Abstract
Background:
While guidelines recommend anticoagulation for all atrial fibrillation (AF) patients ≥75 years, evidence for the net clinical benefit (NCB) of anticoagulant in older adults is sparse. We sought to determine the association between age and NCB of anticoagulation in older adults with AF.
Methods and Results:
We examined adults ≥75 years with incident AF in the Anticoagulation and Risk Factors in Atrial Fibrillation – Cardiovascular Research Network cohort. Using a Markov state transition model, we estimated the lifetime NCB of warfarin and apixaban relative to no treatment in quality-adjusted life years (QALYs). In the decision model, each month patients face a chance of stroke, hemorrhage, or death from a competing cause; the likelihood of each is a function of individual patients’ stroke risk, hemorrhage risk, and life expectancy. We defined minimal clinically relevant lifetime benefit as 0.10 QALYs. In a sensitivity analysis, we examined the effect of competing risks of death on NCB using two models, one including competing risks and the second without competing risks. We included 14946 patients, with a median age of 81 years and median CHA2DS2-VASc score of 4. In the main analysis, after age 87, NCB associated with warfarin decreased below 0.10 lifetime QALYs while NCB associated with apixaban did not decrease below 0.10 lifetime QALYs until after age 92. In sensitivity analyses, over a 3-year horizon, removing competing risks of death resulted in higher NCB (at 90 years, median difference using warfarin 0.010 QALYs, 95% CI 0.009–0.013, median difference using apixaban 0.025 QALYs, 95% CI 0.024–0.026).
Conclusions:
The NCB of anticoagulation decreases with advancing age. The competing risk of death diminishes the NCB of anticoagulation for older AF patients. Physicians should consider competing mortality risks when recommending anticoagulants to older adults with AF.
Introduction
Advancing age affects multiple facets of the risk-benefit assessment of anticoagulation for patients with atrial fibrillation. The increased risk of ischemic stroke conferred by atrial fibrillation increases significantly with older age.1,2 While anticoagulation effectively reduces the risk of ischemic stroke, it increases the risk of hemorrhagic events; importantly, the risk of hemorrhagic events also rises with age.3,4 Decision-making is complicated further because advancing age increases the likelihood of death from a non-atrial fibrillation related cause (i.e., competing risk of death) thereby limiting the likelihood of actualized benefit or harm from atrial fibrillation and anticoagulation.5
Consensus guidelines categorize all patients aged 75 years and older as ‘high risk for ischemic stroke’ thus warranting anticoagulation after a qualitative assessment of hemorrhage risk.6 However, evidence for the net benefit of anticoagulant use in older adults with atrial fibrillation is sparse. Older adults, particularly older frail adults, have been under-represented in randomized trials of anticoagulants for thromboprophylaxis in atrial fibrillation. Observational studies suggest older adults may benefit from anticoagulation but these studies are limited by selection; older patients prescribed anticoagulants are generally healthier than those not prescribed anticoagulants.7,8 Furthermore, few randomized trials or observational studies have examined the effectiveness of anticoagulants accounting for the competing risk of death. A competing risk is an important alternative outcome (e.g., cancer-related death) that alters the probability of the disease-specific outcome (e.g., death from cancer precludes the possibility of ischemic stroke).9 Clinically this is analogous to a physician questioning if a patient with atrial fibrillation and a six-month prognosis due to pancreatic cancer will benefit from anticoagulation. When treating older adults who often have multiple comorbidities, the competing risk of death is an important consideration and one that is under-addressed in atrial fibrillation outcome studies.10
Thus, while clinical guidelines recommend anticoagulation for all adults with atrial fibrillation aged 75 years and older, there is little evidence to guide the use of anticoagulants in these older adults. To examine the net clinical benefit of anticoagulation in older adults, we performed a decision analytic study using a cohort of nearly 15000 adults 75 years and older with incident atrial fibrillation.
Methods
Study Overview
Using the characteristics of 14946 patients aged 75 years and older with incident atrial fibrillation in the Anticoagulation and Risk Factors in Atrial Fibrillation – Cardiovascular Research Network (ATRIA-CVRN) cohort, we estimated the net clinical benefit (NCB) of anticoagulation by age. We used the Atrial Fibrillation Decision Support Tool (AFDST), a well-established 29-state Markov decision analytic model, to estimate the NCB of anticoagulation for each patient in the ATRIA-CVRN cohort in quality-adjusted life years (QALYs).11,12
Population used in Decision Analytic Model
We limited the analysis to adults 75 and older since consensus guidelines recommend anticoagulants to all adults 75 and older.13 The ATRIA-CVRN cohort consists of patients with incident atrial fibrillation in the Kaiser Permanente Northern and Southern California integrated health care delivery systems; we have previously published details of the cohort assembly.14 In brief, patients were included in the cohort if they had a new diagnosis of atrial fibrillation between January 1, 2006, and June 30, 2009, defined by International Classification of Disease, Ninth Revision (ICD-9) codes, and electrocardiographic data with no prior known diagnosis of atrial fibrillation. Clinical characteristics of patients in the cohort were determined by searching inpatient, outpatient, laboratory, and pharmacy databases for the relevant ICD-9 codes, medications, or lab values in the year before the patient’s diagnosis. Since the decision analytic model is not designed to assess the risk of bleeding with concurrent use of anticoagulants and non-aspirin antiplatelet medications (e.g., clopidogrel), we excluded 2028 patients prescribed non-aspirin antiplatelet medications at the time of atrial fibrillation diagnosis.
Main decision analytic model
The AFDST decision analytic model estimates the QALYs that result from treatment strategies including – no antithrombotic therapy, anticoagulation with warfarin, and anticoagulation with any of the currently approved direct oral anticoagulants (DOACs). The net benefit of anticoagulation is calculated as the gain or loss in QALYs for an active treatment compared with no antithrombotic therapy. Since apixaban is the only DOAC to show statistically significant benefit over warfarin in preventing ischemic stroke, we present the results for apixaban.15–18 Because randomized trials of DOACs excluded patients with chronic kidney disease, the benefits and harms in this population are not known.15 As a result, we did not test the apixaban strategy in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73m2 (i.e., chronic kidney disease stage 4 or higher [CKD 4+]).
