Abstract
Introduction/Purpose
Aerobic exercise training (AET) has been shown to improve mitochondrial bioenergetics and upregulate proteins related to lipid metabolism. However, it remains to be determined if these alterations associated with AET persist when measured in energy balance (EB) in the days following the last bout of training.. The purpose of the study was to test the hypothesis that improvements in skeletal muscle mitochondrial function induced by AET observed in previous literature would persist when measured after restoring EB conditions 72 hours removed from the last exercise bout.
Methods
Participants were 14 premenopausal women (age = 31.2 ± 6.7 years, BMI = 26.6 ± 5.1 kg/m2). The AET program required 3 monitored training sessions per week for 8–16 weeks. Skeletal muscle biopsies were obtained at baseline and following 8–16 weeks of AET (≥72 hrs after the last exercise bout). All food was provided for 72 hours prior to biopsies and EB was managed 24 hours prior to testing within ±100 kcal of measured energy requirements using a whole-room calorimeter. Mitochondrial oxidative capacity was quantified in permeabilized muscle fibers from the vastus lateralis.
Results
We found that AET increased coupled respiration (154%) and uncoupled respiration (90%) rates using a fatty acid substrate (palmitoyl carnitine) (P<0.05). However, when rates were normalized to complex IV activity (a marker of mitochondrial content), no significant differences were observed. Additionally, there were no changes in proteins known to mediate mitochondrial biogenesis or lipid transport and metabolism following AET.
Conclusion
8–16 weeks of AET improved mitochondrial capacity under fatty acid substrate when assessed in EB, which appears to be due to mitochondrial biogenesis.
Keywords: aerobic exercise, mitochondrial function, metabolism, energy balance
Introduction
Aerobic exercise training (AET) is well known to confer numerous cardiovascular and metabolic health benefits. These beneficial effects may be mediated in part by improvements in mitochondrial biogenesis and oxidative capacity (1, 2) and upregulation of proteins related to lipid metabolism (3). These findings have implications in the treatment of chronic cardiometabolic diseases, as improved mitochondrial function has been linked to improved insulin sensitivity (4) and lower oxidative stress (5). However, AET can promote both acute and chronic energy deficits and there are a number of independent effects of negative energy balance (EB) on mitochondrial function, including lower ROS emission and increased respiratory capacity (6, 7). Thus, it remains to be determined if improvements in mitochondrial function observed after AET occur independent of negative EB.
Numerous studies have provided evidence to suggest the mechanisms by which AET can induce improvements in mitochondrial function. AET improves the activity of the electron transport system (ETS) in rodents (8) and humans (9, 10). Additionally, a key adaptation to AET is increased mitochondrial content, which is thought to occur in order to meet the energetic demands of endurance training (1) and contribute to increased mitochondrial capacity. Mitochondrial biogenesis is induced in part by peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α) (11), a key protein implicated in mitochondrial function (12) and dynamics (13, 14). AET is also known to enhance mitochondrial capacity to oxidize fatty acids (FA) (3) and can increase the storage of intramyocellular triacylglycerol (15). mRNA levels of key regulatory proteins in FA transport and metabolism, fatty acid translocase (CD36) and carnitine palmitoyltransferase 1B (CPT1B), have been shown to be elevated after a short exercise program (16) and CD36 appears to be an important mediator in the increase in FA metabolism following exercise (17). However, many of these studies are confounded by samples obtained ≤24 hours following the last exercise bout (16, 18) or following weight loss during the course of the AET (10). Given these limitations, it is unknown whether changes in mitochondrial oxidative capacity due to AET persist when measured in carefully controlled EB.
The acute and chronic energetic deficits created by AET interventions may play a role in the improvements observed in mitochondrial function attributed to exercise. Chronic caloric restriction is known to promote greater coupled respiration (19), likely to maximize efficiency of ATP production despite limited substrate availability. The acute depletion of energy reserves from a bout of aerobic exercise is known to promote increased O2 consumption, increased ATP synthesis (20), and decreased uncoupled mitochondrial respiration, likely driven by decreased uncoupling protein 3 (UCP3) (21). Whether weight loss alone is sufficient to alter mitochondrial oxidative capacity is debated (10), however weight loss is well understood to decrease energy expenditure (22).
The purpose of this study was to assess the effects of AET on skeletal muscle mitochondrial function and markers of lipid metabolism when assessed 72 hours removed from the last exercise bout and EB has been restored. We hypothesized that exercise-induced improvements in skeletal muscle mitochondrial function reported in previous literature would persist when EB is controlled. A secondary aim was to assess proteins associated with mitochondrial bioenergetics and lipid metabolism in order to gain insight into potential mechanisms that mediate changes in skeletal muscle mitochondrial function following AET.
