Abstract
Background
Hyperopia in infancy requires accommodative effort to bring images into focus. Prolonged accommodative effort has been associated with an increased risk of strabismus. Strabismus may result in asthenopia and intermittent diplopia, and makes near work tasks difficult to complete. Spectacles to correct hyperopic refractive error is believed to prevent the development of strabismus.
Objectives
To assess the effectiveness of prescription spectacles compared with no intervention for the prevention of strabismus in infants and children with hyperopia.
Search methods
We searched CENTRAL (2018, Issue 12; which contains the Cochrane Eyes and Vision Trials Register); Ovid MEDLINE; Embase.com; three other databases; and two trial registries. We used no date or language restrictions in the electronic search for trials. We last searched the electronic databases on 4 December 2018.
Selection criteria
We included randomized controlled trials and quasi‐randomized trials investigating spectacle intervention or no treatment for children with hyperopia. We required hyperopia to be at least greater than +2.00 diopters (D).
Data collection and analysis
We used standard Cochrane methodological procedures. The primary outcome was the proportion of children with manifest strabismus, as defined by study investigators. Other outcomes included the amblyopia, stereoacuity, and the effect of spectacle use of strabismus and visual acuity. We also collected information on change in refractive error as a measurement of the interference of emmetropization.
Main results
We identified four randomized controlled trials (985 children enrolled who were aged six months to less than 36 months) in this review. Three trials were in the UK with follow‐up periods ranging from one to 3.5 years and one in the US with three years' follow‐up.
Investigators reported both incidence and final status regarding strabismus. Evidence of the incidence of strabismus, measured in 804 children over three to four years in four trials was uncertain although suggestive of a benefit with spectacle use (risk ratio (RR) 0.65, 95% confidence interval (CI) 0.41 to 1.02). We have very low confidence in these results due to high risk of bias, inconsistency, and imprecision. When assessed as the proportion of children with strabismus at the end of three years' follow‐up, we found a similar level of evidence for an effect of spectacles on strabismus as reported in one study (RR 1.00, 95% CI 0.31 to 3.25; 106 children). We have very low confidence in these results because of low sample size and risk of bias.
One trial reported on the risk for developing amblyopia and inadequate stereoacuity after three years in 106 children. There was unclear evidence for a decreased risk of developing amblyopia (RR 0.78, 95% CI 0.31 to 1.93), and limited evidence for a benefit of spectacles for prevention of inadequate stereoacuity (RR 0.38, 95% CI 0.16 to 0.88). We have very low confidence in these findings due to imprecision and risk of bias.
The risk of not developing emmetropization is unclear. One trial reported on the proportion of children not achieving emmetropization at three years' follow‐up (RR 0.75, 95% CI 0.18 to 3.19). One trial suggested spectacles impede emmetropization, and one trial reported no difference. These two trials could not be combined because the methods for assessing emmetropization were different. With the high risk of bias and inconsistency, the certainty of evidence for a risk for impeding or benefiting emmetropization is very low.
Based on a meta‐analysis of four trials (770 children), the risk of having visual acuity worse than 20/30 measured up to three years of age or at the end of three years of follow‐up was uncertain for children with spectacle correction compared with those without correction (RR 0.87, 95% CI 0.64 to 1.18; very low confidence due to risk of bias and imprecision).
Authors' conclusions
The effect of spectacle correction for prevention of strabismus is still unclear. In addition, the use of spectacle on the risk of visual acuity worse than 20/30, amblyopia, and inadequate emmetropization is also unclear. There may be a benefit on prevention of inadequate stereoacuity. However, these effects may have been chance findings or due to bias.
Plain language summary
Glasses to prevent eye misalignment (strabismus) in far‐sighted children
What was the aim of this review? To find out if wearing eyeglasses by far‐sighted children will prevent crossed eyes (strabismus) and associated effects from developing.
Key messages The evidence does not currently support the conclusion that eyeglasses prevent strabismus in far‐sighted children, or that wearing glasses prevents lazy‐eye (amblyopia). This evidence was limited due to some parts of the studies not being done as well as they could and small number of study participants.
What was studied in the review? Infants typically are born far‐sighted, meaning they only have clear vision at distance. As children grow, their eyes also grow to where they can see clearly at both near and far distances. About 9% of children remain very far‐sighted. Being far‐sighted means that to focus on something up close the child must use a great deal of effort. This effort can cause symptoms, such as headaches, seeing double, and eyestrain, as well as cause difficulty doing things up close, such as reading. Children that remain far‐sighted are more likely than children with normal vision to develop crossed eyes (strabismus), which may happen in 3.5% to 5.7% of children, or 10% to 20% of children with high levels of far‐sightedness. Strabismus makes it difficult for the eyes to work together to focus. It is thought that about 50% of children with strabismus develop amblyopia, meaning a child cannot get clear vision even when using glasses. Depth perception, or how two things are related to each other in space, is often affected as well. Doctors often prescribe eyeglasses to prevent the development of strabismus and these other associated problems in far‐sighted children, but it is unclear whether the glasses themselves may prevent the eyes from growing normally.
What were the main results of the review? We identified results from four randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) to determine whether eyeglasses were successful in preventing strabismus in far‐sighted infants compared with no eyeglasses. The trials enrolled infants aged six months to less than 36 months and measured outcomes between the ages of three and four years. The four trials enrolled 985 infants. We found unclear evidence of a difference between the two groups in how often strabismus occurred over the follow‐up period. We also found unclear evidence on whether the prescription of eyeglasses affected depth perception or prevented eyes from developing naturally to clear vision. We have low confidence in these findings because some parts of the studies were not done well and the small number of children in the studies.
How up‐to‐date is this review? December 2018.
Summary of findings
Summary of findings for the main comparison. Spectacle correction compared with no spectacles for infants or children with hyperopia.
| Spectacle correction compared with no spectacles for infants or children with hyperopia | ||||||
|
Patient or population: infants or children with hyperopia Settings: pediatric eye clinics Intervention: spectacle correction Comparison: no spectacles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No spectacles | Spectacles | |||||
| Incidence of strabismus up to 3–4 years of age | 234 per 1000 | 152 per 1000 (96 to 239) | RR 0.65 (0.41 to 1.02) | 804 (4 studies) |
⊕⊝⊝⊝ Very lowa,b,c |
— |
|
Proportion of children with strabismus at 3 years' follow‐up |
94 per 1000 | 94 per 1000 (29 to 306) | RR 1.00 (0.31 to 3.25) | 106 children (1 study) | ⊕⊝⊝⊝ Very lowa,c |
— |
| Proportion of children with amblyopia at 3 years | 170 per 1000 |
133 per 1000 (53 to 328) |
RR 0.78 (0.31 to 1.93) | 106 children (1 study) | ⊕⊝⊝⊝ Very lowa,c |
— |
|
Proportion of children with inadequate stereoacuity at 3 years |
302 per 1000 | 114 per 1000 (48 to 266) | RR 0.38 (0.16 to 0.88) | 106 children (1 study) | ⊕⊝⊝⊝ Very lowa,c |
— |
| Proportion of children without refractive error in the emmetropization range at 3 years | 75 per 1000 |
100 per 1000 (14 to 239) |
RR 0.75 (0.18 to 3.19) | 106 children (1 study) | ⊕⊝⊝⊝ Very lowa,c |
Emmetropization was also reported as:
|
| Visual acuity worse than 20/30 at 3–4 years of age | 183 per 1000 | 159 per 1000 (117 to 216) | RR 0.87 (0.64 to 1.18) | 770 (4 studies) |
⊕⊝⊝⊝ Very lowa,c | — |
| *The basis for the assumed risk (e.g. the mean control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; D: diopter; n: number of participants; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for risk of bias because studies were at high or unclear risk of bias for allocation concealment and high risk of bias for masking. bDowngraded one level because of inconsistent results between studies. cDowngraded two levels for imprecision; wide confidence intervals and less than optimal information size.
Background
Description of the condition
Hyperopia, also known as far‐sightedness or long‐sightedness, is a condition that arises when the image produced by light rays is focused behind the retina. In hyperopia, vision is blurred and requires accommodative effort to produce a clear image. Should the level of hyperopia be too great, accommodation will not be sufficient to overcome the amount of hyperopia without increasing the risk of strabismus and the eventual development of amblyopia (lazy eye), as well as resulting in symptoms such as blurry vision. Prolonged accommodative effort can result in strabismus, a condition commonly known as crossed eyes (also known as squint) (Babinsky 2013). Among full‐term infants, one expects to see low to moderate levels of hyperopia that generally resolve as the child's vision emmetropizes (normalizes) (Goldschmidt 1969), which occurs, in large part, by nine to 12 months of age (Mutti 2004). A proportion of children have such high levels of hyperopia that the vision may not reach normal levels. Ingram 2000 found up to 9% of children retained at least +4.0 diopters (D) of hyperopia at six months old, and estimated that approximately 20% of these children may develop some type of visual problem. Atkinson 1996 estimated that infants with at least +3.50 D of hyperopia were 13 times more likely to develop strabismus by four years of age than infants with less hyperopia, and they were six times more likely to have decreased vision than those infants with less hyperopia or emmetropia or 'normal' vision. While most hyperopic eyes will eventually emmetropize, strabismus and subsequent amblyopia present a real danger to children whose eyes do not normalize. Amblyopia is a loss of vision for which eyeglasses and contact lenses may not restore full vision, though early treatment of children with refractive correction, patching, or drug therapy has been successful (Li 2009; Taylor 2011; Taylor 2012). Amblyopia that has not been identified or treated eventually may lead to vision loss (Holmes 2006). Children with amblyopia also are believed to be at a higher risk of losing vision in the unaffected eye when compared with the general population (Tomilla 1981; van Leeuwen 2007). Because of the more serious consequences of amblyopia, seeking ways to limit the development of strabismus can decrease one of the contributing factors for amblyopia.
After refractive error, strabismus is the next leading cause of eye problems among infants and young children, and has been reported to occur in 3.7% to 5.3% of children (Cotter 1997). If the strabismic child is not given appropriate treatment, strabismus can lead to the development of amblyopia or prevent the development of binocularity, which is the ability of the eyes to work together to produce a single image (Cotter 1997). Strabismus can manifest in different ways. When the eye turns inward to the nose, this condition is called an esotropia, while the eye turning outward is known as exotropia. Sometimes the tropia can be vertical (up or down). Together these are types of manifest strabismus, where the deviation of the eye can be intermittent or constant in frequency. When the strabismus is accommodative in nature and involves an esotropia rather than an exotropia, it is termed accommodative esotropia. Accommodative esotropia results from high levels of hyperopia, an AC/A ratio (accommodative convergence/accommodation ratio that defines how much accommodative convergence occurs for a unit of accommodative response) that is too high, or the two factors working together. Onset is frequently between the ages of two and three years (Donahue 2007). The ability to do near tasks is compromised, with intermittent diplopia (double vision) and asthenopia (eyestrain) among other reported symptoms (Cotter 1997). About 10% to 20% of children with +4.0 D or more of hyperopia develop accommodative esotropia (Atkinson 1996; Dobson 1989). More recent, cross‐sectional data from the Multi‐Ethnic Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies showed that even at +2.0 D of hyperopia, there was a six‐fold increase in the odds of esotropia (Cotter 2011). About half of the children with esotropia become amblyopic according to Donahue 2007.