The decision analytic model tracks each patient’s health status as events occur during each monthly cycle. Health status is captured in states defined by significant clinical events (e.g., an ischemic stroke) and the quality of life in those resulting health states, both of which are affected by treatment strategy. During each month, patients face a chance of experiencing the following clinical events: ischemic stroke, extracranial hemorrhage, intracranial hemorrhage, death from another cause. Using published studies, we calculated patient-specific event probabilities based on their individual comorbidities. We calculated patient-specific CHA2DS2-VASc and ATRIA stroke scores to estimate the risk of ischemic stroke. We used both scores because while clinical guidelines recommend using the CHA2DS2-VASc score, it does not account for the effect of age on stroke beyond 75 years, and it does not perform as well as the ATRIA stroke score.1,19,20 Since alcohol use information is not available in the ATRIA-CVRN cohort, we used the modified HAS-BLED score (hypertension, abnormal liver or renal function, stroke history, bleeding predisposition, labile internal normalized ratio, elderly, drug use) to estimate the probability of extracranial hemorrhage. Using a multivariable regression model developed in the Swedish population registry of untreated patients with atrial fibrillation, we estimated patient-specific risk of intracranial hemorrhage.3
In the decision analytic model, each abovementioned clinical event results in one of several outcomes. Ischemic stroke and hemorrhage may result in death, mild or severe neurologic sequelae, or symptom resolution. Each health state has an associated quality of life determined using patient-reported preferences.21 Since outcomes following hemorrhagic events are worse in anticoagulated patients, we used observational studies to model the differential outcome of hemorrhagic events based on treatment status. Patients’ risk of death from other non-explicitly modeled causes (i.e., unrelated to AF or anticoagulation therapy) is estimated by accounting for excess mortality risk associated with age, sex, and comorbidities including chronic kidney disease, diabetes, hypertension, and prior stroke. Estimated benefits and harms are then summed over the simulated lifespan (i.e., lifetime horizon). We used 0.1 QALY as a minimal clinically significant gain to consider one strategy better than another.7,11,22 We summarize base case values for model parameters in Table 1; we describe the decision tree and health states in Appendix Figures 1 and 2.
Table 1.
Markov Model Inputs, Including, Rates, Outcomes, and Quality of Life
| Parameter | Value | |||
|---|---|---|---|---|
| Ischemic stroke | ||||
| Annual rate of ischemic stroke (off-anticoagulation) by CHA2DS2-VASc Score23 | ||||
| 0 | 0 | |||
| 1 | 1.3 | |||
| 2 | 2.2 | |||
| 3 | 3.2 | |||
| 4 | 4 | |||
| 5 | 6.7 | |||
| 6 | 9.8 | |||
| 7 | 9.6 | |||
| 8 | 6.7 | |||
| 9 | 15.2 | |||
| Annual rate of ischemic stroke (off-anticoagulation) by ATRIA Score1 | ||||
| 0 | 0.08 | |||
| 1 | 0.43 | |||
| 2 | 0.99 | |||
| 3 | 0.73 | |||
| 4 | 0.64 | |||
| 5 | 0.99 | |||
| 6 | 1.91 | |||
| 7 | 2.50 | |||
| 8 | 3.86 | |||
| 9 | 4.33 | |||
| 10 | 6.35 | |||
| 11 | 6.18 | |||
| 12 | 10.95 | |||
| 13 | 7.52 | |||
| 14 | 16.36 | |||
| 15 | 0 | |||
| Efficacy of warfarin24 | 0.68 | |||
| Relative risk of ischemic stroke with apixaban (relative to warfarin)25 | 0.71 | |||
| Extracranial hemorrhage | ||||
| Annual rate of warfarin-associated extracranial bleeding by HAS-BLED score26 | ||||
| 0 | 0 | |||
| 1 | 0.7 | |||
| 2 | 1.9 | |||
| 3 | 2.4 | |||
| 4 | 3.4 | |||
| 5 | 5.7 | |||
| 6 | 15.5 | |||
| Hazard in the untreated (relative to warfarin) 26 | 0.42 | |||
| Hazard of apixaban (relative to warfarin)25 | 0.51 | |||
| Intracranial hemorrhage | ||||
| Annual rate of ICH, based on multivariate hazard ratios (untreated) 3,27 | ||||
| Referent risk | 0.0004 | |||
| Age < 65 | 1.00 | |||
| Age 65–74 | 1.97 | |||
| Age > 75 | 2.43 | |||
| Female | 0.70 | |||
| Prior ischemic stroke | 1.21 | |||
| History of ICH | 8.92 | |||
| History of severe bleed | 3.10 | |||
| History of myocardial infarction | 0.82 | |||
| History of ischemic heart disease | 0.81 | |||
| History of poorly controlled HTN* | 1.32 | |||
| Hazard of warfarin (relative to no treatment)4,28 | 4.1 | |||
| Hazard of apixaban (relative to warfarin)25 | 0.54 | |||
| Subdural hematoma | ||||
| Annual rate of subdural hematoma (untreated)24,28,29 | 0.00027 | |||
| Hazard of anticoagulation (warfarin and apixaban relative to no treatment) 24,29,30 | 5.5 | |||
| Death from other causes (annual rates) | ||||
| Life expectancy31 | Age, sex-specific | |||
| Excess annual mortality rates from selected comorbidities | ||||
| CKD 3a32 | 0.20 | |||
| CKD3b32 | 0.80 | |||
| CKD 432 | 2.20 | |||
| CKD 532 | 4.90 | |||
| Diabetes33 | 0.19 | |||
| CHF, male34 | 0.21 | |||
| CHF, female34 | 0.17 | |||
| Hypertension35 | 0.001 | |||
| Stroke, male36 | 0.05 | |||
| Stroke, female36 | 0.03 | |||
| Probability of outcomes | ||||
| Ischemic stroke outcome probability24,37,38 | ||||
| Death | 0.16 | |||
| Permanent severe disability | 0.30 | |||
| Permanent mild disability | 0.14 | |||
| Good recovery | 0.40 | |||
| Hemorrhage outcome probability off-anticoagulant39–42 | Lobar ICH | Deep ICH | Subdural Hematoma | Extracranial |
| Death | 0.19 | 0.21 | 0.27 | 0.02 |
| Permanent severe disability | 0.43 | 0.44 | 0.07 | |
| Permanent mild disability | 0.20 | 0.19 | 0.40 | |
| Good recovery | 0.19 | 0.17 | 0.26 | 0.98 |
| Hemorrhage outcome probability on-anticoagulant39–42 | Lobar ICH | Deep ICH | Subdural Hematoma | Extracranial |
| Death | 0.38 | 0.41 | 0.27 | 0.05 |
| Permanent severe disability | 0.43 | 0.42 | 0.09 | |
| Permanent mild disability | 0.11 | 0.10 | 0.50 | |
| Good recovery | 0.08 | 0.07 | 0.14 | 0.95 |
| Health states | ||||
| Long-term health state relative utility21 | ||||
| Well | 1.00 | |||
| Well while receiving apixaban | 1.00 | |||
| Well while receiving warfarin | 0.99 | |||
| Mild long-term disability | 0.76 | |||
| Severe long-term disability | 0.11 | |||
| Death | 0.00 | |||
| Short-term health state relative utility | ||||
| ICH† | 0.79 | |||
| Ischemic stroke† | 0.79 | |||
| Extracranial bleed‡ | 0.84 | |||
CHA2DS2-VASc score - congestive heart failure/hypertension/age/diabetes/stroke/vascular disease;
HAS-BLED score – hypertension/abnormal liver or renal function/stroke history/bleeding predisposition/labile INR/elderly/drug or alcohol usage
ICH – intracerebral hemorrhage
HTN – hypertension
SDH – subdural hematoma
CKD – chronic kidney disease
poorly controlled hypertension given by a recorded systolic BP > 160 mmHg in 12 months before AF diagnosis
Assumes quality of life is 0 for the duration of hospitalization. Length of stay for specific cerebrovascular disorders except for transient ischemic attack (diagnosis-related group, 14) is 6.4 days.
