Abstract
Objectives
This study aimed at re-evaluating the strength and shape of the dose-response relationship between the combined (or joint) effect of intensity and duration of cigarette smoking and the risk of head and neck cancer (HNC). We explored this issue considering bivariate spline models, where smoking intensity and duration were treated as interacting continuous exposures.
Materials and Methods
We used individual-level pooled data from 33 case-control studies (18,260 HNC cases and 29,844 controls) participating in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. In bivariate regression spline models, exposures to cigarette smoking intensity and duration (compared with never smokers) were modeled as a linear piecewise function within a logistic regression also including potential confounders. We jointly estimated the optimal knot locations and regression parameters within the Bayesian framework.
Results
For oral-cavity/pharyngeal (OCP) cancers, an odds ratio (OR) >5 was reached after 30 years in current smokers of ~20 or more cigarettes/day. Patterns of OCP cancer risk in current smokers differed across strata of alcohol intensity. For laryngeal cancer, ORs>20 were found for current smokers of ≥20 cigarettes/day for ≥30 years. In former smokers who quit ≥10 years ago, the ORs were approximately halved for OCP cancers, and ~1/3 for laryngeal cancer, as compared to the same levels of intensity and duration in current smokers.
Conclusion
Referring to bivariate spline models, this study better quantified the joint effect of intensity and duration of cigarette smoking on HNC risk, further stressing the need of smoking cessation policies.
Keywords: bivariate spline models, cigarette smoking duration, cigarette smoking intensity, head and neck cancer, INHANCE, laryngeal cancer, oral cavity and pharyngeal cancers
Introductiona
In recent years, tobacco smoking has confirmed its role as the worldwide leading cause of preventable diseases and death. It is responsible for at least 12% of all deaths and of 22% of all cancer deaths worldwide [1]. Tobacco has been strongly associated with head and neck cancer (HNC, i.e., cancers of the oral cavity, pharynx, and larynx), which is the sixth most common cancer type, accounting for nearly 900,000 new cases in 2018 [2].
A dose-response relationship with HNC risk has been reported for smoking intensity (cigarettes per day reported during the exposure period) and duration (years of exposure), as well as for a cumulative smoking exposure measured in pack-years [3,4]. Compared with intensity, smoking duration was shown to provide a greater risk of developing HNC [5,6], as well as, other tobacco-related cancers, such as lung [7,8] and bladder cancers [9,10].
So far, the association between tobacco smoking and HNC risk was evaluated by considering duration and intensity as either separate or interacting predictors. In the latter case, the combined exposure was modeled as either cross-product or pack-years [6,11,12]. Step-functions [4, page 369] were widely used to provide risk estimates for the cross-product of the categorized exposures. This approach assumed a constant rate within each combined category of exposure; however, this assumption may have been too rough and/or leading to some efficiency loss [13–15]. As an alternative, linear-exponential models estimated the excess odds ratio (OR) of HNC within models including pack-years and smoking intensity [6,9]. The pack-year approach was traditionally based on a strong assumption about the effects of smoking intensity and duration; for instance, the effect of smoking ½ pack per day (10 cigarettes) for 40 years was equivalent to smoking 2 packs (40 cigarettes) per day for 10 years. Indeed, both of them reflect a cumulative exposure of 20 pack-years [16]. However, the 2 exposures were measured on different metrics.
Bivariate spline models have the potential to provide a more realistic representation of the association between smoking intensity and duration and HNC risk [15]. First, they allow to model intensity and duration in their original scale of continuous exposures and, in this aspect, splines outperform step-function models. Second, the two exposures are allowed to determine disease risk in their separate or interacting role. Third, a non-linearity in the dose-response relationship may be modeled.
This study re-evaluated the joint effect of intensity and duration of tobacco smoking on HNC risk, by means of bivariate spline models, within the International Head and Neck Cancer Epidemiology (INHANCE) consortium [11].
Materials and methods
Data source
The INHANCE consortium was established in 2004 to elucidate the etiology of HNC through pooled analyses of individual-level data from several studies on a large scale [11]. Several aspects of tobacco smoking and HNC risk have been previously investigated within the consortium [5,6,11,17–19].
From the INHANCE consortium pooled dataset (version 1.5), we extracted all the available case-control studies (35 studies) that collected information on cigarette smoking status, intensity, and duration at individual level (http://www.inhance.utah.edu, last accessed March 25th, 2019). Available data were harmonized at the study coordinating center [11]. In all the studies, information on smoking history was self-reported. While different studies had used different definitions of smoking status, the current study defined as never smokers those individuals who never smoked regularly, or smoked for a very short period, i.e., less than 12 months [11]; likewise, former smokers were defined as those who had abstained from any type of smoking since at least 12 months before cancer diagnosis (cases) or interview (controls).
Details on individual studies, data harmonization, and pooling methods are summarized in eTable 1. Informed consent was obtained from all study subjects. The investigations were approved by the relevant Boards of Ethics, according to the regulation in force at data collection time.
Selection of subjects
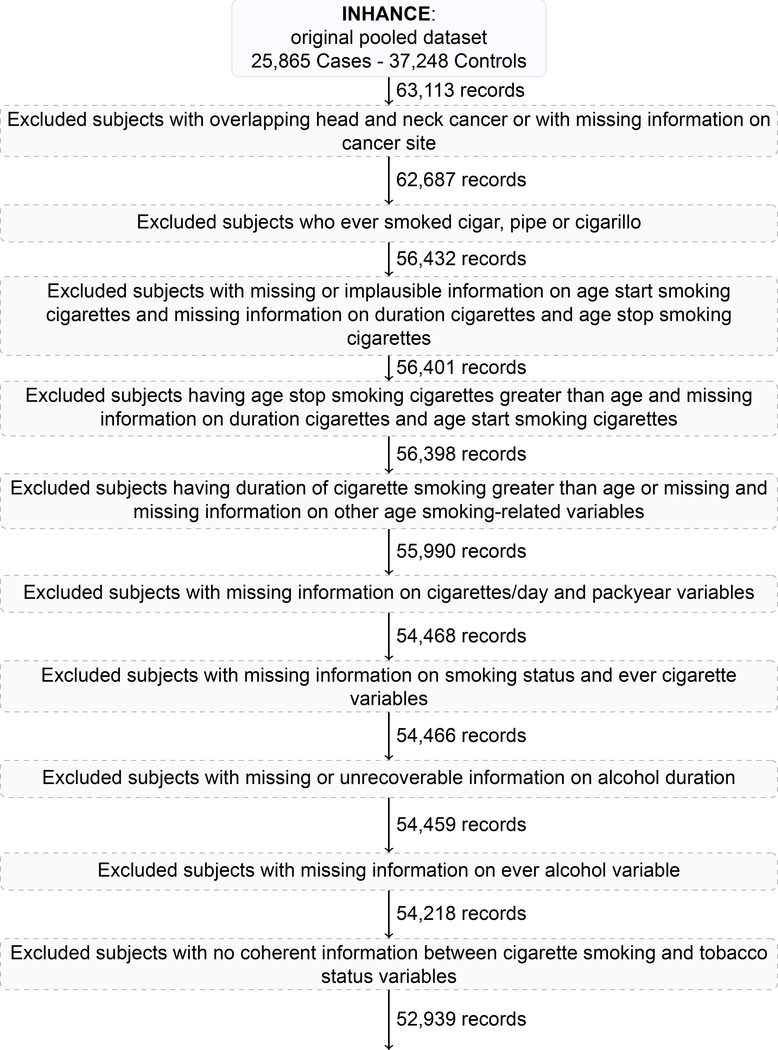
The INHANCE protocol allowed inclusion of invasive cancer cases of the oral cavity, oropharynx, hypopharynx, oral cavity or pharynx not otherwise specified, larynx, or unspecified HNC. Cases with cancers of the salivary glands or of the nasal cavity/ear/paranasal sinuses were excluded [11]. The original study sample included 25,865 HNC cases and 37,248 controls, giving a total of 63,113 subjects.