Methods
Participants
This is a secondary analysis of a study designed to evaluate insulin sensitivity, resting energy expenditure, and blood pressure following a bout of high intensity exercise (23–25). We recruited 14 participants between the age of 21 and 45 years who self-identified as either of European-American (EA) or African-American descent (AA). Participants were not taking oral contraceptives or any medication known to influence blood pressure or metabolism. All participants were normotensive, non-smokers, normoglycemic, and did not engage in routine physical activity (participating in <1 exercise activity per week). All testing was conducted in the first 10 days of the follicular phase of the menstrual cycle. All participants provided written, informed consent and the Institutional Review Board at the University of Alabama at Birmingham approved this study.
Study design
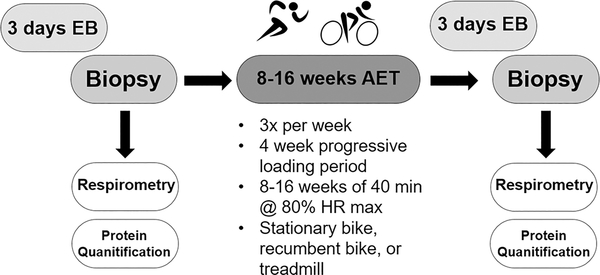
The study design is depicted in Figure 1 and each method is outlined in further detail below. A skeletal muscle biopsy of the vastus lateralis was collected from each participant prior to beginning an AET program. All food was provided to the participants for 72 hours prior to the tissue collection and was made up of ~ 60% carbohydrates, ~15% protein, and ~25% fat by calories. The participants spent the 23 hours immediately prior to the tissue collection in our whole-room indirect calorimeter. Skeletal muscle tissue was used to measure mitochondrial oxidative function and protein levels. Post training evaluations took place after 8 to 16 weeks of exercise training. Training duration varied due to the experimental design of the parent study in which there was a random assignment of test order over 8–16 weeks exercise training. Only the results of the baseline tests (prior to initiation of training) and post training condition in which the subjects participated in no exercise for 72 hours prior to evaluation are included. The other two conditions, either a bout of moderate intensity or a bout of high intensity cycle exercise 22 hours prior to evaluation are not included in this paper (25). There were no differences between the three post training conditions for VO2peak, nor a test order effect for any of the cycling economy, or any of the mitochondrial respiration values, suggesting that duration of training did not affect any variable of interest. The same protocol was used prior to the collection of post-AET muscle biopsies. Again, participants were provided all food for 72 hours and the 23 hours prior to tissue collection were spent in the whole-room calorimeter. Post-AET tissue was collected at least 72 hours removed from the last exercise bout to assess the chronic effect of AET on mitochondrial oxidative function and protein levels in EB.
Figure 1.
Study design.
Energy balance and room calorimetry
Participants were provided with all food for 72 hours prior to tissue collection. We used multiple regression to develop equations designed to maintain energy balance before and during the room calorimeter stay. These equations have previously been used in the following papers (23, 25) Caloric intake for the 48 hours prior to the room calorimetry visit was based on estimates generated from 330 doubly labeled water estimates of free living energy expenditure of sedentary premenopausal women collected in our lab (26, 27). The first equation was: Equation 1 = 750 kcal + [(31.47 * fat-free mass) – (0.31 * fat mass) – (155 * race (race coded 1 for African American and 2 for European American)]. An equation for estimating the room calorimeter energy intake was developed from over 200 room calorimeter visits of premenopausal women (28, 29): Equation 2 = 465 kcal + [(27.8 * fat-free mass) – (2.4* fat mass) – (188*race (race coded 1 for African American and 2 for European American)]. However, we recognized that the estimates may result in overfeeding or underfeeding individual subjects. Therefore, we developed a correction equation for the room calorimeter visit that was based on energy expenditure during the room calorimeter stay up to 5:30 pm. This equation was: [Equation 3 = 9(390 kcal + average energy expenditure in kcal/min between 8:00 AM and 5:30 pm) * 925 kcal) – equation 2 estimate of energy expenditure]. We then adjusted the food intake of the evening meal to match the results of equation.
Aerobic exercise training (AET) program
Each participant trained 3 days per week for 8–16 weeks and was monitored by trained exercise physiologists during each training session. Each exercise session included a 3–5 minute warm-up and cool-down period of light activity. Participants were able to complete the AET using a treadmill, recumbent bike, or stationary bike (at least 50% of the training was performed by cycle ergometry and the remainder of training was optional to the participants). During the first week, participants began training at 67% heart rate max (HRM) for 20 minutes, increasing time and intensity each week until the beginning of the fifth week, when each participant was training at 80% HRM for 40 minutes. This intensity was maintained for the duration of the AET program. We chose this volume of exercise, as we did not want participants to lose a significant amount of weight, in order to control for confounding effects that can occurs with significant weight loss.