Duration of short-term utility loss for major extracranial bleed is 12 months.
Sensitivity analyses
We performed two sensitivity analyses. First, we examined the effect of the competing risk of death on the relationship between age and NCB. We compared the NCB of anticoagulation in a decision model that accounts for disease-specific mortality (i.e., AF and treatment-related mortality) and competing risk of death (i.e., lifetable-based mortality rates for age and sex as well as excess mortality related to comorbidities) to the NCB of anticoagulation in a decision model that just accounts for disease-specific mortality. The difference between the two estimates of NCB is attributable to the effect of competing risks on the estimation of NCB of anticoagulation. The decision analytic model estimates lifetime benefit by running until the patient’s death from a disease-specific cause or competing risks. Removing the competing risk of death would render an estimate of lifetime NCB uninterpretable since we would provide estimates of QALYs accrued over unrealistically long lifespans. Accordingly, for this sensitivity analysis, we estimated NCB over a 3-year window.
Second, we tested the impact of increasing age on risk of ischemic stroke beyond age 75 years. The CHA2DS2-VASc score, used in the main model, groups all patients older than 75 years into a single category. However, studies demonstrate that the risk of stroke continues to increase with advancing age beyond 75 years.1,43 Therefore, in this sensitivity analysis, we increased the base embolic stroke risk by a relative 0.75% for each year beyond age 75.44
Statistical methods
We determined net benefit by computing the gain or loss in QALYs afforded by anticoagulant therapy compared with no oral anticoagulation. Because of the small number of patients older than 95 years (n=180), those older than 95 years were examined together. Since the distribution of net benefit was skewed, we compared estimates of median benefit. When determining if the median benefit was different from the pre-specified threshold of 0.10 QALYs or if a sensitivity analysis resulted in statistically similar results as the main analysis, we used the Sign test. We calculated 95% confidence intervals using 1000 bootstrapped samples. We performed analyses using SAS 9.4 (Cary, NC) and R 3.4.4 (Vienna, Austria). Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the Cardiovascular Research Network managed by the Kaiser Permanente Division of Research at https://cvrn.org/Pages/Collaboration.aspx. This study was approved by the UCSF Human Research Protection Program and the original ATRIA-CVRN cohort study was approved by the institutional review boards of the Kaiser Foundation Research Institute and Kaiser Permanente Southern California. In both cases, no informed consent was required.
Results
Main analysis
We used baseline data from 14946 patients older than 75 years from the ATRIA-CVRN cohort in this analysis (Table 2). Age ranged from 75 to 106 years and 54% were women. At diagnosis of AF, 23% also had chronic heart failure, 88% had hypertension, 3% had a prior ischemic stroke or transient ischemic attack, 27% had diabetes mellitus, and 8% had received dialysis or had an eGFR of < 30 mL/min/1.73m2. Since age greater than 75 years confers 2 points in the CHA2DS2-VASc scoring algorithm, all patients had a CHA2DS2-VASc score of 2 or more at diagnosis.
Table 2:
Baseline Characteristics of the ATRIA-CVRN Cohort Used in the Decision Model
| ATRIA-CVRN Cohort | |
|---|---|
| (n = 14946) | |
| DEMOGRAPHICS | |
| Age, median (range) | 81 (75, 106) |
| Women | 8024 (54%) |
| Race | |
| White | 12500 (84%) |
| Black | 753 (5%) |
| Native American | 23 (0.2%) |
| Asian | 799 (5%) |
| Other | 436 (3%) |
| Unknown | 435 (3%) |
| Hispanic ethnicity | 1495 (10%) |
| COMORBIDITIES | |
| Chronic heart failure | 3472 (23%) |
| Hypertension | 13137 (88%) |
| Poorly controlled hypertension | 3424 (23%) |
| Prior ischemic stroke | 689 (5%) |
| Prior transient ischemic attack | 497 (3%) |
| Vascular disease | |
| Peripheral arterial disease | 780 (5%) |
| Other systemic arterial thromboembolism | 112 (1%) |
| Diabetes mellitus | 4100 (27%) |
| Chronic kidney disease | |
| eGFR < 30 ml/min/1.73 m2 | 940 (6%) |
| Dialysis | 196 (1%) |
| Chronic liver disease | 214 (1%) |
| Bleeding history | |
| Intracranial hemorrhage | 100 (1%) |
| Hospitalized gastrointestinal bleed | 537 (4%) |
| Other hospitalized bleeding | 80 (1%) |
| Prescribed aspirin or NSAID | 3208 (21%) |
| Coronary heart disease | 1412 (9%) |
| Prior acute myocardial infarction | 649 (4%) |
| CHA2DS2-VASc SCORE | |
| 0 | 0 (0%) |
| 1 | 0 (0%) |
| 2 | 688 (5%) |
| 3 | 3476 (23%) |
| 4 | 5900 (39%) |
| 5 | 3081 (21%) |
| 6 | 1288 (9%) |
| 7 | 400 (3%) |
| 8 | 96 (1%) |
| 9 | 18 (0%) |
| HAS-BLED SCORE (modified) | |
| 0 | 0 (0%) |
| 1 | 6266 (42%) |
| 2 | 6037 (40%) |
| 3 | 2177 (15%) |
| 4 | 438 (3%) |
| 5 | 27 (0%) |
| 6 | 2 (0%) |
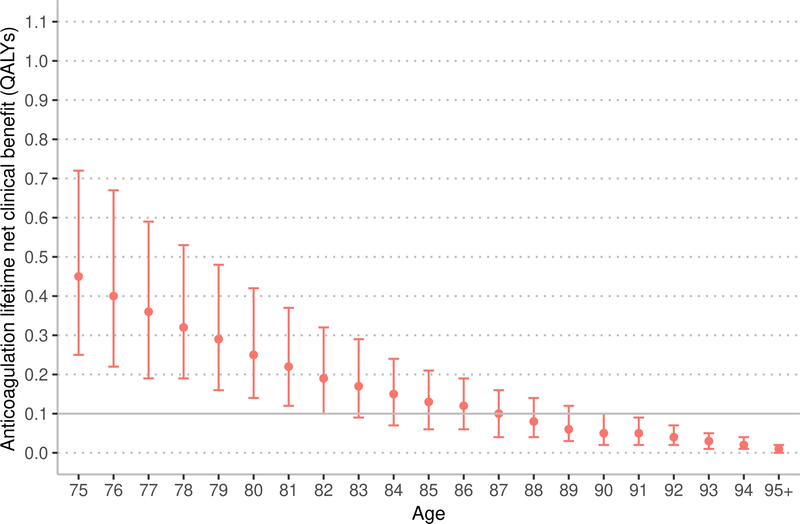
The estimated NCB of anticoagulation with warfarin and apixaban decreased with age (Figure 1). Among patients aged 75 years, using the CHA2DS2-VASc score to estimate stroke risk, warfarin resulted in a median lifetime NCB of 0.45 QALYs (interquartile range [IQR] 0.25 to 0.72 QALYs). Among patients aged 87 years, the median lifetime NCB associated with warfarin decreased to 0.10 QALYs (IQR 0.04 to 0.16 QALYs), which was not significantly different from the prespecified threshold for minimal NCB of 0.10 QALYs (p = 0.28). Among patients aged 75 years eligible for apixaban therapy (e.g., without CKD 4+), using the CHA2DS2-VASc score to estimate stroke risk, apixaban resulted in a median lifetime NCB of 0.74 QALYs (IQR 0.49 to 1.06 QALYs). Among patients aged 92 years, the median lifetime NCB associated with apixaban decreased to 0.10 QALYs (IQR 0.07 to 0.13 QALYs), which was not significantly different from the prespecified threshold for minimal NCB of 0.10 QALYs (p = 0.75).