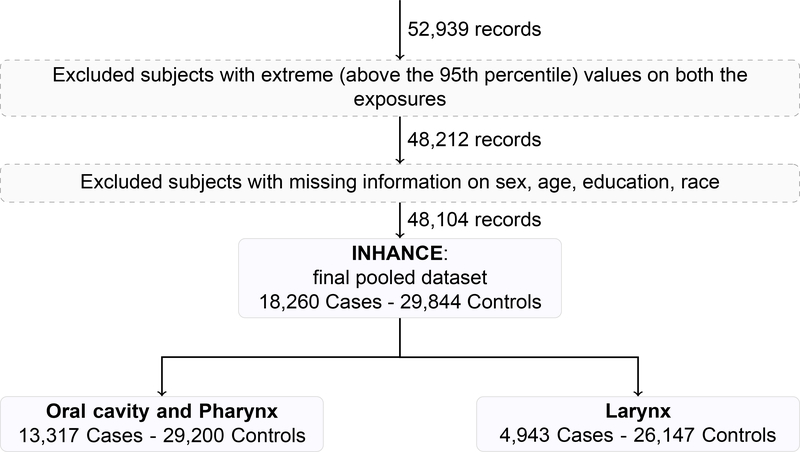
We conducted subjects’ selection according to the following main steps (eAppendix, text and Figure 1), excluding: 1. cases with unspecified (95 subjects) or overlapping HNC (331 subjects); 2. subjects reporting smoking tobacco products other than cigarettes (i.e., cigar, pipe, and cigarillo), to avoid risk distortion due their use [5] (6,255 subjects); 3. subjects with missing information on duration and/or intensity of cigarette smoking (1,897 subjects); 4. all subjects from studies that included only never (i.e., Japan 1988–2000) (822 subjects) or current (i.e., France 1987–1992) smokers (457 subjects), after the previous selection steps.
In order to prevent potential estimation distortion at the highest levels of the exposure distributions (due to small numbers of subjects or information bias in heavy tobacco consumers), further excluded were those subjects reporting the highest 5% of cigarette smoking intensity (>40 cigarettes per day) or duration (>51 years) (14% HNC cases and 6% controls excluded).
After all the described selection steps (eAppendix, text and Figure 1), the analysis included 33 studies [20–53] with 48,104 subjects (18,260 HNC cases and 29,844 controls) (Table 1). When a study reported to have conducted a case-control matching, separate sets of controls were matched for oral cavity and pharynx (OCP) cancers combined and for laryngeal cancer cases. In detail, the analysis included: 5,423 cancers of the oral cavity; 6,261 pharyngeal cancer cases (4,648 oropharyngeal and 1,613 hypopharyngeal cancers cases); 1,633 unspecified oral cavity/pharynx cancers (giving a total of 13,317 OCP cancer cases combined), and 4,943 laryngeal cancers.
Table 1 –
Distribution of cases of oral and pharyngeal cancer, laryngeal cancer, and controls according to selected variables. INHANCE consortium
| Controls | (%) | Oral and pharyngeal cancer cases | (%) | Laryngeal cancer cases | (%) | |
|---|---|---|---|---|---|---|
| 29,844 | 13,317 | 4,943 | ||||
| Sexa | ||||||
| Female | 9,599 | (32) | 3,786 | (28) | 730 | (15) |
| Male | 20,245 | (68) | 9,531 | (72) | 4,213 | (85) |
| Age (years)a | ||||||
| <40 | 2,063 | (7) | 708 | (5) | 94 | (2) |
| 40 to 44 | 2,045 | (7) | 823 | (6) | 207 | (4) |
| 45 to 49 | 3,018 | (10) | 1,692 | (13) | 471 | (10) |
| 50 to 54 | 4,231 | (14) | 2,321 | (17) | 787 | (16) |
| 55 to 59 | 5,044 | (17) | 2,627 | (20) | 1,025 | (21) |
| 60 to 64 | 4,726 | (16) | 2,185 | (16) | 1,011 | (20) |
| 65 to 69 | 4,181 | (14) | 1,489 | (11) | 754 | (15) |
| 70 to 74 | 3,044 | (10) | 922 | (7) | 413 | (8) |
| ≥75 | 1,492 | (5) | 550 | (4) | 181 | (4) |
| Racea | ||||||
| White | 21,462 | (72) | 9,076 | (68) | 3,627 | (73) |
| Black | 976 | (3) | 683 | (5) | 169 | (3) |
| Hispanic | 421 | (1) | 149 | (1) | 41 | (1) |
| Asian and Pacific Islanders | 3,849 | (13) | 1,293 | (10) | 77 | (2) |
| Others and Brazilians | 3,136 | (11) | 2,116 | (16) | 1,029 | (21) |
| Study | ||||||
| Aviano | 802 | (3) | 288 | (2) | 128 | (3) |
| Baltimore | 163 | (1) | 123 | (1) | 29 | (1) |
| Beijing | 377 | (1) | 322 | (2) | 0 | (0) |
| Boston | 473 | (2) | 339 | (3) | 75 | (2) |
| Buffalo | 863 | (3) | 282 | (2) | 113 | (2) |
| Central Europe | 730 | (2) | 238 | (2) | 295 | (6) |
| France Multicen. (1989–1991) | 255 | (1) | 163 | (1) | 247 | (5) |
| France Multicen. (2001–2007) | 2,964 | (10) | 1,343 | (10) | 366 | (7) |
| Germany-Heidelberg | 644 | (2) | 0 | (0) | 172 | (3) |
| Germany-Saarland | 83 | (0) | 53 | (0) | 22 | (0) |
| HOTSPOT | 59 | (0) | 63 | (0) | 0 | (0) |
| Houston | 738 | (2) | 561 | (4) | 119 | (2) |
| International Multicenter | 1,450 | (5) | 1,053 | (8) | 0 | (0) |
| Iowa | 600 | (2) | 344 | (3) | 58 | (1) |
| Italy Multicenter | 2,506 | (8) | 685 | (5) | 421 | (9) |
| Japan (2001–2005) | 2,817 | (9) | 392 | (3) | 71 | (1) |
| Latin America | 1,446 | (5) | 981 | (7) | 612 | (12) |
| Los Angeles | 905 | (3) | 273 | (2) | 70 | (1) |
| Milan (1984–1989) | 1,413 | (5) | 161 | (1) | 215 | (4) |
| Milan (2006–2009) | 669 | (2) | 118 | (1) | 162 | (3) |
| MSKCC | 115 | (0) | 61 | (0) | 20 | (0) |
| New York Multicenter | 1,246 | (4) | 818 | (6) | 202 | (4) |
| North Carolina (1994–1997) | 154 | (1) | 91 | (1) | 31 | (1) |
| North Carolina (2002–2006) | 982 | (3) | 594 | (4) | 283 | (6) |
| Puerto Rico | 410 | (1) | 182 | (1) | 0 | (0) |
| Rome | 350 | (1) | 98 | (1) | 173 | (3) |
| Sao Paulo | 1,519 | (5) | 1,042 | (8) | 406 | (8) |
| Seattle (1985–1995) | 465 | (2) | 317 | (2) | 0 | (0) |
| Seattle-Leo | 371 | (1) | 264 | (2) | 123 | (2) |
| Switzerland | 824 | (3) | 311 | (2) | 104 | (2) |
| Tampa | 789 | (3) | 115 | (1) | 48 | (1) |
| US Multicenter | 936 | (3) | 710 | (5) | 0 | (0) |
| Western Europe | 1,726 | (6) | 932 | (7) | 378 | (8) |
| Educationa | ||||||
| No education | 1,078 | (4) | 726 | (5) | 118 | (2) |
| ≤Junior high school | 10,456 | (35) | 4,674 | (35) | 2,371 | (48) |
| Some high school | 5,330 | (18) | 2,751 | (21) | 922 | (19) |
| High school graduate | 3,883 | (13) | 1,883 | (14) | 717 | (14) |
| Technical school, some college | 4,825 | (16) | 1,989 | (15) | 496 | (10) |
| ≥College graduate | 4,272 | (14) | 1,294 | (10) | 319 | (6) |
| Drinking status | ||||||
| Never user | 8,068 | (27) | 2,279 | (17) | 578 | (12) |
| Former user | 3,072 | (10) | 2,521 | (19) | 833 | (17) |
| Current user | 14,210 | (48) | 6,943 | (52) | 2,442 | (49) |
| Missing | 4,494 | (15) | 1,574 | (12) | 1,091 | (22) |
| Alcohol drinking intensity (number of drinks/day) | ||||||
| 0-<1 | 17,207 | 58) | 4,899 | 37) | 1,433 | (29) |
| 1-<5 | 8,913 | 30) | 4,099 | 31) | 1,757 | (35) |
| ≥5 | 2,925 | 10) | 3,814 | 29) | 1,626 | (33) |
| Missing | 799 | 3) | 505 | 4) | 127 | (3) |
| Alcohol duration (years) | ||||||
| Never drinkers | 8,068 | (27) | 2,279 | (17) | 578 | (12) |
| 0-<20 | 2,984 | (10) | 1,182 | (9) | 297 | (6) |
