Abstract
Background:
Phase 2 cooperative group meningioma trial assessing the safety and efficacy of risk-adaptive management strategies. This is the initial analysis of the high-risk cohort.
Methods and Materials:
High-risk patients were those with a new or recurrent World Health Organization (WHO) grade III meningioma of any resection extent, recurrent WHO grade II of any resection extent, or new WHO grade II after subtotal resection. Patients received intensity-modulated radiotherapy (IMRT) using a simultaneous integrated boost technique (60 Gy high dose and 54 Gy low dose in 30 fractions). Three-year progression-free survival (PFS) was the primary endpoint. Adverse events (AEs) were scored per NCI Common Terminology Criteria for Adverse Events version 3.
Results:
Of 57 enrolled patients, 53 received protocol treatment. Median follow-up was 4.0 years (4.8 years for living patients). Two patients withdrew without progression before year 3; for the remaining 51 patients, 3-year PFS was 58.8%. Among all 53 protocol-treated patients, 3-year PFS was 59.2%. Three-year local control was 68.9%, and overall survival was 78.6%. Of 51 patients, 1 patient (1.9%) experienced a late grade-5 necrosis-related AE. All other acute (23 of 53 patients) and late (21 of 51 patients) AEs were grades 1 to 3.
Conclusions:
Patients with high-risk meningioma treated with IMRT (60 Gy/30) experienced 3-year PFS of 58.8%. Combined acute and late AEs were limited to grades 1 to 3, except for a single necrosis-related grade 5 event. These results support postoperative IMRT for high-risk meningioma and invite ongoing investigations to improve outcomes further. © 2019 Elsevier Inc. All rights reserved.
Summary
We report initial outcomes from the high-risk cohort of NRG Oncology/RTOG-0539, a phase 2 meningioma trial. All patients in the cohort received radiation therapy (RT, 60 Gy in 30 fractions). Three-year PFS was 58.8%, local control was 68.9%, and overall survival was 78.6%. All patients with progression had either in-field (92.9%) or in-field and marginal (7.1%) progression compared with RT planning target volume. Acute and late adverse events were limited to grades 1–3 with the exception of 1 necrosis-related grade 5 event. These results demand further investigations to improve outcomes for patients with high-risk meningioma.
Introduction
Meningiomas are the most common primary intracranial neoplasm, comprising 36.1% of central nervous system tumors; in comparison, glioblastomas account for 15.4%, and gliomas of any grade account for 28.4%.1 The detection of meningioma has increased, likely owing to improved imaging and an aging population rather than to actual increases in incidence.2 With improved adoption of modern World Health Organization (WHO) standards, most recently in 2016,3 the incidence of non-benign (WHO grades II and III) meningioma has increased from less than 10% to 25% to 35%, with WHO grade II tumors accounting for the majority of this increase.1,4–8
Classification and grading changes of such a magnitude confound the interpretation of series using legacy grading criteria, question their relevance to diagnosis with modern grading standards, and complicate consensus in guideline development. The Radiation Therapy Oncology Group launched this nonrandomized phase II cooperative group trial in 2009. Patients were assigned to 1 of 3 risk groups on the basis of 2007 WHO grade, resection extent, and recurrence status.
The present report concerns the high-risk cohort (group 3) composed of patients with a newly diagnosed or recurrent WHO grade III meningioma of any resection extent, a recurrent WHO grade II tumor of any resection extent, or a newly diagnosed WHO grade II meningioma after subtotal resection (STR). In support of this grouping, many reports have found that outcomes with WHO grade II meningioma are worse after either recurrence or STR, approximating those for patients with new or recurrent WHO grade III meningioma.9–19
Methods and Materials
This phase 2 cooperative group trial was approved by the institutional review boards and ethical committees at each participating study site, and documentation was received at the clinical trials central office. Each patient signed an approved informed consent before trial enrollment. The protocol is registered with ClinicalTrials.gov. The ClinicalTrials.gov identifier is NCT00895622.
Selection criteria
Adults 18 years of age or older with a unifocal histologically documented intracranial meningioma, with no history of cranial radiation therapy (RT), with a Zubrod Performance Status score of 0 to 1, and without severe active comorbidity were eligible for enrollment. WHO 2007 standards were used to assign tumor grade and subtype, which were confirmed for each patient with a central pathology review by one of the authors (AP).
Protocol registration
Registration on this phase 2 trial occurred in 2 steps. Step 1 was initial registration, followed by submission of pathology specimens for central review. Step 2 occurred after central pathology review and entailed protocol group assignment, after which protocol-specified treatment began.
Tumor grade and resection extent
This report pertains to the high-risk group (group 3), limited to patients with a newly diagnosed or recurrent WHO grade III meningioma of any resection extent, a recurrent WHO grade II meningioma of any resection extent, or a newly diagnosed WHO grade II meningioma after STR. Resection extent was assigned by the operating neurosurgeon using Simpson criteria20 in concert with postoperative magnetic resonance imaging (MRI) findings. Gross total resection (GTR) was defined as Simpson grades I to III. MRIs were reviewed centrally (see Acknowledgments).
Initial registration and central pathology review had to have been completed within 24 weeks of surgery. Not attaining this time frame was exclusionary. This interval was designed to permit, when desired, imaging at 3 to 4 months after surgery to confirm resection extent adequately and to facilitate, when desired, additional resection. Patients with a recurrent WHO grade II or III tumor not undergoing additional surgery had no such constraints for study participation. In these latter settings, additional resection or biopsy was not a prerequisite. Recurrence or progression was defined radiographically at the enrolling institution, with mandatory documentary MRI submission. If further biopsy or resection was performed, submission of specimens was required. If no additional resection was performed, submission of specimens from the prior resection was required.