VO2peak
This protocol has been adapted from previously published work (27, 30). The testing was done on a cycle ergometer with each participant pedaling at 50 watts for 3 minutes. Every minute thereafter the resistance was increased 20 watts until the subject reached volitional exhaustion. Oxygen uptake and carbon dioxide production were continuously monitored using a MAXX-II metabolic cart (Physio-Dyne Instrument Corp., Massapequa, NY). Heart rate was measured using a POLAR Vantage XL heart rate monitor (Gays Mills, WI). Criteria for achieving VO2peak were heart rate within 10 bpm of estimated maximum, RER of at least 1.10, and plateauing of VO2. All subjects achieved at least one criteria and all but three subjects achieved at least two criteria during each of the four tests.
Body fat quantification
Body composition (fat-free mass (kg) and fat mass (kg) was assessed by dual-energy X-ray absorptiometry (DXA) using a Lunar iDXA densitometer (GE-Lunar Corporation, Madison, WI). Participants wore light clothing and remained supine in compliance with normal testing procedures. Scans were analyzed with enCORE 2011 software (GE Healthcare Lunar, Madison, WI).
Laboratory analyses
Sera were analyzed by the Core Laboratory of the UAB Diabetes Research Center and Center for Clinical and Translational Science. Fasting glucose, total cholesterol, HDL cholesterol, triglycerides, and circulating free FA were measured using a SIRRUS analyzer (Stanbio Laboratory, Boerne, TX). LDL cholesterol was calculated using the Friedewald method (31). Fasting insulin was measured using a TOSOH AIA-II analyzer (TOSOH Corp., South San Francisco, CA).
Mitochondrial respiration measures
This technique has been adapted from previously published methods (32). Immediately following each stay in the room calorimeter (baseline and post-AET), a skeletal muscle biopsy of the vastus lateralis was collected under local anesthesia from each participant. The tissue was immediately cleaned of adipose and connective tissue and a bundle of approximately 20 mg was selected for mitochondrial respirometry. This tissue was transferred to the laboratory on ice in Buffer X buffer containing 50 mM MES, 7.23 mM K2EGTA, 2.77 mM CaK2EGTA, 20 mM imidazole, 0.5 mM DTT, 20 mM taurine, 5.7 mM ATP, 14.3 mM PCr, and 6.56 mM MgCl2-6 H2O (pH 7.1, 290 mOsm). The tissue was then dissected into several smaller muscle bundles (of approximately 3–5 mg wet weight) and each was gently separated with a pair of antimagnetic needle-tipped forceps under magnification. Bundles were treated with 30 μg/ml saponin in Buffer X and incubated on a rotator for 30 min at 4°C. The tissue bundles were washed of saponin for 15 minutes in Buffer Z containing 105mM K-MES, 30 mM KCl, 1 mM EGTA, 10 mM K2HPO4, and 5 mM MgCl2-6 H2O, 5 μM glutamate, 2 μM malate, and 5.0 mg/ml BSA (pH 7.4, 290 mOsm). The samples were transferred to Buffer Z containing 20 mM creatine hydrate and 5 μM of blebbistatin for 10 minutes prior to respirometry experiments (32).
High-resolution respirometry was performed using an Oroboros Oxygraph O2K (Oroboros Instruments, Innsbruck, Austria) containing 2 mL of Buffer Z with creatine and blebbistatin, constantly stirred at 37°C. We assessed mitochondrial O2 consumption using two substrate protocols: 4 mM malate, 9 mM pyruvate, and 2.5 mM succinate (PMS) to drive convergent electron input to complexes I and II of the ETS or 2 mM malate and 40 μM palmitoyl carnitine (PC) to examine mitochondrial fatty acid oxidation. State 3 in each substrate condition was measured after the addition of 1 mM ADP. Cytochrome c (10 μM) was added to assess mitochondrial membrane integrity following the dissection of the myofibers. There was no significant increase in respiration following the addition of cytochrome c. Uncoupled respiration (State 4) was induced by oligomycin (2 μg/mL). Maximal complex IV activity was assessed after the addition of ascorbate (2 mM) and tetramethyl-p-phenylenediamine (TMPD) (0.5 mM), providing a measure of mitochondrial content. The respiratory control ratio (RCR) was calculated as State 3/State 4. The RCR is a commonly used metric for assessing mitochondrial integrity and is highly correlated with coupling efficiency. Oxygen flux was normalized to either the wet weight or complex IV activity of each fiber bundle.
