Abstract
Background
Fetal programming of the endocrine system may be affected by exposure to perfluoroalkyl substances (PFAAs), as they easily cross the placental barrier. In vitro studies suggest that PFAAs may disrupt steroidogenesis. “Mini puberty” refers to a transient surge in circulating androgens, androgen precursors, and gonadotropins in infant girls and boys within the first postnatal months. We hypothesize that prenatal PFAA exposure may decrease the concentrations of androgens in mini puberty.
Objectives
To investigate associations between maternal serum PFAA concentrations in early pregnancy and serum concentrations of androgens, their precursors, and gonadotropins during mini puberty in infancy.
Methods
In the prospective Odense Child Cohort, maternal pregnancy serum concentrations of five PFAAs: Perfluorohexane sulfonic acid (PFHxS), perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), and perfluorodecanoic acid (PFDA) were measured at median gestational week 12 (IQR: 10, 15) in 1,628 women. Among these, offspring serum concentrations of dehydroepiandrosterone (DHEA), dehydroepiandrosterone-sulfate (DHEAS), androstenedione, 17-hydroxyprogesterone (17-OHP), testosterone, luteinizing (LH) and follicle stimulating hormones (FSH) were measured in 373 children (44% girls; 56% boys) at a mean age of 3.9 (±0.9 SD) months. Multivariate linear regression models were performed to estimate associations.
Results
A two-fold increase in maternal PFDA concentration was associated with a reduction in DHEA concentration by −19.6% (95% CI: −32.9%, −3.8%) in girls. In girls, also, the androstenedione and DHEAS concentrations were decreased, albeit non-significantly (p<0.11), with a two-fold increase in maternal PFDA concentration. In boys, no significant association was found between PFAAs and concentrations of androgens, their precursors, and gonadotropins during mini puberty.
Conclusion
Prenatal PFDA exposure was associated with significantly lower serum DHEA concentrations and possibly also with lower androstenedione and DHEAS concentrations in female infants at mini puberty. The clinical significance of these findings remains to be elucidated.
Keywords: Perfluoroalkyl substances, Pregnancy, Mini puberty, Androgens, Adrenal, Developmental toxicity
1. Introduction
Perfluoroalkyl substances (PFAAs) are a group of persistent chemicals with fat, oil, and water repellant properties used in food packaging and textiles ((ATSDR) 2018). PFAA exposure routes are mainly through diet and, for some, drinking water and via inhalation of house dust. Elimination half-lives for PFAAs range from about four to eight years in the human body, and they are measurable in the majority of human samples across many studies ((ATSDR) 2018). Major manufacturers have phased out two of the formerly most used PFAAs, perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), however, they remain in the environment ((ATSDR) 2018; Glynn et al. 2012). Nevertheless, other less studied PFAAs are still in use, such as perfluorodecanoic acid (PFDA), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA) (Stubleski et al. 2016).
PFAAs cross the placental barrier (Monroy et al. 2008), thus leading to early-life exposure in offspring. Fetal life represents a vulnerable window for environmental influence on fetal development, including programming of the endocrine system, which may be disrupted by PFAA exposure. As the infant clears the placental hormones during the early postnatal days, the hypothalamic-pituitary-gonadal (HPG) axis is briefly activated for the first six months of postnatal life, a period referred to as “mini puberty”, after which the HPG axis remains dormant until (pre)puberty (Kuiri-Hanninen et al. 2014). Increased concentrations of androgens, androgen precursors, and gonadotropins are found in neonatal life during mini puberty (Kuiri-Hanninen et al. 2014; Lanciotti et al. 2018). Infant girls reach higher concentrations of dehydroepiandrosterone (DHEA), dehydroepiandrosterone-sulfate (DHEAS), androstenedione, and follicle stimulating hormone (FSH), but lower concentrations of testosterone and luteinizing hormone (LH) compared to boys (Bae et al. 2019; Johannsen et al. 2018; Kuijper et al. 2013; Lanciotti et al. 2018). The fetal adrenal gland consists of a ‘fetal zone’ and secretes androgens, such as DHEA, DHEAS, and androstenedione, but androgen concentrations slowly decline after birth, as the fetal zone disappears within six months of postnatal life (Brosnan 2001; Longcope 1986; Quinn et al. 2018). For girls, the adrenal gland is an important source of androgens in utero (Brosnan 2001; Longcope 1986; Quinn et al. 2018). The postnatal transient surge in androgen hormones may play a role in neurobehavioral development (Lamminmaki et al. 2012) and possibly also in reproductive function (Kuiri-Hanninen et al. 2014; Lanciotti et al. 2018).
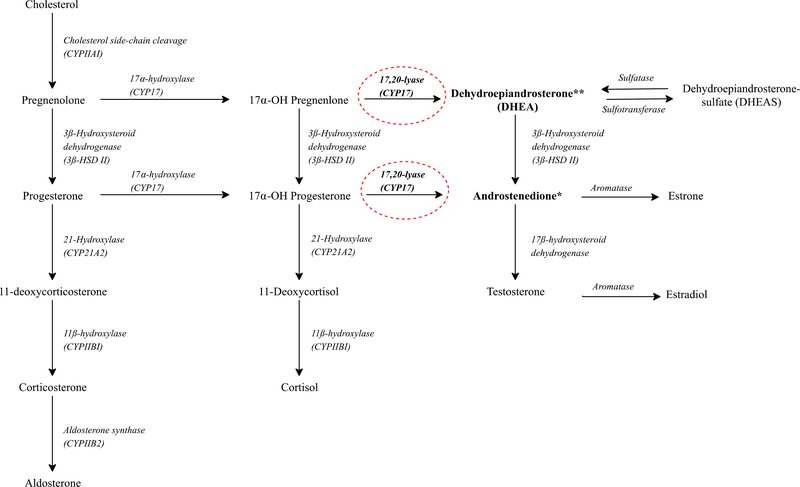
The current epidemiological literature focusing on androgens or gonadotropins in relation to PFAA concentrations is either limited to adolescence or adulthood (Joensen et al. 2013; Kristensen et al. 2013; Petersen et al. 2018; Tsai et al. 2015; Vested et al. 2013) or is based on cross-sectional study designs (Joensen et al. 2013; Lopez-Espinosa et al. 2016; Tsai et al. 2015). A Danish prospective study found that maternal PFOA concentration was associated with higher concentrations of LH and FSH in young adult males (Vested et al. 2013). However, no association was reported between maternal pregnancy PFOA or PFOS concentrations and testosterone or estradiol concentrations in young adult females (Kristensen et al. 2013) or males (Vested et al. 2013). Interestingly, an in vitro study in a human adrenal cortico-carcinoma cell line reported that PFOS had inhibitory effects on the enzyme cytochrome P450 17A1 (CYP17) (Figure 1) (Du et al. 2013). This interaction resulted in decreased concentration of androgens (Du et al. 2013), as CYP17 is a key enzyme involved in the conversion of important androgens, such as DHEA and androstenedione, and is located in the adrenal gland, testis, and ovary (Figure 1) (Boron and Boulpaep 2016). Similarly, an in vivo study in male rats reported that increased PFDA concentration induced decreased plasma concentrations of androgens (Bookstaff et al. 1990).
