Abstract
Background
Cardiac dysfunction and cardiovascular (CV) events are prevalent among patients with chronic kidney disease (CKD) without overt obstructive coronary artery disease (CAD) but the mechanisms remain poorly understood. Coronary microvascular dysfunction (CMD) has been proposed as a link between abnormal renal function and impairment of cardiac function and CV events. We sought to investigate the relationships between CKD, CMD, cardiac dysfunction and adverse CV outcomes.
Methods
Patients undergoing cardiac stress positron emission tomography (PET), echocardiogram and renal function ascertainment at Brigham and Women’s Hospital were studied longitudinally. Patients free of overt coronary (summed stress score < 3 & without history of ischemic heart disease), valvular and end-organ disease were followed for adverse composite outcome of death, hospitalization for myocardial infarction or heart failure. Coronary flow reserve (CFR) was determined from PET. Echocardiograms were used to measure cardiac mechanics: diastolic (lateral and septal E/e’) and systolic [global longitudinal (GLS), radial (GRS) and circumferential strain (GCS)]. Image analyses and event adjudication were blinded. The associations between estimated glomerular filtration rate (eGFR), CFR, diastolic, systolic indices and adverse CV outcomes were assessed in adjusted models and mediation analyses.
Results
352 patients (median age 65 years, 63% women and 22% black) were studied. 35% of patients had eGFR<60 ml/min/1.73 m2, median LVEF of 62% and median CFR of 1.8. eGFR and CFR were associated with diastolic and systolic indices, as well as future CV events (all p<0.05). In multivariable models, CFR but not eGFR was independently associated with cardiac mechanics and CV events. The associations between eGFR, cardiac mechanics and CV events were partly mediated via CFR.
Conclusions
CMD but not eGFR was independently associated with abnormal cardiac mechanics and an increased risk of CV events. CMD may mediate the effect of CKD on abnormal cardiac function and CV events in those without overt CAD.
Journal Subject Terms: Chronic kidney disease, coronary microvascular disease, myocardial mechanics, cardiovascular risk
Keywords: chronic kidney disease, coronary flow reserve, coronary microvascular function, cardiovascular outcomes
Introduction
Approximately 14% of the adult US population has chronic kidney disease (CKD).1 There is clear evidence of a graded association between the severity of CKD and adverse cardiovascular (CV) risk,2 which begins early in the natural history of the disease even when serum creatinine is within normal limits.3 In fact, patients with early CKD are more likely to die of CV disease than progress to ESRD.4 The mechanisms mediating increased risk of CV mortality and morbidity in CKD patients are not well understood. Although CKD clusters with conventional atherosclerotic risk factors, age-adjusted CV mortality is several fold higher in patients with CKD than in the general population.5 Interestingly, sudden cardiac death (SCD) and heart failure related deaths are more common than the classic type 1 myocardial infarction (MI) related deaths in CKD patients,5 which is in keeping with the relatively lower prevalence of obstructive coronary artery disease (CAD) on autopsy and coronary angiography.6, 7 These findings collectively suggest that other mechanisms may contribute to cardiovascular risk in CKD patients. One such mechanism may involve the effects of CKD and its associated risk factors on coronary epicardial and microcirculatory dysfunction, thereby increasing the risk of subclinical myocardial ischemia and injury, myocardial dysfunction, heart failure and, ultimately, mortality.8-12
We designed this study to test the hypothesis that coronary microvascular dysfunction (CMD) is associated with abnormalities in myocardial structure and function in patients across a spectrum of eGFR, and that this may help explain the increased risk of heart failure and death related with worsening renal function.
Methods
The analytic methods will be/have been made available in the supplement to other researchers for purposes of reproducing the results or replicating the procedure. The study was reviewed and approved by the Partners Institutional Review Board. Informed consent was waived as all data were collected as part of standard clinical care.
Patient population
We included consecutive patients referred to the Brigham and Women’s Hospital between January 1, 2006 and December 31, 2016 for stress myocardial perfusion positron emission tomography (PET) who also underwent 2-dimensional (2-D) echocardiography and serum creatinine determination within 90 days of the PET study. Patients with known coronary artery disease, as defined by a history of prior revascularization (percutaneous coronary intervention or coronary artery bypass grafting) and/or MI or imaging evidence of flow-limiting coronary artery disease (summed stress score>2 on PET) were excluded as were those with any of the following: severe valvular heart disease, infiltrative cardiomyopathy, congenital heart disease, history of active malignancy or end-stage liver or lung disease, history of organ transplantation and poor quality echocardiogram (Supplemental Figure 1).