Radiation therapy
Patients were assigned to receive intensity-modulated RT (IMRT) using simultaneous integrated boost: the higher-dose volume 60 Gy in 30 fractions and lower-dose volume 54 Gy in the same 30 fractions. RT was initiated in each case within the requisite time frame.
The gross tumor volume (GTV) was the tumor bed on postoperative MRI inclusive of any nodular enhancement. This definition was applied even in the recurrent setting without additional surgery, in which case the tumor bed was the previous operative site. Reference to prior imaging was recommended for GTV definition but was not strictly mandated. Neither cerebral edema nor dural tail was specifically included within the GTV. Based on the GTV, 2 clinical target volumes (CTVs) were defined. The CTV60 was the GTV plus 1.0 cm, and the CTV54 was the GTV plus 2.0 cm. It was permissible to reduce the CTV54 margins to 1.0 cm (corresponding to the CTV60 margin) around natural barriers such as the skull, excepting hyperostotic or invaded bone, which were included within the GTV. Planning target volume (PTV) margins of 0.3 to 0.5 cm were added to the CTVs.
Each organ at risk (OAR) was delineated with a planning risk volume (PRV) defined as the OAR plus 0.3 cm. In the event that an OAR was in immediate proximity to a PTV, such that OAR doses could not be constrained within protocol limits, a second PTV (termed the PTVPRV) was permissible and was defined as the overlap between the PTV and the PRV of concern. Dose to the PTVPRV was to be as close as permissible to the respective PTV dose (eg, 60 Gy for PTV60, 54 Gy for PTV54) while not exceeding the predefined normal tissue dose constraint.
Patient assessment
Pretreatment evaluation included a history and physical examination, documentation of steroid and other hormonal agent use and dose, and brain MRI (T1 before and after contrast, T2, and fluid-attenuated inversion recovery) within 12 weeks before step 2 registration. Preoperative and postoperative MRI was essential for new diagnoses, as was postoperative MRI within 12 weeks of surgery. Confirmatory imaging was permitted provided that registration and central pathology review were completed within 24 weeks of surgery. In the setting of recurrent or progressive meningioma without salvage surgery, MRI documentation was required. The determination of progression was at the discretion of the enrolling site, whereas centrally confirmed progression was used for statistical analysis.
A posttreatment clinical assessment was required 1 month after RT, every 3 months for 3 years, and then at least yearly for 10 years. Mini-Mental State Examination and documentation of corticosteroid and other hormonal agent use followed the same schedule. Response was evaluated according to criteria similar to RECIST (Response Evaluation Criteria in Solid Tumors), modified to better apply to meningioma. Continual lack of evidence of disease was ascribed when there was no measurable meningioma, stable disease when measurable tumor remained unchanged or increased in maximum diameter less than 20%, and progressive disease when tumor increased in any diameter by 20% or more. Adverse event (AE) evaluations, per Common Terminology Criteria for Adverse Events version 3, were at least every 3 months for 3 years from registration and then yearly for 10 years. Brain MRI was stipulated at 3 months after RT, at least every 6 months for 3 years, and thereafter at least yearly for 10 years.
Statistical methodology
The primary endpoint was 3-year PFS. Considering historical data, 3-year PFS with surgery and RT was estimated at 50%.21–23 With a 1-sided significance level of 0.05, a sample of 50 eligible patients would provide a statistical power of >90% to detect a 20% absolute increase using a 1-sample test on proportion. This required accrual of 55 patients to adjust for a 10% ineligibility rate. PFS was measured from step 1 registration to progression or death, or the date of last follow-up on which the patient was alive and free of progression.
Secondary endpoints reported herein are overall survival (OS), the incidence of grade 2+ adverse events, and adherence to protocol-specific radiation therapy guidelines. Secondary endpoints to be separately or subsequently reported are concordance between parent and central histopathologic diagnosis, subtyping, and grading; histopathologic correlates of PFS (eg, light microscopy, immunohistochemical analysis, molecular, and microarray analyses); and imaging (MRI) predictors of progression via central neuroradiology review.
OS was measured from study entry to death or to the date of last follow-up on which the patient was alive. Survival outcomes were estimated using the Kaplan-Meier method.24 Time to tumor progression was estimated using the cumulative incidence function, with death without progression as a competing risk. Grade 2+ acute and late AEs for dermatologic/skin (excluding alopecia), neurologic, and ocular or visual categories were reported. Acute AEs were those that occurred ≤90 days from the start of RT, and late AEs occurred >90 days from the start of RT.
Results
Patient characteristics, protocol enrollment, and treatment delivery
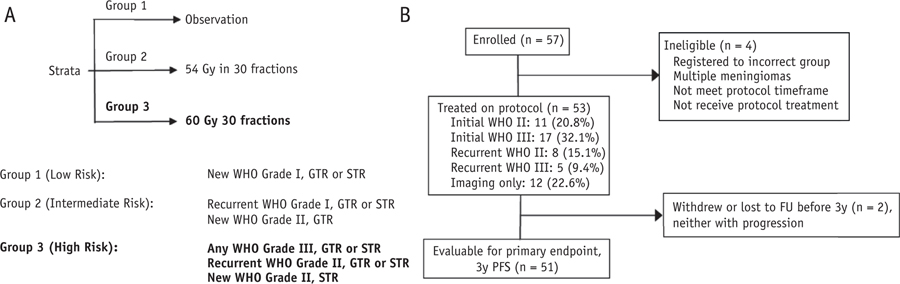
The study was activated June 19, 2009, and accrual for the high-risk group (group 3) was completed August 24, 2012. The analysis date for this report was December 3, 2016. Pathologic concordance findings were published separately.6 The protocol schema is shown in Figure 1A along with a CONSORT diagram (Fig. 1B) of the 57 patients who enrolled in group 3, of whom 53 (93.0%) were eligible and treated with protocol-specified RT (Table 1), and 51 (89.5%) were fully evaluable for the primary endpoint of 3-year PFS. Patient, tumor, and histopathologic characteristics shown in Table 1.