Immunoblotting
A portion of muscle tissue collected at baseline and following the completion of the AET protocol was snap frozen in liquid N2 and stored at −80°C until analysis. Muscle samples of approximately 30 mg were powdered in a liquid N2-cooled mortar and pestle and homogenized in 6 μL/mg muscle of ice-cold lysis buffer (150mM NaCl, 50 mM Tris-HCl, 5mM EDTA, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, and 0.5% NP-40 at pH 7.4) with protease (Sigma P2714) and phosphatase (Sigma P0044) inhibitors. The samples were centrifuged at 15,000 g for 15 min at 4°C and the supernatant was collected. Protein content of the supernatant was quantified using a BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Thirty-seven micrograms of protein was treated with 4x NuPAGE LDS Sample Buffer (Novex, Carlsbad, CA) and 10x NuPAGE Reducing Agent (Novex) and incubated at 70°C for 10 minutes. The samples were electrophoresed in an SDS polyacrylamide gel (4–20%) at 100 v on ice. The gels were blotted to polyvinylidene fluoride membranes using a semi-dry transfer method at 25 v for 12 min using a Pierce Power Blotter (Thermo Fisher Scientific). The membranes were blocked under conditions optimized for each antibody (2–5% milk and/or 2–5% BSA in PBS with 0.1% Tween 20 (PBST)) for 1 hr at room temperature (RT) with gentle agitation. An appropriate dilution of primary antibody (Ab) was added for incubation overnight at 4°C with gentle agitation. We probed for the following: rabbit polyclonal Ab against CD36 (1:1000, Santa Cruz Biotechnology, sc-9154), and UCP3 (1:500, Abcam, ab3477); rabbit monoclonal Ab against α-tubulin (1:1000, Cell Signaling, #2125), and CPT1B (1:1000, Abcam, ab134135); and a goat polyclonal Ab against PGC1α (1:1000, Abcam, ab106814). Horseradish peroxidase-conjugated secondary Abs were used at 1:50,000 in 0.5% of selected blocking agent in PBST for 1 hr at RT with gentle agitation. Bands were visualized by chemiluminescent detection using ECL Western Blotting Substrate (Pierce Biotechnology) in a ChemicDoc XRS (Bio-Rad Laboratories). Band densitometry was quantified by Image Lab software (Version 4.1) (Bio-Rad Laboratories). Values shown were obtained after normalization to the reference protein α-tubulin.
Statistical analyses
Not all data were available for all participants. Each table and figure accurately represents the number of participants assessed for each measure. Descriptive characteristics are reported as mean ± SD. Changes in variables between baseline and post-AET were assessed using two-tailed t-tests for paired samples and depicted as mean ± SD. An alpha level of 0.05 was used to determine statistical significance. All statistical analyses were conducted using SPSS Statistics for Macintosh Version 22.0 (IBM Corp., Armonk, NY).
Results
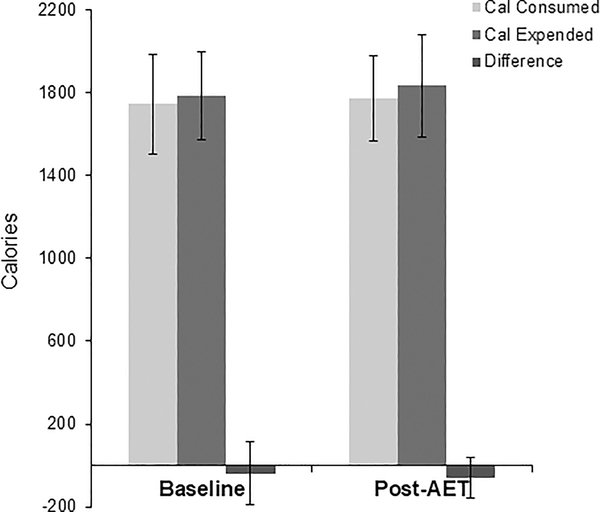
Demographic, physical, and metabolic characteristics of the study participants at baseline and following AET are shown in Table 1. Body mass index (BMI) ranged from 20.1 to 35.0 kg/m2 and age from 21 to 40 years. There were 8 EA participants and 6 AA participants. There were no significant changes in weight, BMI, or body fat between baseline and post-AET conditions. There was a 0.6 kg increase in fat-free mass (P<0.05). VO2peak improved significantly following the AET protocol (P<0.05). There were no differences in energy intake and energy expenditure during the stay in the room calorimeter (Figure 2).