Renal Function
Estimated GFR (eGFR) was calculated using the Chronic Kidney Disease (CKD) Epidemiology Collaboration formula.13 CKD was defined as eGFR< 60 ml/min/1.73 m2.
Quantification of coronary vascular function
Coronary vascular function was quantified in all patients using a whole-body PET/computed tomography scanner (PET/CT Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI). Myocardial blood flow (MBF, in mL/min/g) was measured at rest and during maximal hyperemia by a standard intravenous infusion of adenosine, dipyridamole or regadenoson using either 13N-ammonia or 82Rubidium as the flow tracers, as described previously.14 The image acquisition and post processing techniques for quantification of myocardial blood flow (MBF) and flow reserve did not change over the study period. Previous studies have demonstrated that equivalence of dipyridamole, regadenoson, and adenosine as vasodilators for myocardial perfusion imaging and quantitative myocardial blood flow.15, 16 Furthermore, the use of different vasodilator stress agents has not affected the value of quantitative myocardial blood flow by PET for risk stratification.15, 17-19 The heart rate, blood pressure, and 12-lead electrocardiogram were recorded at baseline and every minute during and after the vasodilator infusion.
Image analysis:
For semi-quantitative assessment of myocardial scarring and ischemia, 17-segment visual interpretation of gated myocardial perfusion images was performed by experienced operators using a standard five-point scoring system.20 Rest left ventricular ejection fraction (LVEFs) was calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; Ann Arbor, Michigan).
Absolute regional and global MBF was quantified at rest and at peak hyperemia using a validated two-compartment kinetic model, as described previously.21 Per-patient CFR was calculated as the ratio of MBF during maximal hyperemia over that at rest for each coronary territory and for the entire left ventricle. This method for quantitation of MBF is highly reproducible. In our laboratory, the intra-class correlation coefficient for CFR among four readers is 0.94 (95% confidence interval (CI) 0.88–0.98), indicating excellent reproducibility.18
Quantification of cardiac structure and function
Left ventricular diastolic and systolic function was quantified from 2-D echocardiograms. The echocardiograms were acquired as recommended by the American Society of Echocardiography (ASE) and in a manner consistent with standard practices for patient comfort and position. All echocardiographic readers were blinded to the results of PET imaging. No cardiovascular events occurred between echocardiography and PET imaging.
Echocardiograms with views to: (1) assess LV diastolic function, and (2) ensure optimal imaging for off-line LV deformation analysis with speckle-tracking software were analyzed with following methods:
Diastolic function:
peak early mitral annular relaxation velocity (e’) was measured from both the septal and lateral aspects of the mitral annulus from the apical 4-chamber view.22 Mitral inflow velocity (E) was assessed by pulsed wave Doppler from the apical 4-chamber view by positioning the sample volume at the tip of the mitral leaflets.22
Systolic function:
Deformational indices were estimated using B-mode speckle-tracking analysis, which was performed off-line using commercially available software (Cardiac Performance Analysis, Tomtec system, Munich, Germany).23 The endocardial border was traced at an end-diastolic frame in apical 4– 2- & 3- chamber views and at an end-systolic frame in short axis view, where end-diastole is defined by the QRS complex from the electrocardiogram (ECG) or the frame just before mitral valve opening. Adequate tracking of speckles along the endocardial and the epicardial borders throughout the cardiac cycle was visually assessed. Peak global longitudinal (GLS), radial (GRS) and circumferential strain (GCS) curves were then computed automatically and provided as global and segmental data including 6 segments in each view24. LV volumes were determined by the modified Simpson’s method in the apical 4 and 2 chamber views, and LVEF was calculated from volumes in the standard manner.25
LV mass was calculated by the ASE recommended formula for estimation of LV mass from LV linear dimensions and indexed to body surface area (LVMI), and relative wall thickness (RWT) was calculated in accordance with ASE guidelines, this information was used to identify those with abnormal LV geometry.25
The intra-class correlation coefficient for echocardiographic parameters were good, the details are presented in Supplemental Table 1.
Circulating biomarkers
N-terminal pro–B-type natriuretic peptide (NTproBNP) was measured using an electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN) in clinical laboratory, and values within 90 days of cardiac PET study were used in the analysis.