Figure 1.
Steroidogenesis
** Higher exposure to maternal PFDA concentration was associated with decreased levels of dehydroepiandrosterone (DHEA) in girls.
* Higher exposure to maternal PFDA concentration was non-significantly associated with decreased levels of androstenedione in girls.
Circles with dashed lines represent hypothetical potential PFDA target sites in the steroidogenesis.
A 2-column fitting image
We hypothesized that exposure to PFAAs may decrease the concentrations of androgens in infancy, and we aimed to examine the association among 373 mother-child pairs in the Odense Child Cohort (OCC) from which we had maternal serum PFAA concentrations in early pregnancy and offspring concentrations of androgens, their precursors, LH, and FSH during mini puberty.
2. Materials and methods
2.1. Study population
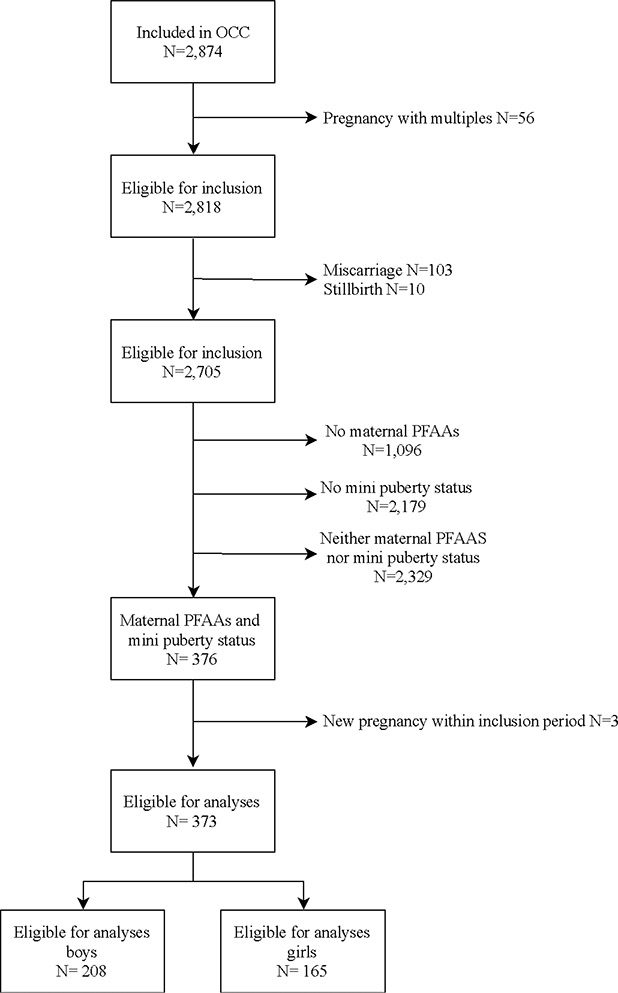
The present exploratory study is part of OCC (N= 2,874), a longitudinal birth cohort conducted in Denmark (Kyhl et al. 2015). Eligible women for study participation resided in the Municipality of Odense, Region of Southern Denmark and were recruited in early pregnancy (gestational age (GA) <16 weeks) between 2010 and 2012. Following enrolment, the pregnant women were requested to donate a blood sample for PFAA analysis (N=1,628) and respond to a questionnaire on current health status and lifestyle. Families were invited to a clinical examination of the infant at four months of age. All parents of children participating at the clinical examination at age four months were approached to consent for blood sample collection. The selection of children for hormone analysis (N=526) was based on the availability of data regarding the child’s anogenital distance at age four months and maternal oral glucose tolerance test during pregnancy (Figure 2).
Figure 2.
Flowchart of participant exclusion and inclusion
A 2-column fitting image
In this study, we excluded multiple pregnancies (N=56), miscarriages (N=103), stillbirths (N=10), and mother-child pairs without measured maternal serum PFAA concentrations or infant serum hormone status (N=2,329) (Figure 2). Three women were pregnant more than once within the inclusion period, and only the first pregnancy was included in the present study (Figure 2). No other exclusion criteria for infants were applied, beside the previously mentioned.
2.2. PFAA assessment
Assessment of maternal serum PFAA concentrations included the following compounds: PFHxS, PFOS, PFOA, PFNA, and PFDA. Serum PFAA concentrations were assessed in 1,628 serum samples obtained at inclusion (median GA (IQR): 12 (10, 15) weeks). Maternal serum samples were pipetted into polypropylene cryotubes and stored at −80°C of maximum 3 years before analysis (Dalsager et al. 2016). Analyses were performed in two analytical series. First, 649 blood samples were analyzed between September 2011 and September 2013, and finally the remaining 979 samples were analyzed in January 2019. All PFAA concentrations were estimated using the same online solid-phase extraction followed by liquid chromatography and triple quadruple mass spectrometry (LC-MS/MS) at the Department of Environmental Medicine, University of Southern Denmark (Jensen et al. 2015). Throughout the analyses, the within-batch coefficients of variation (CVs) were <3% and the between-batch CVs for both sets of data were <10.5%. Quality control and quality assessment were based on certified reference material from the National Institute of Standards and Technology (NIST 1958) (Vorkamp et al. 2014). The Department of Environmental Medicine participates in the German External Quality Assessment Scheme (G-EQUAS) regarding analyses of biological materials organized by The German Society of Occupational Medicine (Vorkamp et al. 2014). The Limit of Quantification (LOQ) was 0.03 ng/mL for all compounds. PFOS, PFOA, PFNA, and PFDA were detectable in all samples in this study (LOQ >0.03 ng/mL), while 0.5% (2/373) of the included women had a PFHxS concentration below the LOQ that was reported as LOQ/2 (Table 1).
Table 1.