Description of the intervention
Hyperopia is often treated with a spectacle correction to remove or reduce the need for accommodation to view distance and near targets (Donahue 2007; Rubin 2006), and, thus, may help to prevent the development of strabismus (Rubin 2006). No other interventions have been proposed to manage hyperopic infants in an effort to prevent the development of strabismus (AOA 2008).
How the intervention might work
Treatment of hyperopia with the intention of preventing accommodative esotropia is a topic of disagreement. Using a spectacle correction to reduce the need for accommodation when fixating at is distance (i.e. reducing the accommodative effort needed to bring an image into focus) is a proposed treatment. In addition, reduced stimulation of accommodative convergence is an added benefit. The angle of existing esotropia will be reduced or perhaps eliminated. The expectation is that removing accommodative strain will allow regular binocular vision to develop during emmetropization, allowing for a decrease and eventual cessation of the spectacle correction (Rubin 2006). Although spectacle correction may be critical to prevent the development of esotropia, spectacles may prevent normal emmetropization. Atkinson 2000 found that any lag in the emmetropization of infants wearing a partial correction (undercorrection) for hyperopia at nine or 18 months had disappeared by three years, and that these children had similar refractive errors to children who were untreated. Others, however, found that children with a spectacle correction for their hyperopia did not emmetropize (Ingram 2000; Mulvihill 2000; Repka 1989). It is speculated that emmetropization is affected by the absence of accommodative demand that comes about from wearing a full spectacle correction (Birch 2005; Mutti 2009). As a result of this speculation, some ophthalmologists adopt a partial correction of the hyperopia to reduce the need for accommodation while providing some signal for emmetropization.
Why it is important to do this review
The conversion rate to amblyopia is high among children with esotropia, threatening long‐term visual health and productivity. Hyperopia presents a strain on the visual system causing symptoms such as blurred vision and asthenopia, as well as possibly adversely affecting the learning and academic growth of the highly hyperopic child. The spectacle prescription is meant to correct the hyperopia to reduce the accommodative effort required to bring images into focus. It reduces stimulation of accommodative convergence as an added benefit. Whether reduced stimulation of accommodative convergence with spectacles lowers the risk of future strabismus is an important but unanswered question. However, if we treat every infant with hyperopia with spectacles, we could be treating 80% to 90% of these children unnecessarily (Birch 2005). Because of the differing evidence regarding the effectiveness of treating these children with spectacles and the concerns about interference with emmetropization, straightforward recommendations are difficult to make. To that end, high‐quality data describing the effects, if any, of treatment with spectacles, and the level of treatment required to be effective, are necessary.
Objectives
To assess the effectiveness of prescription spectacles compared with no intervention for the prevention of strabismus in infants and children with hyperopia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized controlled trials.
Types of participants
We included trials with children from three months old to four years old at entry, with an age of primary outcome assessment at or before seven years of age. We based the risk of development of strabismus on the criteria as defined by the included studies. Typically, the criteria include high hyperopia which is at least +2.0 D of refractive error. The trial investigators must have diagnosed hyperopia by cycloplegic retinoscopy. Other conditions that could be included were anisometropia or astigmatism. There were no gender restrictions. We excluded studies that included children with existing strabismus, amblyopia, or other ocular pathology.
Types of interventions
We included trials in which spectacle correction for any level of hyperopia greater than +2.00 D was compared with no spectacle correction. We also planned, when available, to include trials that used a sham correction in the control group and trials that had compared full hyperopic spectacle correction with partial hyperopic spectacle correction.
Types of outcome measures
Primary outcomes
Proportion of children with manifest strabismus, as defined by study investigators, in the spectacle correction group compared with the proportion of children with strabismus in the no treatment group after a treatment period of a minimum of three years.
Because no child started with strabismus, studies may have reported this outcome directly as the proportion of children with strabismus at the end of the follow‐up period or as the incidence of strabismus during the follow‐up period. Strabismus was commonly identified by one of the following methods: unilateral cover test, or the Hirschberg test (with or without spectacle correction), in conjunction with the 4‐D prism test for suppression or microtropia. We considered other clinically meaningful treatment periods as reported by included studies in this review.
We considered manifest strabismus as a dichotomous variable and did not attempt to stratify outcomes according to final deviation or visual acuity (VA), or whether participants wore spectacles at the time of final assessment.
Secondary outcomes
Amblyopia, defined as a difference of three lines of VA between eyes.
Stereoacuity as defined by the study using the appropriate criteria for the test chosen (i.e. Randot Stereoacuity Test or a similar measure). These were also measured after a minimum treatment period of three years. When reported from studies, we also considered how outcome results (such as strabismus and VA) were affected when accounting for compliance with spectacle correction.
Change in refractive error, as a measurement of the interference of emmetropization, defined as the expected change anticipated to achieve emmetropization, given the level of refractive error at baseline, both continuously and as the proportion of children who did not achieve a refractive error in the emmetropic range.
Quality of life.
Proportion of participants with inadequate VA (worse than 20/30). (This was a post‐hoc decision as an intermediate assessment of the natural development of vision as only one included trial evaluated amblyopia and stereoacuity.)
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for randomized controlled trials and controlled clinical trials. There were no restrictions to language or year of publication.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 12) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 4 December 2018; Appendix 1).
MEDLINE Ovid (1946 to 4 December 2018; Appendix 2).
Embase.com (1947 to 4 December 2018; Appendix 3).
PubMed (1948 to 4 December 2018; Appendix 4).
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 4 December 2018; Appendix 5).
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (last searched 3 April 2014; Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 4 December 2018; Appendix 7).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 4 December 2018; Appendix 8).
Searching other resources
We searched the reference lists of reports from trials we identified to look for additional trials. We did not conduct manual searches of conference proceedings or abstracts specifically for this review. In the first version of the review, we also used the Science Citation Index‐Expanded database (September 2013) to identify additional studies that may have cited trials that we included in this review.
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts resulting from the literature searches, according to the inclusion criteria. We classified the records as 'definitely relevant', 'possibly relevant', or 'definitely not relevant'. After adjudication, we retrieved the full‐text reports for records classified as 'definitely relevant' or 'possibly relevant' by both review authors and then assessed the studies for inclusion, labeling each study as 'include' or 'exclude'. We did not need to contact trial authors of studies for further clarification as all studies could be included or excluded after examining the full‐text reports. We excluded studies labeled as 'exclude' by both review authors after the review of full‐texts and documented the reasons for exclusion in the Characteristics of excluded studies table. We resolved any disagreements through discussion at all stages of the study selection process.
Data extraction and management
Two review authors independently extracted data using a form based on templates developed and piloted by the Cochrane Eyes and Vision. One review author entered data into Review Manager 5 (Review Manager 2014), and a second review author verified the data entered. We resolved discrepancies in data extraction and data entry by discussion.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for sources of bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and resolved disagreements through discussion. We evaluated the studies for the following criteria: sequence generation and allocation concealment (selection bias), masking of participants/personnel (performance bias), masking of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. We reported our judgment for each criterion as 'low risk', 'high risk', or 'unclear risk' of bias. We contacted study investigators for the present review. In case of failure to communicate with the primary investigators, or if there is no response within four weeks, we will assess the risk of bias on the basis of the available information and will update it as more information becomes available.
We reported adequacy of sequence generation and allocation concealment. Methods of sequence generation that we considered to be at low risk of bias included use of random number tables, computer‐generated randomization, and coin tosses. We considered any method of allocation concealment that provided reasonable confidence that the investigators concealed the allocation sequence from participating physicians and participants (children and parents) at low risk of bias (such as centralized randomization and use of sequential opaque envelopes).
We assessed masking (blinding) of participants and personnel in the included studies. Although masking is not possible when spectacles are compared with no spectacles, masking is possible if the comparison of spectacles was with sham spectacles or partial spectacle correction.
We noted masking (blinding) of outcome assessors by study outcome or group of outcomes (e.g. primary and secondary outcomes) in the included studies.
For incomplete outcome data, we examined the proportion of participants lost to follow‐up, reasons for loss to follow‐up, and methods of analysis. We assessed whether follow‐up for treatment and control arms were similar and whether there were missing data for outcomes of interest. We considered studies at low risk of bias when there were no missing data or when missing data were correctly imputed and when the reasons for missing data were adequately addressed. We plan to note the method of data imputation if reported in any future included studies.
We assessed risk of bias for selective outcome reporting. We considered a study at low risk of bias when trial authors reported all prespecified outcomes of interest in the published report in the same manner as described in the study protocol. We considered the risk of bias to be unclear whenever the protocol was not available. Whenever the published report described different outcomes in the protocol and published report, or when the protocol was not available, different outcomes were reported in the Methods and Results section, we considered the trial as having high risk of bias.
We examined included studies for other sources of bias and considered studies at low risk of bias when there was no evidence of research misconduct and no potential for bias based on study methodology.
Measures of treatment effect
We calculated a summary risk ratio (RR) with corresponding 95% confidence interval (CI) for dichotomous outcomes, including the primary outcome of the presence or absence of strabismus at three years and secondary outcomes of amblyopia, stereoacuity, the presence or absence of emmetropization, and VA above a clinically meaningful cut‐point (i.e. 20/30 or worse). For continuous outcomes (e.g. change in refractive error to determine if emmetropization had occurred), we calculated mean differences (MD) with corresponding 95% CIs in changes from baseline when reported by included studies as well as the MD at a time point. For continuous outcomes, reported for spectacle compliant, spectacle non‐compliant, and control groups separately, we averaged the means and standard deviations (SD) to calculate effect estimates for the as‐randomized analyses (spectacle compliant and non‐compliant versus controls).
Unit of analysis issues
The unit of analysis was the child, as strabismus is a malfunction of the binocular system. Cross‐over trials are not included in this review because the status of the condition under study may change following an intervention, making a second intervention meaningless.
Dealing with missing data
We did not contact primary authors of included studies because no relevant data were missing. If any data were missing, such as SDs and intention‐to‐treat data, we would have contacted primary authors. If data were missing, we would not have imputed them, but analyzed them as available.
Assessment of heterogeneity
We assessed clinical and methodologic heterogeneity by examining variations in participant characteristics, inclusion/exclusion criteria, and methods of assessment of primary and secondary outcomes. We calculated the I2 statistic (%) to determine the percentage of variation in effect estimates due to heterogeneity and not chance; a value above 50% suggested substantial statistical heterogeneity. We also examined the result of the Chi2 test for heterogeneity and the degree of overlap in CIs of included studies. Poor overlap also may suggest heterogeneity.
Assessment of reporting biases
The small number of studies included in each meta‐analysis prevented a meaningful interpretation of funnel plots. If studies are added to the meta‐analyses in the future updates, we will examine funnel plots to assess small‐study effects, which could be due to reporting biases. We will use this method in conjunction with study characteristics or other factors that may contribute to asymmetry of the funnel plot.
Data synthesis
In the protocol of this review, we stated that we would not combine results in meta‐analysis when the I2 statistic suggested substantial statistical heterogeneity (i.e. I2 greater than 50%) (Jones 2009). However, because we considered the included trials were similar clinically and methodologically, we combined the results in meta‐analyses for strabismus and VA outcomes. We used a random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
We further evaluated heterogeneity through subgroup analyses of children with high levels of hyperopia (+3.50 D or greater) at baseline. We may include additional subgroup analyses with strictly hyperopia versus hyperopia and additional risk factors as we include new studies in review updates.
Sensitivity analysis
We conducted analyses based on the available‐case data for participants as randomized (assigned treatment group). We will consider the impact of excluding industry‐funded studies and unpublished studies if we include such studies in review updates.