| 20-<40 | 9,427 | (32) | 5,422 | (41) | 1,848 | (37) |
| 40-<60 | 6,013 | (20) | 2,920 | (22) | 1,435 | (29) |
| ≥60 | 412 | (1) | 176 | (1) | 123 | (2) |
| Missing | 2,940 | (10) | 1,338 | (10) | 662 | (13) |
Statistical analysis
The bivariate regression spline models [54] used to investigate the dose–response relationship between HNC and the joint exposure to smoking intensity and duration was described extensively in eAppendix – Statistical Analysis and in Di Credico’s PhD Thesis [55]. We assumed a generalized semi-parametric logistic regression model where the two exposures were entered as a joined piecewise polynomial of a linear degree with constraints for continuity at each join point (called knot), together with potential confounders (i.e., age, sex, race, study, education, drinking status, drinking intensity, and drinking duration) [15]. Knots represented change points in the slope of the risk surface. The set of spline regression parameters described the shape of the risk surface. We further assumed that the knot locations for any of the two exposures were unknown parameters to be estimated, up to a maximum of 2 knots allowed for each exposure. At the maximum level of complexity, the risk surface was allowed to have 2 change-points in the slope for any exposure and 9 different areas with a possibly changing slope.
The optimal knot locations and regression parameters were jointly estimated within the Bayesian approach. Prior distributions expressed a priori knowledge (here vague) on plausible values of knot locations and regression parameters [56,57]. Their posterior distribution combined prior information and available data through the Bayes theorem. A joint posterior distribution was simulated for each cancer site, smoking-status stratum (current/former smokers), and combination of number of knots using the Markov Chain Monte Carlo-type NUTS (No-U-Turn Sampler) [58] algorithm. For each combination of site and stratum, the best model was selected among the convergent models with sensible knot locations (≤95th percentile of either exposures) as the one that minimized the Wanatabe-Akaike Information Criterion (WAIC) [59,60]. The optimal knot locations, the ORs, and their 95% credible intervals (CIs) were derived from the posterior distribution corresponding to the best model. The ORs were presented through three-dimensional mesh plots that displayed the surface of risk for any combination of cigarette smoking intensity and duration, and through two-dimensional contour plots that showed iso-risk curves identifying the combinations of the two exposures with the same OR. Also explored were trade-offs between intensity and duration in two-dimensional contour plots by comparison of ORs for a fixed cumulative exposure (i.e., pack-years).
To evaluate potential modifying effects of some covariates, analyses were carried out in strata of alcohol drinking intensity (i.e., <1, 1-<5, ≥5 drinks per day) for current smokers, and years since quitting (i.e., <10, ≥10 years) for former smokers. Separate results for the risk of laryngeal cancer across alcohol drinking intensity strata were presented, due to absence of heterogeneity of the risk surfaces across strata for current smokers. As a comparison, risk estimates were further estimated according to the Bayesian step-function regression model.
When possible, all the models were fitted with the full set of potential confounders shown in Table 1; moreover, “Never smokers” were assumed as the reference category. Calculations were carried out using the open-source Stan program [61] within the open-source R program [62,63].
Results
Table 1 shows selected characteristics of cases and controls, according to the variables included in the models as potential confounders. Approximately 70% of the subjects were white. Studies from Europe contributed with approximately 44% of subjects; 31% of subjects were from the United States, whereas the remaining ones were from Latin America (14%) and Asia (11%). Six studies provided only cases of OCP cancer.
Table 2 shows the distribution of cigarette smoking habits for OCP cancer, laryngeal cancer, and controls. Never smokers were 21% of OCP and 7% of laryngeal cancers, versus 45% of controls. Current smokers were 66% of laryngeal cancer cases, 57% of OCP cancer cases, and 26% of controls. The prevalence of former smokers was similar in cases and controls, but the percentage of subjects who quit cigarette smoking ≥10 years ago was ~52% among HNC cases and 72% among controls.
Table 2 –
Distribution of cases of oral and pharyngeal cancer, laryngeal cancer, and controls according to selected aspects of cigarette smoking habits. INHANCE consortium
| Controls | (%) | Oral and pharyngeal cancer cases | (%) | Laryngeal cancer cases | (%) | |
|---|---|---|---|---|---|---|
| Cigarette smoking status | ||||||
| Never user | 13,347 | (45) | 2,791 | (21) | 330 | (7) |
| Former user | 8,792 | (29) | 2,909 | (22) | 1,353 | (27) |
| Current user | 7,705 | (26) | 7,617 | (57) | 3,260 | (66) |
| Cigarette smoking intensity (number of cigarettes/day) | ||||||
| Never user | 13,347 | (45) | 2,791 | (21) | 330 | (7) |
| ≥1–15 | 7,199 | (24) | 2,814 | (21) | 1,054 | (21) |
| >15–25 | 6,166 | (21) | 4,483 | (34) | 2,083 | (42) |
| >26–40 | 3,132 | (10) | 3,229 | (24) | 1,476 | (30) |
| Cigarette smoking duration (years) | ||||||
| Never user | 13,347 | (45) | 2,791 | (21) | 330 | (7) |
| ≥1–25 | 7,214 | (24) | 2,019 | (15) | 604 | (12) |
| >25–35 | 4,360 | (15) | 3,370 | (25) | 1,330 | (27) |
| >35–51 | 4,923 | (16) | 5,137 | (39) | 2,679 | (54) |
| Time since quitting cigarette smoking (years) (for formers only)a | ||||||
| ≥1-<10 | 2,300 | (26) | 1,310 | (45) | 626 | (46) |
| ≥10 | 3,655 | (72) | 1,522 | (52) | 711 | (53) |
| Missing | 137 | (2) | 77 | (3) | 16 | (1) |
Proportions were calculated among former smokers only.