Clinical outcomes
A Clinical Endpoints Committee (CEC) reviewed and adjudicated the primary composite endpoint (all cause death, non-fatal MI, and hospitalizations for heart failure). The members of CEC were blinded to image analyses. Time to first event major adverse cardiovascular event (MACE), defined as a composite of all cause death, and hospitalization for nonfatal MI or heart failure was analyzed. Time to first event was analyzed. Ascertainment of clinical endpoints were determined by CEC from the longitudinal medical record, Partners Healthcare Research Patient Data Registry, the National Death Index, mail surveys and telephone calls. The details of clinical outcomes are presented in the supplement (Supplemental methods). The follow up was censored on December 31st, 2017.
Statistical Analysis
Baseline characteristics were reported as frequency with percentage (%) for categorical variables and median with interquartile ranges for continuous variables. We used χ2 and Wilcoxon rank sum tests to evaluate for differences in categorical and continuous baseline characteristics, respectively. Poisson regression models were used to estimate the annualized rate of adverse events and its components. Global MBF and CFR values were used in all analyses.
Unadjusted and multivariable-adjusted relationships between eGFR & CFR, diastolic indices (Lateral E/e’ and Septal E/e’), systolic indices (GLS, GRS and GCS), marker of LV wall stress (NTproBNP) and adverse composite clinical endpoint were evaluated using appropriate linear, Poisson and Cox proportional hazard models while accounting for non-linearity of relationships using restricted cubic splines. All the adjusted models included demographic factors (age, sex, race), clinical factors (history of hypertension, diabetes, peripheral vascular disease, stroke, diabetes, body mass index (BMI), LVMI, LVEF and eGFR as well as CFR. The variables and number of knots were selected based on optimal values of the Akaike information criterion after including clinically important covariates. We also tested for a statistical interaction between CFR and eGFR in the multivariate model in the Poisson model. Cox proportional hazards models were used to determine the effect of abnormal eGFR26 (<60 ml/min/1.73m2) and abnormal CFR (<1.5). The cox proportional hazard (PH) assumptions test based on Schoenfeld residuals was used to verify non-violation of PH assumption in the adjusted model.
To understand the interplay between LV structure, myocardial mechanics and coronary microvascular dysfunction on future outcomes, we performed an exploratory analysis where we stratified patients by abnormal geometry, diastolic dysfunction (E/e’<15), systolic dysfunction (GLS<−17%) and CFR (<1.5) and compared the rate of adverse events as well as a composite of heart failure admissions and non-fatal MI. The cutoff for GLS was median values in our cohort, whereas ASE definitions were used to define abnormal geometry25, cutoffs for E/e’ and CFR were based on previous studies.10, 18
Mediation analysis (i.e. path analysis)-which tests a putative causal relation among variables—was also performed to test whether renal function exerts its effect on cardiovascular disease via microvascular dysfunction. eGFR was chosen as measure of renal function, CFR was chosen as marker of microvascular disease whereas measures of diastolic/systolic function, NTproBNP and clinical composite endpoint were chosen as markers of cardiovascular disease.27 The details of statistical analysis are provided in the supplement (Supplementary methods).
Two-sided p-values < 0.05 were considered significant. Stata software version 15.1 (StataCorp, College Station, Texas) and R (version 3.6.0) were used for analyses. The results are presented in accordance with STROBE checklist (Supplemental table 2).
Results
Baseline characteristics
The final study cohort consisted of 352 patients. The distribution of baseline characteristics by categories of CKD is summarized in Table 1. The median (Q1–Q3) age of patients in the overall cohort was 65 (55–75) years, 63% were women, and 22% were Black. One-third of the patients had CKD 3 or higher. The median (Q1–Q3) LVEF was 62% (55–68%) by echocardiography and 59% (50–66%) by PET. More than three-quarters of patients had a history of hypertension, approximately two-thirds had dyslipidemia and one-third had diabetes mellitus. More than 70% of the patients had an abnormally remodeled left ventricle.