Summary of findings and assessment of the certainty of the evidence
We summarized the findings of the review based on the guidelines in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b). We judged the confidence we had in the evidence for each outcome using the GRADE Working Group approach. Using this approach, we classified the certainty of evidence for each outcome of this review as 'high', 'moderate', 'low', or 'very low' and documented reasons for our judgments.
Results
Description of studies
We presented studies included in this review in the Characteristics of included studies table. We described excluded studies in the Characteristics of excluded studies table.
Results of the search
The 2014 version of this review identified 401 records through electrical searches (Jones‐Jordan 2014). Of 14 records that were reviewed in full‐text, we excluded five with reasons, and included nine records of four trials (Atkinson 1996; Hyperopia Treatment Study 1; Ingram 1985; Ingram 1990). An updated search in December 2018 resulted in 89 additional records after removal of duplicates. After assessing titles and abstracts for eligibility, five records were identified for full‐text screening. Two records of one trial were the same study as previously included as a protocol (Hyperopia Treatment Study 1), and three records of two trials were excluded with reason (NCT00574717; Pediatric Eye Disease Investigator Group 2016; Characteristics of excluded studies table). In summary, the updated search yielded results from one study that had been identified as a protocol in the last version of this review (Figure 1). This updated review contains four included studies.
1.
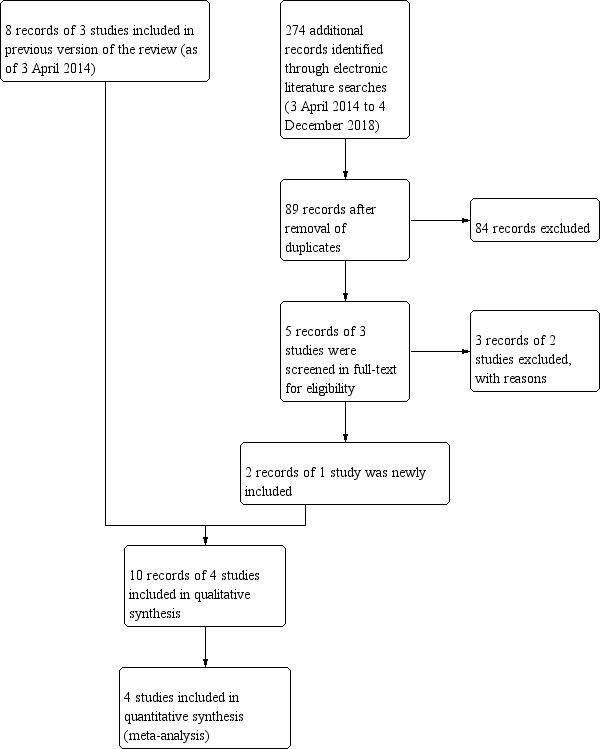
Study flow diagram as of 4 December 2018.
Included studies
Participant selection
Three included trials enrolled children beginning at six and up to and including 12 months of age (Atkinson 1996; Ingram 1985; Ingram 1990), with the remaining trial including children from 12 months up to, but not including, 36 months of age (Hyperopia Treatment Study 1). There were 985 children enrolled across all four trials. To account for the broad inclusion criteria with respect to minimum eligible hyperopia levels, which ranged from +2.00 D in both meridians (Ingram 1985), +4.00 D or more in any meridian (Atkinson 1996; Ingram 1990), to +3.00 D to +6.00 D spherical equivalent (Hyperopia Treatment Study 1), we conducted prespecified subgroup analyses in which we analyzed treatment outcomes for children with hyperopia less than +3.50 D and hyperopia +3.50 D or more separately. This analysis made it possible to evaluate treatment effects in children within a similar range of hyperopia at baseline.
Interventions
All trials included in this review assigned children to receive spectacle correction or no spectacle correction. Table 2 provides a description of the parameters used for prescribing spectacle correction that differed between studies. Trial investigators used a consistent prescription of 2 D less than the participant's current refraction in two trials (Ingram 1985; Ingram 1990). In another trial, spectacle correction was influenced to a certain degree by the amount of hyperopia (Atkinson 1996), and in the remaining trial, the correction was considered partial plus, a correction by 1.0 D plus correction for astigmatism. Three studies were similar in their follow‐up protocols (every three to six months), as well as referrals for strabismus treatment should an incident case have appeared. In these trials, investigators modified spectacle prescriptions as necessary in response to refractive error changes. The remaining trial scheduled follow‐up visits at six‐month intervals at which they were assessed for deterioration (Hyperopia Treatment Study 1). Deterioration was defined as presence of manifest strabismus, distance VA below age normal values, loss of 2 or more logMAR lines of vision if VA was 20/25 or worse in the better‐seeing eye or 3 or more logMAR lines if VA was 20/20 or better in the better seeing eye, stereoacuity at near below age normal values, or non‐protocol treatment received in the absence of meeting any other deterioration criteria. If esotropia was present, then spectacles were prescribed. Spectacles were not undercorrected for hyperopia by more than 1.0 D, had full correction for anisometropia, and correction for astigmatism within 0.5 D of full correction. Additional treatment was allowed at the investigator discretion.
1. Spectacle prescription by study.
| Study | Spectacle treatment method |
| Atkinson 1996 | Sphere: 1.00 D less than the least hyperopic meridian with no prescription given for < 1.50 D Cylinder: < 2 years old – half of the astigmatic error over 2.50 D; 2–3.5 years – half of the astigmatic error; > 3.5 years – full astigmatic error |
| Ingram 1985 | Each meridian minus 2.00 D |
| Ingram 1990 | Each meridian minus 2.00 D |
| Hyperopia Treatment Study 1 | Partial plus: glasses with sphere cut symmetrically by 1.0 D from cycloplegic refraction and full astigmatism |
D: diopter.
Outcomes
Three trials reported using the cover test to assess the presence of strabismus (assumed to be unilateral cover test) (Atkinson 1996; Hyperopia Treatment Study 1; Ingram 1990), with one trial also using the prism and alternate cover test (Hyperopia Treatment Study 1). Children were between three and four years of age at the end of three of these trials. We considered that the methods used to measure strabismus were sufficiently consistent across the included trials to allow quantitative summary meta‐analyses of these outcomes. One trial also assessed the proportion of children with strabismus at the end of three years' follow‐up (Hyperopia Treatment Study 1), so we conducted a separate analysis for strabismus for this trial.
Only one trial measured amblyopia (Hyperopia Treatment Study 1). This trial also assessed stereo acuity using the Randot Preschool Stereoacuity Test.
Three trials evaluated emmetropization (Atkinson 1996; Hyperopia Treatment Study 1; Ingram 1990). The trials used different methods to determine whether the child's vision had emmetropized, which may not necessarily have measured the actual developmental changes in the eye, and effect estimates may not have been comparable across trials. One trial recorded the mean refractive error at baseline and follow‐up in both "fixing eyes" and "non‐fixing eyes", and set a cut‐point of greater than +3.5 D in any meridian to determine whether emmetropization had not occurred (Ingram 1990). Another trial analyzed the change in the most hyperopic meridian, which was not always the same at each follow‐up examination (Atkinson 1996). The last trial determined emmetropization in two ways (Hyperopia Treatment Study 1). They compared the proportion of children who did not achieve a refractive error in the emmetropic range after three years, and the difference in spherical equivalent refractive error in both the more and less hyperopic eye between baseline and three years' follow‐up.
One trial assessed VA using Cambridge Crowding Cards with failure defined as worse than 20/40 (i.e. 6/12) (Atkinson 1996). Two trials used the Sheridan Gardiner test (Atkinson 1996; Ingram 1990), one trial used the Snellen test (Ingram 1990), and one trial used the Amblyopia Treatment Study single‐surround HOTV VA testing protocol (Hyperopia Treatment Study 1). All trials assessed VA at the last study visit; this visit was variable in three trials (Atkinson 1996; Ingram 1985; Ingram 1990), and after three years of follow‐up in the fourth (Hyperopia Treatment Study 1), so we included all trials in a meta‐analysis.
Outcome examiners in two trials determined compliance with treatment (Ingram 1985; Ingram 1990). Investigators of one trial considered children who wore the prescribed spectacles for greater than 50% of waking hours as compliant based on parent interviews and questionnaires (Atkinson 1996), while those in the remaining trial asked parents to report on children's use of spectacles (Hyperopia Treatment Study 1).
None of the trials measured quality of life in these children or their parents.
Funding sources
One trial was funded by regional sources, the Medical Research Council of Great Britain and a grant from the East Anglia Regional Health Authority (Atkinson 1996), and one by the National Eye Institute, National Institutes of Health (Hyperopia Treatment Study 1). Ingram 1985 and Ingram 1990 did not report funding sources.
Excluded studies
We reviewed and excluded two studies at this update totaling seven with previously excluded five studies (Characteristics of excluded studies table). One study did not investigate the use of spectacle correction as an intervention (Althaus 1994), and one study included older participants (Pediatric Eye Disease Investigator Group 2016). The other five studies were not randomized or quasi‐randomized trials (Anker 2004; MacEwen 2008; NCT00574717; Nischal 2008; Roth 1989).
Risk of bias in included studies
Figure 2 presents summary information on the risk of bias for the trials included in this review.
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
We judged Ingram 1990 at low risk of bias for random sequence generation because the study investigators used a "random number table" to generate the random sequence. We also judged the Hyperopia Treatment Study 1 at low risk of bias for random sequence generation because the Jaeb Center prepared the stratified blocked random sequence by computer. We judged Atkinson 1996 at high risk of bias because some of the infants were alternately assigned to the treated or untreated group. Ingram 1985 did not specify the method for randomization, therefore, we judged the risk to be unclear.
None of the four trials provided sufficient details describing how participants were allocated to treatment groups and we judged them to have an unclear risk of bias for allocation concealment.
Masking of participants and personnel (performance bias)
All studies compared spectacles versus no spectacles. Because no sham or control spectacles were used, we judged performance bias as high in all the included studies.
Masking of outcome assessors (detection bias)
Three trials did not address masking of people who assessed VA and strabismus and were thus at unclear risk of bias. Examiners were masked in the Hyperopia Treatment Study 1 trial and so this trial was at low risk of detection bias.
Incomplete outcome data
We judged three of the included trials at high risk of bias for incomplete outcome data. Atkinson 1996 did not adequately outline the number of participants from the baseline visit onward and the numbers reported in the results were inconsistent among analyses; thus, the data available did not allow us to determine the number of participants who dropped out. Ingram 1990 did not clearly identify participants allocated to the treatment and no treatment groups, and was not specific about the participants dropping out. Ingram 1985 did not specify how many participants were allocated to each group or the number of participants lost to follow‐up according to treatment group. The study authors reported 149 eligible children did not enter the study but did not explain why. In Hyperopia Treatment Study 1, 24 children did not complete the three‐year primary outcome examination. However, the protocol prespecified that the primary outcome would be analyzed using modified intention to treat without adjustment for missing values. A secondary analysis was completed using multiple imputation to account for missing values. We judged this trial to have low risk of bias.
Selective reporting
We judged two trials at unclear risk of bias for selective reporting because we did not identify study protocols (Atkinson 1996; Ingram 1990). We judged Ingram 1985 at high risk of bias due to a change in the study outcome because of concerns regarding the ability to measure VA and amblyopia in 3.5 year olds correctly. We judged the Hyperopia Treatment Study 1 trial at low risk of bias because all outcomes that were reported in the protocol were reported in the publication.