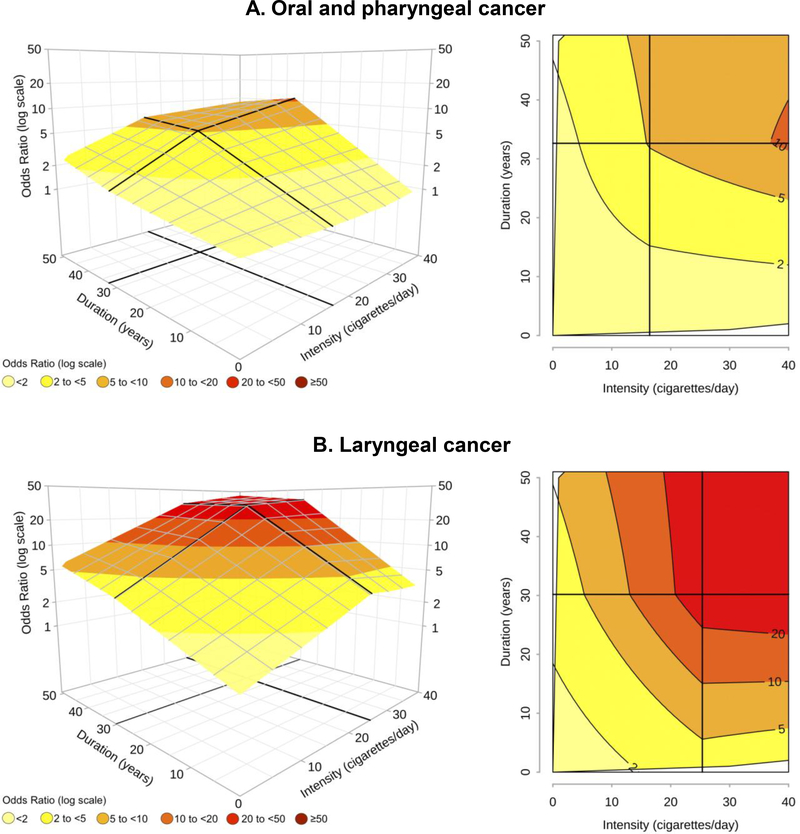
Figure 2 shows the mesh and contour plots for cancers of the OCP and larynx among current cigarette smokers. Knot locations were indicated by thicker black lines; the intersections of the lines defined the areas where the risk surface had different slopes. For both cancer sites, the best model was characterized by one knot for duration (at 33 years for OCP cancer and 30 years for laryngeal cancer) and one knot for intensity (at 16 and 25 cigarettes/day, respectively). Smoking duration modified OCP cancer risk at any levels of intensity (Figure 2A). In contrast, for any duration up to 10 years, the ORs were always <2, regardless of the intensity of cigarette smoking. In addition, at a fixed value of 20 pack-years, an intensity of 40 cigarettes/day and a duration of 10 years led to an OR of ~2, whereas the OR was equal to ~4 with a duration of 40 years in smokers of 10 cigarettes/day.
Figure 2 – Odds ratiosa,b of oral and pharyngeal cancer and laryngeal cancer in current smokers, for the joint effect of intensity (cigarettes/day) and duration (years) of cigarette smoking estimated through bivariate spline models. INHANCE consortium.
a Fitted models included adjustment for age, sex, race, study, education, drinking status, drinking intensity, and drinking duration. The reference category was defined as “Never smokers”. b On the grid, black thicker lines represent knot locations: 16 cigarettes/day and 33 years of duration for oral and pharyngeal cancer and 25 cigarettes/day and 30 years of duration for laryngeal cancer, respectively. Dark grey lines in contour plots indicate iso-risk curves at defined levels of risk.
For laryngeal cancer, ORs>20 were found for intensities of >20 cigarettes/day and durations of >28 years (Figure 2B). Moreover, ORs>10 of laryngeal cancer were reached by current smokers of >20 cigarettes/day only when duration was >20 years; however, ORs>10 were not reached for any duration <15 years and any level of intensity. Finally, the OR was 6.2 for smokers of 40 cigarettes/day for 10 years, but it was higher (between 9 and 10) for 20 cigarettes/day smoked for 20 years, or for 10 cigarettes/day smoked for 40 years.
The shape of the risk surface and the values of the ORs were also very similar across major OCP cancer subsites (i.e., oral cavity and oropharyngeal cancers) (eFigure 2).
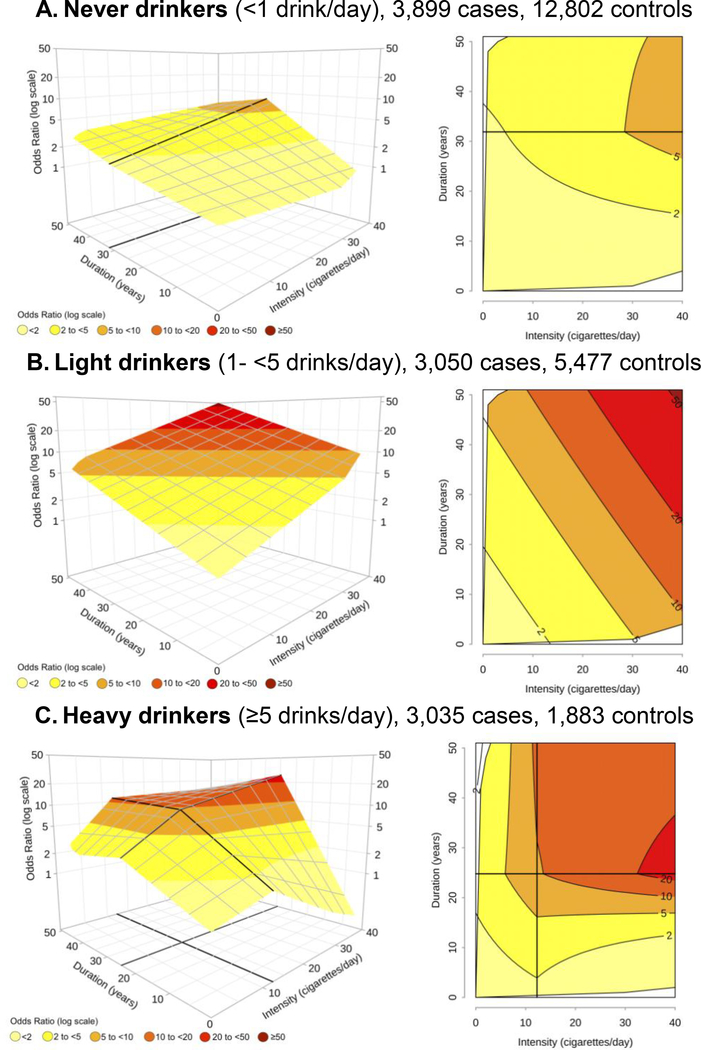
Figure 3 shows the joint effect of current smoking intensity and duration in strata of alcohol consumption. Among never drinkers (<1 drink/day), the shape of the risk surface was similar to the one presented for all alcohol intensities together (Figure 2A), but the ORs were generally lower; all the ORs were < 2 for durations ≤15 years and any intensity, whereas an OR>5 was observed only after ~25 years of duration or more (Figure 3A). However, the shape of the surface and/or the ORs of the joint effect of duration and intensity were different when light (Figure 3B) and heavy (Figure 3C) drinkers were considered.
