Scholar]
- 12.Todgham AE, Schulte PM, Iwama GK. Cross-tolerance in the tidepool sculpin: the role of heat shock proteins. Physiol Biochem Zool. 2005;78: 133–144. 10.1086/425205 [DOI] [PubMed] [Google Scholar]
- 13.Hilton Z, Wellenreuther M, Clements K. Physiology underpins habitat partitioning in a sympatric sister‐species pair of intertidal fishes. Funct Ecol. 2008;22: 1108–1117. [Google Scholar]
- 14.Mandic M, Speers-Roesch B, Richards JG. Hypoxia tolerance in sculpins is associated with high anaerobic enzyme activity in brain but not in liver or muscle. Physiol Biochem Zool. 2013;86: 92–105. 10.1086/667938 [DOI] [PubMed] [Google Scholar]
- 15.Speers-Roesch B, Mandic M, Groom DJE, Richards JG. Critical oxygen tensions as predictors of hypoxia tolerance and tissue metabolic responses during hypoxia exposure in fishes. J Exp Mar Biol Ecol. 2013;449: 239–249. [Google Scholar]
- 16.Rogers NJ, Urbina MA, Reardon EE, McKenzie DJ, Wilson RW. A new analysis of hypoxia tolerance in fishes using a database of critical oxygen level (Pcrit). Conserv Physiol. 2016;4: cow012 10.1093/conphys/cow012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claireaux G, Chabot D. Responses by fishes to environmental hypoxia: integration through Fry's concept of aerobic metabolic scope. J Fish Biol. 2016;88: 232–251. 10.1111/jfb.12833 [DOI] [PubMed] [Google Scholar]
- 18.McArley TJ, Hickey AJ, Herbert NA. Chronic warm exposure impairs growth performance and reduces thermal safety margins in the common triplefin fish (Forsterygion lapillum). J Exp Biol. 2017;220: 3527–3535. 10.1242/jeb.162099 [DOI] [PubMed] [Google Scholar]
- 19.Norin T, Malte H, Clark TD. Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J Exp Biol. 2014;217: 244–251. 10.1242/jeb.089755 [DOI] [PubMed] [Google Scholar]
- 20.Khan J, Pether S, Bruce M, Walker S, Herbert N. Optimum temperatures for growth and feed conversion in cultured hapuku (Polyprion oxygeneios)—Is there a link to aerobic metabolic scope and final temperature preference? Aquaculture. 2014;430: 107–113. [Google Scholar]
- 21.Cumming H, Herbert N. Gill structural change in response to turbidity has no effect on the oxygen uptake of a juvenile sparid fish. Conserv Physiol. 2016;4: cow033 10.1093/conphys/cow033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schurmann H, Steffensen J. Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol. 1997;50: 1166–1180. [Google Scholar]
- 23.Cook DG, Iftikar FI, Baker DW, Hickey AJ, Herbert NA. Low-O2 acclimation shifts the hypoxia avoidance behaviour of snapper (Pagrus auratus) with only subtle changes in aerobic and anaerobic function. J Exp Biol. 2013;216: 369–378. 10.1242/jeb.073023 [DOI] [PubMed] [Google Scholar]
- 24.Behrens JW, Steffensen JF. The effect of hypoxia on behavioural and physiological aspects of lesser sandeel, Ammodytes tobianus (Linnaeus, 1785). Mar Biol. 2007;150: 1365–1377. [Google Scholar]
- 25.Perkins S, Alexander L, Nairn J. Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophys Res Lett. 2012;39. [Google Scholar]
- 26.Somero GN. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine 'winners' and 'losers'. J Exp Biol. 2010;213: 912–920. 10.1242/jeb.037473 [DOI] [PubMed] [Google Scholar]
- 27.Mandic M, Todgham AE, Richards JG. Mechanisms and evolution of hypoxia tolerance in fish. Proc Biol Sci. 2009;276: 735–744. 10.1098/rspb.2008.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards JG. Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. J Exp Biol. 2011;214: 191–199. 10.1242/jeb.047951 [DOI] [PubMed] [Google Scholar]
- 29.Clark TD, Sandblom E, Cox GK, Hinch SG, Farrell AP. Circulatory limits to oxygen supply during an acute temperature increase in the Chinook salmon (Oncorhynchus tshawytscha). Am J Physiol Regul Integr Comp Physiol. 2008;295: R1631–R1639. 10.1152/ajpregu.90461.2008 [DOI] [PubMed] [Google Scholar]
- 30.Hilton Z, Clements KD, Hickey AJ. Temperature sensitivity of cardiac mitochondria in intertidal and subtidal triplefin fishes. J Comp Physiol B. 2010;180: 979–990. 10.1007/s00360-010-0477-7 [DOI] [PubMed] [Google Scholar]
- 31.Devor DP, Kuhn DE, O’Brien KM, Crockett EL. Hyperoxia does not extend critical thermal maxima (CTmax) in white-or red-blooded Antarctic notothenioid fishes. Physiol Biochem Zool. 2016;89: 1–9. 10.1086/684812 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.