Other potential sources of bias
We did not identify other potential sources of bias for the four trials.
Effects of interventions
See: Table 1
Strabismus
All four trials contributed data to conduct as‐randomized analyses for the incidence of strabismus over a period of three years. Three trials reported available data only, comprising 674 children and not accounting for 181 children (21%) with missing data (Atkinson 1996; Ingram 1985; Ingram 1990). The reason for missing data was mainly due to dropout or participants. The fourth trial contributed data from 130 children as determined using a Kaplan Meier survival analysis and these 130 children were included in the meta‐analysis (Hyperopia Treatment Study 1). This trial also reported the presence of strabismus at three years' follow‐up, including 106/130 children originally enrolled in the trial.
When we combined the four trials (804 children) that measured incidence of strabismus into a summary meta‐analysis based on as‐randomized data, spectacle correction led to a 35% reduction in the risk of developing strabismus up to three years of age compared with no correction (RR 0.65, 95% CI 0.41 to 1.02; I2 = 53%; Analysis 1.1). The RR for the subgroup of 615 children with hyperopia +3.50 D or greater (using a subset of children from Ingram 1985 and children from the remaining studies) was 0.70 (95% CI 0.44 to 1.14; I2 = 55%; Analysis 1.1). We have very low confidence in these results due to high risk of bias, imprecision, and inconsistency.
1.1. Analysis.

Comparison 1 Spectacle correction versus no spectacle correction, Outcome 1 Incidence of strabismus up to 3–4 years of age.
When looking at the presence of strabismus at the end of three years of follow‐up reported in Hyperopia Treatment Study 1, the proportion of children with manifest strabismus in the spectacle group (5/53) and in the control group (5/53) were identical. This finding agrees with the lack of evidence for an effect of spectacles on strabismus as reported looking at the incidence of strabismus in the previous three years (RR 1.00 95% CI 0.31 to 3.25; Analysis 1.2). We have very low confidence in this result due to risk of bias and imprecision.
1.2. Analysis.

Comparison 1 Spectacle correction versus no spectacle correction, Outcome 2 Proportion of children with manifest strabismus.
Amblyopia
One trial reported the presence of amblyopia (Hyperopia Treatment Study 1). Amblyopia was present at three‐year follow‐up for 7/53 children in the spectacle group and 9/53 children in the control group (RR 0.78 95% CI 0.31 to 1.93; Analysis 1.3). Because this result was from a single small trial, we have very low confidence in the findings (high risk of bias, imprecision).
1.3. Analysis.

Comparison 1 Spectacle correction versus no spectacle correction, Outcome 3 Proportion of participants with amblyopia.
Stereoacuity
One trial assessed stereoacuity in 106 children at the end of three years' follow‐up using the Randot Preschool Stereoacuity test (Hyperopia Treatment Study 1). Children whose stereoacuity was below age normal values were considered failures. There were six stereoacuity failures in 53 children in the spectacle correction group and 16 failures among the 53 children in the control group (RR 0.38, 95% CI 0.16 to 0.88; Analysis 1.4). We have very low confidence in this result because it was derived from a single study with high risk of bias and imprecision.
1.4. Analysis.

Comparison 1 Spectacle correction versus no spectacle correction, Outcome 4 Proportion of participants with inadequate stereoacuity.
Emmetropization
Three trials reported emmetropization (Atkinson 1996; Hyperopia Treatment Study 1; Ingram 1990). Given the variety of methods used to evaluate emmetropization, we could not conduct summary meta‐analyses of this outcome including all trials. Instead, we have provided a narrative summary of the results from three of the trials individually.
When dichotomized with refractive error +3.5 D or greater at follow‐up, indicating emmetropization had not occurred, one trial (287 children) showed a RR of 1.32 (95% CI 0.97 to 1.80) when comparing 60/144 children in the spectacle group with 45/143 children in the control group for which emmetropization had not occurred (Ingram 1990). When evaluating the mean change in refractive error from baseline to final follow‐up (mean of 3.2 years), there was an MD of 0.22 D (95% CI –0.20 to 0.64) in the "fixing eye" between 100 children in the spectacle group (mean change –1.12 D, SD 1.65) and 89 children in the control group (mean change –1.34 D, SD 1.30) among children without strabismus at the follow‐up visit. Among children with strabismus, the MD was –0.24 D (95% CI –0.96 to 0.48) between 47 children in the spectacle group (mean change –0.40 D, SD 2.13) and 53 children in the control group (mean change –0.16 D, SD 1.40) (Ingram 1990).
The second trial reported no difference between the spectacle group and control group after accounting for compliance with treatment (Atkinson 1996). At three years of age, the refractive error in the most hyperopic meridian was +3.4 D in the spectacle group and +3.1 D in the control groups. Using SDs inferred in the text describing the results for each group, the difference in the mean refractive error between 44 children in the spectacle group (mean +3.4 D, SD 1.4) and 37 children in the control group (mean +3.1 D, SD 1.5) was 0.3 D (95% CI –0.34 to 0.94). The trend was similar when we accounted for compliance with a refractive error of +3.3 D in the spectacle compliant group. The investigators reported a statistically non‐significant difference in refractive error at three years for both of the above comparisons.
The remaining trial reported emmetropization in two ways (Hyperopia Treatment Study 1). First, they reported it as the mean change in spherical equivalent from baseline to the three‐year follow‐up visit by the more or less hyperopic eye. The difference in spherical equivalent refractive error between groups was 0.15 (95% CI –0.51 to 0.84) for the more hyperopic eye and 0.22 (95% CI –0.43 to 0.86) in the less hyperopic eye. Second, they reported the proportion of children who did not achieve a refractive error in the emmetropic range at the three‐year follow‐up visit. Three of 53 children in the spectacle group and 4/53 in the control group failed to present with refraction in the emmetropic range at the three‐year follow‐up visit (RR 0.75, 95% CI 0.18 to 3.19; Analysis 1.5). The confidence in this finding is low due to risk of bias and imprecision.
1.5. Analysis.

Comparison 1 Spectacle correction versus no spectacle correction, Outcome 5 Proportion of participants who did not achieve a refractive error in the emmetropic range.
Quality of life
None of the studies reported quality of life.
Visual acuity
All trials reported VA at visits up to the age of three to four years or at the end of three years of follow‐up. Investigators of three trials assessed VA by considering vision worse than 20/30 (i.e. 6/9) to be inadequate (Atkinson 1996; Ingram 1985; Ingram 1990), while in the Hyperopia Treatment Study 1, VA worse than age‐adjusted values at the three‐year follow‐up visit was considered inadequate. Among these four studies (770 children), there was unclear evidence of an effect of spectacles on VA compared with no spectacles (RR 0.87, 95% CI 0.64 to 1.18; I2 = 0%; Analysis 1.6). If we exclude those children who had less than +3.50 D of hyperopia at baseline from Ingram 1985, the summary meta‐analysis (three trials, 581 children) revealed an RR of 0.84 (95% CI 0.59 to 1.20; I2 = 15%; Analysis 1.6). We judged this evidence as very low, being downgraded for high risk of bias and imprecision.
1.6. Analysis.
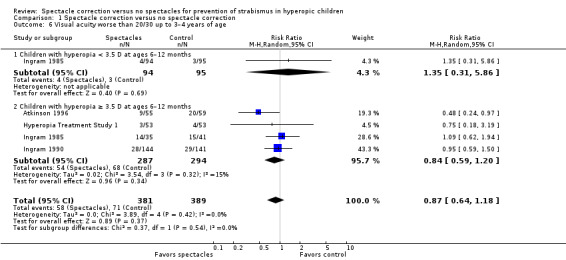
Comparison 1 Spectacle correction versus no spectacle correction, Outcome 6 Visual acuity worse than 20/30 up to 3–4 years of age.
Discussion
Summary of main results
We included four trials in this review from which 804 hyperopic children provided follow‐up data to determine the effects of spectacle correction for preventing strabismus. Three trials allocated infants between the ages of six and 12 months to a spectacle treatment group or an untreated group and evaluated the incidence of strabismus and assessed VA and emmetropization when the children were between three and four years of age. The remaining trial included 130 children from ages of 12 to less than 36 months and provided data on the incidence of strabismus and VA based on age normal values between the ages of four and five years. This trial also provided data on the presence of strabismus, amblyopia, spherical equivalent within emmetropic range, and failure to meet age‐based norms for stereoacuity in the same time frame as above. Based on our assessment of these trials, there is unclear evidence that spectacle correction is associated with a lower risk of developing strabismus or preventing inadequate VA in infants or children with hyperopia. There is some indication that spectacles provide a benefit in achieving stereoacuity, although this was reported in only a single study. Three studies considered the potential adverse effect of spectacle treatment, the inhibition of emmetropization. The results from one trial suggested that spectacles impede emmetropization (as defined by an increase in risk of remaining hyperopic) (Ingram 1990); a second trial reported no difference in the rate of refractive error change (Atkinson 1996); and a third trial reported no difference in the spherical equivalent that would indicate emmetropization at the end of follow‐up (at age four to five years) (Hyperopia Treatment Study 1). Although children who were allocated to the spectacle group were less likely to develop strabismus across the included studies, this may have been a chance finding, or due to bias. Due to high risks of bias and poor reporting of the majority of included trials, the true effect of spectacle correction for hyperopia on strabismus is still uncertain.
Overall completeness and applicability of evidence
Clinically, the four included studies were not substantially heterogeneous. For presence of strabismus, although there appeared to be some statistical heterogeneity across trials, the CIs of individual studies overlapped and the I2 value was around our prespecified threshold of 50% (I2 = 53%). Thus, we combined the findings in a meta‐analysis. For VA worse than 20/30, there was no statistical heterogeneity (I2 = 15%). We combined the findings in a meta‐analysis for presence of strabismus at three to four years of age and VA worse than 20/30. There was additional methodologic variation in the way investigators of trials evaluated emmetropization, preventing meta‐analysis of this outcome. In trials, the amount of refractive error change that occurred seemed to result in incomplete emmetropization (i.e. the resulting refractive error had not reached a 'normal' level) in some children.
It is important to consider children similar in risk and possible etiology because highly hyperopic children are thought to be less likely to emmetropize (Mutti 2009), and therefore are likely to benefit from treatment. In subgroup analyses, we considered only those children with at least +3.50 D hyperopia at baseline. The subgroup analyses did not show that wearing spectacles decreased the risk of developing strabismus or of poor VA in children who were highly hyperopic.
One potential issue when considering these trials is the possible impact the time of treatment initiation may have had. Ingram 1985 began with infants who were one year old, but in a subsequent study (Ingram 1990), treatment initiation was moved to six months of age because they suspected the sensitive period was earlier. Atkinson 1996 enrolled infants between six and eight months of age and the Hyperopia Treatment Study 1 enrolled children between one and two years of age. Therefore, comparing varying ages may be obscuring the possibility that a sensitive period may exist.
The trials used similar treatments (spectacles) and controls (untreated) and the amount of correction was reasonably consistent across all trials (Table 2). Both Ingram 1985 and Ingram 1990 used a +2.00 D decrease in the refractive correction for spectacle prescription as opposed to the +1.00 D decrease in refractive correction used by the other trial (Atkinson 1996), while the Hyperopia Treatment Study 1 provided partial plus hyperopic correction (i.e. sphere cut symmetrically by 1.00 D and full astigmatism correction).