Fig. 1.
(A) Schema for the protocol and (B) CONSORT diagram for high-risk meningioma patients (group 3). Abbreviations: FU = follow-up; GTR = gross-total resection; PFS = progression-free survival; STR = subtotal resection. Figure 1A is modified from Rogers et al.42 Used with permission from Journal of Neurosurgery. Figure 1B is used with permission from NRG Oncology.
Table 1.
Study A group 3 (high-risk meningioma) patient and tumor characteristics (n = 53)
| Characteristic | n | % |
|---|---|---|
| Age, years | ||
| ≤49 | 11 | 20.8 |
| 50–59 | 15 | 28.3 |
| 60–69 | 15 | 28.3 |
| ≥70 | 12 | 22.6 |
| Sex | ||
| Female | 28 | 52.8 |
| Male | 25 | 47.2 |
| Zubrod performance status score | ||
| 0 | 28 | 52.8 |
| 1 | 25 | 47.2 |
| Neurologic function | ||
| No symptoms | 23 | 43.4 |
| Minor symptoms | 21 | 39.6 |
| Moderate symptoms | 9 | 17.0 |
| Race | ||
| American Indian or Alaska Native | 1 | 1.9 |
| Asian | 2 | 3.8 |
| Black or African American | 10 | 18.9 |
| Pacific Islander | 1 | 1.9 |
| White | 37 | 69.8 |
| Unknown or not reported | 2 | 3.8 |
| Histology | ||
| Initial WHO grade II (STR) | 11 | 20.8 |
| Initial WHO grade III (GTR or STR) | 17 | 32.1 |
| Recurrent WHO grade II (GTR or STR) | 8 | 15.1 |
| Recurrent WHO grade III (any) | 5 | 9.4 |
| Recurrent WHO grade II/III by imaging only* | 12 | 22.6 |
| Aggressive features (n = 53) | ||
| ≤4 mitoses per 10 high-power fields | 37 | 69.8 |
| ≥20 mitoses per 10 high-power fields | 16 | 30.2 |
| Brain invasion | 26 | 49.1 |
| Sheeting | 40 | 75.5 |
| Small cells | 22 | 41.5 |
| Macronucleoli | 35 | 66.0 |
| Hypercellularity | 41 | 77.4 |
| Spontaneous necrosis | 32 | 60.4 |
| Anaplasia | 16 | 30.2 |
| Variant histology (focal, diffuse) | ||
| Chordoid | 4/1 | 7.5 / 1.9 |
| Clear cell | 7/0 | 13.2 / 0 |
| Papillary | 9/2 | 17.0 / 3.8 |
| Rhabdoid | 3/2 | 5.7 / 3.8 |
| Recurrence status | ||
| Initial diagnosis | 28 | 52.8 |
| Recurrent | 25 | 47.2 |
| Extent of resection | ||
| Initial, Simpson 1 | 6 | 11.3 |
| Initial, Simpson 2 | 6 | 11.3 |
| Initial, Simpson 3 | 4 | 7.5 |
| Initial, Simpson 4 | 12 | 22.6 |
| Recurrent, Simpson 1 | 3 | 5.7 |
| Recurrent, Simpson 2 | 4 | 7.5 |
| Recurrent, Simpson 3 | 2 | 3.8 |
| Recurrent, Simpson 4 | 4 | 7.5 |
| Recurrent, imaging only | 12 | 22.6 |
| Lateralization | ||
| Right | 20 | 37.7 |
| Left | 26 | 49.1 |
| Bilateral | 7 | 13.2 |
Abbreviations: = GTR = gross-total resection; STR = subtotal resection; WHO = World Health Organization.
Patients with recurrent meningioma by imaging only must have had a central pathology review–documented WHO grade II or III meningioma previously.
IMRT was mandatory for protocol enrollment, and 52 patients (98.1%) were treated accordingly; however, 1 patient (1.9%) received 3-dimensional conformal RT and represented a protocol deviation. All patients receiving IMRT were treated per protocol or with acceptable variation. A PTVPRV was used in 21 patients (40.4%, 21 of 52 for whom such data was available), principally to limit optic apparatus or brainstem dose, or both.
The median follow-up for eligible patients was 4.0 years (range, 0.2–6.3 years) and 4.8 years (range, 0.2–6.3 years) for those still alive. Of 53 patients receiving protocol treatment, 2 patients (3.8%) withdrew <3 years after study entry, both without disease progression.
Progression-free survival
Considering the 51 patients evaluable for the primary endpoint, 3-year PFS was 58.8%, comparable to the literature-based estimate of 50% (P = .13). Within 3 years, 21 of 51 patients (41.2%) experienced progression or died: 16 patients (31.4%) with tumor progression and 5 patients (9.8%) without progression. After 3 years, 5 additional PFS events occurred: 3 patients (5.9%) with disease progression and 2 patients (3.9%) without progression. For 53 patients receiving protocol-specified treatment, 3-year PFS was 59.2%, and 5-year PFS was 47.2% (Fig. 2A).
Fig. 2.

(A) Progression-free survival (PFS) and (B) time to progression for the 53 patients at high risk receiving protocol-specified treatment. The numbers accompanying each curve show annual actuarial values, from 1 to 5 years after study registration. For PFS, progression or death are considered events. Used with permission from NRG Oncology.