Maternal pregnancy serum PFAA and serum androgens, androgen precursors, and gonadotropins concentrations in infants at four months of age
| Girls (N=165) |
Boys (N=208) |
||||
|---|---|---|---|---|---|
| LOQ | n<LOQ (%) | Median (5th, 95th percentile) a | n<LOQ (%) | Median (5th, 95th percentile) a | |
| Serum-PFHxS | 0.03 ng/mL | 0% | 0.30 (0.15, 0.66) | 0.5% | 0.34 (0.13, 0.69) |
| Serum-PFOS | 0.03 ng/mL | 0% | 8.07 (4.21, 15.50) | 0% | 8.33 (4.09, 15.67) |
| Serum-PFOA | 0.03 ng/mL | 0% | 1.70 (0.67, 3.70) | 0% | 1.64 (0.73, 3.76) |
| Serum-PFNA | 0.03 ng/mL | 0% | 0.66 (0.38, 1.43) | 0% | 0.67 (0.36, 1.40) |
| Serum-PFDA | 0.03 ng/mL | 0% | 0.26 (0.16, 0.45) | 0% | 0.26 (0.14, 0.51) |
| DHEA | 0.88 nM | 0% | 4.97 (2.23, 13.69) | 0.5% | 4.50 (1.65, 12.32)* |
| DHEAS | 48 nM | 7.9% | 173.59 (24.00, 622.65) | 7.7% | 161.81 (24.00, 585.06) |
| Androstenedione | 0.18 nM | 13.9% | 0.33 (0.09, 0.75) | 19.7% | 0.31 (0.09, 0.65)* |
| 17-OHP | 0.19 nM | 0.6% | 0.95 (0.38, 2.52) | 0.5% | 1.23 (0.46, 2.66)* |
| Testosterone | 0.10 nM | 83.6% | 0.05 (0.05, 0.14) | 1.4% | 3.23 (0.43, 8.32)* |
| LH | 0.05 IU/L | 0% | 0.15 (0.05, 1.12) | 0% | 2.00 (0.54, 5.28)* |
| FSH | 0.10 IU/L | 0% | 4.56 (2.15, 12.10) | 0% | 1.45 (0.56, 2.96)* |
Note: Data are presented as median (5th, 95th percentile).
17-OHP, 17-hydroxyprogesterone; DHEA, dehydroepiandrosterone; DHEAS, Dehydroepiandrosterone-sulfate, FSH, Follicle-Stimulating Hormone; LH, luteinizing hormone; LOQ, Limit of Quantification, PFDA, Perfluorodecanoic acid; PFHxS, Perfluorohexane sulfonic acid; PFOS, Perfluorooctane sulfonic acid; PFOA, Perfluorooctanoic acid; Perfluorooctane sulfonic acid; PFNA, Perfluorononanoic acid.
Values below limit of quantification (LOQ) were substituted by LOQ/2.
p-value<0.05, when comparing PFAA concentrations and hormone concentration between boys and girls using Wilcoxon rank sum test.
2.3. Hormone analyses
Non-fasting blood samples were drawn from an antecubital vein from 526 infants at the four-month clinical examination (mean age (±SD): 3.9 (±0.9) months). Subsequently, blood samples were clotted and centrifuged, and serum was stored at −20°C for a maximum of 5 years before analysis. Serum concentrations of DHEA, DHEAS, androstenedione, 17-hydroxyprogesterone (17-OHP), and testosterone were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Soeborg et al. 2013) at the Department of Growth and Reproduction, Rigshospitalet, University of Copenhagen, Denmark. Serum LH and FSH concentrations were analyzed with solid-phase, two-site chemiluminescent immunometric assay (Immulite®, Siemens Healthcare Diagnostic, Marburg, Germany) at The University Hospital of Southern Denmark, Esbjerg, Denmark. The interassay coefficients of variations were 11% for DHEA, 5% for DHEAS, 8% for androstenedione, 9% for 17-OHP, 9% for testosterone, 7% for LH, and 6% for FSH. The hormone specific LOQs (Johannsen et al. 2018) were as follows: DHEA=0.88 nM, DHEAS=48 nM, androstenedione=0.18 nM, 17-OHP=0.19 nM, testosterone=0.10 nM, LH=0.05 IU/L, and FSH=0.10 IU/L. Values below the LOQ were replaced with the hormone-specific LOQ/2. The percentage of samples below LOQ in girls and boys are presented in Table 1. Additionally, the testosterone / LH ratio was calculated for boys.
2.4. Covariates
Maternal age at delivery and parity were derived from hospital records using maternal civil registration numbers. Pre-pregnancy BMI, smoking status (yes; no) during pregnancy, breastfeeding duration (months) for the current pregnancy, and educational level (high school or less; high school + 1–4 years; high school + >4 years) were extracted from questionnaires. If educational information was missing in questionnaires, they were retrieved from hospital records. Maternal ethnicity (European; non-European) was based on information on her mother’s country of birth acquired from Odense Municipality. Child characteristics, including sex and gestational age at birth, were obtained from hospital records.
At four months of age, child weight and length were measured at a clinical examination by three health care professionals. Weight was measured without clothing using an electronic scale (Seca 717, Seca, Hamburg, Germany), and recumbent length was determined to the nearest millimeter (Seca 416, Seca, Hamburg, Germany). BMI (in kg/m2) was calculated as weight (kg) divided by the length squared (m2). Age (month-by-month) and sex-specific standard deviation score (SDS) for BMI was calculated according to 2014 Danish reference data (Tinggaard et al. 2014).
2.5. Ethical approval
The study was performed in accordance with the Helsinki Declaration II and approved by the Regional Scientific Ethical Review Committee for Southern Denmark (Project ID S-20090130) and the Danish Data Protection Agency (j.no. 18/33119). All participants received written and oral information and provided their written consent for participation in the study.
2.6. Statistical analyses
Maternal serum PFAA concentrations, infant serum concentrations of DHEA, DHEAS, androstenedione, 17-OHP, testosterone, and LH and FSH were non-normally distributed and reported as median (5%, 95% percentiles) in subgroups according to maternal and child characteristics. More than 80% of the girls had testosterone concentrations below the LOQ (Table 1), and testosterone was therefore not included as an outcome for girls. Differences in maternal PFAA concentrations and offspring concentrations of androgens, androgen precursors, and gonadotropins between subgroups of maternal and infant descriptive characteristics were analyzed using Kruskal-Wallis or Wilcoxon rank sum test. Characteristics of included mother-child pairs were compared to the rest of the women in OCC, and maternal and infant characteristics between girls and boys were investigated, using t-test (normally distributed variables) or Wilcoxon’s rank sum test (non-normally distributed variables) between groups, and Chi square test for categorical variables Correlations between PFAAs were tested by pairwise Spearman correlations.
Associations between maternal PFAA concentrations and infant serum concentrations of androgens, their precursors, and LH and FSH were investigated using linear regression models. As maternal PFAA concentrations and infant concentrations of DHEA, DHEAS, androstenedione, 17-OHP, and gonadotropins were ln-transformed, the β-estimates were transformed to express percent change in infant hormone concentration for a two-fold increase in the maternal PFAA concentration. In boys, β-estimates were transformed to express change in testosterone concentration (nmol/L) for a two-fold increase in the maternal PFAA concentration, as only PFAA concentrations were ln-transformed.
Confounders and intermediate factors were identified based on a priori review of published evidence and using directed acyclic graphs (DAGs) (Figure S1). Maternal serum PFAA concentrations have been found to change across categories of parity (Brantsaeter et al. 2013) and sex of the child (Dalsager et al. 2016), and PFAAs have been associated with gestational age (Meng et al. 2018). Parity, age at examination, and sex of the child may be strong predictors for the infant serum concentrations of androgens, androgen precursors, and gonadotropins (Kuijper et al. 2013). Hence, we adjusted for age of the child at examination time (continuous), maternal parity (nulliparous; parous), and sex of the child (girl; boy).