In general, the studies did not consider the level of astigmatism. Current literature suggests that the amount of infant astigmatism seems to have little to no effect on emmetropization of spherical equivalent refractive error (Ehrlich 1997; Mutti 2004).
Assessing treatment compliance is difficult to ascertain given the potential for recall bias. Determining what constitutes compliance is also a problem. It is possible that there is a certain amount of time that spectacles should be worn to have a clinically meaningful effect. Each study used a different assessment of compliance. Ingram 1990 simply stated that "some children obviously wore the glasses consistently, but it was apparent that some wore them irregularly and others not at all" as their definition of compliance. It appears that Ingram 1985 used something similar to classify participants as compliant. Atkinson 1996 questioned the parent and classified a child as compliant whenever the parent indicated that the child wore the spectacles at least 50% of the time, while the Hyperopia Treatment Study 1 measured compliance by parent report of use of spectacles at least 75% of the time. One option for a future trial would be one that assesses treatment groups assigned to varying wearing times (e.g. four hours per day, eight hours per day, 12 hours per day) compared with a no correction group to allow for the determination of an effect of the amount of time correction was worn.
In terms of the outcomes, all trials used similar assessments at a similar time, so comparing them was reasonable; however, the data contributing to this review, given the potential attrition bias mentioned above, do not provide a reliable assessment of the effect of spectacle correction. Only one study report included any information regarding race, gender, and family history of amblyopia or strabismus (Hyperopia Treatment Study 1).
Quality of the evidence
We observed several potential biases in the design (no use of a proper randomization sequence and no masking of those who assessed treatment outcomes) and conduct (large proportion of participants lost to follow‐up as well as incomplete compliance with the intervention) in three of the included trials (Atkinson 1996; Ingram 1985; Ingram 1990), and biases associated with lack of masking and possible allocation concealment in the fourth trial (Hyperopia Treatment Study 1). Three of the four included trials had a high degree of uncertainty in the presence of potential biases due to insufficient descriptions of important methodologic design issues. One trial used alternate assignment for participants to treatment groups, so we judged the risk of bias as high. Two trials specified the methods of sequence generation, so we judged the risk as low and another trial did not specify the method for randomization. None of the three trials indicated how treatment assignments were allocated to ensure investigators and study participants did not have prior knowledge of their treatment assignment. We judged all trials at high risk of performance bias as they all compared spectacles versus no treatment. Only one trial specified masking of people assessing VA and strabismus (Hyperopia Treatment Study 1), but the risk of detection bias remained unclear in the other three trials. The Hyperopia Treatment Study 1 was also stopped early due to slow recruitment. We considered three of the four trials included in this review to be at a high risk of bias for incomplete outcome data (Figure 2). Due to the high loss to follow‐up in all trials and lack of details regarding missing participant data, the data were not complete and do not provide an accurate estimate of the overall treatment effect of spectacle correction for preventing strabismus (i.e. very low certainty of evidence) (Table 1). Added to the high risk of bias is the imprecision observed across all trials and meta‐analytic findings, further reducing the confidence we have in these results.
Potential biases in the review process
Two review authors independently reviewed titles, abstracts, and full‐text reports of potentially eligibly trials, and extracted data and assessed risk of bias for all trials included in this review, which should have minimized review bias. During the review process, we added a post‐hoc secondary outcome for VA. We are aware of no other potential biases in the review process.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other systematic reviews on this subject. One retrospective, observational study of hyperopic children identified in a preschool screening found no statistically significant difference between the percentage of children who developed amblyopia among children who received some type of spectacle correction and children who did not (21% with spectacle correction versus 38% with no spectacle correction); however, the study authors concluded the results were consistent with a benefit of spectacle correction on reducing amblyopia risk. However, only 16 children received no treatment so the sample size was insufficient to support inferences (Colburn 2010). Anker and coworkers in a follow‐up study to Atkinson's First Cambridge Study, reported on a non‐randomized study of spectacle correction in children with hyperopia of +4.0 D or greater. Correction did not reduce the occurrence of strabismus after three years, but did seem to improve VA over no treatment (Anker 2004). Current preferred practice guidelines show uncertainty with regard to when to prescribe spectacles for hyperopic children due to the lack of empirical data. The American Optometric Association Optometric Clinical Practice Guidelines suggest that in the absence of amblyopia or strabismus, practitioners begin treating hyperopia in children from birth to 10 years of age at levels of hyperopia of +5.0 D or greater, with consideration made for other coexisting factors that are relevant in the clinician's judgment. They recommend a partial prescription with monitoring (AOA 2008). The American Academy of Ophthalmology Pediatric Ophthalmology/Strabismus Panel has issued age‐tiered guidelines for prescribing correction for hyperopia. In children under one year of age with no manifest deviation, they recommend correction beginning at +6.0 D of hyperopia. For children ages one to two years, they recommend prescribing spectacles for hyperopia of +5.0 D or greater, and for children ages two to three years of age, they recommend refractive correction for +4.50 D and greater of hyperopia (AAO 2012).
Leat presented several recommendations for prescribing spectacle correction based upon the literature, mainly the studies cited in this review, evaluating potential treatment effects in hyperopic children (Leat 2011). Based on the expected distribution of refractive error at various ages, the trial author recommended partial correction when the refractive error fell outside the normal range of the age group. After one year of age, a partial prescription was suggested starting at +3.50 D of hyperopia. Ciner recommended prescribing at greater than +2.0 D for children less than five years of age (Ciner 1990), while and Marsh‐Tootle and Frazier suggested a prescription for those greater than +3.0 D up to the age of three years (Marsh‐Tootle 2006).
Based upon the guidelines, recommendations, and the results of this review, there is uncertainty in the benefit of spectacle correction and the preferred amount of correction, which is another area that warrants investigation to be able to assess the efficacy of spectacle treatment for the prevention of strabismus. There is also some variation in the level of hyperopia at which to prescribe spectacle correction, although the guidelines agree that there is a level that is 'high' hyperopia beyond which refractive error is unlikely to resolve on its own.
Authors' conclusions
Implications for practice.
The limited number of trials, all with potentially significant risk of bias affecting the treatment effect estimates, makes it impossible to draw firm conclusions regarding the most appropriate course of action with respect to spectacle correction for preventing the development of strabismus in hyperopic infants. Although children who were allocated to the spectacle group were less likely to develop strabismus, this may have been a chance finding or due to bias. Because of the high/unclear risk of bias of included trials, the true effect of spectacle correction for hyperopia on strabismus is still uncertain. The suggestion of positive effects on later visual acuity remains inconclusive until further clinical trial results are available. The information is, at best, equivocal from the trials that have been reported in the literature.
Implications for research.
More clinical trials with attention to improved study design are needed to provide a better foundation for determining the effect of spectacle correction to prevent strabismus in hyperopic children. Properly randomizing participants to either a treatment or control group and careful concealment of the treatment allocation has been lacking in research completed to date. In addition, participants need to be followed adequately and every attempt must be made to schedule final outcome visits for these children. Highly hyperopic children make up a very small percentage of the infant population, so minimizing loss to follow‐up in these trials is necessary to achieve adequate power. Estimating sample size with adjustment for attrition is also recommended. An adequately powered clinical trial is likely to require multiple sites to address the issue of incidence of strabismus with and without spectacle correction for hyperopia. Although the Hyperopia Treatment Study 1 has provided more information on this topic, additional trials are required with similar entry criteria and interventions to provide sufficient evidence to answer these research questions.
Future research should carefully consider the issue of compliance to spectacle wear in these infants. Because of their age, education and encouragement of the parents is key to increasing the percentage who comply with the treatment. Alternatives to make it easier to keep the babies in their correction (glasses straps, contact lenses, etc.) should make it easier for parents to comply as well. As the effectiveness of treatment depends on actually receiving the treatment, this area is worth considerable effort prior to launching a trial.
What's new
| Date | Event | Description |
|---|---|---|
| 12 June 2019 | New search has been performed | Issue 4 2020: An updated search yielded results from a study previously identified as a protocol. Three new outcomes were added to the review, including the proportion of children with manifest strabismus, amblyopia, or inadequate stereoacuity after three years of follow‐up, as well as additional information to previous outcomes. The overall finding that whether there is a benefit in using spectacles in hyperopic children is still unclear. |
| 12 June 2019 | New citation required and conclusions have changed | Issue 4 2020: One new study added: Hyperopia Treatment Study 1. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 8, 2014
| Date | Event | Description |
|---|---|---|
| 19 August 2014 | Amended | Minor amendments (added GRADE ratings to abstract). |
Acknowledgements
We would like to thank Iris Gordon, Information Specialist, Cochrane Eyes and Vision (CEV), for devising and conducting the electronic searches. We also thank the CEV editorial team for their advice and assistance during the review process. We thank the following peer reviewers for their comments: Sandy Finestone (Association of Cancer Patient Educators), Elise Ciner (Pennsylvania College of Optometry), and Scott Lambert (Stanford University Medical Center).