The numbers of patients within various subgroups are too small for statistically reliable comparisons. However, among 21 evaluable patients with GTR per central neuroradiology review, 3-year PFS was 57.1%, and 5-year PFS was 52.4%. After STR (n = 11), these results were 53.0% and 39.8%, respectively.
Patients with high-risk (either subtotally resected or recurrent) WHO grade II meningioma (n = 29, STR or recurrent) experienced a 57.1% 3-year PFS, versus 61.8% with WHO grade III (n = 24, new or recurrent, any resection extent). Five-year PFS for WHO grade II and grade III patients was 39.5% and 57.0%, respectively.
Time to progression
As depicted in Figure 2B, actuarial progression risk for the entire cohort was 31.1% at 3 years and 37.6% at 5 years. For patients with GTR by central review, 3-year progression risk was 33.3%, and 5-year risk was 38.1% (n = 21). With STR by central review, 3- and 4-year progression rates were each 47% (n = 11), with too few patients to estimate 5-year progression accurately. Realizing that WHO grade II patients in the high-risk cohort had either STR or recurrent tumor, 3-year progression was 35.7% for those with central pathology-determined WHO grade II (n = 29) versus 25.5% for those with WHO grade III (n = 24); 5-year progression was 43.6% and 30.2%, respectively.
Sites of progression
As determined by enrolling sites, 16 patients experienced disease progression within 3 years, including 14 (87.5%) exclusively within the 90% isodose surrounding the 60 Gy PTV, 1 (6.25%) marginal to the same isodose but within the larger 54 Gy PTV, and 1 (6.25%) scored as clinically progressive but died before imaging confirmation. Per central neuroradiology review, 14 experienced disease progression within 3 years, including 13 (92.9%) exclusively within the 54-Gy PTV and 1 (7.1%) with both in-field and marginal progression.
The discordance between study site and centrally reported progression included 1 patient scored by the site with imaging progression but who had not experienced progression per central review. The remaining patient, detailed earlier, had site-reported clinical progression but no imaging for central review. Both patients were scored as having experienced disease progression clinically.
Among 53 patients treated according to protocol, 7 patients (13.2%) had skull base meningioma, 44 patients (83.0%) had non–skull base meningioma, and 2 patients (3.8%) had site unspecified. Each of the 14 meningiomas with central review–determined progression or recurrence involved the convexity or falx; only 1 patient had progression that included the skull base.
Overall survival
Among the 53 patients receiving protocol therapy, 3-year OS was 78.6%, and 5-year OS was 59.1% (Fig. 3). Among 21 patients with GTR determined by central review who were evaluable for OS, 3-year OS was 81.0% and 5-year OS was 75.2%. For 11 patients with STR by central review, 3-year OS was 70.7%, and 5-year OS was 47.1%. With resection extent judged by the enrolling site, after GTR (n = 22) OS was 85.7% at 3 years and 75.6% at 5 years, and after STR (n = 18) it was 66.7% at 3 years and 53.3% at 5 years.
Fig. 3.

Overall survival for the 53 patients at high risk receiving protocol-specified treatment. The numbers accompanying the curve are annual actuarial results, from 1 to 5 years after study registration. Eighteen deaths were observed at the time of analysis. Used with permission from NRG Oncology.
Grade III versus recurrent WHO grade II subgroup comparisons
A post hoc analysis comparing newly diagnosed WHO grade III (n = 17) and recurrent WHO grade II (n = 8) was completed. PFS, OS, and time to progression were each numerically inferior for patients with recurrent WHO grade II meningioma: 3-year PFS was 45.0% with recurrent WHO grade II versus 64.7% with initial WHO III, and 5-year PFS was 30.0% versus 58.2%, respectively. Three-year OS for recurrent WHO grade II was 71.4% versus 82.4% for initial WHO grade III. Corresponding 5-year OS outcomes were 47.6% and 76.0%, respectively. Regarding time to progression, the 3-year cumulative incidence for recurrent WHO grade II was 55.0%, compared with 23.5% for initial WHO grade III. At 5 years, the respective progression rates were 70.0% and 30.0% (Fig. 4).
Fig. 4.
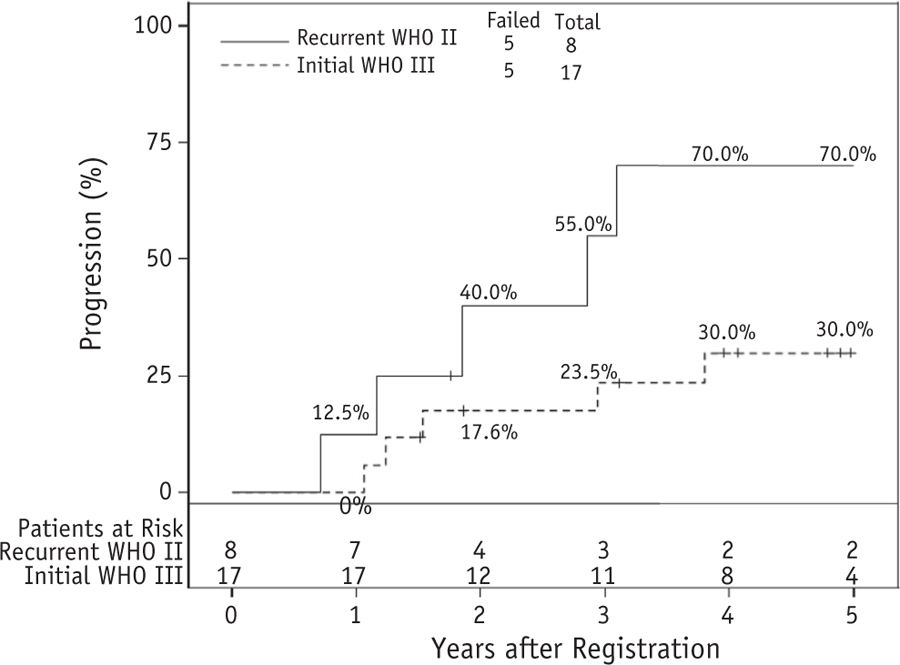
Time to progression comparing patients with recurrent World Health Organization (WHO) grade II to those with initially diagnosed WHO grade III meningioma. The percentages accompanying the curves are annual actuarial results, from 1 to 5 years after study registration. Used with permission from NRG Oncology.