As there are sex differences in hormone concentrations during mini puberty (Kuijper et al. 2013), effect modification by child sex on the association between maternal PFAA concentrations and offspring hormone concentrations was evaluated by including an interaction term (between PFAA concentration and sex) in the crude and adjusted models. These models were used to obtain sex-specific results.
In sensitivity analyses, the adjusted linear regression models for infant hormone serum concentrations at four months of age were additionally adjusted for gestational age, BMI SDS at four months, and breastfeeding, as these factors might mediate the association between maternal PFAA exposure and offspring hormone concentrations (Figure S1). Finally, analyses were reanalyzed among term births only, as a prolonged and augmented surge in reproductive hormones has been described in premature infants when compared with full-term infants (Kuiri-Hanninen et al. 2011a; Kuiri-Hanninen et al. 2011b).
Model assumptions of all models were validated through comprehensive residual analyses, and multicollinearity was assessed using variance inflation factors. A two-sided significance level of 5% was used. Data were analyzed using STATA/IC version 15.1 (StataCorp, College Station, TX, USA).
3. Results
A total of 373 mother-child dyads with both maternal PFAA concentrations and infant hormone status at four months of age were eligible for inclusion in the final analyses (165 girls, 208 boys).
Of the 373 mother-child pairs, mothers had a mean age of 30.1 (± 4.4 SD) years at the time of parturition and were predominantly nulliparous (58.1%) and of European origin (98.7%), 18.2% were in the highest educational group (completed high school +> 4 years), and 1.3% smoked during pregnancy (Table S1). The included children (44% girls; 56% boys) were born after a median gestation of 40.1 (5th; 95th percentile: 36.9; 41.7) weeks, their hormone status was evaluated at a mean age of 3.9 (± 0.9 SD) months, and they had a mean BMI SDS of 0.05 (± 0.96 SD) at the four-month clinical examination. The included girls and boys were comparable regarding maternal age, parity, maternal BMI, smoking, education, ethnicity, gestational age, breastfeeding, infant age and BMI at blood sampling (Table 2). The included mother-child pairs were similar to the rest of the participants in OCC in regard to maternal age, educational level, gestational age, and sex of the child, but they smoked less, had a higher pre-pregnancy BMI, and were more often nulliparous and of European origin (data now shown).
Table 2.
Maternal and offspring characteristics
| Variables | All | Girls | Boys | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | p-value | |
| Total | 373 (100) | 165 (100) | 208 (100) | |
| Maternal characteristics | ||||
| Maternal age (years) | ||||
| Mean (±SD) | 30.1 (±4.4) | 30.1 (±4.5) | 30.0 (±4.2) | 0.95 |
| <25 | 38 (10.1) | 18 (10.9) | 20 (9.6) | |
| 25–29 | 129 (34.6) | 54 (32.7) | 75 (36.1) | |
| 30–34 | 148 (39.7) | 71 (43.0) | 77 (37.0) | |
| >35 | 58 (15.5) | 22 (13.3) | 36 (17.3) | 0.53 |
| Parity | ||||
| Nulliparous | 217 (58.1) | 92 (55.8) | 125 (60.1) | |
| Parous | 156 (41.8) | 73 (44.2) | 83 (39.9) | 0.40 |
| BMI | ||||
| Median (p5, p95) | 23.9 (19.3; 32.9) | 23.8 (19.2; 32.1) | 24.2 (19.5; 34.7) | 0.55 |
| Smoking | ||||
| Yes | 5 (1.3) | 3 (1.8) | 2 (1.0) | |
| No | 368 (98.7) | 162 (98.2) | 206 (99.0) | 0.48 |
| Education | ||||
| High school or less | 103 (27.6) | 41 (24.8) | 62 (29.9) | |
| High school + 1–4 years | 199 (53.4) | 88 (53.3) | 111 (53.3) | |
| High school + >4 years | 68 (18.2) | 34 (20.7) | 34 (16.3) | 0.42 |
| Missing | 3 (0.8) | 2 (1.2) | 1 (0.5) | |
| Ethnicity | ||||
| European | 368 (98.7) | 163 (98.8) | 205 (98.6) | |
| Non-European | 5 (1.3) | 2 (1.2) | 3 (1.4) | 0.84 |
| Child Characteristics | ||||
| Gestational age (weeks) | ||||
| Median (p5, p95) | 40.1 (36.9; 41.7) | 40.1 (36.7; 41.7) | 40.1 (37.0; 41.9) | 0.89 |
| <40 | 162 (43.4) | 70 (42.4) | 92 (44.2) | |
| >40 | 211 (56.6) | 95 (57.6) | 116 (55.8) | 0.73 |
| Breastfeeding at infant blood sampling | ||||
| No | 91 (24.4) | 34 (20.6) | 57 (27.4) | |
| Yes | 227 (60.9) | 102 (61.8) | 125 (60.1) | 0.22 |
| Missing | 55 (15.7) | 29 (17.6) | 26 (12.5) | |
| Body mass index SDS at four months of age | ||||
| Mean (±SD) | 0.05 (±1.0) | −0.4 (±0.93) | 0.13 (±0.98) | 0.10 |
| <−1 | 53 (14.2) | 27 (16.4) | 26 (12.5) | |
| −1 >1 | 261 (70.0) | 117 (70.9) | 144 (69.2) | |
| >1 | 56 (15.0) | 20 (12.1) | 36 (17.3) | 0.27 |
| Missing | 3 (0.8) | 1 (0.6) | 2 (1.0) | |
| Age at 3 months examination | ||||
| Mean (±SD) | 3.8 (±0.9) | 3.8 (±0.9) | 3.8 (±0.8) | 0.68 |
| 0–4 | 219 (58.7) | 92 (55.8) | 127 (61.1) | |
| 4–5 | 124 (33.3) | 57 (34.5) | 67 (32.2) | |
| >5 | 30 (8.0) | 16 (9.7) | 14 (6.7) | 0.45 |
P-value, when comparing maternal and infant characteristics between girls and boys, using t-test (normally distributed variables) or Wilcoxon’s rank sum test (non-normally distributed variables) between groups, and Chi square test for categorical variable
Nulliparous women had significantly higher concentrations of all five PFAAs compared to parous women, and women giving birth before gestational week 40 had significantly higher concentrations of PFHxS, PFOS, PFOA, and PFNA concentrations compared to women giving birth after gestational week 40 (Table S1). PFOS and PFOA concentrations decreased with increasing maternal age (Table S1), and the estimates were similar and in the same direction when stratifying according to nulliparous and parous women, but not statistically significant. Finally, PFDA concentration was lower in women with offspring below −1 BMI SDS at four months (Table S1). A weak to strong intercorrelation was found between the five PFAA concentrations, as the Spearman correlation coefficients ranged from 0.28 (PFHxS and PFDA) to 0.73 (PFNA and PFDA) (Table S2).