The 2020 review update was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Strabismus] explode all trees #2 strabism* or squint* #3 MeSH descriptor: [Esotropia] explode all trees #4 esotrop* #5 MeSH descriptor: [Exotropia] explode all trees #6 exotrop* #7 hypertrop* #8 hypotrop* #9 cyclotrop* #10 heterophor* #11 esophor* #12 exophor* #13 hyperphor* #14 hypophor* #15 cyclophor* #16 phoria* #17 tropia* #18 esodeviation* #19 (cross NEXT eye*) OR crosseye* #20 exodeviation* #21 {OR #1‐#20} #22 MeSH descriptor: [Hyperopia] explode all trees #23 hyperop* or hypermetrop* #24 far next sight* #25 long next sight* #26 farsight* #27 longsight* #28 {OR #22‐#27} #29 MeSH descriptor: [Eyeglasses] explode all trees #30 eyeglass* OR (eye NEXT glass*) OR spectacle* or glasses #31 (#29 or #30) #32 (#21 and #28 and #31)
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp strabismus/ 13. (strabism* or squint*).tw. 14. exp esotropia/ 15. esotrop*.tw. 16. exp exotropia/ 17. exotrop*.tw. 18. hypertrop*.tw. 19. hypotrop*.tw. 20. cyclotrop*.tw. 21. heterophor*.tw. 22. exp esophoria/ 23. esophor*.tw. 24. exp exophoria/ 25. exophor*.tw. 26. hyperphor*.tw. 27. hypophor*.tw. 28. cyclophor*.tw. 29. phoria*.tw. 30. tropia*.tw. 31. esodeviation*.tw. 32. (cross eye* or crosseye*).tw. 33. exodeviation*.tw. 34. or/12‐33 35. exp hyperopia/ 36. (hyperop* or hypermetrop*).tw. 37. (far adj1 sight*).tw. 38. (long adj1 sight*).tw. 39. farsight*.tw. 40. longsight*.tw. 41. or/35‐40 42. exp eyeglasses/ 43. (eyeglass* or eye glass* or spectacle* or glasses).tw. 44. or/42‐43 45. 34 and 41 and 44 46. 11 and 45
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'strabismus'/exp #34 (strabism* OR squint*):ab,ti,kw #35 'convergent strabismus'/exp #36 esotrop*:ab,ti,kw #37 'divergent strabismus'/exp #38 exotrop*:ab,ti,kw #39 hypertrop*:ab,ti,kw #40 hypotrop*:ab,ti,kw #41 cyclotrop*:ab,ti,kw #42 heterophor*:ab,ti,kw #43 esophor*:ab,ti,kw #44 exophor*:ab,ti,kw #45 hyperphor*:ab,ti,kw #46 hypophor*:ab,ti,kw #47 cyclophor*:ab,ti,kw #48 phoria*:ab,ti,kw #49 tropia*:ab,ti,kw #50 esodeviation*:ab,ti,kw #51 ((cross NEXT eye*) OR crosseye*):ab,ti,kw #52 exodeviation*:ab,ti,kw #53 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 #54 'hypermetropia'/exp #55 (hyperop* OR hypermetrop*):ab,ti,kw #56 (far NEAR/1 sight*):ab,ti,kw #57 (long NEAR/1 sight*):ab,ti,kw #58 farsight*:ab,ti,kw #59 longsight*:ab,ti,kw #60 #54 OR #55 OR #56 OR #57 OR #58 OR #59 #61 'spectacles'/exp #62 (eyeglass* OR (eye NEXT glass*) OR spectacle* or glasses):ab,ti,kw #63 #61 OR #62 #64 #53 AND #60 AND #63 #65 #32 AND #64
Appendix 4. PubMed search strategy
1. ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) 2. (strabism*[tw] OR squint*[tw]) NOT Medline[sb] 3. esotrop*[tw] NOT Medline[sb] 4. exotrop*[tw] NOT Medline[sb] 5. hypertrop*[tw] NOT Medline[sb] 6. hypotrop*[tw] NOT Medline[sb] 7. cyclotrop*[tw] NOT Medline[sb] 8. heterophor*[tw] NOT Medline[sb] 9. esophor*[tw] NOT Medline[sb] 10. exophor*[tw] NOT Medline[sb] 11. hyperphor*[tw] NOT Medline[sb] 12. hypophor*[tw] NOT Medline[sb] 13. cyclophor*[tw] NOT Medline[sb] 14. phoria*[tw] NOT Medline[sb] 15. tropia*[tw] NOT Medline[sb] 16. esodeviation*[tw] NOT Medline[sb] 17. (cross eye*[tw] OR crosseye*[tw]) NOT Medline[sb] 18. exodeviation*[tw] NOT Medline[sb] 19. #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 20. (hyperop*[tw] OR hypermetrop*[tw]) NOT Medline[sb] 21. (far[tw] AND sight*[tw]) NOT Medline[sb] 22. (long[tw] AND sight*[tw]) NOT Medline[sb] 23. farsight*[tw] NOT Medline[sb] 24. longsight*[tw] NOT Medline[sb] 25. #20 OR #21 OR #22 OR #23 OR #24 26. (eyeglass*[tw] OR eye glass*[tw] OR spectacle*[tw] OR glasses[tw]) NOT Medline[sb] 27. #19 AND #25 AND #26 28. #1 AND #27
Appendix 5. LILACS search strategy
(Strabism$ OR Estrabism$ OR squint$ OR MH:C10.292.562.887$ OR MH:C11.590.810$ OR esotrop$ OR exotrop$ OR hypertrop$ OR hypotrop$ OR cyclotrop$ OR heterophor$ OR esophor$ OR exophor$ OR hyperphor$ OR hypophor$ OR cyclophor$ OR phoria$ OR tropia$ OR esodeviation$ OR (cross eye$) OR crosseye$ OR Exodeviation$) AND (Hyperop$ OR Hiperop$ OR MH: C11.744.479$ OR (far sight$) OR (long sight$) OR farsight$ OR longsight$) AND (eyeglass$ OR Anteojos OR Óculos OR (eye glass$) OR spectacle$ OR glasses)
Appendix 6. metaRegister of Controlled Trials search strategy
(Strabismus OR Esotropia OR Exotropia) AND (Hyperopia)
Appendix 7. ClinicalTrials.gov search strategy
(Strabismus OR Squint OR Esotropia OR Exotropia OR Hypertropia OR Hypotropia OR Cyclotropia OR Heterophoria OR Esophoria OR Exophoria OR Hyperphoria OR Hypophoria OR Cyclophoria OR Phoria OR Tropia OR esodeviation OR crosseye OR exodeviation) AND (Hyperopia OR Hypermetropia OR farsighted OR longsighted)
Appendix 8. ICTRP search strategy
Strabismus AND Hyperopia OR Squint AND Hyperopia OR Esotropia AND Hyperopia OR Exotropia AND Hyperopia OR Hypertropia AND Hyperopia OR Hypotropia AND Hyperopia OR Cyclotropia AND Hyperopia OR Heterophoria AND Hyperopia OR Esophoria AND Hyperopia OR Exophoria AND Hyperopia OR Hyperphoria AND Hyperopia OR Hypophoria AND Hyperopia OR Cyclophoria AND Hyperopia OR Phoria AND Hyperopia OR Tropia AND Hyperopia OR esodeviation AND Hyperopia OR crosseye AND Hyperopia OR exodeviation AND Hyperopia OR Strabismus AND Hypermetropia OR Squint AND Hypermetropia OR Esotropia AND Hypermetropia OR Exotropia AND Hypermetropia OR Hypertropia AND Hypermetropia OR Hypotropia AND Hypermetropia OR Cyclotropia AND Hypermetropia OR Heterophoria AND Hypermetropia OR Esophoria AND Hypermetropia OR Exophoria AND Hypermetropia OR Hyperphoria AND Hypermetropia OR Hypophoria AND Hypermetropia OR Cyclophoria AND Hypermetropia OR Phoria AND Hypermetropia OR Tropia AND Hypermetropia OR esodeviation AND Hypermetropia OR crosseye AND Hypermetropia OR exodeviation AND Hypermetropia Strabismus AND Hypermetropia OR Squint AND Hypermetropia OR Esotropia AND Hypermetropia OR Exotropia AND Hypermetropia OR Hypertropia AND Hypermetropia OR Hypotropia AND Hypermetropia OR Cyclotropia AND Hypermetropia OR Heterophoria AND Hypermetropia OR Esophoria AND Hypermetropia OR Exophoria AND Hypermetropia OR Hyperphoria AND Hypermetropia OR Hypophoria AND Hypermetropia OR Cyclophoria AND Hypermetropia OR Phoria AND Hypermetropia OR Tropia AND Hypermetropia OR esodeviation AND Hypermetropia OR crosseye AND Hypermetropia OR exodeviation AND Hypermetropia OR Strabismus AND Farsighted OR Squint AND Farsighted OR Esotropia AND Farsighted OR Exotropia AND Farsighted OR Hypertropia AND Farsighted OR Hypotropia AND Farsighted OR Cyclotropia AND Farsighted OR Heterophoria AND Farsighted OR Esophoria AND Farsighted OR Exophoria AND Farsighted OR Hyperphoria AND Farsighted OR Hypophoria AND Farsighted OR Cyclophoria AND Farsighted OR Phoria AND Farsighted OR Tropia AND Farsighted OR esodeviation AND Farsighted OR crosseye AND Farsighted OR exodeviation AND Farsighted
Strabismus AND Longsighted OR Squint AND Longsighted OR Esotropia AND Longsighted OR Exotropia AND Longsighted OR Hypertropia AND Longsighted OR Hypotropia AND Longsighted OR Cyclotropia AND Longsighted OR Heterophoria AND Longsighted OR Esophoria AND Longsighted OR Exophoria AND Longsighted OR Hyperphoria AND Longsighted OR Hypophoria AND Longsighted OR Cyclophoria AND Longsighted OR Phoria AND Longsighted OR Tropia AND Longsighted OR esodeviation AND Longsighted OR crosseye AND Longsighted OR exodeviation AND Longsighted
Data and analyses
Comparison 1. Spectacle correction versus no spectacle correction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of strabismus up to 3–4 years of age | 4 | 804 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.02] |
| 1.1 Children with hyperopia < 3.5 D at ages 6–12 months | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.05] |
| 1.2 Children with hyperopia ≥ 3.5 D at ages 6–12 months | 4 | 615 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.44, 1.14] |
| 2 Proportion of children with manifest strabismus | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Proportion of participants with amblyopia | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Proportion of participants with inadequate stereoacuity | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Proportion of participants who did not achieve a refractive error in the emmetropic range | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Visual acuity worse than 20/30 up to 3–4 years of age | 4 | 770 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.18] |
| 6.1 Children with hyperopia < 3.5 D at ages 6–12 months | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.31, 5.86] |
| 6.2 Children with hyperopia ≥ 3.5 D at ages 6–12 months | 4 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Atkinson 1996.
| Methods | Initially 3166 children were screened, which was approximately 74% of the total eligible infant population. Of those screened, 9.3% were identified for additional follow‐up Number randomized (total and per group): 177 children; number in each group not reported Number analyzed (total and per group):
Infants provided outcome data that were analyzed 2 different ways:
Losses to follow‐up: number lost to follow‐up not reported, no sufficient baseline information to calculate number lost to follow‐up from the number with follow‐up data available Unit of randomization: individual Unit of analysis: strabismus: individual child; VA: individual child (pass/fail) |
|
| Participants |
Country: UK Age: children initially were screened at 6–8 months of age; eligible children identified from screening were followed up within 9 months of age Gender: not reported Inclusion/exclusion criteria: infants attending well‐baby visits ages 6–8 months were referred for follow‐up if the photorefraction revealed marked hyperopia, myopia, or anisometropia or if significant strabismus or any ocular pathology was evident Equivalence of baseline characteristics: not reported Diagnoses in participants: screening procedure included cycloplegia with 1% cyclopentolate, isotropic photorefraction; infants identified to have hyperopia (> +3.5 D), myopia (≥ –2.0 D), anisometropia (1.5 D difference between corresponding axes), strabismus, or any other ocular pathology from photorefraction were followed up with retinoscopy |
|
| Interventions |
Intervention 1: spectacle correction:
Prescriptions were checked frequently, at which time the child's wearing of the spectacles was monitored by close, sympathetic questioning of the parents. Intervention 2: no spectacle correction Length of follow‐up:
|
|
| Outcomes | Incidence of strabismus was diagnosed with the cover test. Single‐letter acuity was measured with the Sheridan‐Gardiner test at 3 m and Cambridge Crowding Cards with spectacle correction worn as appropriate. Emmetropization was evaluated by measuring the refractive error in the most hyperopic meridian Intervals at which outcome assessed: repeated follow‐up visits at 4‐ to 6‐month intervals Adverse effects: not reported |
|
| Notes |
Type of study: published Funding: Medical Research Council of Great Britain, and a grant from the East Anglia Regional Health Authority Declaration of interest: not reported Study period: children born in 1981–1983 were screened and followed up until 7 years of age Subgroup analyses: according to compliance with wearing spectacles |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Spectacle correction was tested as a preventive intervention by randomly assigning infants in the hyperopic group to 'spectacles' and 'non‐spectacles' groups". Quote: "Infants identified as having significant hyperopia but with no meridian greater than +6D were alternately assigned to the treated or untreated group". |
| Allocation concealment (selection bias) | Unclear risk | Assignment by alternation allows next assignment to be known. |
| Masking of participants and personnel | High risk | Participants and personnel were not masked, which could have been attempted with use of sham spectacles. |
| Masking of outcome assessors (detection bias) | Unclear risk | Masking of outcome assessors was not specified. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study did not report baseline numbers for each treatment group. The numbers reported in the results were inconsistent between analyses, which did not allow us to determine the number of participants who dropped out. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. |
| Other bias | Low risk | No other sources of bias identified. |
Hyperopia Treatment Study 1.