Table 1.
Clinical characteristics of participants (n=14).
| Baseline | Post-AET | P | |
|---|---|---|---|
| Age (years) | 31.2 ± 6.7 | - | - |
| Weight (kg) | 72.2 ± 12.8 | 72.5 ± 12.5 | 0.656 |
| BMI | 26.6 ± 5.1 | 26.7 ± 5.0 | 0.717 |
| Total Body Fat (kg)† | 39.7 ± 4.0 | 39.8 ± 4.6 | 0.373 |
| Fat Free Mass (kg)† | 43.0 ± 5.6 | 43.6 ± 6.0 | 0.026* |
| Total Cholesterol (mg/dL)† | 180.4 ± 38.2 | 182.3 ± 36.1 | 0.649 |
| Triglycerides (mg/dL)† | 94.5 ± 39.6 | 103.0 ± 45.1 | 0.163 |
| HDL (mg/dL)† | 65.2 ± 19.4 | 65.9 ± 19.7 | 0.577 |
| LDL (mg/dL)† | 96.3 ± 26.5 | 95.8 ± 25.0 | 0.885 |
| Fasting Glucose (mg/dL)† | 91.3 ± 9.9 | 90.1 ± 7.8 | 0.620 |
| Fasting Insulin (μIU/mL)† | 10.5 ± 5.9 | 9.9 ± 4.4 | 0.680 |
| VO2max (L/min) | 1.7 ± 0.3 | 1.9 ± 0.3 | 0.043* |
| Relative VO2max (mL/kg/min) | 24.6 ± 4.7 | 26.4 ± 3.9 | 0.048* |
n=13
P<0.05.
Figure 2.
Energy intake, energy expenditure, and difference calculated during room calorimetry. n=12. No significant differences between baseline and post training.
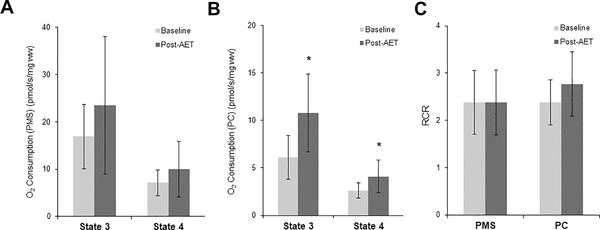
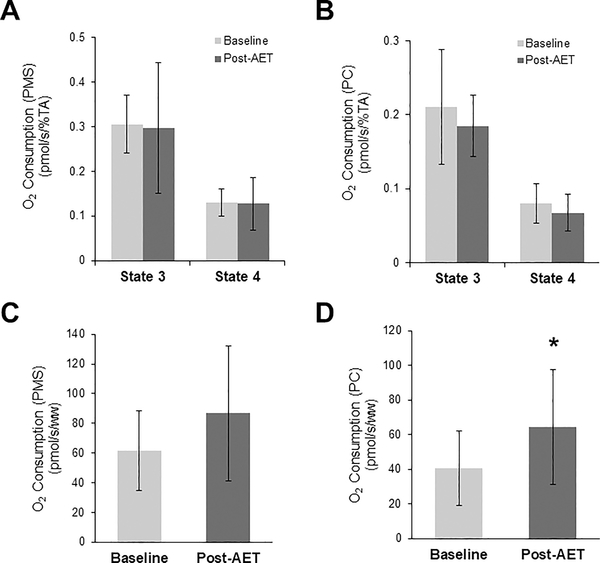
Following AET, there were no changes in mitochondrial respiration normalized to fiber weight when supported by PMS (Figure 3A), but there were significant increases in both State 3 and State 4 respiration rates supported by PC (Figure 3B). There were no changes in the RCR following AET supported by PMS or PC (Figure 3C). When mitochondrial respiration measures were normalized to maximal complex IV activity (a marker of mitochondrial content), there were no changes in mitochondrial function following AET supported by either PMS (Figure 4A) or PC (Figure 4B).
Figure 3.
Oxygen consumption under State 3 and State 4 conditions normalized to fiber weight supported by PMS (A) and PC (B). (C) Respiratory control ratio (RCR) supported by PMS or PC. n=11, *P<0.05.
Figure 4.