Figure 3 – Odds ratiosa,b of oral and pharyngeal cancer in current smokers by alcohol drinking intensity, for the joint effect of intensity (cigarettes/day) and duration (years) of cigarette smoking, estimated through bivariate spline models. INHANCE consortium.
a Fitted models included adjustment for age, sex, race, study, education, and alcohol drinking status. The reference category was defined as “Never smokers”, in each strata of alcohol drinking intensity. b On the grid, black thicker lines represent knot locations: 32 years of duration for never drinkers, and 12 cigarettes/day and 25 years of duration for heavy drinkers, respectively. Dark grey lines in contour plots indicate iso-risk curves at defined levels of risk.
As a comparison, the ORs and their corresponding 95% CIs were estimated for each cancer site in current smokers within the Bayesian logistic regression model that assumed the presence of step functions (Table 3, main ORs). These estimates were compared with the range of OR estimates derived from the spline models for each joint category of duration and intensity (Table 3, bracketed ORs). For both cancer sites, all Min-Max ranges included the OR estimates obtained from the Bayesian logistic regression, and this replication reassured that the spline model was valid. In addition, within the examined categories, the step-function intervals widely overlapped with the Min-Max ranges of the ORs from the spline models, but failed to capture the ORs variability. For instance, for OCP cancer in current smokers of 26–40 cigarettes/day for 36–51 years, the categorical OR was 8.4 (95% CI: 8.0–8.9), whereas the OR varied from 7.1 to 10.6 under the spline model approach for the same combined category (Table 3). The same pattern emerged at any combination of duration and intensity for both cancer sites for former smokers who quit ≥10 years ago (eTable 2).
Table 3 –
Odds ratios (ORs)a and 95% credible intervals (CIs) of oral and pharyngeal cancer and laryngeal cancer in current smokers, for the joint effect of intensity (cigarettes/day) and duration (years) of cigarette smoking estimated through step-function as compared with results from bivariate spline models. INHANCE consortium
| Intensity (cigarettes/day) | ||||||
|---|---|---|---|---|---|---|
| Cancer type | 1–15 | 16–25 | 26–40 | |||
| Ca:Co | OR (95%CI) [Min-Max]b | Ca:Co | OR (95%CI) [Min-Max]b | Ca:Co | OR (95%CI) [Min-Max]b | |
| Oral cavity and pharynx | ||||||
| Duration (years) | ||||||
| 1–25 | 322:1,046 | 1.5 (1.4–1.7) | 371:663 | 2.7 (2.4–2.9) | 191:270 | 3.3 (2.8–3.9) |
| [0.9–3.1] | [0.9–4.2] | [0.8–5.9] | ||||
| 26–35 | 583:911 | 2.9 (2.7–3.1) | 1,138:954 | 5.1 (4.9–5.4) | 849:466 | 7.2 (6.7–7.8) |
| [1.4–4.8] | [3.5–6.9] | [4.6–11.0] | ||||
| 36–51 | 912:1,175 | 3.7 (3.5–3.9) | 1,838:1,409 | 6.0 (5.8–6.2) | 1,413:694 | 8.4 (8.0–8.9) |
| [1.6–5.8] | [5.2–7.1] | [7.1–10.6] | ||||
| Larynx | ||||||
| Duration (years) | ||||||
| 1–25 | 75:908 | 3.8 (3.3–4.4) | 112:576 | 9.4 (8.3–10.5) | 70:247 | 14.8 (12.2–17.8) |
| [1.1–8.8] | [2.3–20.1] | [2.8–23.1] | ||||
| 26–35 | 183:812 | 7.3 (6.9–7.9) | 423:845 | 17.2 (16.8–17.6) | 319:415 | 28.5 (27–30.2) |
| [2.9–12.6] | [10.1–29.6] | [22.4–37.7] | ||||
| 36–51 | 394:1,064 | 8.9 (8.8–9.1) | 966:1,274 | 19.5 (19.3–19.8) | 718:616 | 33.9 (33.5–34.3) |
| [3.9–15.3] | [13.9–31.0] | [30.9–43.2] | ||||
Fitted models included adjustment for age, sex, race, study, education, drinking status, drinking intensity, and drinking duration. The reference category was defined as “Never smokers”. The reference category included 2,791 cases and 13,139 controls for the analysis on oral and pharyngeal cancer and 330 cases and 11,433 controls for that on laryngeal cancer.
Min and Max represent the lowest and the highest OR values estimated for any combinations of intensity and duration by bivariate spline models.
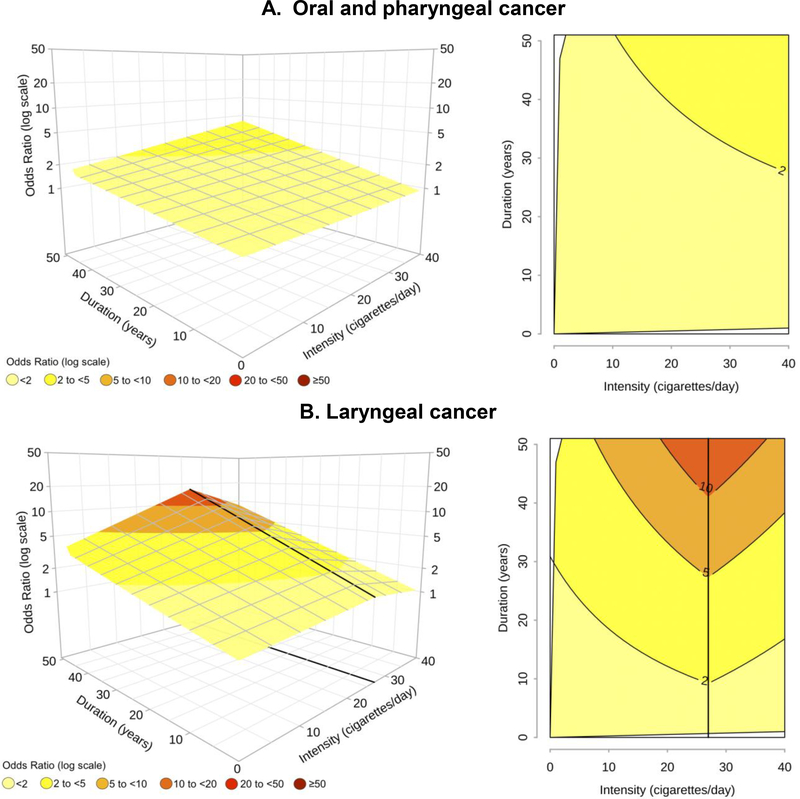
Among former smokers who quit ≥10 years ago, no ORs>4 were observed for OCP cancer (Figure 4A), and the ORs were approximately halved, as compared to the same levels of duration and intensity in current smokers (Figure 2A). A mean risk reduction of 2/3 was also found for laryngeal cancer in long-term former smokers, with ORs declines that varied from 1/3 to 4/5 depending on the different combinations of intensity and duration (Figure 4B). These estimates were derived from models that included one knot for intensity (27 cigarettes/day) for laryngeal cancer and no knots for OCP cancer. Results on the combined set of former smokers (regardless of time since quitting) were similarly presented in eFigure 3.
Figure 4 – Odds ratioa,b of oral and pharyngeal cancer and laryngeal cancer in former smokers who quit ≥10 years ago, for the joint effect of intensity (cigarettes/day) and duration (years) of cigarette smoking estimated through bivariate spline models. INHANCE consortium.
a Fitted models included adjustment for age, sex, race, study, education, drinking status, drinking intensity, and drinking duration. The reference category was defined as “Never smokers”. b On the grid, the black thicker line represents the knot location: 27 cigarettes/day for laryngeal cancer. Dark grey lines in contour plots indicate iso-risk curves at defined levels of risk.