Table 1:
Baseline Characteristics
| Overall (N=352) |
Preserved (eGFR≥60) (N=236) |
CKD stage 3 or higher (eGFR<60) (N=116) |
p-value* | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 65.2 (55.4, 75.1) | 61.8 (52.8, 69.9) | 73.3 (64.1, 81.4) | <0.001 |
| Female | 222 (63.1%) | 151 (64.0%) | 71 (61.2%) | 0.61 |
| Black | 76 (21.6%) | 51 (21.6%) | 25 (21.6%) | 0.37 |
| Clinical parameters | ||||
| Hypertension | 273 (77.6%) | 169 (71.6%) | 104 (89.7%) | <0.001 |
| Hyperlipidemia | 225 (62.2%) | 140 (59.3%) | 85 (73.3%) | 0.010 |
| Diabetes | 116 (33.0%) | 71 (30.1%) | 45 (38.8%) | 0.10 |
| Peripheral vascular disease | 22 (6.3%) | 9 (3.8%) | 13 (11.2%) | 0.007 |
| Stroke | 20 (5.7%) | 13 (5.5%) | 7 (6.0%) | 0.84 |
| Chronic obstructive pulmonary disease | 41 (11.6%) | 27 (11.4%) | 14 (12.1%) | 0.86 |
| Tobacco use | 24 (6.8%) | 18 (7.6%) | 6 (5.2%) | 0.39 |
| Family history of CAD | 72 (20.5%) | 54 (22.9%) | 18 (15.5%) | 0.11 |
| Dialysis | 7 (2.0%) | 0 | 7 (6.0%) | <0.001 |
| Body mass index, kg/m2 | 29.4 (25.4, 35.7) | 30.1 (25.8, 35.9) | 27.8 (24.7, 35.2) | 0.043 |
| Positron emission tomography parameters | ||||
| Left ventricular ejection fraction, % | 59.0 (50.0, 66.0) | 60.0 (52.0, 67.0) | 58.0 (46.0, 65.0) | 0.044 |
| Rest myocardial blood flow, ml/min/g | 1.0 (0.8, 1.3) | 1.1 (0.8, 1.3) | 1.0 (0.8, 1.3) | 0.62 |
| Stress myocardial blood flow, ml/min/g | 1.9 (1.4, 2.6) | 2.1 (1.6, 2.7) | 1.7 (1.2, 2.2) | <0.001 |
| Coronary flow reserve | 1.8 (1.4, 2.3) | 1.9 (1.5, 2.5) | 1.5 (1.3, 2.1) | <0.001 |
| Echocardiography parameters | ||||
| Diastolic function | ||||
| Septal E/e’ ratio | 11.7 (8.8, 16.2) | 10.8 (8.6, 14.6) | 14.1 (9.6, 19.5) | <0.001 |
| Lateral E/e’ ratio | 8.8 (6.5, 13.0) | 8.3 (6.1, 11.4) | 9.8 (7.1, 15.8) | <0.001 |
| Systolic function | ||||
| Peak GLS, % | −17.0 (−21.6, −13.4) | −18.6 (−22.2, −14.6) | −15.1 (−19.3, −10.9) | <0.001 |
| Peak GRS, % | 31.5 (22.0, 45.7) | 34.6 (24.6, 48.1) | 25.5 (19.2, 39.7) | <0.001 |
| Peak GCS, % | −24.3 (−29.8, −18.5) | −25.7 (−30.9, −20.1) | −21.3 (−27.7, −15.5) | <0.001 |
| LV structure/geometry | ||||
| LVESV, milliliters | 30.0 (21.9, 45.0) | 30.0 (21.3, 45.0) | 30.8 (22.0, 50.5) | 0.45 |
| LVEDV, milliliters | 81.9 (64.0, 108.3) | 81.1 (63.5, 109.0) | 82.8 (65.3, 106.8) | 0.71 |
| LV mass, grams | 172.0 (132.1, 226.4) | 169.9 (132.3, 224.6) | 179.6 (130.7, 233.1) | 0.49 |
| LV mass index, grams/m2 | 89.0 (71.4, 111.6) | 88.0 (70.1, 110.6) | 93.9 (73.1, 119.5) | 0.13 |
| RWT ratio | 0.44 (0.38-0.53) | 0.44 (0.38-0.52) | 0.45 (0.39, 0.53) | 0.58 |
| LV remodeling | ||||
| Normal | 98 (29.9%) | 70 (31.8%) | 28 (25.9%) | 0.20 |
| Eccentric hypertrophy | 37 (11.3%) | 21 (9.5%) | 16 (14.8%) | |
| Concentric remodeling | 122 (37.2%) | 86 (39.1%) | 36 (33.3%) | |
| Concentric hypertrophy | 71 (21.6%) | 43 (19.5%) | 28 (25.9%) | |
| Circulating biomarkers | ||||
| Natural log NTproBNP | 6.3 (4.9, 7.6) | 5.3 (4.7, 7.0) | 7.1 (6.1, 9.1) | <0.001 |