There was no statistically significant difference in exposure concentrations for the five PFAAs between girls and boys (Table 1). Girls had significantly higher concentrations of DHEA, androstenedione, and FSH, but lower concentrations of 17-OHP and LH compared to boys (Table 1). In girls, 17-OHP decreased with increasing maternal age, and DHEA and DHEAS concentrations decreased with increasing offspring age at the four-month clinical examination, but borderline significant for the DHEAS (p=0.06) (Table S3). In boys, DHEAS concentrations increased with offspring BMI SDS at age four months, while testosterone and testosterone / LH ratio were higher when being breastfed at the time of blood sampling, and all hormone concentrations decreased with increasing age of the child at the four-month clinical examination, but borderline significant for DHEA (p=0.08) and DHEAS (p=0.07) (Table S3).
After adjustment for the confounders, a two-fold increase in maternal PFDA concentration was associated with a decrease by −10.7% (95% CI: −20.0%, −0.4%) in offspring DHEA concentration at four months of age (Table 3a). Trends of interaction between PFDA and sex was found (p=0.15). In girls, a two-fold increase in maternal PFDA concentration was strongly associated with a reduction by −19.6% (95% CI: −32.9%, −3.8%) in DHEA concentrations after adjustment, while PFHxS, PFOS, PFOA, and PFNA were non-significantly and inversely associated with DHEA (Table 3a). A two-fold increase in maternal PFDA was also non-significantly (p<0.11) associated with decreased androstenedione and DHEAS concentrations in girls after adjustment (Tables 3a–b). In boys, no significant association was found between maternal PFAA concentrations and DHEA, DHEAS, or androstenedione concentrations (Tables 3a–b). Maternal PFAA concentrations were not significantly associated with offspring concentrations of 17-OHP, testosterone, testosterone / LH ratio, or gonadotropins in girls or in boys (Table 3b and Tables S4–5).
Table 3a.
Percentage change in infant serum concentrations of DHEA and androstenedione during mini puberty for a two-fold increase in maternal serum PFAA concentrations
| Compound | DHEA |
Androstenedione |
||||
|---|---|---|---|---|---|---|
| All (N=373) | Girls (N=165) | Boys (N=208) | All (N=373) | Girls (N=165) | Boys (N=208) | |
| % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | |
| PFHxS (ng/mL) | ||||||
| Crude a | 3.0 (−4.3; 10.7) | −0.8 (−11.3; 10.9) | 5.7 (−3.7; 16.1) | 6.3 (−1.3, 14.5) | 6.7 (−4.9, 19.6) | 6.1 (−3.6, 16.8) |
| Adjusted b | 2.8 (−4.5; 10.7) | −1.0 (−11.6; 10.9) | 5.6 (−4.0; 16.1) | 6.0 (−1.7, 14.3) | 6.3 (−5.4, 19.4) | 5.8 (−4.1, 16.6) |
| PFOS (ng/mL) | ||||||
| Crude a | −3.8 (−13.4; 6.9) | −9.0 (−22.0; 6.3) | 0.8 (−12.1; 15.7) | 3.2 (−7.4, 14.5) | 1.3 (−13.6, 18.8) | 4.4 (−9.6, 20.7) |
| Adjusted b | −4.5 (−14.1; 6.3) | −9.4 (−22.5; 5.9) | −0.2 (−13.4; 15.1) | 2.3 (−8.3, 14.1) | 0.6 (−14.3, 18.2) | 3.7 (−10.4, 20.0) |
| PFOA (ng/mL) | ||||||
| Crude a | −3.1 (−10.5, 5.0) | −3.2 (−13.5, 8.3) | −2.4 (−12.5, 8.9) | 0.6 (−7.2, 9.2) | 6.3 (−5.2, 19.4) | −4.0 (−14.2, 7.4) |
| Adjusted b | −4.2 (−12.3, 4.5) | −4.7 (−15.5, 7.4) | −3.7 (−14.3, 8.1) | −0.5 (−9.1, 8.8) | 4.8 (−7.3, 18.5) | −5.3 (−15.9, 6.6) |
| PFNA (ng/mL) | ||||||
| Crude a | −2.9 (−12.4, 7.7) | −3.1 (−17.1, 13.2) | −2.7 (−15.1, 11.6) | 2.7 (−7.6, 14.2) | 2.6 (−12.6, 20.3) | 2.9 (−10.6, 18.4) |
| Adjusted b | −3.5 (−13.2, 7.3) | −4.0 (−18.1, 12.6) | −3.1 (−15.7, 11.2) | 1.9 (−8.5, 13.6) | 1.5 (−13.8, 19.5) | 2.3 (−11.3, 17.9) |
| PFDA (ng/mL) | ||||||
| Crude a | −10.5 (−19.8, −0.06) * | −18.8 (−32.1, −3.0) * | −5.1 (−17.3, 8.9) | −7.6 (−17.5, 3.4) | −13.1 (−27.7, 4.4) | −4.1 (−16.8, 10.4) |
| Adjusted b | −10.7 (−20.0, −0.4) * | −19.6 (−32.9, −3.8) * | −5.1 (−17.3, 8.9) | −8.0 (−17.8, 2.9) | −14.3 (−28.8, 3.2) | −4.2 (−16.9, 10.4) |
Table 3b.