Discussion
Our large pooled analysis showed that cigarette smoking duration and intensity did not increase HNC risk to the same extent, but the effect was greater for longer durations. At the highest combined levels of cigarette intensity and duration, subjects may reach ORs>10 of OCP cancer and ORs>40 of laryngeal cancer, as compared to never smokers. Bivariate regression spline models have been proven successful in exploring the separate and joint effects of intensity and duration of cigarette smoking. Therefore, when possible, models that allow this differential impact of intensity and duration on the risk should be considered [8,12,16].
Results from the present study were consistent with previous findings showing no threshold effect on HNC risk of shorter durations or lower intensities of tobacco smoking [13,64]. Indeed, the literature supported the presence of a dose-response relationship between cigarette smoking and HNC risk over the entire range of consumption [5,13]. Our contribution additionally suggested that the dose-response relationship with OCP and laryngeal cancer risk was still far from being linear, with a steeper increase for intermediate consumptions and a possible plateaux indicating a ‘saturation effect’ in smokers with >20 years of duration and >30 cigarettes/day.
Our results also strengthen the evidence [18,65] on the impact of smoking cessation on HNC cancer risk, with the OR in former smokers who quit ≥10 years ago being more than halved for OCP cancer and ~1/3 lower for laryngeal cancer, in comparison with current smokers. These results convey to the general population and to public health professionals the valuable message that it is never too late to give up cigarette smoking, as quitting provides far smaller risks of developing HNC cancer for OCP and, most of all, for laryngeal cancers. This is in accordance with another recently published meta-analysis of observational studies on laryngeal cancer based on 14,292 cases from 3 cohort and 15 case-control studies [65].
When the combined effect of smoking intensity and duration was modelled as a cross-product term, an interaction term between alcohol consumption and cigarette smoking was traditionally added to the models [3,4]. In our study this effect was explored by stratified regression spline models estimated within categories of alcohol consumption; we compared the shape of the risk surface and the ORs values from the iso-risk curves across the three stratified analyses. However, our results also showed that alcohol acts as a substantial modifier of the association between OCP cancer risk and the joint effect of cigarette intensity and duration [4,17].
Major strengths of our study included a large sample size, international representativeness, and valid information on potential confounding factors. In addition, we applied a novel Bayesian approach to jointly estimate the optimal knot locations and the ORs of HNC for the joint effect of intensity and duration in a bivariate context. After examining various frequentist solutions [66,67], we opted for the Bayesian approach that allowed to put the knots where the data suggested, once we had taken the entire set of confounding variables into consideration. Within the Bayesian framework, we were also able to choose the optimal number of knots (up to 2) and knot locations by model comparison based on information criteria, instead of just comparing models with fixed number and location of the knots. Finally, we could incorporate in the model selection process evidence from the literature on the maximum number of changes in the risk pattern, which is unlikely to be higher than 2. Indeed, cigarette smoking is supposed to have a protective or null effect on the risk at lower levels, a saturation effect at the highest intensity levels [68], as well as the expected increase previously described at the intermediate levels of consumption. The proposed Bayesian approach is applicable to other epidemiologic scenarios where continuous exposures in their potential interaction affect disease risk.
Among study limitations, the retrospective study design and the self-reported smoking history were the most relevant ones. Even if a large amount of literature has suggested acceptable correlations between self-reported smoking intensity and cotinine levels in blood or urine [69], inaccurate self-reporting may occur. Discrepancies between self-reported and objective information were more likely among long-term heavy smokers [70]; higher values of intensity and duration were therefore more prone to inaccurate reporting. Furthermore, smoking intensity may have varied over time and by age of exposure. However, its estimates were often based on the self-reported average number of cigarettes per day; these two aspects may have led to appreciable errors in measuring the true mean intensity of exposure over one’s lifetime. These issues may well be serious here, given the continuous nature of the exposures considered in spline models. To reduce information bias and residual confounding at the extreme values of the exposure distributions, we excluded subjects reporting higher (>95th percentiles) cigarette intensity and/or duration from the present analysis [13,15,71]. In addition, to avoid bias due to the use of other tobacco products, we excluded subjects reporting use of tobacco products other than cigarettes. We were also obliged to combine different subsites of OCP cancer, although differences in aetiology (i.e., the causal role of human papillomavirus in oropharyngeal cancer) have been demonstrated [34]. However, results were similar for major subsites, although based on less stable models. Finally, our Bayesian approach was computationally time consuming, asking for several hours of server computing for each model fitted.
Results from the present study confirmed previous evidence on the main contribution of cigarette smoking duration in influencing HNC risk and on the important reduction in HNC risk observed in former smokers who quit ≥10 or 15 years ago. Both results strongly support an even wider diffusion of public interventions to encourage smokers to quit and help them succeed.
Supplementary Material
Figure 1 –
Flow chart of subjects’ selection
Acknowledgements
The authors would like to thank Xavier Castellsagué who collected data in the IARC International Multicenter study and passed away in 2016. We thank Mrs Luigina Mei for editorial assistance.
Funding: This work was supported by grants from the: National Institutes of Health (NIH) [no grant number provided for the INHANCE Pooled Data Project, grant numbers P01CA068384, K07CA104231 for the New York Multicenter study, grant numbers R01CA048996, R01DE012609 for the Seattle (1985–1995) study, grant number TW001500 for the Fogarty International Research Collaboration Award (FIRCA) supporting the Iowa study, grant number R01CA061188 for the North Carolina (1994–1997) study, grant numbers P01CA068384, K07CA104231, R01DE013158 for the Tampa study, grant numbers P50CA090388, R01DA011386, R03CA077954, T32CA009142, U01CA096134, R21ES011667 for the Los Angeles study, grant numbers R01ES011740, R01CA100264 for the Houston study, grant numbers R01CA078609, R01CA100679 for the Boston study, grant number R01CA051845 for the MSKCC study, grant number R01CA030022 for the Seattle-Leo study, grant number DE016631 for the Baltimore study]; National Cancer Institute (NCI) at the National Institutes of Health (NIH) [grant number R03CA113157 for the INHANCE Pooled Data Project, no grant number provided for the Intramural Programs supporting the Puerto Rico and the US Multicenter studies, grant number R01CA90731–01 for the North Carolina (2002–2006) study]; National Institute of Dental and Craniofacial Research (NIDCR) at the National Institutes of Health (NIH) [grant number R03DE016611 for the INHANCE Pooled Data Project, grant numbers R01DE011979, R01DE013110 for the Iowa study, no grant number provided for the Intramural Program supporting the Puerto Rico study]; Italian Association for Research on Cancer (AIRC) [no grant number provided for the Milan (1984–1989) study, for the Aviano study, for the Italy Multicenter study and for the Rome study, grant number 10068 for the Milan study (2006–2009)]; Italian League against Cancer [no grant number provided for the Aviano and Italy Multicenter studies]; Italian Ministry of Research [no grant number provided for the Aviano and Italy Multicenter studies]; the Swiss Research against cancer/Oncosuisse [grant numbers KFS-700, OCS-1633 for the Swiss study]; World Cancer Research Fund [no grant number provided for the Central Europe study]; European Commission [grant number IC18-CT97–0222 (INCO-DC Program) for the Latin America study, grant number IC15-CT98–0332 (INCO-COPERNICUS Program) for the Central Europe study]; Veterans Affairs Merit Review Funds [no grant number provided for the Iowa study]; National Institute of Environmental Health Sciences (NIEHS) [grant number P30ES010126 for the North Carolina (1994–1997) study, grant number P30ES010126 for the North Carolina (2002–2006) study]; Alper Research Program for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center [no grant number provided for the Los Angeles study]; Fondo para la Investigacion Cientifica y Tecnologica Argentina (FONCYT) [no grant number provided for the Latin America study]; Institut Hospital del Mar d’Investigacions Mediquès (IMIM) [no grant number provided for the Latin America study]; Fundação de Amparo à Pesquisa no Estado de São Paulo (FAPESP) [grant number 01/01768–2 for the Latin America study, grant numbers GENCAPO 04/12054–9, 10/51168–0 for the Sao Paulo study]; Fondo de Investigaciones Sanitarias (FIS) of the Spanish Government [grant number FIS 97/0024, FIS 97/0662, BAE 01/5013 for the International Multicenter study]; International Union Against Cancer (UICC) [no grant number provided for the International Multicenter study]; Yamagiwa-Yoshida Memorial International Cancer Study Grant [no grant number provided for the International Multicenter study]; European Community (5th Framework Programme) [grant number QLK1-CT-2001–00182 for the Western Europe study]; Ministry of Science, Research and Arts Baden-Wurttemberg [no grant number provided for the Germany-Saarland study]; German Ministry of Education and Research [grant number 01GB9702/3 for the Germany-Heidelberg study]; Scientific Research grant from the Ministry of Education, Science, Sports, Culture and Technology of Japan [grant number 17015052 for the Japan (2001–2005) study]; Third-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare of Japan [grant number H20–002 for the Japan (2001–2005) study]; Johns Hopkins Richard Gelb Cancer Prevention Award [no grant number provided for the HOTSPOT study]; Italian Foundation for Cancer Research (FIRC) [no grant number provided for the Milan study (2006–2009)]; Italian Ministry of Education - PRIN 2009 Program [grant number X8YCBN for the Milan study (2006–2009)]; VE was supported by Università degli Studi di Milano ‘Young Investigator Grant Program 2017’.