| NTproBNP, pg/mL | 548.0 (133.0, 1950.0) | 198.0 (108.0, 1089.0) | 1250.5 (468.0, 8603.0) | <0.001 |
| Serum creatinine , mg/dl | 1.0 (0.8, 1.2) | 0.8 (0.7, 1.0) | 1.4 (1.2, 2.3) | <0.001 |
| eGFR, mL/min/1.73 m2 | 72.3 (51.1, 90.5) | 85.9 (71.9, 98.4) | 41.0 (25.4, 51.0) | <0.001 |
| Cardiovascular medications | ||||
| Calcium channel Blockers | 99 (28.1%) | 56 (23.7%) | 43 (37.1%) | 0.009 |
| Beta Blockers | 202 (57.4%) | 120 (50.8%) | 82 (70.7%) | <0.001 |
| Angiotensin converting enzyme inhibitors | 129 (36.6%) | 86 (36.4%) | 43 (37.1%) | 0.91 |
| Aspirin | 213 (60.5%) | 135 (57.2%) | 78 (67.2%) | 0.070 |
| Lipid lowering therapy | 216 (61.4%) | 134 (56.8%) | 82 (70.7%) | 0.012 |
Values are median (interquartile range) or n (%).
CKD=Chronic kidney disease, eGFR = estimated glomerular filtration rate using chronic kidney disease epidemiology collaboration (CKD-EPI) formula, bpm= beats per minute, E = Early wave of mitral inflow, e’= early diastolic mitral annular velocity, LV= Left ventricle, ESV= end systolic volume, EDV= end diastolic volume, RWT= relative wall thickness, m/sec= meters/second, GLS= peak global longitudinal strain, GRS= peak global radial strain, peak global circumferential strain, NTproBNP = N-terminal pro B-type natriuretic peptide, kg/m2= kilograms per square meter, ml/min/g= milliliters per minute per gram, pg/mL= picogram per milliliter, mL/min= milliliters per minute.
Comparison between the groups are based on the chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Compared to patients with preserved eGFR (≥60 ml/min/1.73 m2), those with CKD 3 or greater had higher prevalence of hypertension and diabetes and lower BMI (all p<0.05), lower stress MBF (1.7 vs. 2.1 mL/min/g, p<0.001) and lower CFR (1.5 vs. 1.9, p<0.001) (Table 1), here reflecting coronary microvascular dysfunction. Rest and stress MBF and CFR were comparable across all three coronary artery territories. Measurements of eGFR and CFR were modestly correlated (r=0.26, p<0.001), this correlation was independent of clinical important confounders (Supplemental Table 3).
Association between eGFR, coronary flow reserve and left ventricular mechanics
Compared to patients with preserved eGFR (≥ 60 ml/min/1.73 m2), those with CKD stage 3 or greater had higher lateral E/e’(14.1 vs. 10.8, p<0.001) and septal E/e’(8.3 vs. 9.8, p<0.001), reflecting increased left ventricular filling pressure, as well as impaired GLS (−15.1% vs. −18.6%, p<0.001), GCS (−21.3% vs. −25.3%, p<0.001) and GRS (25.5% vs. 34.6%, p<0.001), reflecting systolic dysfunction (Table 1).
In unadjusted models, both eGFR and CFR were associated with measures of diastolic function (i.e. lateral and septal E/e’), such that lower eGFR and CFR were associated with worse diastolic function (all p-trends <0.05) (Table 2). Likewise, lower eGFR and CFR were associated with worse systolic strain (GLS, GRS and GCS) (all p-trends <0.05) (Table 2).
Table 2.