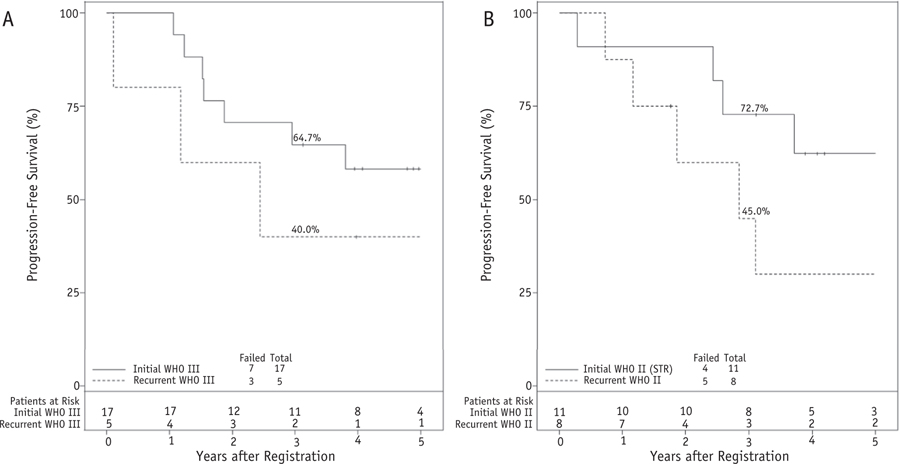
Comparing outcomes within the same WHO grade (Fig. 5), patients with newly diagnosed WHO grade III (n = 17) experienced 3-year PFS of 64.7% versus 40.0% with recurrent WHO grade III (n = 5; Fig. 5A). Progression risk was also compared for newly diagnosed STR WHO grade II (n = 11) versus recurrent WHO grade II (n = 8); the 3-year PFS outcomes were 72.7% and 45.0%, respectively (Fig. 5B). No formal testing was performed for these subgroup comparisons because sample sizes were limited.
Fig. 5.
(A) Progression-free survival (PFS) comparing patients with recurrent versus newly diagnosed World Health Organization (WHO) grade III and (B) recurrent versus subtotally resected (STR), newly diagnosed WHO grade II meningioma. The percentages accompanying the curves are actuarial. For PFS, progression or death are considered events. No formal testing was performed in these subgroups because of the small sample size. Used with permission from NRG Oncology.
Adverse events
According to the prespecified analysis of AEs definitely, probably, or possibly related to protocol treatment, acute and late AEs were limited to grades 1, 2, or 3, with the exception of 1 necrosis-related late grade 5 event. This patient developed cerebral necrosis identified 362 days after protocol therapy and died 14 days thereafter, reportedly because of treatment-related events. The most commonly reported AEs were dermatologic (60.3%, nearly all grade 1), constitutional (45.3% nearly all grade 1 or 2), neurologic (47.2%, predominantly grades 1 or 2 with a single late grade 5), pain related (37.7%, nearly all grade 1 and 2), and gastrointestinal (20.8%, nearly all grade 1). Functional outcomes were measured using Zubrod performance status score, mini-mental state examination (MMSE), and neurologic function score. The distributions of the outcomes at baseline, end of RT, and year 3 are shown in Table 2. For each of these measures, the majority of patients had either stable or improved status at the end of RT and year 3. Table 3 summarizes the highest-grade adverse events definitely, probably, or possibly related to protocol therapy by specific event term.
Table 2.
Functional outcome scores: Neurologic function, MMSE, and Zubrod performance status scores
| Time Point |
|||
|---|---|---|---|
| Measure | Baseline | End of RT | Year 3 |
| Neurologic function | n = 53 | n = 53 | n = 36 |
| No symptoms | 23 (43.4%) | 28 (52.8%) | 12 (33.3%) |
| Minor symptoms | 21 (39.6%) | 15 (28.3%) | 5 (13.9%) |
| Moderate symptoms | 9 (17.0%) | 2 (3.8%) | 5 (13.9%) |
| Severe symptoms | 0 | 0 | 1 (2.8%) |
| Unknown | 0 | 8 (15.1%) | 13 (36.1%) |
| MMSE total score | n = 51 | n = 47 | n = 27 |
| Median | 29 | 29 | 30 |
| Range | 11–30 | 19–30 | 24–30 |
| First and third quartiles | 27, 30 | 27, 30 | 29, 30 |
| Zubrod performance status score | n = 53 | n = 53 | n = 36 |
| 0 | 28 (52.8%) | 31 (58.5%) | 15 (41.7%) |
| 1 | 25 (47.2%) | 12 (22.6%) | 4 (11.1%) |
| 2 | 0 | 4 (7.5%) | 3 (8.3%) |
| 3 | 0 | 0 | 2 (5.6%) |
| 4 | 0 | 1 (1.9%) | 1 (2.8%) |
| Unknown | 0 | 5 (9.4%) | 11 (30.6%) |
Abbreviations: MMSE = Mini-Mental State Examination; RT = radiation therapy.
Table 3.