Oxygen consumption under State 3 and State 4 conditions normalized to maximal complex IV activity supported by PMS (A) and PC (B), and maximal complex IV activity supported respiration PMS (C) and PC (D). n=12
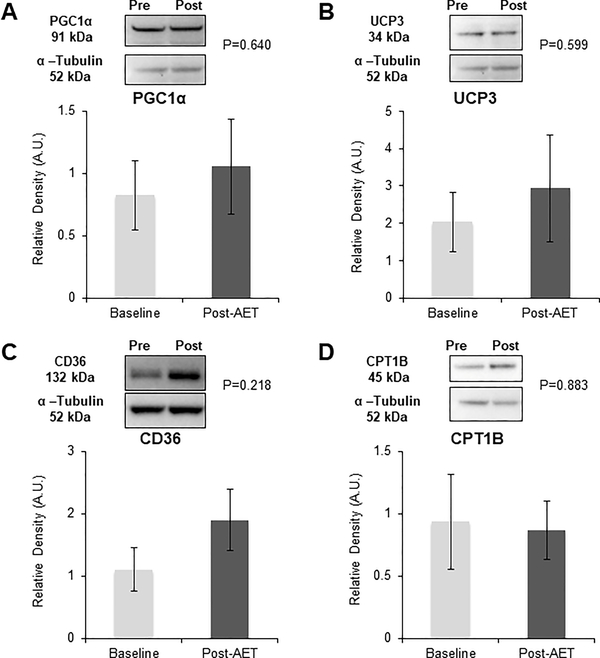
There were no changes in any proteins measured following the AET program when assessed under energetic balance (PGC1α, UCP3, CD36, or CPT1B) (Figure 5).
Figure 5.
Skeletal muscle protein expression at baseline and post-AET was measured using Western blotting. Values are normalized to α-tubulin and are presented as mean ± SEM. (A) PGC1α, n=11; (B) UCP3, n=11; (C) CD36, n=11; (D) CPT1B, n=5.
Discussion
Mitochondrial oxidative capacity and content are known to improve following AET (1, 2). This study tested the hypothesis that these improvements would persist 72 hours after the last exercise bout when EB had been restored. There were no differences between caloric intake and energetic expenditure in the 23 hours prior to each tissue collection and no changes in weight or REE during the course of the AET. We demonstrated that mitochondrial respiratory capacity supported by PMS was unchanged when measured in an energetically balanced state following AET. However, when mitochondrial oxidation was supported by a FA substrate, we observed an enhanced mitochondrial oxidative capacity and elevated uncoupled respiration. These changes were no longer apparent when these data were normalized for a marker of mitochondrial content, suggesting that the improvements in mitochondrial capacity were due to mitochondrial biogenesis induced by AET, and not intrinsic changes to existing mitochondria. Finally, there were no changes in proteins known to mediate mitochondrial biogenesis or lipid transport and metabolism.
The results from this study suggest that improvements in mitochondrial FA oxidation may be due primarily to mitochondrial biogenesis. Although, we cannot rule out other potential mechanism that may be responsible for changes in FA oxidation. Chronic AET is well understood to induce mitochondrial biogenesis. Improvements in mitochondrial content have been observed after only 7 weeks of training (33), but can be reversed in as little as 6 weeks after the conclusion of an exercise program (34). Acute exercise is known to promote enhanced expression of p38 MAPK and thus activation of PGC1α, an important signaling cascade for mediating mitochondrial biogenesis (35). Despite this, we found no increase in PGC1α protein levels following AET. However, this is likely because increases in PGC1α are transient and protein levels in this study were measured in EB and more than 72 hours following the last exercise bout. This is supported by two previous studies in which transient increases were observed immediately following successive exercise bouts (36) and 24 hours following a single exercise bout (37). We are aware of no study that has observed increased mitochondrial content following an acute bout of exercise. We hypothesize that mitochondrial biogenesis due to exercise is regulated in a more chronic manner and that transient increases in PGC1α from acute AET are likely sufficient to induce mitochondrial biogenesis associated with chronic AET.
All other proteins measured in the present study are known to be induced by AET, however, like PGC1α, no changes were apparent when assessed in EB and 72 hours removed from the last exercise bout. UCP3 expression is increased following AET in rodents, but only as a function of increased mitochondrial content (38). This study did not consider the effect of energy balance on UCP3 expression. Elevated expression of mRNA for both CD36 and CPT1B has been observed when measured after a short exercise training program (16). Additionally, previous investigators have shown that CD36 is an important mediator in the increase in FA oxidation following exercise, independent of mitochondrial biogenesis (17). However, it is possible that the timing of sample collection plays an important role in mediating these results, as others have also found (in agreement with our findings) no changes in CD36 or CPT1B protein expression when measured 24 hours after an exercise bout (39).