| Methods |
Number randomized: 130 (65 to spectacles; 65 to observation) Number analyzed: 106 (53 to spectacles; 53 to observation) for measurement of outcomes at the end of three years' follow‐up or 130 (65 to spectacles; 65 to observation) for measurement of outcomes during study Losses to follow‐up: 24 children did not complete the 3‐year primary outcome examination, 12 in each group Unit of randomization: participant Unit of analysis: participant Number of centers: 39 Date of first enrolment: February 2012 Length of follow‐up (planned and actual): 3 years, planned and actual Sample size estimation: sample size of 286 was chosen to provide 90% power to detect a difference in failure rates at 3 years given expected 3‐year failure rates of 10% in the spectacles and 25% in the observation groups and a type I error rate of 5%. Quality of life measures (record verbatim): none reported |
|
| Participants |
Country: US Age: mean age (SD): 1.8 years (0.6) for the spectacles group and 1.8 (0.6) for the observation group Gender: overall, there were 68 (52%) girls; 33 (51%) in the spectacles group and 35 (54%) in the observation group Included criteria: age 12 to <36 months; spherical equivalent refractive error between +3.00 and +6.00 (by cycloplegic refractions) in either eye; astigmatism ≤ 1.50 D in both eyes; spherical equivalent anisometropia ≤ 1.50 D; gestational age ≥ 32 weeks; investigator willing to prescribe glasses per protocol or observe hyperopia untreated for 3 years unless specific criteria for deterioration confirmed; parent understood the protocol and was willing to accept randomization to either spectacles or no spectacles initially, and was willing to wear spectacles as prescribed or accept that spectacles would not be prescribed by the investigator unless specific deterioration criteria were confirmed; parent had telephone (or access to telephone) and was willing to be contacted by Jaeb Center staff; relocation outside of area of an active Pediatric Eye Disease Investigator Group site for this study within the next 36 months was not anticipated. Excluded criteria: measurable manifest strabismus at distance (3 m) or near (1/3 m) by cover/uncover testing. Heterophoria was eligible; previously documented strabismus (parental report must have been confirmed by investigator); clinical evidence of manifest or latent nystagmus; prior treatment of refractive error with spectacles or contact lenses unless duration of spectacles or contact lens wear was ≤ 1 week and occurred > 2 months before enrollment; prior intraocular, refractive, or extraocular surgery; prior treatment for amblyopia; prior therapy for vergence/accommodation; parental concerns over learning or development; ocular comorbidity that may reduce VA; symptoms of blur or asthenopia; developmental delay, diagnosed by pediatrician or Individualized Education Program; known neurologic anomalies (e.g. cerebral palsy, Down syndrome). Pretreatment difference: none detected at baseline |
|
| Interventions |
Intervention 1: spectacle correction Intervention 2: observation |
|
| Outcomes |
Primary outcome: failure. Participant was considered to have met failure criteria if any of the following criteria were met during testing:
Children who met deterioration criteria during 3 years of follow‐up were classified as meeting failure criteria if only if ≥ 1 failure criteria were met at the 3‐year outcome examination. Secondary outcome: none reported Other measured outcomes: cumulative proportion of children with deterioration prior to 3 years, including manifest strabismus, amblyopia, mean change in refractive error, participants who did not achieve a refractive error in the emmetropic range, inadequate VA (< 20/30). |
|
| Notes |
Funding: National Eye Institute, National Institutes of Health Authors name: Marjean T Kulp Institution: Jaeb Center for Health Research Email: pedig@jaeb.org Address: 15310 Amberly Drive Suite 350Tampa, FL 33647 Trial registration number: NCT01515475 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned with equal probability, using a permuted block design stratified by clinical site, to either prescribed spectacles or observation (with spectacles prescribed only if confirmation of prespecified deterioration criteria). |
| Allocation concealment (selection bias) | Unclear risk | No information provided about how personnel enrolling participants received the allocation. |
| Masking of participants and personnel | High risk | Participants and personnel were not masked, which could have been attempted with use of sham spectacles. |
| Masking of outcome assessors (detection bias) | Low risk | 3‐year primary outcome exam was masked. At other visits, initial exams were unmasked and if deterioration suspected, a masked exam was completed to confirm or refute the presence of deterioration based on the suspect finding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24 children did not complete the 3‐year primary outcome examination, 12 in each group. Per protocol, a modified intention‐to‐treat analysis was completed for the primary analysis by not adjusting for missing values. A secondary analysis was completed using multiple imputation to account for missing values. Secondary outcomes were completed using the modified intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes specified in the protocol all appeared to be reported in the trial publication. |
| Other bias | Low risk | No other sources of bias noted. |
Ingram 1985.
| Methods |
Number randomized (total and per group): 306 children entered the trial
Number analyzed (total and per group): 265 children
Losses to follow‐up: 41 Unit of randomization: individual Unit of analysis: squint – individual child; VA – single eye with the lowest VA |
|
| Participants |
Country: UK Age: not reported Gender: not reported Inclusion/exclusion criteria: refractive criteria for entry into the trial were bilateral spherical hypermetropia (≥ +2.0 DS ) or anisometropia (≥ +1.0 D sphere or cylinder), or both Equivalence of baseline characteristics: not reported Diagnoses in participants: children from the general study population underwent refraction after cycloplegia with 1% cyclopentolate or 1% atropine. The refractive criteria for entry into the trial were bilateral spherical hypermetropia (≥ +2.0 D sphere) or anisometropia (≥ +1.0 D sphere or cylinder), or both. |
|
| Interventions |
Intervention 1: spectacle correction: "the prescription of the glasses was based on the cycloplegic retinoscopy with 2.00 D subtracted from each meridian of each eye. Spectacles were prescribed until refraction came within normal limits, that is, < +2.00 DS right and left, < +1.50 D cyl [cylinder], < 1.00 DS or cyl anisometropia". Intervention 2: no spectacles If strabismus (squint) was detected during follow‐up, children were given conventional treatment. Additional occlusion was also offered to both groups throughout follow‐up Length of follow‐up:
|
|
| Outcomes | Incidence of strabismus (squint) and VA in the worse eye Intervals at which outcome assessed: every 3 months until the age of 3.5 years Other issues with outcome assessment: quote: "The end result was to be decided on the basis of: (a) presence or absence of esotropia/intermittent esotropia; (b) presence or absence of amblyopia at the age of 3½. This has not proved possible in practice". Quote: "Therefore the end result has been recorded as the last known visual acuity of the eye with lower acuity, with spectacle correction if necessary, and after occlusion had been given and often some time after occlusion had stopped". Adverse effects: not reported |
|
| Notes |
Type of study: published Funding: not reported Declaration of interest: not mentioned Study period: not reported Subgroup analyses: according to compliance with wearing spectacles |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the trial required that her child would be randomly allocated to wearing glasses or not, but that she had to decide whether she wished to enter the trial before the random decision for 'treatment' or 'no treatment' was taken". Comment: method for random sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not specified. |
| Masking of participants and personnel | High risk | Participants and personnel not masked, which could have been attempted with use of sham spectacles. |
| Masking of outcome assessors (detection bias) | Unclear risk | Unknown as to whether the person who assessed VA and strabismus was also the outcomes assessor. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Some of the children who were lost to follow‐up moved from the area, but some information has been obtained from colleagues elsewhere in the UK. Others dropped out but reattended after a final recall about the age of 3½ years or returned spontaneously because of squint or poor performance at a school vision screening test. A final vision was recorded for 265 of the 306 children who originally entered the trial". Comment: the trial authors did not report the number of participants initially allocated to each treatment group, or the number of participants lost to follow‐up according to treatment group. Study authors reported 149 eligible children did not enter the study but did not explain why. |
| Selective reporting (reporting bias) | High risk | Quote: "The end result was to be decided on the basis of 1) presence or absence of esotropia/amblyopia at the age 3½. This has not proved possible in practice". Comment: investigators reported the presence of esotropia/amblyopia that was recorded at last known visit. |
| Other bias | Low risk | No other sources of bias identified. |
Ingram 1990.
| Methods |
Number randomized (total and per group): total: 372 children; number in each group was not reported Number analyzed (total and per group): results were reported for 289 children ages 3.5 years of age who were assessed for the presence of strabismus (squint) and VA with cycloplegic refraction
Losses to follow‐up: 56 children dropped out of the study early and others throughout follow‐up for a total of 87. Unit of randomization: individual Unit of analysis: strabismus (squint): individual child; VA: 'worse eye' of individual child |
|
| Participants |
Country: UK Age: children born between July 1978 and July 1981 who were 6 months old at time of visit Gender: not reported Inclusion/exclusion criteria: ≥ +4.0 D meridional hypermetropia. 1 child was excluded because of congenital nystagmus Equivalence of baseline characteristics: not reported Diagnoses in participants: children within the general screening population undergoing retinoscopy with cycloplegia with 1% cyclopentolate refractions ≥ +4.0 D hypermetropia. A 'refraction' figure was calculated to determine the degree of hypermetropia by subtracting 1.75 D from 1‐m retinoscopy in the most hypermetropic meridian |
|
| Interventions |
Intervention 1: prescription spectacles based on subtracting 2.0 D from each meridian of the retinoscopy Intervention 2: no spectacles Additional treatment with spectacles, occlusion, operation as necessary was offered to children who developed squint throughout the follow‐up period Length of follow‐up:
|
|
| Outcomes | Presence of strabismus (squint) assessed with the cover test, and VA measured on either the linear Sheridan‐Gardiner or Snellen tests with correction if worn. Emmetropization was initially determined by dichotomizing refractive error, with > +3.5 D indicating emmetropization had not occurred. A secondary analysis of emmetropization included the change in the mean refractive error from baseline to follow‐up. Intervals at which outcome assessed: every 3 months Adverse effects: not reported |
|
| Notes |
Type of study: published Funding: not reported Declaration of interest: not reported Study period: not reported Subgroup analyses: according to compliance with wearing spectacles |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a table of random numbers, all cases with an odd number were prescribed glasses (2.00 D less than the retinoscopy figure for each meridian in each eye) for constant wear and those with even numbers were not prescribed glasses". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not specified. |
| Masking of participants and personnel | High risk | Participants and personnel were not masked, which could have been attempted with use of sham spectacles. |
| Masking of outcome assessors (detection bias) | Unclear risk | Unknown as to whether the person who assessed VA and strabismus was masked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 56 children dropped out of study but number per treatment group were not reported. The investigators did not provide reasons for loss to incomplete outcome data. 87 children were unaccounted for the reporting of the results. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. |
| Other bias | Low risk | No other sources of bias identified. |
D: diopter; n: number; VA: visual acuity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Althaus 1994 | Did not investigate effects of spectacle correction. |
| Anker 2004 | Not a randomized or quasi‐randomized trial. |
| MacEwen 2008 | Not a randomized or quasi‐randomized trial. |
| NCT00574717 | Not a randomized or quasi‐randomized trial. |
| Nischal 2008 | Not a randomized or quasi‐randomized trial. |
| Pediatric Eye Disease Investigator Group 2016 | Not population of interest. |
| Roth 1989 | Not a randomized or quasi‐randomized trial. |
Differences between protocol and review
We intended to handsearch journals not included in electronic bibliographic databases, but did not conduct these searches in the implementation of the review. We decided searching CENTRAL was adequate to identify potentially eligible studies that we would have identified through handsearching. We also added a post‐hoc secondary outcome for visual acuity. Although the protocol stated we would not conduct meta‐analyses when the I2 value was 50% or greater, we considered the included studies were sufficiently similar with respect to clinical and methodologic factors to perform meta‐analyses for strabismus and visual acuity outcomes.