Highest-grade adverse event by specific event term definitely, probably, or possibly related to protocol therapy
| Group III (n = 53) |
|||||
|---|---|---|---|---|---|
| n and (%) of patients by grade | |||||
| Category and Term | 1 | 2 | 3 | 4 | 5 |
| Constitutional symptoms | 13 (21.7) | 10 (16.7) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Fatigue | 13 (21.7) | 10 (16.7) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal | 9 (15.0) | 1 (1.7) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Dysphagia | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal, soft tissue | 0 (0.0) | 1 (1.7) | 2 (3.3) | 0 (0.0) | 0 (0.0) |
| Extraocular muscle paresis | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Gait abnormal | 0 (0.0) | 1 (1.7) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Neurology | 8 (13.3) | 12 (20.0) | 4 (6.7) | 0 (0.0) | 1 (1.7) |
| Central nervous system necrosis | 0 (0.0) | 2 (3.3) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Cognitive disturbance | 2 (3.3) | 2 (3.3) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Confusion | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Memory impairment | 3 (5.0) | 5 (8.3) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Mini mental status examination abnormal | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Peripheral sensory neuropathy | 2 (3.3) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Seizure | 0 (0.0) | 5 (8.3) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Speech disorder | 0 (0.0) | 2 (3.3) | 2 (3.3) | 0 (0.0) | 0 (0.0) |
| Tremor | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Pain | 13 (21.7) | 6 (10.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| Headache | 9 (15.0) | 5 (8.3) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
Adverse events were graded with Common Terminology Criteria for Adverse Events version 3.0. including only categories and terms with at least one grade 3, 4, or 5.
Discussion
We hereby report results of patients with high-risk meningioma managed within a prospective phase II multi-institutional cooperative group trial, which incorporated central neuropathology review and a uniform management strategy. High-risk meningioma included newly diagnosed or recurrent WHO grade III (any resection extent), recurrent WHO grade II (any resection extent), or newly diagnosed WHO grade II tumor after STR. To date, this grouping appears appropriate, with no significant subgroup differences in PFS or OS. It has been difficult to estimate precise recurrence risks for high-risk meningioma. Grading standards have changed considerably over time. We approximated 3-year PFS at 50%.23,25,26 Three-year PFS for the present high-risk cohort was 58.8%, comparable to the predetermined estimate (P = .13).
WHO grade III comprises only 1% to 2% of meningiomas.3 At recurrence, WHO grade III meningioma is modestly more common, but 75% or more of meningiomas do not exhibit grade progression at recurrence.27 Given the relative rarity of WHO III, it is questionable whether a phase 3 trial could accrue sufficient patients to provide timely and definitive evidence vis-à-vis adjuvant RT. However, the majority of retrospective evidence supports adjuvant RT, including older studies23,25 and more recent reports using modern grading standards.18,26,28–32
The high-risk group also included newly diagnosed STR WHO grade II meningioma. There is no consensus that early RT is required after STR of a WHO grade II meningioma. Surveys of neurosurgeons in the United Kingdom and Germany found that, respectively, 41% and 26% do not recommended adjuvant therapy after STR.33,34
However, a National Cancer Database review of 2515 patients with atypical meningioma reported improved 3-year OS with early RT, 93.5% versus 87% with STR alone.35 Other retrospective studies have found significant differences in PFS with STR and RT versus STR alone.14,35,36 Conversely, some studies have not identified improvements with postoperative RT after STR of a WHO grade II meningioma.26,32,37–39 Considerable difficulties remain in interpreting available retrospective literature. Sample sizes have often been small, RT methods less conformal than modern intensity modulated or image-guided therapy, doses lower than presently considered optimal, selection criteria for observation versus RT indistinct, and differentiation between newly diagnosed and recurrent meningioma ambiguous or disregarded.
In this analysis and pera central neuroradiology review, 14 patients experienced disease progression within 3 years, including 13 patients (92.9%) exclusively within the 54 Gy PTV, and 1 patient (7.1%) with both infield and marginal progression. At present, this does not appear to advocate for changes in target volume dimensions, yet it might argue against target expansion specifically to cover a dural tail; however, our findings suggest the possible applicability of higher doses. The trial defined GTV as the tumor bed on postoperative MRI inclusive of any nodular enhancement without inclusion of cerebral edema or dural tail. Two CTVs were used: a CTV60 with a 1-cm margin and a CTV54 with a 2-cm margin. Subsequent PTV margins were 0.3 to 0.5 cm. One consideration might be 60 Gy to the full 2-cm margin, although this must be tempered by the fact that 1 protocol patient’s death, 1 year after treatment, was attributed to in-field necrosis. Another option might be IMRT (eg, 54 Gy in 30 fractions) with a radiosurgery boost. These conjectures, although conceptually reasonable, do not follow as a consequence of this trial alone. Further investigation is required.
In the present trial, a post hoc analysis was performed to compare recurrent and initially diagnosed tumors of the same grade. The analysis revealed that PFS, OS, and local control were numerically inferior in the recurrent setting; this is corroborated by retrospective studies.13,15,16 Our separate post hoc analysis comparing recurrent WHO grade II with new WHO grade III found that 5-year progression risk was 30.0% for new WHO grade III versus 70.0% for recurrent WHO grade II. Although numerically striking, this difference was not statistically significant. It was not entirely unanticipated. We concluded, on the basis of the available body of retrospective data, that patients with recurrent WHO grade II meningioma would have outcomes as poor as those with newly diagnosed WHO grade III primaries. This conclusion was the justification for including them in the high-risk cohort.