The increase in mitochondrial oxidative capacity (State 3) measured under a FA substrate may reflect a greater capacity to utilize FA substrate prompted by AET. In addition, respiration uncoupled from ATP synthesis (State 4) was increased when supported by FA substrate after AET. Uncoupled respiration is commonly thought to be a mechanism to limit the production of mitochondrial-derived reactive oxygen species (ROS), and is known to be induced by both FA substrate (40) and ROS production (41). Given that there were no changes in the RCR, the overall proportion of coupled to uncoupled respiration remained similar before and after AET, and thus overall efficiency was unchanged. Greater mitochondrial FA oxidative capacity may have the potential to decrease cellular storage of FA in the form of intramyoceullar triglyceride (42) or ceramides and diacylglycerols (43), and prevent the cardiometabolic complications associated with them.
There were a number of strengths associated with the present study design. The AET program required participants to attend three sessions per week in our training facility and trained personnel monitored the participants during each session. The provision of all food consumed by the participants for 72 hours prior to the collection of each biopsy sample and 23 hour energy expenditure measured by room calorimeter ensured a high degree of control over EB. We acknowledge a primary limitation within this study was the lack of a true control group for which EB was not controlled. Additionally, there are a number of methods with which to quantify mitochondrial content not used in the present study (e.g. mtDNA copy number, citrate synthase activity). These measures may have provided additional insight into the mechanisms of mitochondrial biogenesis that appear to be responsible for improvements in mitochondrial capacity. Additionally, these data may only reflect the population in which they were collected (generally healthy premenopausal women), and not other subgroups, including men, aging persons, or those with cardiometabolic disease.
In conclusion, an 8–16-week AET program was sufficient to improve mitochondrial respiratory capacity under a fatty acid substrate load even when measured in EB, as evidenced by no weight loss during. After normalization of respiratory capacity to a marker for mitochondrial content, these changes were no longer apparent, suggesting that the improvements in mitochondrial capacity after AET were due primarily to mitochondrial biogenesis.
Acknowledgements
This work was supported by the National Institutes of Health (2R01DK049779-11A1) and the UAB Center for Exercise Medicine (5T32HD071866-04). The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of this study do not constitute endorsement by ACSM. The authors gratefully acknowledge the work of the UAB Bioanalytical Redox Biology Core and the Clinical and Translational Science and the Clinical Research Unit for their assistance with the muscle biopsies. In addition, we thank Mr. David Bryan and Mr. Brandon Kane for their work coordinating this study.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports medicine (Auckland, N.Z.). 2003;33(11):783–93. [DOI] [PubMed] [Google Scholar]
- 2.Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. Journal of applied physiology (Bethesda, Md. : 1985). 1996;80(6):2250–4. [DOI] [PubMed] [Google Scholar]
- 3.Melanson EL, MacLean PS, Hill JO. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exercise and sport sciences reviews. 2009;37(2):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koliaki C, Roden M. Alterations of Mitochondrial Function and Insulin Sensitivity in Human Obesity and Diabetes Mellitus. Annual review of nutrition. 2016;36:337–67. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Experimental biology and medicine (Maywood, N.J.). 2013;238(5):450–60. [DOI] [PubMed] [Google Scholar]
- 6.Sreekumar R, Unnikrishnan J, Fu A et al. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. American journal of physiology. Endocrinology and metabolism. 2002;283(1):E38–43. [DOI] [PubMed] [Google Scholar]
- 7.Ruetenik A, Barrientos A. Dietary restriction, mitochondrial function and aging: from yeast to humans. Biochimica et biophysica acta. 2015;1847(11):1434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva LA, Pinho CA, Scarabelot KS et al. Physical exercise increases mitochondrial function and reduces oxidative damage in skeletal muscle. European journal of applied physiology. 2009;105(6):861–7. [DOI] [PubMed] [Google Scholar]
- 9.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of Exercise on Mitochondrial Content and Function in Aging Human Skeletal Muscle. The journals of gerontology. Series A, Biological sciences and medical sciences. 2006;61(6):534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. American journal of physiology. Endocrinology and metabolism. 2005;288(4):E818–25. [DOI] [PubMed] [Google Scholar]