Associations between LV mechanics, wall stress, cardiovascular outcomes, renal function and coronary flow reserve
| Measure | Association with CFR | Association with eGFR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shape | Correlation Coefficient |
Partial Correlation Coefficient |
Unadjusted p-value |
Adjusted p-value |
Shape | Correlation Coefficient |
Partial Correlation Coefficient |
Unadjusted p-value |
Adjusted p- value |
|
| Diastolic indices | ||||||||||
| Lateral E/e’ | L-shaped | −0.29 | −0.14 | <0.001 | 0.001 | L-shaped | −0.28 | −0.05 | 0.001 | 0.263 |
| Septal E/e’ | L-shaped | −0.29 | −0.17 | <0.001 | 0.004 | L-shaped | −0.27 | −0.02 | 0.001 | 0.058 |
| Systolic indices | ||||||||||
| GLS | Reverse sigmoid | −0.56 | −0.53 | <0.001 | 0.001 | Linear | −0.27 | −0.10 | 0.001 | 0.162 |
| GRS | Sigmoid | 0.32 | 0.26 | <0.001 | 0.001 | Linear | 0.19 | 0.10 | 0.002 | 0.250 |
| GCS | Reverse sigmoid | −0.39 | −0.38 | <0.001 | 0.001 | Linear | −0.19 | −0.06 | 0.001 | 0.584 |
| Markers of LV wall stress | ||||||||||
| Natural log NTproBNP | L-shaped | −0.34 | −0.21 | <0.001 | 0.012 | L-shaped | −0.43 | −0.27 | 0.001 | 0.003 |
| Adverse cardiovascular event | ||||||||||
| Composite clinical endpoint (MACE) | L-shaped | NA | NA | <0.001 | 0.015 | L-shaped | NA | NA | 0.001 | 0.116 |
Adjusted regression models included CFR, eGFR, age, gender, race, hypertension, hyperlipidemia, diabetes, peripheral vascular disease, stroke, left ventricular mass indexed and left ventricular ejection fraction. For continuous outcomes (Lateral E/e’, Septal E/e’, GLS, GRS, GCS and Natural log NTproBNP linear regression restricted spline models were used) whereas for MACE Poisson regression restricted cubic spline models were used). Correlation and Partial correlation coefficients after accounting for aforementioned variables are presented as strength of association.
CFR = Coronary flow reserve, eGFR= estimated glomerular filtration rate using chronic kidney disease epidemiology collaboration (CKD-EPI) formula, E = Early wave of mitral inflow, e’= early diastolic mitral annular velocity, GLS= peak global longitudinal strain, GRS= peak global radial strain, GCS = peak global circumferential strain, NTproBNP = N-terminal pro B-type natriuretic peptide, NA= not applicable, MACE = major adverse cardiovascular event (composite of death, non-fatal myocardial infarction and heart failure)
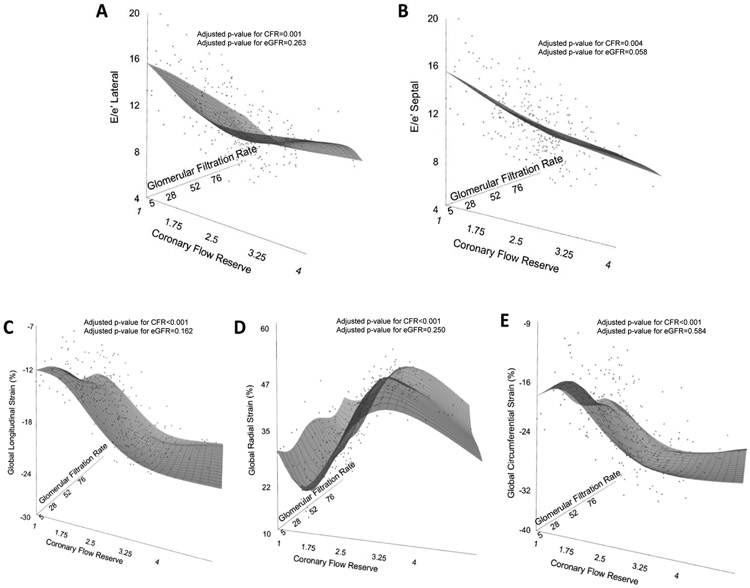
In the multivariable models including both CFR and eGFR, only the association between CFR, but not eGFR, with lateral and septal E/e’ (Figure 1, A & B) as well as measures of systolic deformation indices (Figure 1 C, D & E) remained significant, indicating that variability in diastolic and systolic indices was explained independently by variability in CFR, but not renal function.
Figure 1:
Relationship between cardiac mechanics (diastolic and systolic indices), eGFR and CFR using a three dimensional scatter plot and restricted cubic spline linear regression plane (Black grid on gray surface).