Contributions of authors
Conceiving the review: LJJ.
Designing the review: LJJ.
Co‐ordinating the review: LJJ.
Data collection for the review
Designing search strategies: CEV, LJJ, DOM
Undertaking searches: CEV
Screening search results: LJJ, CEV@US
Organizing retrieval of papers: LJJ, CEV@US
Screening retrieved papers against inclusion criteria: LJJ, XW, RS
Appraising risk of bias in trials: LJJ, XW, RS
Extracting data from papers: LJJ, XW, RS
Writing to authors of papers for additional information: LJJ
Providing additional data about papers: LJJ
Obtaining and screening data on unpublished studies: LJJ
Data management for the review
Entering data into Review Manager 5: LJJ, XW, RS
Analysis of data: LJJ, XW, RS
Interpretation of data
Providing a methodologic perspective: LJJ, XW, RS
Providing a clinical perspective: DOM
Providing a policy perspective: LJJ, DOM
Providing a consumer perspective: LJJ
Writing the review: LJJ, XW, RS
Providing general advice on the review: LJJ
Guarantor of the review: LJJ
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Eye Institute, National Institutes of Health, USA.
The Cochrane Eyes and Vision US Project is funded by Grant 1 U01 EY020522
-
National Institute of Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology
- This review update was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
LJJ: none. XW: none. RS: none. DOM: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Atkinson 1996 {published data only}
- Atkinson J, Anker S, Bobier W, Braddick O, Durden K, Nardini M, et al. Normal emmetropization in infants with spectacle correction for hyperopia. Investigative Ophthalmology and Visual Science 2000;41(12):3726‐31. [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Nardini M, Anker S. Infant hyperopia: detection, distribution, changes and correlates‐outcomes from the Cambridge infant screening programs. Optometry and Vision Science 2007;84(2):84‐96. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Robier B, Anker S, Ehrlich D, King J, et al. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo‐ and videorefractive screening. Eye 1996;10(Pt 2):189‐98. [DOI] [PubMed] [Google Scholar]
Hyperopia Treatment Study 1 {published data only}
- Kulp MT, Holmes JM, Dean TW, Suh DW, Kraker RT, Wallace DK, et al. A randomized clinical trial of immediate versus delayed glasses for moderate hyperopia in 1‐ and 2‐year‐olds. Ophthalmology 2019;126(6):876‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01515475. Glasses versus observation for moderate hyperopia in young children (HTS1). clinicaltrials.gov/show/NCT01515475 (first received 24 January 2012).
Ingram 1985 {published data only}
- Ingram RM, Walker C, Wilson JM, Arnold PE, Lucas J, Dally S. A first attempt to prevent amblyopia and squint by spectacle correction of abnormal refractions from age 1 year. British Journal of Ophthalmology 1985;69(11):851‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingram 1990 {published data only}
- Ingram RM, Arnold PE, Dally S, Lucas J. Emmetropisation, squint, and reduced visual acuity after treatment. British Journal of Ophthalmology 1991;75(7):414‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM, Arnold PE, Dally S, Lucas J. Results of a randomised trial of treating abnormal hypermetropia from the age of 6 months. British Journal of Ophthalmology 1990;74(3):158‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM, Gill LE, Lambert TW. Effect of spectacles on changes of spherical hypermetropia in infants who did, and did not, have strabismus. British Journal of Ophthalmology 2000;84(3):324‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM, Gill LE, Lambert TW. Reduction of astigmatism after infancy in children who did and did not wear glasses and have strabismus. Strabismus 2001;9(3):129‐35. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Althaus 1994 {published data only}
- Althaus K, Bischoff P. Video refraction measurement in the first year of life [Videorefraktionsmessung im ersten Lebensjahr]. Klinische Monatsblatter fur Augenheilkunde 1994;205(3):133‐7. [DOI] [PubMed] [Google Scholar]
Anker 2004 {published data only}
- Anker S, Atkinson J, Braddick O, Nardini M, Ehrlich D. Non‐cycloplegic refractive screening can identify infants whose visual outcome at 4 years is improved by spectacle correction. Strabismus 2004;12(4):227‐45. [DOI] [PubMed] [Google Scholar]
MacEwen 2008 {published data only}
- MacEwen CJ, Lymburn EG, Ho WO. Is the maximum hypermetropic correction necessary in children with fully accommodative esotropia?. British Journal of Ophthalmology 2008;92(10):1329‐32. [DOI] [PubMed] [Google Scholar]
NCT00574717 {published data only}
- NCT00574717. Enhancement of emmetropization in hyperopic infants. clinicaltrials.gov/ct2/show/study/NCT00574717 (first received 17 December 2007).
Nischal 2008 {published data only}
Pediatric Eye Disease Investigator Group 2016 {published data only}
- Pediatric Eye Disease Investigator Group. Clinical factors associated with moderate hyperopia in preschool children with normal stereopsis and visual acuity. Journal of AAPOS 2016;20(5):455‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 1989 {published data only}
- Roth A. Medical treatment – optical correction [Traitement medical – correction optique]. Bulletin des Societes d'Ophtalmologie de France 1989;89(1):113‐5. [PubMed] [Google Scholar]
Additional references
AAO 2012
- American Academy of Ophthalmology Pediatric Ophthalmology/Strabismus Panel. Preferred Practice Pattern® Guidelines: Pediatric Eye Evaluations. San Francisco (CA): American Academy of Ophthalmology, 2012. [Google Scholar]
AOA 2008
- American Optometric Association. Optometric Clinical Practice Guidelines: Care of the Patient with Hyperopia. St Louis (MO): American Optometric Association, 2008. [Google Scholar]
Atkinson 2000
- Atkinson J, Anker S, Bobier W, Braddick O, Durden K, Nardini M, et al. Normal emmetropization in infants with spectacle correction for hyperopia. Investigative Ophthalmology and Visual Science 2000;41(12):3726‐31. [PubMed] [Google Scholar]
Babinsky 2013
- Babinsky E, Candy TR. Why do only some hyperopes become strabismic?. Investigative Ophthalmology and Visual Science 2013;54(7):4941‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birch 2005
- Birch EE, Fawcett SL, Morale SE, Weakley DR Jr, Wheaton DH. Risk factors for accommodative esotropia among hypermetropic children. Investigative Ophthalmology and Visual Science 2005;46(2):526‐9. [DOI] [PubMed] [Google Scholar]
Ciner 1990
- Ciner EB. Management of refractive error in infants, toddlers, and preschool children. In: Scheiman MM editor(s). Problems in Optometry. Vol. 3, Philadelphia (PA): Lippincott, 1990:394‐419. [Google Scholar]
Colburn 2010
- Colburn JD, Morrison DG, Estes RL, Li C, Lu P, Donahue SP. Longitudinal follow‐up of hypermetropic children identified during preschool vision screening. Journal of AAPOS 2010;14(3):211‐5. [DOI] [PubMed] [Google Scholar]
Cotter 1997
- Cotter SA, Frantz KA. Strabismus: detection, diagnosis and classification. In: Moore BD editor(s). Eye Care for Infants and Young Children. Boston (MA): Butterworth‐Heinemann, 1997:123‐54. [Google Scholar]
Cotter 2011
- Cotter SA, Varma R, Tzrczy‐Hornoch K, McKean‐Cowdin R, Lin J, Wen G, et al. Risk factors associated with childhood strabismus. Ophthalmology 2011;118(11):2251‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dobson 1989
- Dobson V, Sebris SL. Longitudinal study of acuity and stereopsis in infants with or at‐risk for esotropia. Investigative Ophthalmology and Visual Science 1989;30(6):1146‐58. [PubMed] [Google Scholar]
Donahue 2007
- Donahue SP. Clinical practice. Pediatric strabismus. New England Journal of Medicine 2007;356(10):1040‐7. [DOI] [PubMed] [Google Scholar]
Ehrlich 1997
- Ehrlich DL, Braddick OJ, Atkinson J, Anker S, Weeks F, Hartley T, et al. Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optometry and Vision Science 1997;74(10):822‐43. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Goldschmidt 1969
- Goldschmidt E. Refraction in the newborn. Acta Ophthalmologica 1969;47(3):570‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne, JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holmes 2006
- Holmes JM, Clarke MP. Amblyopia. Lancet 2006;367(9519):1343‐51. [DOI] [PubMed] [Google Scholar]
Ingram 2000
- Ingram RM, Gill LE, Lambert TW. Effect of spectacles on changes of spherical hypermetropia in infants who did, and did not, have strabismus. British Journal of Ophthalmology 2000;84(3):324‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leat 2011
- Leat SJ. To prescribe or not prescribe? Guidelines for spectacle prescribing in infants and children. Clinical and Experimental Optometry 2011;94(6):514‐27. [DOI] [PubMed] [Google Scholar]
Li 2009
- Li T, Shotton K. Conventional occlusion versus pharmacologic penalization for amblyopia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006460.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsh‐Tootle 2006
- Marsh‐Tootle WL, Frazier MG. Infants, toddlers, and children. In: Benjamin, WJ editor(s). Borish's Clinical Refraction. 2nd Edition. Philadelphia (PA): Butterworth‐Heinemann, 2006:1395‐460. [Google Scholar]
Mulvihill 2000
- Mulvihill A, MacCann A, Flitcroft I, O'Keefe M. Outcome in refractive accommodative esotropia. British Journal of Ophthalmology 2000;84(7):746‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutti 2004
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, et al. Refractive astigmatism and the toricity of ocular components in human infants. Optometry and Vision Science 2004;81(10):753‐61. [DOI] [PubMed] [Google Scholar]
Mutti 2009
- Mutti DO, Michell L, Jones JA, Friedman NE, France SL, Lin WK, et al. Accommodation, acuity, and their relationship to emmetropization in infants. Optometry and Vision Science 2009;86(6):666‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Repka 1989
- Repka MX, Wellish K, Wisnicki HJ, Guyton DL. Changes in the refractive error of 94 spectacle‐treated patients with acquired accommodative esotropia. Binocular Vision 1989;4(1):15‐21. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubin 2006
- Rubin SE. Bringing the management of accommodative esotropia into sharp focus. American Journal of Ophthalmology 2006;141(5):914‐5. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Taylor 2011
- Taylor K, Elliott S. Interventions for strabismic amblyopia. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD006461.pub3] [DOI] [PubMed] [Google Scholar]
Taylor 2012
- Taylor K, Powell C, Hatt SR, Stewart C. Interventions for unilateral and bilateral refractive amblyopia. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD005137.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomilla 1981
- Tomilla V, Tarkkanen A. Incidence of loss of vision in the healthy eye in amblyopia. British Journal of Ophthalmology 1981;65(8):575‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Leeuwen 2007
- Leeuwen R, Eijkemans MJ, Vingerling JR, Hofman A, Jon PT, Simonsz HJ. Risk of bilateral visual impairment in individuals with amblyopia: the Rotterdam study. British Journal of Ophthalmology 2007;91(11):1450‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Jones 2009
- Jones L, Mutti DO. Spectacle correction versus no spectacles for prevention of strabismus in hyperopic children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones‐Jordan 2014
- Jones‐Jordan L, Wang X, Scherer RW, Mutti DO. Spectacle correction versus no spectacles for prevention of strabismus in hyperopic children. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD007738.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