Footnotes
Abbreviations: CI: credible interval; HNC: head and neck cancer; INHANCE: International Head and Neck Cancer Epidemiology; NUTS: No-U-Turn Sampler; OCP: oral cavity and pharynx; OR: odds ratio; WAIC: Wanatabe-Akaike Information Criterion.
References
- [1].World Health Organization. WHO global report: mortality attributable to tobacco. Geneva: WHO; 2012. [Google Scholar]
- [2].Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2018). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; Available from: https://gco.iarc.fr/today, accessed [21 March 2019]. [Google Scholar]
- [3].IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 38 Tobacco Smoking. Lyon: IARC Press; 1986. [Google Scholar]
- [4].IARC. IARC Monographs on the evaluation of carcinogenic risks to Humans, Volume 83: Tobacco smoke and involuntary smoking. Lyon: IARC Press; 2004. [PMC free article] [PubMed] [Google Scholar]
- [5].Lubin JH, Purdue M, Kelsey K, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and risk of neck cancer: a pooled analysis of case-control studies. Am J Epidemiol 2009;170:937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lubin JH, Gaudet MM, Olshan AF, et al. Body mass index, cigarette smoking, and alcohol consumption and cancers of the oral cavity, pharynx, and larynx: modeling odds ratios in pooled case-control data. Am J Epidemiol 2010;171:1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Doll R An epidemiological perspective of the biology of cancer. Cancer Res 1978;38:3573–83. [PubMed] [Google Scholar]
- [8].Vlaanderen J, Portengen L, Schüz J, et al. Effect modification of the association of cumulative exposure and cancer risk by intensity of exposure and time since exposure cessation: a flexible method applied to cigarette smoking and lung cancer in the SYNERGY Study. Am J Epidemiol 2014;179:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lubin JH, Alavanja MC, Caporaso N et al. Cigarette smoking and cancer risk: modeling total exposure and intensity. Am J Epidemiol 2007;166:479–89. [DOI] [PubMed] [Google Scholar]
- [10].Baris D, Karagas MR, Verrill C, et al. A case-control study of smoking and bladder cancer risk: emergent patterns over time. J Natl Cancer Inst 2009;101:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 2007;99:777–89. [DOI] [PubMed] [Google Scholar]
- [12].Lubin JH, Caporaso NE. Misunderstandings in the misconception on the use of pack-years in analysis of smoking. Br J Cancer 2013;108:1218–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Polesel J, Talamini R, La Vecchia C, et al. Tobacco smoking and the risk of upper aerodigestive tract cancers: A reanalysis of case-control studies using spline models. Int J Cancer 2008;122:2398–402. [DOI] [PubMed] [Google Scholar]
- [14].Desquilbet L, Mariotti F. Dose–response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–57. [DOI] [PubMed] [Google Scholar]
- [15].Dal Maso L, Torelli N, Biancotto E, et al. Combined effect of tobacco smoking and alcohol drinking in the risk of head and neck cancers: A re-analysis of case-control studies using bi-dimensional spline models. Eur J Epidemiol 2016;31:385–93. [DOI] [PubMed] [Google Scholar]
- [16].Peto J That effects of smoking should be measured in pack-years: misconceptions 4. Br J Cancer 2012;107:406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hashibe M, Brennan P, Chuang S-C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 2009;18:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and neck cancer risk. Int J Epidemiol 2010;39:182–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wyss A, Hashibe M, Chuang SC, et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled. Am J Epidemiol 2013;178:679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Franceschi S, Talamini R, Barra S, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res 1990;50:6502–7. [PubMed] [Google Scholar]
- [21].Negri E, La Vecchia C, Franceschi S, Tavani A. Attributable risk for oral cancer in northern Italy. Cancer Epidemiol Biomarkers Prev 1993;2:189–93. [PubMed] [Google Scholar]
- [22].Bosetti C, Gallus S, Trichopoulou A, et al. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev 2003;12:1091–4. [PubMed] [Google Scholar]
- [23].Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S, Monnier P. Food groups and risk of oral and pharyngeal cancer. Int J Cancer 1998;77:705–9. [DOI] [PubMed] [Google Scholar]
- [24].Hashibe M, Boffetta P, Zaridze D. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev 2006;15:696–703. [DOI] [PubMed] [Google Scholar]
- [25].Muscat JE, Richie JP Jr., Thompson S, Wynder EL. Gender differences in smoking and risk for oral cancer. Cancer Res 1996;56:5192–7. [PubMed] [Google Scholar]
- [26].Rosenblatt KA, Daling JR, Chen C, Sherman KJ, Schwartz SM. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res 2004;64:4049–54. [DOI] [PubMed] [Google Scholar]
- [27].Smith EM, Hoffman HT, Summersgill KS, Kirchner HL, Turek LP, Haugen TH. Human papillomavirus and risk of oral cancer. Laryngoscope 1998;108:1098–103. [DOI] [PubMed] [Google Scholar]
- [28].Olshan AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev 2000;9:185–91. [PubMed] [Google Scholar]
- [29].Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis 2002;23:1229–34. [DOI] [PubMed] [Google Scholar]
- [30].Cui Y, Morgenstern H, Greenland S, et al. Polymorphism of xeroderma pigmentosum group G and the risk of lung cancer and squamous cell carcinomas of the oropharynx, larynx and esophagus. Int J Cancer 2006;118:714–20. [DOI] [PubMed] [Google Scholar]
- [31].Zhang Z, Shi Q, Liu Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of methionine synthase and methionine synthase reductase and risk of squamous cell carcinoma of the head and neck: a case-control analysis. Cancer Epidemiol Biomarkers Prev 2005;14:1188–93. [DOI] [PubMed] [Google Scholar]
- [32].Hayes RB, Bravo-Otero E, Kleinman DV, et al. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control 1999;10:27–33. [DOI] [PubMed] [Google Scholar]
- [33].Szymańska K, Hung RJ, Wünsch-Filho V, et al. Alcohol and tobacco, and the risk of cancers of the upper aerodigestive tract in Latin America: a case-control study. Cancer Causes Control 2011;22:1037–46. [DOI] [PubMed] [Google Scholar]
- [34].Herrero R, Castellsagué X, Pawlita M, et al. IARC Multicenter Oral Cancer Study Group. Human papillomavirus and the risk of Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. [DOI] [PubMed] [Google Scholar]
- [35].Peters ES, McClean MD, Liu M, Eisen EA, Mueller N, Kelsey KT. The ADH1C polymorphism modifies the risk of squamous cell carcinoma of the head and neck associated with alcohol and tobacco use. Cancer Epidemiol Biomarkers Prev 2005;14:476–82. [DOI] [PubMed] [Google Scholar]