Since the opening of this trial, additional studies have confirmed relatively poor long-term outcomes for patients with recurrent atypical meningioma. Four series, in composite with 1058 patients, and with lengthy follow-up (53 months to 25 years) have reported no long-term survivors among patients with recurrent WHO grade II meningioma.13,16,40,41 One of these studies, with 25-year follow-up on living patients, encountered no survivors beyond 16 years among those with WHO grade II meningioma, recurrent or otherwise. These findings call attention to optimal initial management for patients with high-risk meningioma, and they draw into question assertions that patients with newly diagnosed atypical meningioma can be adequately managed in a less aggressive fashion, relying on salvage therapy as needed.
To date, no other cooperative-group prospective trials of surgery and adjuvant RT in the management of high-risk meningioma have been published. Early outcomes for the intermediate-risk cohort of the present trial were published recently,42 as were initial results from the EORTC 22042–26042 trial.43 These phase 2 cooperative-group efforts could guide clinical decision making, but additional follow-up on the present data set and higher-level evidence with larger data sets and longer follow-up are still needed.
NRG Oncology has opened a phase 3 trial (BN003); randomizing newly diagnosed, GTR WHO grade II meningioma patients to observation versus early RT, photons, or protons. A similar study, the ROAM trial, is available in Europe and at many other sites outside of the United States. A phase 2 trial (Alliance AO71401) evaluating targeted systemic management for progressive meningioma is also available. Enrolling appropriate patients on such clinical trials is encouraged, of course, as are investigations into other therapeutic options for patients with high-risk meningioma.
Conclusion
Patients with high-risk meningioma experienced a 3-year PFS rate of 58.8% after treatment including RT (60 Gy in 30 fractions). Per central review, 92.9% of recurrences were within the RT planning target volume. Higher RT doses, such as highly conformal, radiosurgery, or brachytherapy boosts, may be applicable but would, of course, require further study. It is anticipated that advances in the molecular characterization of aggressive meningioma will lead to improved management. These endpoints remain for future analysis with this trial as its data mature and and for the work of other investigators. The results of this trial support postoperative RT for high-risk meningioma, but they confirm suboptimal outcome and intimate opportunities for innovation to improve long-term outcomes for these patients.
Acknowledgments—
The authors thank Bruce Dean, MD (1951–2015), the neuroradiologist at Barrow Neurological Institute who reviewed magnetic resonance images to detail the extent of tumor resection, and the staff members of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
This study was supported by National Cancer Institute grants U10CA180868 and U10CA180822.
This protocol (NRG Oncology/RTOG 0539) is registered with ClinicalTrials.gov (https://clinicaltrials.gov). The ClinicalTrials.gov identifier is NCT00895622.
This cooperative group trial was approved by the institutional review boards at each participating site, with documentation received at headquarters. Each patient signed an approved informed consent form before enrollment.
Footnotes
Disclosures: Dr Mehta reports personal fees from Varian, Agenus, Insys, Remedy, IBA, Oncoceutics, Astra Zeneca, Celgene, Tocagen, Abbvie, and Monteris. Dr Vogelbaum reports personal fees, grants, and other financial support from Varian, Elekta, Radialogica, Robinson, and Infuseon Therapeutics. Dr Alleman reports personal fees from Ontario Hospital Insurance Plan. Grant or research support was provided by Sanofi-Aventis (XL765); Astrazeneca (AZD 0156); EMD-Serono (M2698, Pimasertib); Eli Lilly (numerous durgs); Novartis (numerous durgs); Deciphera Pharmaceuticals DCC2618, altiratinib), Mundipharma (EDO-S101), Paid Consultant: Celldex (rindopepimit); Deciphera Pharmaceuticals, AbbVie (ABT-414), FivePrime Therapeutics, Inc. (cabiralizumab), GW Pharma (CBD/THC), Carthera (ultrasound device). Eli Lilly, Boston Biomedical Inc., Kairos Venture Investments, Syneos Health, Monteris, Advisory Boards: Genentech (bevacizumab); Celldex (see above); Foundation Medicine, Inc. (diagnostics, genomic testing platform); Novogen (GDC-0084); Deciphera (see above); Astrazeneca (see above), Insys Therapeutics (CBD), Kadmon (KD019), Merck (keytruda), Eli Lilly, Other Relevant Financial or Material Interests: DSMB: VBL Therapeutics (VB111); DSMB: Novella (ICT-107); VBI Vaccines, Inc.,Stock Ownership: Ziopharm Oncology, Gilead, Company Employment (Spouse): Ziopharm Oncology (de Groot).
Open payments of lunch meals were delivered to multiprovider private practice office from various general neurology product and pharmaceutical representatives September 2017 to August 2018, with no relationship to neuro-oncology or meningioma. (Ashby).