Panel A & B: Diastolic indices (Lateral and Septal E/e’), eGFR and CFR
Panel C, D & E: Systolic indices (GLS, GRS & GCS), eGFR and CFR
Adjusted linear regression models included CFR (coronary flow reserve), eGFR (estimated glomerular filtration rate), age, sex, race, hypertension, hyperlipidemia, diabetes, peripheral vascular disease, stroke, indexed left ventricular mass, and resting left ventricular ejection fraction
E = Early wave of mitral inflow, e’= early diastolic mitral annular velocity, GLS= peak global longitudinal strain, GRS= peak global radial strain, GCS = peak global circumferential strain
We also evaluated the associations between renal function, CFR and indices of myocardial mechanics in those with a history of diabetes. The effect estimates and statistical significance for association of CFR and eGFR with measures of myocardial mechanics remained unchanged in this sub-group analysis as compared to overall population indicating that associations hold true in diabetics as well as non-diabetics (Table 2 & Supplemental Table 4)
Association between eGFR, coronary flow reserve and LV wall stress
As measures of diastolic and systolic LV mechanics worsened with impaired renal and coronary microvascular function, we hypothesized that NTproBNP- a biomarker of LV wall stress- would also show similar associations with eGFR and CFR. Indeed, patients with CKD stage 3 or greater had higher NTproBNP as compared to those with preserved eGFR (p<0.001) (Table 1). In unadjusted and adjusted models, both eGFR and CFR were independently associated with elevated NTproBNP levels (p-trend<0.05) (Table 2 and Supplemental Figure 2).
Association between eGFR, coronary flow reserve and clinical outcomes
Over a median follow-up of 4.4 years (Q1–Q3, 1.2–7.7 years), 108 patients met the primary composite endpoint of major adverse cardiac events (MACE) including death or hospitalization for non-fatal myocardial infarction or heart failure (Table 3). Individual components of the composite endpoint increased with worsening renal function (Table 3).
Table 3.
Clinical endpoints* stratified by renal function
| Overall (N=352) |
Preserved (eGFR≥60) (N=236) |
CKD stage 3 or higher (eGFR<60) (N=116) |
p-value** | |
|---|---|---|---|---|
| n events/ annualized event rate** | ||||
| Total composite clinical endpoint (MACE) |
108/ 6.6% | 56/ 4.6% | 52/ 12.5% | <0.001 |
| Death | 74/ 4.1% | 40/ 3.0% | 34/ 7.0% | <0.001 |
| Hospitalization for nonfatal myocardial infarction | 18/ 1.0% | 12/ 0.9% | 6/ 1.1% | 0.559 |
| Hospitalization for heart failure | 41/ 2.7% | 19/1.7% | 22/6.2% | <0.001 |
Median (Q1-Q3) follow-up time was 4.4 (1.2, 7.7) years. Time to first event was analyzed.
Annualized event rates and p-values were calculated using Poisson regression.
CKD=Chronic kidney disease, MACE = major adverse cardiovascular event (composite of death, non-fatal myocardial infarction and heart failure).