Percentage change in infant serum concentrations of DHEAS and 17-hydroxyprogesterone (17-OHP) during mini puberty for a two-fold increase in maternal serum PFAA concentrations
| Compound (ng/mL) | DHEAS |
17-OHP |
||||
|---|---|---|---|---|---|---|
| All (N=373) | Girls (N=165) | Boys (N=208) | All (N=373) | Girls (N=165) | Boys (N=208) | |
| % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | % Change (95 % CI) | |
| PFHxS (ng/mL) | ||||||
| Crude a | 7.0 (−3.5; 18.6) | 4.9 (−10.8; 23.2) | 8.5 (−5.2; 24.3) | 1.7 (−5.0, 8.9) | −2.2 (−11.9, 8.7) | 4.4 (−4.4, 14.0) |
| Adjusted b | 5.2 (−5.5; 17.0) | 2.9 (−12.7, 21.1) | 6.8 (−6.9, 22.5) | 1.1 (−5.7, 8.4) | −2.8 (−12.6, 8.2) | 3.9 (−5.0, 13.6) |
| PFOS (ng/mL) | ||||||
| Crude a | 0.4 (−13.6; 16.8) | −8.3 (−26.7; 14.7) | 8.1 (−11.8; 32.4) | 2.6 (−7.1, 13.4) | 2.7 (−11.2, 18.8) | 3.0 (−9.7; 17.6) |
| Adjusted b | −2.1 (−16.0; 14.1) | −10.4 (−28.4, 12.2) | 5.3 (−14.2, 29.3) | 2.2 (−7.5, 12.9) | 2.1 (−11.9, 18.2) | 2.3 (−10.5, 17.0) |
| PFOA (ng/mL) | ||||||
| Crude a | −0.2 (−11.0, 11.8) | 5.9 (−10.0, 24.6) | −5.5 (−19.3, 10.7) | 3.5 (−4.0, 11.5) | 3.9 (−6.5, 15.5) | 2.4 (−7.6, 13.5) |
| Adjusted b | −5.1 (−16.3, 7.7) | 0.4 (−15.5, 19.3) | −9.9 (−23.7, 6.4) | 2.3 (−5.8, 11.0) | 3.0 (−7.9, 15.2) | 1.6 (−8.8, 13.2) |
| PFNA (ng/mL) | ||||||
| Crude a | −2.6 (−16.0, 13.0) | −1.5 (−21.3, 23.4) | −3.4 (−20.8, 17.7) | 2.6 (−7.0, 13.1) | 3.9 (−10.2, 20.2) | 1.6 (−10.6, 15.5) |
| Adjusted b | −5.5 (−18.8, 10.0) | −5.4 (−24.8, 18.9) | −5.6 (−22.6, 15.2) | 1.8 (−7.8, 12.4) | 2.8 (−11.4, 19.4) | 1.0 (−11.2, 15.0) |
| PFDA (ng/mL) | ||||||
| Crude a | −11.1 (−24.1, 4.1) | −16.0 (−35.0, 8.8) | −7.7 (−24.1, 12.1) | −1.6 (−11.4, 9.2) | −5.5 (−20.1, 11.8) | 0.7 (−11.5, 14.7) |
| Adjusted b | −12.3 (−25.1, 2.7) | −18.9 (−37.5, 5.2) | −8.3 (−24.8, 11.9) | −2.1 (−11.7, 8.6) | −6.5 (−21.2, 10.8) | 0.6 (−11.6, 14.6) |
Note: CI, Confidence interval; DHEA, dehydroepiandrosterone; DHEAS, Dehydroepiandrosterone-sulfate; PFDA, Perfluorodecanoic acid; PFHxS, Perfluorohexane sulfonic acid; PFOA, Perfluorooctanoic acid; PFOS, Perfluorooctane sulfonic acid; PFNA, Perfluorononanoic acid
Adjusted for age of the child at examination time.
Adjusted for age of the child at examination time, maternal parity, and sex of the child (not in sex-specific analyses).
p-value<0.05
In sensitivity analyses, estimates from the multiple linear regressions did not change markedly after additionally adjusting for the BMI SDS of child at four months, breastfeeding, gestational age, or if preterm births (< 37 GA) (N=19) were excluded (data not shown).
4. Discussion
This is the first cohort study investigating associations between maternal serum PFAA concentrations in early pregnancy and offspring concentrations of androgens, androgen precursors, and gonadotropins during mini puberty. Only in girls, we found that higher maternal pregnancy PFDA concentrations were significantly associated with decreased concentration of DHEA and non-significantly (p<0.11) with reduced concentrations of androstenedione and DHEAS during mini puberty. Otherwise, no significant association was found between maternal serum PFAA concentrations and concentrations of 17-OHP, testosterone, and gonadotropins in girls or in boys during mini puberty.
Our findings are supported by in vitro studies performed in a steroidogenesis assay using a human adrenal cortico-carcinoma cell line (Du et al. 2013; Rosenmai et al. 2013). Rosenmai et al. demonstrated reduced concentrations of DHEA and androstenedione and increased concentration of estrone induced by exposure to concentrations of PFAA precursors, referred to as polyfluoroalkyl phosphate surfactants (PAPS) (Rosenmai et al. 2013). Du et al. also reported decreased concentrations of androgens and increased concentration of estradiol after PFOS exposure (Du et al. 2013). To the best of our knowledge, there is no in vivo study on fetal adrenal gland and concentrations of androgens during mini puberty in relation to concentrations of PFAAs and PAPS. In a different setting, an in vivo study in male rats found that higher exposure to PFDA resulted in decreased concentrations of plasma androgens and unchanged LH concentrations (Bookstaff et al. 1990). Our findings and the results reported by others (Du et al. 2013; Rosenmai et al. 2013) are suggestive of a disruption of the enzyme-regulated steroidogenesis, possibly involving the enzyme CYP17 (Figure 1), which is expressed in the adrenal gland, testis, and ovary (Boron and Boulpaep 2016). CYP17 is a significant bifunctional enzyme with 17,20-lyase and 17-alpha-hydroxylase activities, and CYP17 (17,20-lyase) plays a pivotal role in the synthesis of the essential androgens, DHEA and androstenedione (Figure 1, dashed circles) (Boron and Boulpaep 2016; Longcope 1986). In accordance, Du et al. (Du et al. 2013) reported a significant decrease in the gene expression of CYP17 after exposure to PFOS, thereby resulting in decreased androgen concentrations. An increased aromatization of androgens into estrogens by the aromatase enzyme should also be considered as a potential contributing mechanism (Du et al. 2013; Rosenmai et al. 2013). Our observation of a significant association between PFDA and DHEA in girls could be explained by the female reliance of androgen secretion from the adrenal gland, especially DHEA (Arlt et al. 1998; Longcope 1986). It is well-known that females are more dependent on adrenal androgen production compared to males, as the main production of androgen for males occur in the testes (Antoniou-Tsigkos A 2000, South Dartmouth, MDText.com, Inc.; 2000; Longcope 1986).
We observed no significant association between prenatal PFAA concentrations and testosterone or DHEA in boys at four months of age. This is in agreement with findings from another prospective Danish study reporting no association between maternal PFOS and PFOA in late pregnancy and testosterone in young adult men (Vested et al. 2013) and women (Kristensen et al. 2013). Three cross-sectional studies (Joensen et al. 2013; Lopez-Espinosa et al. 2016; Tsai et al. 2015) indicated that higher exposure to PFOS was associated with lower concentration of testosterone in American boys and girls aged six to nine years (Lopez-Espinosa et al. 2016), lower testosterone concentration in 12–17 year old Taiwanese girls (Tsai et al. 2015), and lower concentration of total and calculated free testosterone concentration in adult Danish men (median age of 19 years) (Joensen et al. 2013). The inverse associations between PFOS concentrations and testosterone concentrations reported in cross sectional studies (Joensen et al. 2013; Lopez-Espinosa et al. 2016; Tsai et al. 2015) are different to findings from ours and other (Kristensen et al. 2013; Vested et al. 2013) prospective studies. Results could be affected by different study designs and stages in life, as mini puberty and pre-puberty may differ in more ways, than we currently are aware of, thus cautious comparisons must be made. We demonstrated no significant association between maternal PFAA concentrations in early pregnancy and LH and FSH in neither girls nor boys at four months. This is in line with observations in young women from the prospective Danish study (Kristensen et al. 2013), where no association was found between prenatal PFOA or PFOS concentrations and LH and FSH, but, in adult men, concentrations of both LH and FSH increased significantly across tertiles of prenatal PFOA concentrations (Vested et al. 2013). Pregnant women in the Danish cohort (Kristensen et al. 2013; Vested et al. 2013) were recruited in the late 1980s, where a two-fold higher concentration of PFOA (3.8 ng/mL) and PFOS (21.2 ng/mL) was found in comparison to concentrations in our study. In a Faroese cross-sectional study (Petersen et al. 2018), higher PFOS concentration was associated with increased concentrations of LH but not FSH in adult men (median age of 20 years), nevertheless, no measurement of prenatal exposure was available.