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 2014;16(suppl 4):1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery 2005;57:1088–1095; [discussion: 1088–1095]. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803–820. [DOI] [PubMed] [Google Scholar]
- 4.Perry A, Stafford SL, Scheithauer BW, et al. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 1997;21:1455–1465. [DOI] [PubMed] [Google Scholar]
- 5.Perry A, Scheithauer BW, Stafford SL, et al. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer 1999;85:2046–2056. [DOI] [PubMed] [Google Scholar]
- 6.Rogers CL, Perry A, Pugh S, et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro Oncol 2016;18:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus 2008;24:E3. [DOI] [PubMed] [Google Scholar]
- 8.Backer-Grondahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol 2012;5:231–242. [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor BW Jr., Marcus RB Jr., Friedman WA, et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys 1988;15:299–304. [DOI] [PubMed] [Google Scholar]
- 10.Miralbell R, Linggood RM, de la Monte S, et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol 1992;13:157–164. [DOI] [PubMed] [Google Scholar]
- 11.Peele KA, Kennerdell JS, Maroon JC, et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology 1996;103:1761–1766; [discussion: 1766–1767]. [DOI] [PubMed] [Google Scholar]
- 12.Cao X, Hao S, Wu Z, et al. Treatment response and prognosis after recurrence of atypical meningiomas. World Neurosurg 2015;84:1014–1019. [DOI] [PubMed] [Google Scholar]
- 13.Talacchi A, Muggiolu F, De Carlo A, et al. Recurrent atypical meningiomas: combining surgery and radiosurgery in one effective multimodal treatment. World Neurosurg 2016;87:565–572. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Somasundaram A, Opalak C, et al. Effectiveness of salvage radiation therapy following subtotal and gross total resection of atypical meningiomas. Int J Radiat Oncol Biol Phys 2016;96:E104–105. [Google Scholar]
- 15.Bagshaw HP, Burt LM, Jensen RL, et al. Adjuvant radiotherapy for atypical meningiomas. J Neurosurg 2017;126:1822–1828. [DOI] [PubMed] [Google Scholar]
- 16.Kessel KA, Fischer H, Oechnser M, et al. High-precision radiotherapy for meningiomas: Long-term results and patient-reported outcome (PRO). Strahlenther Onkol 2017;193:921–930. [DOI] [PubMed] [Google Scholar]
- 17.Ryu HS, Moon KS, Lee KH, et al. Recurred intracranial meningioma: a retrospective analysis for treatment outcome and prognostic factor. Brain Tumor Res Treat 2017;5:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg 2015;122:4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers L, Vogelbaum M, Gilbert M. Intracranial meningiomas of atypical (WHO Grade II) histology. J Neurooncology 2010;99(3):393–405. [DOI] [PubMed] [Google Scholar]
- 20.Simpson D The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry A Meningiomas In: McLendon R, Rosenblum M, Bigner DD, editors. Russell & Rubinstein’s Pathology of Tumors of the Nervous System. 7th ed. London: Hodder Arnold; 2006. pp. 427–474. [Google Scholar]
- 22.Combs SE, Schulz-Ertner D, Debus J, et al. Improved correlation of the neuropathologic classification according to adapted world health organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol Biol Phys 2011;81:1415–1421. [DOI] [PubMed] [Google Scholar]
- 23.Dziuk TW, Woo S, Butler EB, et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol 1998;37:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 25.Milosevic MF, Frost PJ, Laperriere NJ, et al. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys 1996;34:817–822. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson MD, Waqar M, Farah JO, et al. Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci 2016;28:87–92. [DOI] [PubMed] [Google Scholar]
- 27.McGovern SL, Aldape KD, Munsell MF, et al. A comparison of World Health Organization tumor grades at recurrence in patients with non-skull base and skull base meningiomas. J Neurosurg 2010;112:925–933. [DOI] [PubMed] [Google Scholar]
- 28.Sun SQ, Hawasli AH, Huang J, et al. An evidence-based treatment algorithm for the management of WHO grade II and III meningiomas. Neurosurg Focus 2015;38:E3. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramanian SK, Sharma M, Silva D, et al. Longitudinal experience with WHO grade III (anaplastic) meningiomas at a single institution. J Neurooncol 2017;131:555–563. [DOI] [PubMed] [Google Scholar]
- 30.Sughrue ME, Rutkowski MJ, Aranda D, et al. Factors affecting outcome following treatment of patients with cavernous sinus meningiomas. J Neurosurg 2010;113:1087–1092. [DOI] [PubMed] [Google Scholar]
- 31.Zhao P, Hu M, Zhao M, et al. Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg Rev 2015;38:101–107; [discussion: 107]. [DOI] [PubMed] [Google Scholar]
- 32.Durand A, Labrousse F, Jouvet A, et al. WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol 2009;95: 367–375. [DOI] [PubMed] [Google Scholar]
- 33.Marcus HJ, Price SJ, Wilby M, et al. Radiotherapy as an adjuvant in the management of intracranial meningiomas: are we practising evidence-based medicine? Br J Neurosurg 2008;22:520–528. [DOI] [PubMed] [Google Scholar]
- 34.Simon M, Bostrom J, Koch P, et al. Interinstitutional variance of postoperative radiotherapy and follow up for meningiomas in Germany: impact of changes of the WHO classification. J Neurol Neurosurg Psychiatry 2006;77:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Kaprealian TB, Suh JH, et al. Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro Oncol 2017;19:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shakir SI, Souhami L, Petrecca K, et al. Prognostic factors for progression in atypical meningioma. J Neurosurg 2018;1–9. [DOI] [PubMed]
- 37.Park HJ, Kang HC, Kim IH, et al. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol 2013;115:241–247. [DOI] [PubMed] [Google Scholar]
- 38.Champeaux C, Houston D, Dunn L. Atypical meningioma. A study on recurrence and disease-specific survival. Neurochirurgie 2017;63:273–281. [DOI] [PubMed] [Google Scholar]
- 39.Goyal LK, Suh JH, Mohan DS, et al. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys 2000;46:57–61. [DOI] [PubMed] [Google Scholar]
- 40.de Almeida AN, Pereira BJA, Aguiar PHP, Paiva WS, Cabrera HN, da Silva CC, Teixeira MJ, Marie SKN. Clinical outcome, tumor recurrence, and causes of death: a long-term follow-up of surgically treated meningiomas. World Neurosurg 2017;102:139–143. [DOI] [PubMed] [Google Scholar]
- 41.Pettersson-Segerlind J, Orrego A, Lönn S, Mathiesen T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg 2011;76:564–571. [DOI] [PubMed] [Google Scholar]
- 42.Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg 2018;129:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber DC, Ares C, Villa S, et al. Phase II trial: adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: a phase-II parallel non-randomized and observation study (EORTC 22042–26042). Radiother Oncol 2018;128:260–265. [DOI] [PubMed] [Google Scholar]