Figure 1:
Marker indicates median lifetime net clinical benefit for age, bar indicates interquartile range. QALYs – quality-adjusted life years. Ischemic stroke risk was estimated using the CHA2DS2-VASC score.
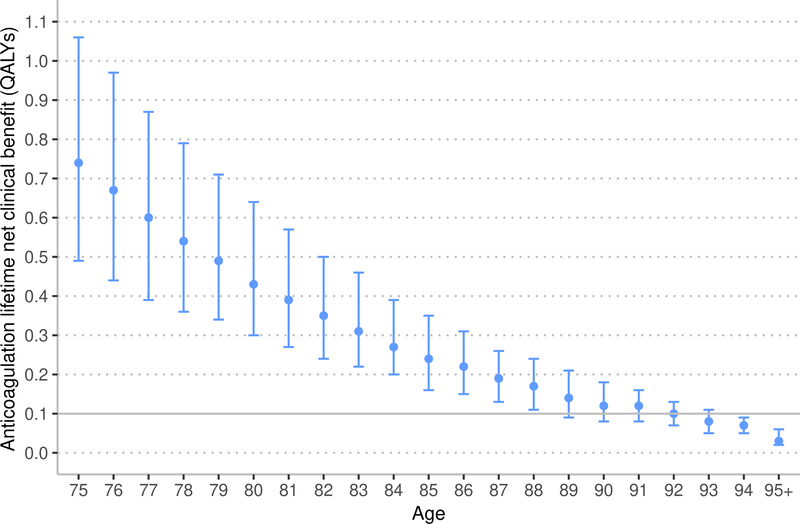
The ATRIA stroke score accounts for increasing stroke risk after 75 years of age. Using the ATRIA score, the estimated NCB of anticoagulation with warfarin and apixaban decreased with age (Figure 2). Among patients aged 75 years, using the ATRIA score, warfarin resulted in a median lifetime NCB of 0.36 QALYs (IQR 0.19 to 0.68 QALYs). Among patients aged 88 years, the median lifetime NCB associated with warfarin decreased to 0.10 QALYs (IQR 0.05 to 0.16 QALYs), which was not significantly different from the prespecified threshold for minimal NCB of 0.10 QALYs (p = 0.93). Among patients aged 75 years eligible for apixaban therapy (e.g., without CKD 4+), using the ATRIA score, apixaban resulted in a median lifetime NCB of 0.64 QALYs (IQR 0.46 to 1.03 QALYs). Among patients aged 93 years, the median lifetime NCB associated with apixaban decreased to 0.09 QALYs (IQR 0.05 to 0.11 QALYs), which was less than the prespecified threshold for minimal NCB of 0.10 QALYs (p = 0.01).
Figure 2:
Marker indicates median lifetime net clinical benefit for age, bar indicates interquartile range. QALYs – quality-adjusted life years. Ischemic stroke risk was estimated using the ATRIA score.
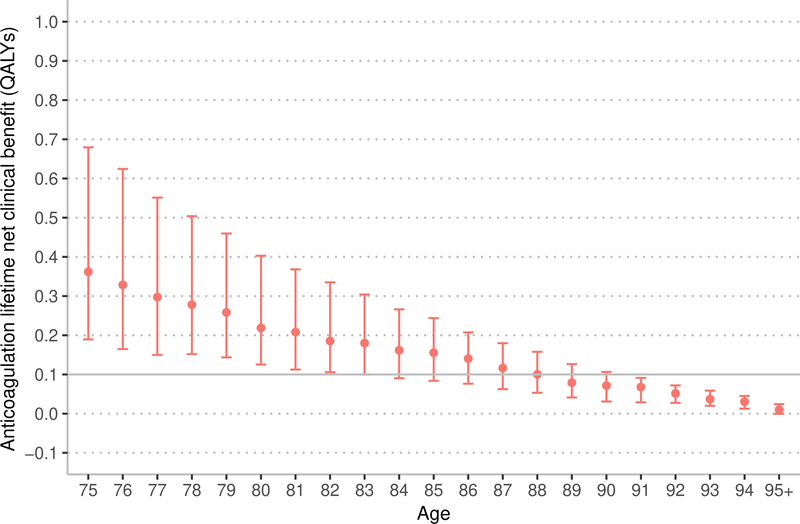
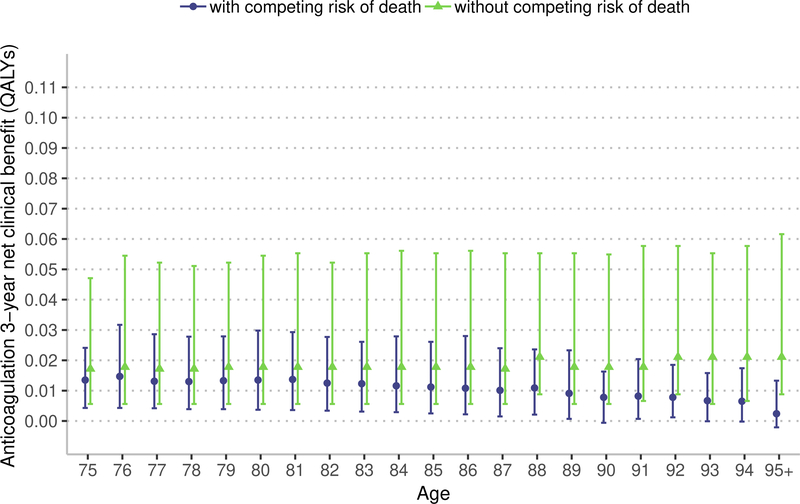
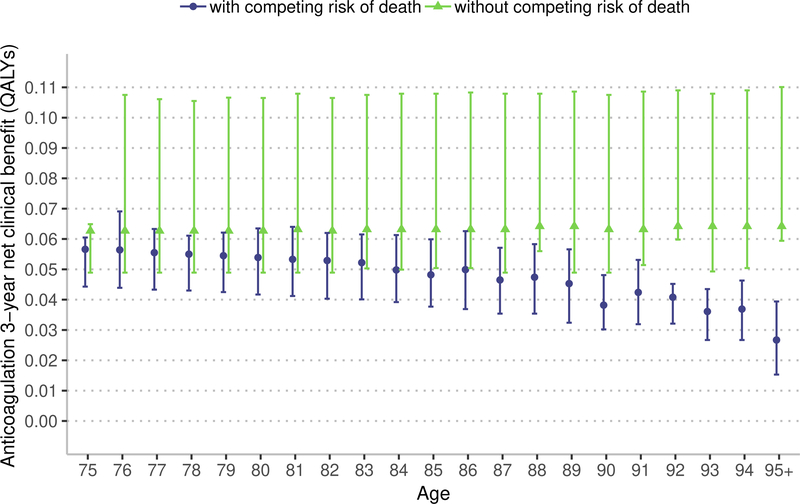
Sensitivity analysis: competing risk of death
Removing the competing risk of death from the estimation of NCB results in a higher estimated NCB; a difference that is more pronounced with apixaban and at older ages (Figure 3). Among patients aged 75 years, over a 3-year window, warfarin resulted in a median NCB of 0.013 QALYs (IQR 0.004 to 0.024) accounting for the competing risk of death and 0.017 QALYs (IQR 0.005 to 0.047) without the competing risk of death (median difference, 0.004 QALYs, 95% confidence interval [CI] 0.002 to 0.004 QALYs). Among patients aged 90 years, over a 3-year window, warfarin resulted in a median NCB of 0.008 QALYs (IQR −0.001 to 0.016 QALYs) accounting for the competing risk of death and 0.018 QALYs (IQR 0.006 to 0.047 QALYs) without the competing risk of death (median difference, 0.010 QALYs, 95% CI 0.009 to 0.013 QALYs).
Figure 3:
Marker indicates median 3-year net clinical benefit for age, bar indicates interquartile range. QALYs – quality-adjusted life years. The model with competing risk of death accounts for disease-specific mortality and competing risk of death from non-AF related causes. The model without competing risks just accounts for disease-specific mortality. In the main model we use a lifetime estimate of net clinical benefit. In this sensitivity analysis, we estimate net clinical benefit over 3-years because removing the competing risk of death would result in a model with an unrealistically long lifespan and generate an uninterpretable estimate of lifetime net clinical benefit.