The maternal serum PFDA concentration in early pregnancy in this study was comparable to serum PFDA concentration at first trimester in pregnant women from the Danish National Birth Cohort (Ernst et al. 2019), although the latter was conducted earlier. Maternal pregnancy PFHxS, PFOS, and PFOA concentrations were lower compared to a British cohort study also relying on early pregnancy blood samples collected in the early 1990ties (Maisonet et al. 2015), whereas our PFNA concentration was somewhat higher. PFOS and PFOA concentrations were lower than in a Danish cohort study using late pregnancy PFAA assessment (Kristensen et al. 2013; Vested et al. 2013) obtained in the late 1980s.
The major strength of the present study is the longitudinal study design with maternal PFAAs obtained in early pregnancy and unique data on infant hormone status during mini puberty. In our study, maternal concentrations of legacy PFAAs were measured in early pregnancy around the most sensitive stage of fetal adrenal and gonad development. Whereas other prospective studies (Kristensen et al. 2013; Vested et al. 2013) determined PFAA concentrations in late pregnancy putatively resulting in lowered maternal serum concentration, as PFAAs bind to proteins in plasma, and plasma volume and glomerular filtration rate in the kidneys increase during pregnancy (Hussein and Lafayette 2014; Monroy et al. 2008). Our single-time point measurement of exposure largely mirrors the PFAA concentrations throughout pregnancy and mini puberty due to the stable and long half-lives of PFAAs in humans ((ATSDR) 2018). So, children highly exposed to PFAA concentrations during pregnancy will most likely still be highly exposed during mini puberty, thus more likely to reveal effects of PFAAs on the steroidogenesis in children at mini puberty. Additionally, humans are continuously exposed to these chemicals, hence it stresses the necessity to follow-up these children at adrenarche and pubarche in order to assess putative long-term associations with PFAAs later in childhood. Misclassification is most likely minimal, and any misclassification would be non-differential, as the three trained health care professionals conducting the clinical child examinations and the parents were blinded to maternal PFAA concentrations and infant concentrations of androgens, androgen precursors, and gonadotropin.
Some potential limitations should be noted. We acknowledge the risk of chance findings due to multiple comparison bias, as associations were not adjusted for multiple testing. However, the study was conducted with an exploratory approach based on a priori hypothesis. The investigated associations may have been affected by residual confounding from other unknown or unmeasured covariates related to maternal PFAA exposure and mini puberty, such as exposure to correlated endocrine disrupting chemicals during infancy, diet, or genetic disposition. The gonadotropins and hormones in girls and boys peak around three months of age during mini puberty and hereafter slowly decline (Kurtoglu and Bastug 2014; Lanciotti et al. 2018). The somewhat wide age span at the time of infant examination and the moderate sample size may have introduced the risk of type II error, when investigating associations between maternal PFAA concentrations and infant concentrations of androgens and gonadotropins during mini puberty. Finally, the included mothers smoked less, had a higher pre-pregnancy BMI, were more often nulliparous and of European origin compared to the rest of the women in OCC, but this potential lack of complete representativity is unlikely to have affected the association between maternal PFAA concentrations and infant concentrations of androgens during mini puberty.
5. Conclusion
Increased maternal serum PFDA concentration in early pregnancy is associated with significantly decreased serum DHEA in girls at age four months of age. The potential disruption of in utero PFDA exposure on offspring steroidogenesis during infancy underscores the necessity of a follow-up of children at adrenarche and pubarche in order to assess putative long-term associations with PFAAs later in childhood. Moreover, it would be clinically relevant to investigate time to pregnancy and prevalence of premature ovarian failure in relation to PFAA exposure.
Supplementary Material
Acknowledgments
The families in OCC are acknowledged for their participation and commitment to the study. The health care professionals at the Hans Christian Andersen’s Children’s Hospital and the technicians at the Department of Environmental Medicine are acknowledged for their careful examination of the children and analysis of PFAA concentrations in serum samples, respectively. The laboratories at Dept. of Growth and Reproduction, Rigshospitalet and the Hospital of South West Jutland are acknowledged for their thorough analyses of androgens, androgen precursors, and gonadotropins in offspring.
Funding
This work was supported by the Danish Council for Independent Research, Medical Sciences (4004-00352B and 8020-00123B), Odense University Hospital, the Region of Southern Denmark, the Municipality of Odense, the Mental Health Service of the Region of Southern Denmark, the Danish Council for Strategic Research, Program Commission on Health, Food and Welfare (2101-08-0058), Odense University Hospital Research Foundation, Odense Patient data Explorative Network (OPEN), Novo Nordisk Foundation (grant no. NNF15OC00017734 and NNF17OC0029404), the Foundation for Research Collaboration between Rigshospitalet and Odense University Hospital, and the Health Foundation (Helsefonden). P. Grandjean is supported by a grant from the National Institutes of Health (ES026596). None of the funding sources had any involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- 17-OHP
17-hydroxyprogesterone
- BMI
Body mass index
- CI
Confidence interval
- CV
Coefficient of variation
- DAG
Directed acyclic graph
- DHEA
Dehydroepiandrosterone
- DHEAS
Dehydroepiandrosterone-sulfate
- FSH
Follicle stimulating hormone
- GA
Gestational age
- LH
Luteinizing hormone
- LOQ
Limit of Quantification
- OCC
Odense Child Cohort
- PFAAs
Perfluoroalkyl substances
- PFDA
Perfluorodecanoic acid
- PFHxS
Perfluorohexane sulfonic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonic acid
- PFNA
Perfluorononanoic acid
- SDS
Standard deviation score
- T/LH ratio
Testosterone/luteinizing hormone ratio
Footnotes
Competing Financial Interests
The authors declare they have no actual or potential competing financial interests.
Ethics
The study was performed in accordance with the Helsinki Declaration II and approved by the Regional Scientific Ethical Review Committee for Southern Denmark (Project ID S-20090130) and the Danish Data Protection Agency (j.no. 18/33119). All participants received written and oral information and provided their written consent for participation in the study.