- 11.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochimica et biophysica acta. 2011;1813(7):1269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zechner C, Lai L, Zechner JF et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell metabolism. 2010;12(6):633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominy JE, Puigserver P. Mitochondrial Biogenesis through Activation of Nuclear Signaling Proteins. Cold Spring Harbor Perspectives in Biology. 2013;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabrowska A, Venero JL, Iwasawa R et al. PGC-1α controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging. 2015;7(9):629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horowitz JF, Klein S. Lipid metabolism during endurance exercise. The American journal of clinical nutrition. 2000;72(2 Suppl):558s–63s. [DOI] [PubMed] [Google Scholar]
- 16.Tunstall RJ, Mehan KA, Wadley GD et al. Exercise training increases lipid metabolism gene expression in human skeletal muscle. American journal of physiology. Endocrinology and metabolism. 2002;283(1):E66–72. [DOI] [PubMed] [Google Scholar]
- 17.McFarlan JT, Yoshida Y, Jain SS et al. In Vivo, Fatty Acid Translocase (CD36) Critically Regulates Skeletal Muscle Fuel Selection, Exercise Performance, and Training-induced Adaptation of Fatty Acid Oxidation. The Journal of biological chemistry. 2012;287(28):23502–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefai E, Blanc S, Momken I et al. Exercise training improves fat metabolism independent of total energy expenditure in sedentary overweight men, but does not restore lean metabolic phenotype. Int J Obes (Lond). 2017;41(12):1728–36. [DOI] [PubMed] [Google Scholar]
- 19.Asami DK, McDonald RB, Hagopian K et al. Effect of aging, caloric restriction, and uncoupling protein 3 (UCP3) on mitochondrial proton leak in mice. Experimental gerontology. 2008;43(12):1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell metabolism. 2013;17(2):162–84. [DOI] [PubMed] [Google Scholar]
- 21.Fernström M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. The Journal of Physiology. 2004;554(Pt 3):755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. International journal of obesity (2005). 2015;39(8):1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter GR, Moellering DR, Carter SJ et al. Potential Causes of Elevated REE after High-Intensity Exercise. Med Sci Sports Exerc. 2017;49(12):2414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter SJ, Goldsby TU, Fisher G et al. Systolic blood pressure response after high-intensity interval exercise is independently related to decreased small arterial elasticity in normotensive African American women. Appl Physiol Nutr Metab. 2016;41(5):484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher G, Gower BA, Ovalle F, Behrens CE, Hunter GR. Acute Effects of Exercise Intensity on Insulin Sensitivity under Energy Balance. Med Sci Sports Exerc. 2019;51(5):988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE. Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr. 2002;75(3):499–504. [DOI] [PubMed] [Google Scholar]
- 27.Hunter GR, Fisher G, Neumeier WH, Carter SJ, Plaisance EP. Exercise Training and Energy Expenditure following Weight Loss. Medicine and science in sports and exercise. 2015;47(9):1950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treuth MS, Hunter GR, Williams M. Effects of exercise intensity on 24-h energy expenditure and substrate oxidation. Med Sci Sports Exerc. 1996;28(9):1138–43. [DOI] [PubMed] [Google Scholar]
- 29.Weinsier RL, Hunter GR, Zuckerman PA, Darnell BE. Low resting and sleeping energy expenditure and fat use do not contribute to obesity in women. Obes Res. 2003;11(8):937–44. [DOI] [PubMed] [Google Scholar]
- 30.Hunter GR, Bickel CS, Del Corral P et al. Age, muscle fatigue, and walking endurance in pre-menopausal women. European journal of applied physiology. 2011;111(4):715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 32.Perry CG, Kane DA, Lin CT et al. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. The Biochemical journal. 2011;437(2):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292(3):R1271–8. [DOI] [PubMed] [Google Scholar]
- 34.Henriksson J, Reitman JS. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta physiologica Scandinavica. 1976;97(3):392–7. [DOI] [PubMed] [Google Scholar]
- 35.Akimoto T, Pohnert SC, Li P et al. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280(20):19587–93. [DOI] [PubMed] [Google Scholar]
- 36.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene NP, Fluckey JD, Lambert BS, Greene ES, Riechman SE, Crouse SF. Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women. Am J Physiol Endocrinol Metab. 2012;303(10):E1212–21. [DOI] [PubMed] [Google Scholar]
- 38.Jones TE, Baar K, Ojuka E, Chen M, Holloszy JO. Exercise induces an increase in muscle UCP3 as a component of the increase in mitochondrial biogenesis. American journal of physiology. Endocrinology and metabolism. 2003;284(1):E96–101. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. Journal of applied physiology (Bethesda, Md. : 1985). 2005;98(5):1745–52. [DOI] [PubMed] [Google Scholar]
- 40.Skulachev VP. Anion carriers in fatty acid-mediated physiological uncoupling. Journal of bioenergetics and biomembranes. 1999;31(5):431–45. [DOI] [PubMed] [Google Scholar]
- 41.Echtay KS, Roussel D, St-Pierre J et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415(6867):96–9. [DOI] [PubMed] [Google Scholar]
- 42.Guo ZK. Intramyocellular lipid kinetics and insulin resistance. Lipids Health Dis. 2007;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annual review of biochemistry. 2006;75:367–401. [DOI] [PubMed] [Google Scholar]