Among patients aged 75 years, over a 3-year window, apixaban resulted in a median NCB of 0.056 QALYs (IQR 0.044 to 0.061) accounting for the competing risk of death and 0.063 QALYs (IQR 0.049 to 0.065) without the competing risk of death (median difference, 0.007 QALYs, 95% CI 0.006 to 0.008 QALYs). Among patients aged 90 years, over a 3-year window, apixaban resulted in a median NCB of 0.038 QALYs (IQR 0.030 to 0.048) accounting for the competing risk of death and 0.063 QALYs (IQR 0.049 to 0.108 QALYs) without the competing risk of death (median difference, 0.025 QALYs, 95% CI 0.024 to 0.026). As demonstrated in Figure 3, the NCB of anticoagulation remains relatively stable with advancing age when the competing mortality risk, including those associated with advancing age, are removed. Thus, these sensitivity analyses indicate that the competing mortality risk due to advancing age is an important factor in limiting the NCB of anticoagulation in adults over 75 years.
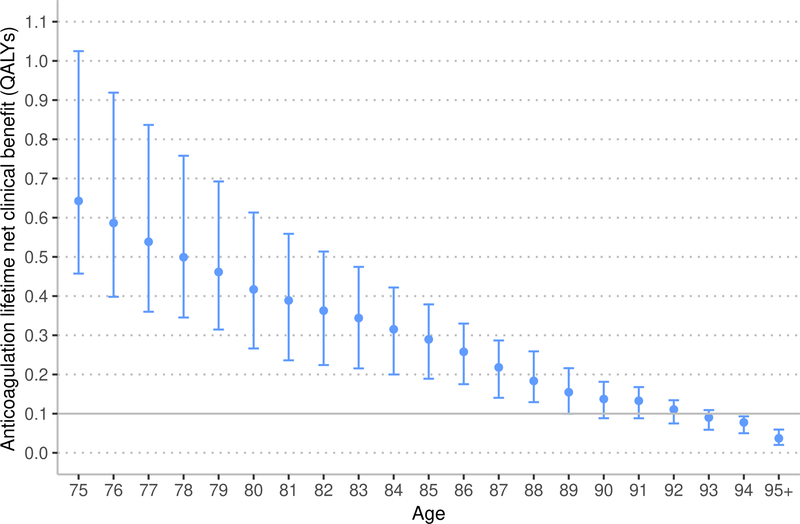
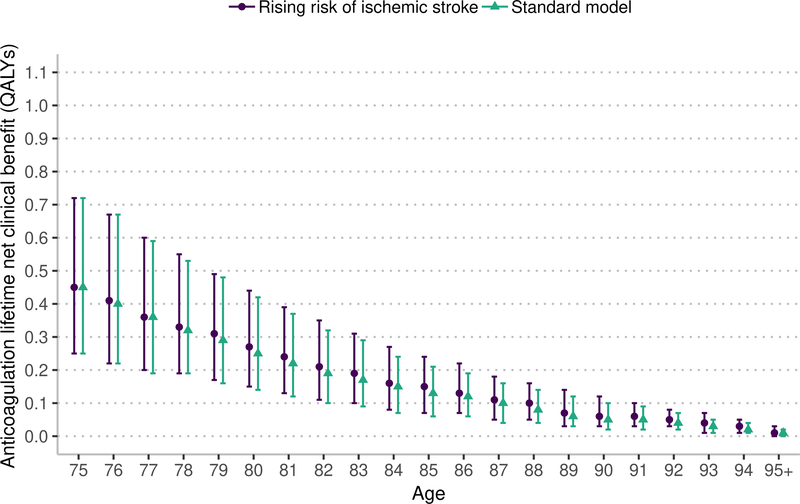
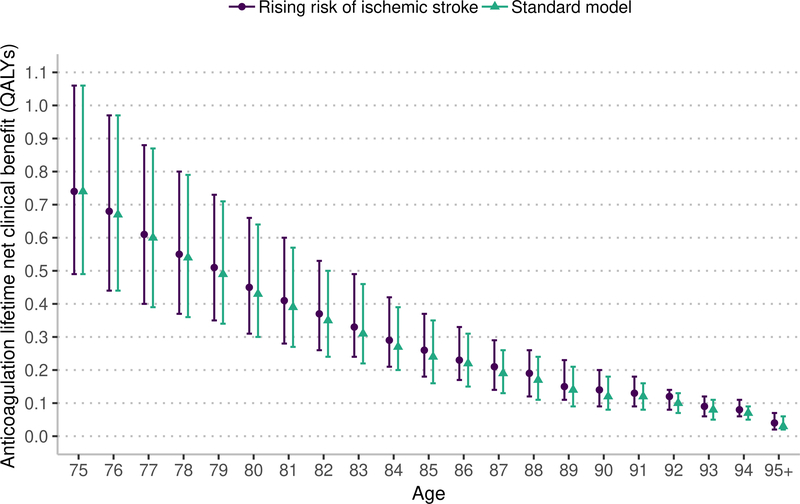
Sensitivity analysis: risk of ischemic stroke with age
This sensitivity analysis uses the same lifetime horizon as the main model. Under the assumption that the risk of ischemic stroke increases with age beyond 75 years, the lifetime NCB from both warfarin and apixaban anticoagulation is nearly the same as in analyses assuming that ischemic stroke risk is stable beyond 75 years (Figure 4). Under the assumption of rising ischemic stroke risk, warfarin and apixaban resulted in a higher NCB that was statistically significant but not clinically significant (warfarin median increase 0.011, 95% CI 0.011 to 0.012 QALYs; apixaban median increase 0.013, 95% CI 0.012 to 0.013 QALYs).
Figure 4:
Marker indicates median lifetime net clinical benefit for age, bar indicates interquartile range. QALYs – quality-adjusted life years. The rising risk of ischemic stroke increases the base stroke risk by 0.75% annually based on the risk model by Hijazi et at.43,44
Discussion
Our study provides evidence that the net clinical benefit (NCB) of anticoagulation decreases with advancing age. We found that warfarin results in minimal lifetime net benefit for the median patient beyond the age of 87 years, while apixaban yields minimal lifetime net benefit after age 92 years. Second, we found that the decline in NCB with advancing age is strongly associated with competing risks of death. Third, we found that the declining benefit of anticoagulation with advancing age is only modestly affected when accounting for the age-related increase in ischemic stroke risk.
In these analyses, we used quality-adjusted life years (QALYs) as a summary measure of NCB. In prior reports, investigators have measured NCB using stroke equivalents in a non-decision analytic approach.28,45–47 In this study, we employed a formal decision analytic model that incorporates life expectancy and is inclusive of all relevant outcome events, weighted by published utility weightings. Nevertheless, QALYs are not routinely used in clinical practice. To ground the notion of 0.1 QALYs over a lifetime as “minimal benefit,” in Table 3, we list the QALYs gained with selected clinical interventions. For instance, increasing from conventional dose to high dose statin in patients with stable coronary disease results in a lifetime benefit of 0.1 QALYs per person.49
Table 3.
Gain in Quality-adjusted Life Years for Selected Medical Interventions
| Selected Interventions | Lifetime QALY gain |
|---|---|
| Lung cancer screening (age 55–75 years with 30 pack-year history)48 | 0.02 |
| High vs. conventional dose statin for stable coronary artery disease49 | 0.10 |
| Aspirin for primary prevention with new diagnosis of diabetes50 | 0.19 |
| High vs. conventional dose statin following acute coronary syndrome49 | 0.35 |
All studies cited use a lifetime window. QALYs – quality-adjusted life years.