- [36].Gallì P, Cadoni G, Volante M, et al. A case-control study on the combined effects of p53 and p73 polymorphisms on head and neck cancer risk in an Italian population. BMC Cancer 2009;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282. [PubMed] [Google Scholar]
- [38].Boing AF, Ferreira Antunes JL, de Carvalho MB, et al. How much do smoking and alcohol consumption explain socioeconomic inequalities in head and neck cancer risk? J Epidemiol Community Health 2010;65:709–14. [DOI] [PubMed] [Google Scholar]
- [39].Schantz SP, Zhang ZF, Spitz MS, Sun M, Hsu TC. Genetic susceptibility to head and neck cancer: interaction between nutrition and mutagen sensitivity. Laryngoscope 1997;107:765–81. [DOI] [PubMed] [Google Scholar]
- [40].Rogers MA, Thomas DB, Davis S, Vaughan TL, Nevissi AE. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev 1993;2:305–12. [PubMed] [Google Scholar]
- [41].Lagiou P, Georgila C, Minaki P, et al. Alcohol-related cancers and genetic susceptibility in Europe: the ARCAGE project: study samples and data collection. Eur J Cancer Prev 2009;18:76–84. [DOI] [PubMed] [Google Scholar]
- [42].Anderson KS, Gerber JE, D’Souza G, et al. Biologic predictors of serologic responses to HPV in oropharyngeal cancer: The HOTSPOT study. Oral Oncol 2015;51:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Twardella D, Loew M, Rothenbacher D, Stegmaier C, Ziegler H, Brenner H. The diagnosis of a smoking-related disease is a prominent trigger for smoking cessation in a retrospective cohort study. J Clin Epidemiol 2006;59:82–9. [DOI] [PubMed] [Google Scholar]
- [44].Dietz A, Ramroth H, Urban T, Ahrens W, Becher H. Exposure to cement dust, related occupational groups and laryngeal cancer risk: results of a population based case-control study. Int J Cancer 2004;108:907–11. [DOI] [PubMed] [Google Scholar]
- [45].Suzuki T, Wakai K, Matsuo K, et al. Effect of dietary antioxidants and risk of oral, pharyngeal and laryngeal squamous cell carcinoma according to smoking and drinking habits. Cancer Sci 2006;97:760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Divaris K, Olshan AF, Smith J, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control 2010;21:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Menvielle G, Luce D, Goldberg P, Leclerc A. Smoking, alcohol drinking, occupational exposures and social inequalities in hypopharyngeal and laryngeal cancer. Int J Epidemiol 2004;33:799–806. [DOI] [PubMed] [Google Scholar]
- [48].Jayaprakash V, Rigual NR, Moysich KB et al. Chemoprevention of Head and Neck Cancer With Aspirin. Arch Otolaryngol Head Neck Surg 2006;132:1231–6. [DOI] [PubMed] [Google Scholar]
- [49].Luce D, Stücker I. ICARE Study Group. Investigation of occupational and environmental causes of respiratory cancers (ICARE): a multicenter, population-based case-control study in France. BMC Public Health 2011;11:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].D’souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papilloma virus and oropharyngeal cancer. N Engl J Med 2007;10;356:1944–56. [DOI] [PubMed] [Google Scholar]
- [51].Zheng TZ, Boyle P, Hu HF, et al. Dentition, oral hygiene, and risk of oral cancer: a case-control study in Beijing, People’s Republic of China. Cancer Causes Control 1990;1:235–41. [DOI] [PubMed] [Google Scholar]
- [52].Bravi F, Bosetti C, Filomeno M, et al. Foods, nutrients and the risk of oral and pharyngeal cancer. British Journal of Cancer 2013;109:2904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lubin JH, Kogevinas M, Silverman D, et al. Evidence for an intensity-dependent interaction of NAT2 acetylation genotype and cigarette smoking in the Spanish Bladder Cancer Study. Int J Epidemiol 2007;36:236–41. [DOI] [PubMed] [Google Scholar]
- [54].Ruppert D, Wand M, Carroll R. Semiparametric Regression (Cambridge Series in Statistical and Probabilistic Mathematics). Cambridge: Cambridge University Press; 2003. [Google Scholar]
- [55].Di Credico G Some developments in semiparametric and cross-classified multilevel models. Ph.D. Thesis Padua University: Italy; 2018 [Google Scholar]
- [56].Gelman A, Stern HS, Carlin JB, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis. Chapman and Hall/CRC; 2013. [Google Scholar]
- [57].Gelman A, Jakulin A, Pittau MG, Su YS. A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat 2008;2:1360–83. [Google Scholar]
- [58].Hoffman MD, Gelman A. The No-U-turn sampler: adaptively setting path lengths in Hamiltonian Monte Carlo. J Mach Learn Res 2014;15:1593–623. [Google Scholar]
- [59].Watanabe S Asymptotic equivalence of Bayes cross validation and widely applicable information criterion in singular learning theory. J Mach Learn Res 2010;11:3571–94. [Google Scholar]
- [60].Gelman A, Hwang J, Vehtari A Understanding predictive information criteria for Bayesian models. Stat Comput 2014;24:997–1016. [Google Scholar]
- [61].Stan Development Team. Stan Modeling Language Users Guide and Reference Manual. Version 2.17.0, 2017.
- [62].Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat 1996;5:299–314. [Google Scholar]
- [63].R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: Austria; 2018. [Google Scholar]
- [64].Berthiller J, Straif K, Agudo A, et al. Low frequency of cigarette smoking and the risk of head and neck cancer in the INHANCE consortium pooled analysis. Int J Epidemiol 2016;45:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zuo JJ, Tao ZZ, Chen C, et al. Characteristics of cigarette smoking without alcohol consumption and laryngeal cancer: overall and time-risk relation. A meta-analysis of observational studies. Eur Arch Otorhinolaryngol 2017;274:1617–31. [DOI] [PubMed] [Google Scholar]
- [66].Molinari N, Durand JF, Sabatier R. Bounded optimal knots for regression splines. Comput Stat Data Anal 2004;45:159–78. [Google Scholar]
- [67].Mao W, Zhao LH. Free-knot polynomial splines with confidence intervals. J R Statist Soc B 2003;65( Part 4):901–19. [Google Scholar]
- [68].Schöllnberger H, Manuguerra M, Bijwaard H, et al. Analysis of epidemiological cohort data on smoking effects and lung cancer with a multi-stage cancer model. Carcinogenesis 2006;27:1432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2001;153:807–14. [DOI] [PubMed] [Google Scholar]
- [70].Morales NA, Romano MA, Michael Cummings K, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control 2013;24:1223–30. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rothman KJ, Greenland S, Lash T. Modern epidemiology. Third Edition. Philadelphia: Lippincott Williams & Wilkins; 2008; pp. 303–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.