Reference list
- (ATSDR) AfTSaDR. 2018. Toxicological profile for perfluoroalkyls. Atlanta, GA: US Department of Health and Human Services, Public Health Service. [Google Scholar]
- Antoniou-Tsigkos A ZE, Ghizzoni L, Mastorakos M. 2000, South Dartmouth, MDText.com, Inc.; 2000. Adrenal androgens. Endotextorg—Adrenal Disease and Function [Google Scholar]
- Arlt W, Justl HG, Callies F, Reincke M, Hubler D, Oettel M, et al. 1998. Oral dehydroepiandrosterone for adrenal androgen replacement: Pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. The Journal of clinical endocrinology and metabolism 83:1928–1934. [DOI] [PubMed] [Google Scholar]
- Bae YJ, Zeidler R, Baber R, Vogel M, Wirkner K, Loeffler M, et al. 2019. Reference intervals of nine steroid hormones over the life-span analyzed by lc-ms/ms: Effect of age, gender, puberty, and oral contraceptives. The Journal of steroid biochemistry and molecular biology:105409. [DOI] [PubMed] [Google Scholar]
- Bookstaff RC, Moore RW, Ingall GB, Peterson RE. 1990. Androgenic deficiency in male rats treated with perfluorodecanoic acid. Toxicol Appl Pharmacol 104:322–333. [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. 2016. Medical physiology. Third edition ed. Philadelphia, PA:Elsevier. [Google Scholar]
- Brantsaeter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, et al. 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant norwegian women. Environ Int 54:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan PG. 2001. The hypothalamic pituitary axis in the fetus and newborn. Semin Perinatol 25:371–384. [DOI] [PubMed] [Google Scholar]
- Dalsager L, Christensen N, Husby S, Kyhl H, Nielsen F, Host A, et al. 2016. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1–4years among 359 children in the odense child cohort. Environ Int 96:58–64. [DOI] [PubMed] [Google Scholar]
- Du G, Hu J, Huang H, Qin Y, Han X, Wu D, et al. 2013. Perfluorooctane sulfonate (pfos) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem 32:353–360. [DOI] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, et al. 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the danish national birth cohort. Environ Health Perspect 127:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, et al. 2012. Perfluorinated alkyl acids in blood serum from primiparous women in sweden: Serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol 46:9071–9079. [DOI] [PubMed] [Google Scholar]
- Hussein W, Lafayette RA. 2014. Renal function in normal and disordered pregnancy. Curr Opin Nephrol Hypertens 23:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Andersen LB, Kyhl HB, Nielsen F, Christesen HT, Grandjean P. 2015. Association between perfluorinated compound exposure and miscarriage in danish pregnant women. PloS one 10:e0123496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, et al. 2013. Pfos (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum Reprod 28:599–608. [DOI] [PubMed] [Google Scholar]
- Johannsen TH, Main KM, Ljubicic ML, Jensen TK, Andersen HR, Andersen MS, et al. 2018. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. The Journal of clinical endocrinology and metabolism 103:3028–3037. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, et al. 2013. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod 28:3337–3348. [DOI] [PubMed] [Google Scholar]
- Kuijper EA, Ket JC, Caanen MR, Lambalk CB. 2013. Reproductive hormone concentrations in pregnancy and neonates: A systematic review. Reproductive biomedicine online 27:33–63. [DOI] [PubMed] [Google Scholar]
- Kuiri-Hanninen T, Kallio S, Seuri R, Tyrvainen E, Liakka A, Tapanainen J, et al. 2011a. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. The Journal of clinical endocrinology and metabolism 96:3432–3439. [DOI] [PubMed] [Google Scholar]
- Kuiri-Hanninen T, Seuri R, Tyrvainen E, Turpeinen U, Hamalainen E, Stenman UH, et al. 2011b. Increased activity of the hypothalamic-pituitary-testicular axis in infancy results in increased androgen action in premature boys. The Journal of clinical endocrinology and metabolism 96:98–105. [DOI] [PubMed] [Google Scholar]
- Kuiri-Hanninen T, Sankilampi U, Dunkel L. 2014. Activation of the hypothalamic-pituitary-gonadal axis in infancy: Minipuberty. Horm Res Paediatr 82:73–80. [DOI] [PubMed] [Google Scholar]
- Kurtoglu S, Bastug O. 2014. Mini puberty and its interpretation. Turk pediatri arsivi 49:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhl HB, Jensen TK, Barington T, Buhl S, Norberg LA, Jorgensen JS, et al. 2015. The odense child cohort: Aims, design, and cohort profile. Paediatr Perinat Epidemiol 29:250–258. [DOI] [PubMed] [Google Scholar]
- Lamminmaki A, Hines M, Kuiri-Hanninen T, Kilpelainen L, Dunkel L, Sankilampi U. 2012. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm Behav 61:611–616. [DOI] [PubMed] [Google Scholar]
- Lanciotti L, Cofini M, Leonardi A, Penta L, Esposito S. 2018. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front Endocrinol (Lausanne) 9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcope C 1986. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab 15:213–228. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. 2016. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6–9 years of age: A cross-sectional analysis within the c8 health project. Environ Health Perspect 124:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Calafat AM, Marcus M, Jaakkola JJ, Lashen H. 2015. Prenatal exposure to perfluoroalkyl acids and serum testosterone concentrations at 15 years of age in female alspac study participants. Environ Health Perspect 123:1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. 2018. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the danish national birth cohort. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, et al. 2008. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res 108:56–62. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Jorgensen N, Nielsen F, Grandjean P, Jensen TK, et al. 2018. Reproductive function in a population of young faroese men with elevated exposure to polychlorinated biphenyls (pcbs) and perfluorinated alkylate substances (pfas). Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T, Greaves R, Badoer E, Walker D. 2018. Dhea in prenatal and postnatal life: Implications for brain and behavior. Vitam Horm 108:145–174. [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, Nielsen FK, Pedersen M, Hadrup N, Trier X, Christensen JH, et al. 2013. Fluorochemicals used in food packaging inhibit male sex hormone synthesis. Toxicol Appl Pharmacol 266:132–142. [DOI] [PubMed] [Google Scholar]
- Soeborg T, Frederiksen H, Fruekilde P, Johannsen TH, Juul A, Andersson AM. 2013. Serum concentrations of dhea, dheas, 17alpha-hydroxyprogesterone, delta4-androstenedione and testosterone in children determined by turboflow-lc-ms/ms. Clin Chim Acta 419:95–101. [DOI] [PubMed] [Google Scholar]
- Stubleski J, Salihovic S, Lind L, Lind PM, van Bavel B, Karrman A. 2016. Changes in serum levels of perfluoroalkyl substances during a 10-year follow-up period in a large population-based cohort. Environ Int 95:86–92. [DOI] [PubMed] [Google Scholar]
- Tinggaard J, Aksglaede L, Sorensen K, Mouritsen A, Wohlfahrt-Veje C, Hagen CP, et al. 2014. The 2014 danish references from birth to 20 years for height, weight and body mass index. Acta Paediatr 103:214–224. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Lin CY, Lin CC, Chen MH, Hsu SH, Chien KL, et al. 2015. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int J Hyg Environ Health 218:437–443. [DOI] [PubMed] [Google Scholar]
- Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, et al. 2013. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect 121:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorkamp K, Nielsen F, Kyhl HB, Husby S, Nielsen LB, Barington T, et al. 2014. Polybrominated diphenyl ethers and perfluoroalkyl substances in serum of pregnant women: Levels, correlations, and potential health implications. Arch Environ Contam Toxicol 67:9–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.