While this study finds that the NCB of anticoagulation declines with advancing age, prior observational studies have found the opposite. Prior studies are limited by selection bias (i.e., those treated are more likely to benefit than the untreated)7 and often do not account for the competing risk of death (i.e., death from non-atrial fibrillation related causes).51 The decision analytic model used in this study minimizes selection bias by estimating treatment effects for all patients and intrinsically accounts for the competing risk of death. The results demonstrate that the competing risk of death is an important consideration when estimating the NCB of anticoagulation therapy. We find failing to account for competing risks likely overestimates the NCB of anticoagulation, an effect that is more pronounced at older ages and with more effective anticoagulants.
The results of this study have important implications for the clinical care of older adults with atrial fibrillation. If using the CHA2DS2-VASc stroke risk score, consensus guidelines recommend anticoagulant use for all adults 75 years and older with atrial fibrillation.13 This study of adults 75 years and older finds that while most are likely to benefit, the NCB decreases every year beyond 75. Further, the results indicate that competing risks (e.g., life-limiting conditions such as end-stage kidney disease) are a major determinant of reduced NCB at a population level. These results, however, must be translated by clinicians to the care of individual patients. For example, the results indicate that for half of 92-year-olds with atrial fibrillation, apixaban may not confer a net benefit. Clinicians must translate if an individual patient’s comorbidity burden will limit the benefits of anticoagulants. This information can then inform a shared decision-making encounter so that the anticoagulation decision advances individual patients’ health priorities.
Our study has limitations inherent to the study design and data available. First, the cohort that we examined through the decision analytic model was created from among those insured through Kaiser Permanente Northern California and Southern California. While such a patient sample has broad age, gender, and racial/ethnic diversity and is highly representative of the California adult population, it may not be completely generalizable to all populations in the U.S. or internationally. However, use of a cohort of atrial fibrillation patients that includes outpatients is more generalizable than samples of atrial fibrillation patients selected entirely from hospitals.3,52 Second, we incorporate multiple estimates of probabilities from varied sources, and there is uncertainty associated with these estimates. While the decision analytic model does not formally incorporate this variation when measuring benefit, we have performed extensive sensitivity analyses outlined in prior publications.11 Third, while we endeavored to use high-quality epidemiologic studies to inform the rates, probabilities, and outcomes used decision analytic model, not all risk estimates may be transportable to the ATRIA-CVRN cohort. Fourth, we did not test the model assumption from Friberg et al.3 that the risk of ICH does not increase beyond age 75. Since an increasing risk of ICH would result in lower NCB, the results of this study represent a conservative estimate of NCB. Finally, this study design could not account for individual patient preferences. We used population averages that physicians and patients must adapt for individual treatment decisions.
In conclusion, we found that the net clinical benefit of anticoagulation decreases with age, and for the typical patient, provides minimal benefit after age 87 years with warfarin and 92 years when using apixaban. Competing risks have an important influence on this declining net clinical benefit. Clinicians should consider competing risks when discussing the potential benefit of anticoagulants with older adults with atrial fibrillation. Future work should focus on incorporating competing risks into estimates of the net clinical benefit of anticoagulation and anticoagulation clinical decision aids.
Supplementary Material
What is known:
Because aging increases the risk of ischemic stroke, guidelines recommend anticoagulation for all patient 75 years old and older with atrial fibrillation
However, aging also increases the risk of anticoagulant-associated bleeding complications and the risk of death from other causes (e.g., cancer)
How these competing risks affect the net clinical of anticoagulation in older adults is not well described
What the study adds:
Examining a large cohort of patients with atrial fibrillation using a simulation model, we found that the net clinical benefit of anticoagulant use decreases with age beyond 75 years
The risk of death from other causes (e.g., cancer) have an important influence on the declining net clinical benefit of anticoagulant use
Acknowledgments
Sources of Funding
Funded by National Center for Advancing Translational Sciences (NIH – UL1TR000077-05), National Heart, Lung, Blood Institute (U19HL91179, 1RC2HL101589), National Institutes on Aging (R01 AG15478). Dr. Singer was supported, in part, by the Eliot B. and Edith C. Shoolman Fund of Massachusetts General Hospital. Dr. Shah was supported, in part, by the Division of Hospital Medicine, University of California, San Francisco. The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
Disclosures
Mark Eckman has current or recent investigator-initiated grant funding from the Heart Rhythm Society through a grant from Boehinger-Ingelheim Pharmaceuticals, Inc. (BIPI), the National Center for Advancing Translational Sciences (NIH – UL1TR000077-05), the National Institute of Child Health and Human Development (NIH – R01HD094213), Virus Action Coalition of the Centers for Disease Control, Merck, Pfizer Educational Group, Bristol-Myers Squibb/Pfizer Education Consortium, and the Cystic Fibrosis Foundation. Alan Go has a research grant through Kaiser Permanente Northern California Division of Research from iRhythm Technologies. Kristi Reynolds has received research support through Kaiser Permanente Southern California Department of Research & Evaluation from iRhythm Technologies. Daniel Singer is supported, in part, from the Eliot B. and Edith C. Shoolman Fund of Massachusetts General Hospital. He receives research support from Bristol-Myers Squibb and Boehringer Ingelheim. He serves as a consultant or an advisory board member for Bristol-Myers Squibb, Boehringer Ingelheim, CVS Health, Johnson and Johnson, Merck, and Pfizer.
References
- 1.Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, Reynolds K, Go AS. A New Risk Scheme to Predict Ischemic Stroke and Other Thromboembolism in Atrial Fibrillation: The ATRIA Study Stroke Risk Score. J Am Heart Assoc. 2013;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 3.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 4.Fang MC, Go AS, Hylek EM, Chang Y, Henault LE, Jensvold NG, Singer DE. Age and the Risk of Warfarin-Associated Hemorrhage: The Anticoagulation and Risk Factors In Atrial Fibrillation Study. Journal of the American Geriatrics Society. 2006;54:1231–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashburner JM, Go AS, Chang Y, Fang MC, Fredman L, Applebaum KM, Singer DE. Influence of Competing Risks on Estimating the Expected Benefit of Warfarin in Individuals with Atrial Fibrillation Not Currently Taking Anticoagulants: The Anticoagulation and Risk Factors in Atrial Fibrillation Study. J Am Geriatr Soc. 2017;65:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 7.Chao T-F, Liu C-J, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, Tuan T-C, Liao J-N, Chung F-P, Chen T-J, Lip GYH, Chen S-A. Oral Anticoagulation in Very Elderly Patients With Atrial Fibrillation: A Nationwide Cohort Study. Circulation. 2018;138:37–47. [DOI] [PubMed] [Google Scholar]
- 8.Ray WA. Evaluating Medication Effects Outside of Clinical Trials: New-User Designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 9.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 10.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing Risk of Death: An Important Consideration in Studies of Older Adults. Journal of the American Geriatrics Society. 2010;58:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckman MH, Wise RE, Speer B, Sullivan M, Walker N, Lip GYH, Kissela B, Flaherty ML, Kleindorfer D, Khan F, Kues J, Baker P, Ireton R, Hoskins D, Harnett BM, Aguilar C, Leonard A, Prakash R, Arduser L, Costea A. Integrating Real-Time Clinical Information to Provide Estimates of Net Clinical Benefit of Antithrombotic Therapy for Patients With Atrial Fibrillation. Circulation: Cardiovascular Quality and Outcomes. 2014;7:680–686. [DOI] [PubMed] [Google Scholar]
- 12.Eckman MH, Wise RE, Naylor K, Arduser L, Lip GYH, Kissela B, Flaherty M, Kleindorfer D, Khan F, Schauer DP, Kues J, Costea A. Developing an Atrial Fibrillation Guideline Support Tool (AFGuST) for shared decision making. Current Medical Research and Opinion. 2015;31:603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.January Craig T, Wann L Samuel Calkins Hugh, Chen Lin Y., Cigarroa Joaquin E., Cleveland Joseph C., Ellinor Patrick T., Ezekowitz Michael D., Field Michael E., Furie Karen L., Heidenreich Paul A., Murray Katherine T., Shea Julie B., Tracy Cynthia M., Yancy Clyde W. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 14.Freeman JV, Reynolds K, Fang M, Udaltsova N, Steimle A, Pomernacki NK, Borowsky LH, Harrison TN, Singer DE, Go AS. Digoxin and Risk of Death in Adults With Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology. 2015;8:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L. Apixaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 16.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. New England Journal of Medicine. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM, the ROCKET AF Steering Committee. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. New England Journal of Medicine. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 19.Aspberg S, Chang Y, Atterman A, Bottai M, Go AS, Singer DE. Comparison of the ATRIA, CHADS2, and CHA2DS2-VASc stroke risk scores in predicting ischaemic stroke in a large Swedish cohort of patients with atrial fibrillation. Eur Heart J. 2016;37:3203–3210. [DOI] [PubMed] [Google Scholar]
- 20.van den Ham HA, Klungel OH, Singer DE, Leufkens HGM, van Staa TP. Comparative Performance of ATRIA, CHADS2, and CHA2DS2-VASc Risk Scores Predicting Stroke in Patients With Atrial Fibrillation. Journal of the American College of Cardiology. 2015;66:1851–1859. [DOI] [PubMed] [Google Scholar]
- 21.Gage BF. The Effect of Stroke and Stroke Prophylaxis With Aspirin or Warfarin on Quality of Life. Archives of Internal Medicine. 1996;156:1829. [PubMed] [Google Scholar]
- 22.Eckman MH, Costea A, Attari M, Munjal J, Wise RE, Knochelmann C, Flaherty ML, Baker P, Ireton R, Harnett BM, Leonard AC, Steen D, Rose A, Kues J. Atrial fibrillation decision support tool: Population perspective. American Heart Journal. 2017;194:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lip GYH, Frison L, Halperin JL, Lane DA. Identifying Patients at High Risk for Stroke Despite Anticoagulation: A Comparison of Contemporary Stroke Risk Stratification Schemes in an Anticoagulated Atrial Fibrillation Cohort. Stroke. 2010;41:2731–2738. [DOI] [PubMed] [Google Scholar]
- 24.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 25.Graham DJ, Baro E, Zhang R, Liao J, Wernecke M, Reichman ME, Hu M, Illoh O, Wei Y, Goulding MR, Chillarige Y, Southworth MR, MaCurdy TE, Kelman JA. Comparative Stroke, Bleeding, and Mortality Risks in Older Medicare Patients Treated with Oral Anticoagulants for Nonvalvular Atrial Fibrillation. The American Journal of Medicine. 2019;132:596–604.e11. [DOI] [PubMed] [Google Scholar]
- 26.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 27.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic Therapy to Prevent Stroke in Patients Who Have Nonvalvular Atrial Fibrillation. Annals of Internal Medicine. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 28.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, Go AS. The Net Clinical Benefit of Warfarin Anticoagulation in Atrial Fibrillation. Ann Intern Med. 2009;151:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wintzen AR, de Jonge H, Loeliger EA, Bots GT a. M. The risk of intracerebral hemorrhage during oral anticoagulant treatment: A population study. Ann Neurol. 1984;16:553–558. [DOI] [PubMed] [Google Scholar]
- 30.Hart RG, Boop BS, Anderson DC. Oral Anticoagulants and Intracranial Hemorrhage: Facts and Hypotheses. Stroke. 1995;26:1471–1477. [DOI] [PubMed] [Google Scholar]
- 31.Products - Life Tables - Homepage [Internet]. 2017. [cited 2018 Mar 12];Available from: https://www.cdc.gov/nchs/products/life_tables.htm
- 32.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. New England Journal of Medicine. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 33.Tancredi M, Rosengren A, Svensson A- M, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373:1720–1732. [DOI] [PubMed] [Google Scholar]
- 34.Roger Véronique L Epidemiology of Heart Failure. Circulation Research. 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WRITING GROUP MEMBERS, Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive Summary: Heart Disease and Stroke Statistics—2010 Update: A Report From the American Heart Association. Circulation. 2010;121:948–954. [DOI] [PubMed] [Google Scholar]
- 36.Brønnum-Hansen Henrik, Davidsen Michael, Thorvaldsen Per Long-Term Survival and Causes of Death After Stroke. Stroke. 2001;32:2131–2136. [DOI] [PubMed] [Google Scholar]
- 37.Sherman DG. Occurrence and Characteristics of Stroke Events in the Atrial Fibrillation Follow-up Investigation of Sinus Rhythm Management (AFFIRM) Study. Archives of Internal Medicine. 2005;165:1185–1191. [DOI] [PubMed] [Google Scholar]
- 38.Penado S, Cano M, Acha O, Hernández JL, Riancho JA. Stroke severity in patients with atrial fibrillation. The American Journal of Medicine. 2002;112:572–574. [DOI] [PubMed] [Google Scholar]
- 39.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The Effect of Warfarin and Intensity of Anticoagulation on Outcome of Intracerebral Hemorrhage. Archives of Internal Medicine. 2004;164:880–884. [DOI] [PubMed] [Google Scholar]
- 40.Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, Singer DE. Death and Disability from Warfarin-Associated Intracranial and Extracranial Hemorrhages. The American Journal of Medicine. 2007;120:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hylek EM. Risk Factors for Intracranial Hemorrhage in Outpatients Taking Warfarin. Annals of Internal Medicine. 1994;120:897–902. [DOI] [PubMed] [Google Scholar]
- 42.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, Koudstaal PJ, Chang Y, Hellemons B. Oral Anticoagulants vs Aspirin in Nonvalvular Atrial Fibrillation: An Individual Patient Meta-analysis. JAMA. 2002;288:2441–2448. [DOI] [PubMed] [Google Scholar]
- 43.Hijazi Z, Lindbäck J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RAH, White HD, Granger CB, Wallentin L. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldgren J, Hijazi Z, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Granger CB, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Wallentin L. Performance and Validation of a Novel Biomarker-Based Stroke Risk Score for Atrial Fibrillation. Circulation. 2016;134:1697–1707. [DOI] [PubMed] [Google Scholar]
- 45.Connolly SJ. Net Clinical Benefit of Adding Clopidogrel to Aspirin Therapy in Patients With Atrial Fibrillation for Whom Vitamin K Antagonists Are Unsuitable. Annals of Internal Medicine. 2011;155:579–586. [DOI] [PubMed] [Google Scholar]
- 46.Friberg L, Rosenqvist M, Lip GYH. Net Clinical Benefit of Warfarin in Patients With Atrial FibrillationCLINICAL PERSPECTIVE: A Report From the Swedish Atrial Fibrillation Cohort Study. Circulation. 2012;125:2298–2307. [DOI] [PubMed] [Google Scholar]
- 47.Banerjee A, Lane DA, Torp-Pedersen C, Lip GYH. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: A modelling analysis based on a nationwide cohort study: Thrombosis and Haemostasis. 2011;107:584–589. [DOI] [PubMed] [Google Scholar]
- 48.Black WC, Gareen IF, Soneji SS, Sicks JD, Keeler EB, Aberle DR, Naeim A, Church TR, Silvestri GA, Gorelick J, Gatsonis C. Cost-Effectiveness of CT Screening in the National Lung Screening Trial. New England Journal of Medicine. 2014;371:1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan PS, Nallamothu BK, Gurm HS, Hayward RA, Vijan S. Incremental benefit and cost-effectiveness of high-dose statin therapy in high-risk patients with coronary artery disease. Circulation. 2007;115:2398–2409. [DOI] [PubMed] [Google Scholar]
- 50.Li R, Zhang P, Barker LE, Hoerger TJ. Cost-Effectiveness of Aspirin Use Among Persons With Newly Diagnosed Type 2 Diabetes. Diabetes Care. 2010;33:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alnsasra H, Haim M, Senderey AB, Reges O, Leventer-Roberts M, Arnson Y, Leibowitz M, Hoshen M, Avgil-Tsadok M. Net clinical benefit of anticoagulant treatments in elderly patients with nonvalvular atrial fibrillation: Experience from the real world. Heart Rhythm. 2018;16:31–37. [DOI] [PubMed] [Google Scholar]
- 52.Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen A- MS, Gislason GH, Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.