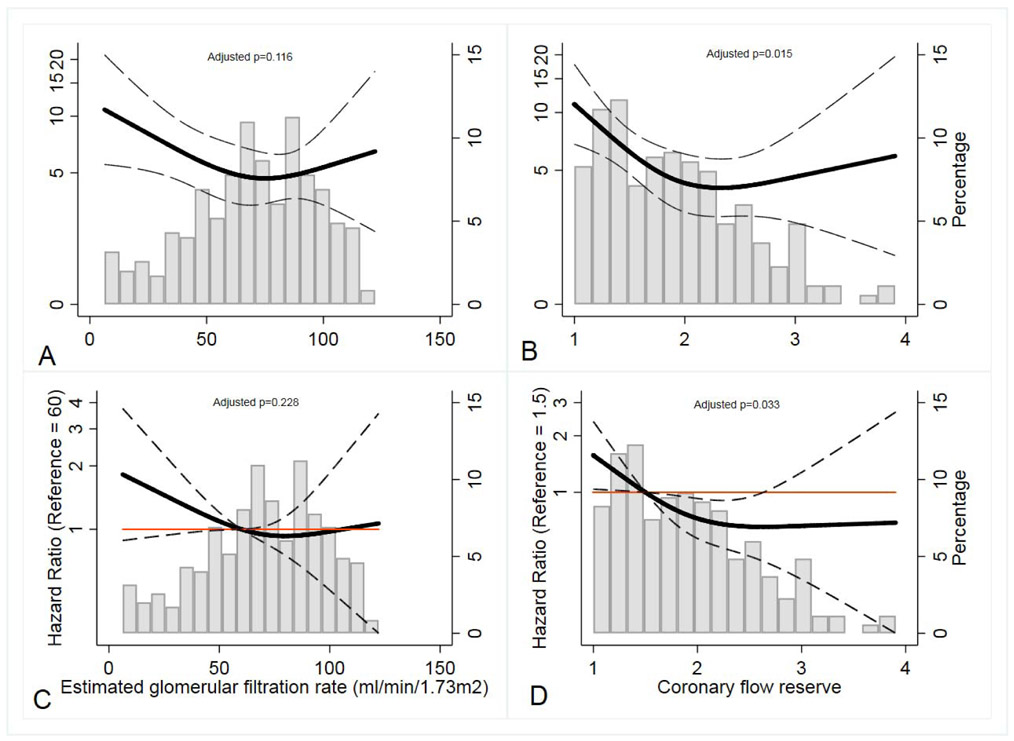
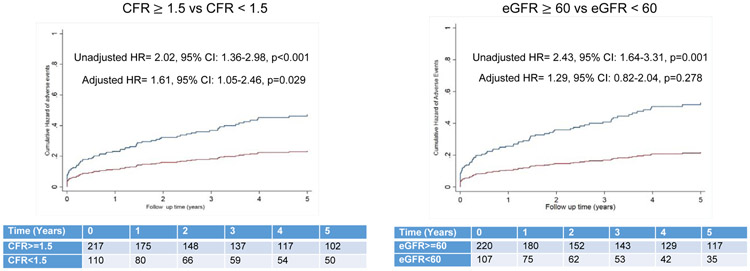
In unadjusted models, there was a significant association between the primary composite endpoint and eGFR and CFR (both p-trend<0.001). However, in multivariable models including both CFR and eGFR, only CFR (p-trend=0.015), but not eGFR (p-trend=0.116), was significantly associated with MACE (Figure 2 A & B). No significant interaction was observed between CFR and eGFR on the occurrence of MACE (p=0.840). In a stratified analysis by abnormal eGFR (<60 ml/min/1.73m2) and severely abnormal CFR (<1.5), only abnormal CFR (adjusted Hazard Ratio (HR): 1.61: 95% CI: 1.05–2.46, p=0.029), but not eGFR (adjusted HR= 1.29, 95% CI: 0.82–2.04, p=0.278), remained significantly associated with MACE (Figure 3). These results were unchanged after imputation of the missing LVMI and BMI data in 25 patients (Supplemental Table 5 & 6).
Figure 2:
Relationship between MACE, eGFR & CFR
Panel A: MACE and eGFR (Poisson model)
Panel B: MACE and CFR (Poisson model)
Panel C: MACE and eGFR (Cox proportional hazard model)
Panel D: MACE and CFR (Cox proportional hazard model)
Adjusted models included CFR (coronary flow reserve), eGFR (estimated glomerular filtration rate), age, sex, race, hypertension, hyperlipidemia, diabetes, peripheral vascular disease, stroke, indexed left ventricular mass, and resting left ventricular ejection fraction. Restricted cubic spline Poisson and Cox proportional hazard model regression model estimates with 95% confidence intervals are shown in black. The orange line in the Cox proportional hazard model is line of reference. (Gray histogram bars, secondary y-axis display % population with corresponding values of eGFR and CFR).
MACE= major adverse cardiovascular event (composite of death, non-fatal myocardial infarction and heart failure).
Figure 3.
Cumulative Hazard of MACE in Unadjusted Models stratified by abnormal CFR (<1.5) and abnormal eGFR (<60 ml/min/1.73 m2).
Hazard ratio for adjusted model was derived from model included CFR (coronary flow reserve), eGFR (estimated glomerular filtration rate), age, sex, race, hypertension, hyperlipidemia, diabetes, peripheral vascular disease, stroke, indexed left ventricular mass, and resting left ventricular ejection fraction.
MACE = major adverse cardiovascular event (composite of death, non-fatal myocardial infarction and heart failure).
To explore the potential confounding of the association between ESRD and microvascular dysfunction, we also performed a sensitivity analysis of the association between renal function and MACE after excluding the 7 patients with end stage renal disease. We observed that the association between eGFR, CFR and MACE did not change after excluding the 7 ESRD patients.
Risk stratification across categories of LV structure/function and coronary flow reserve
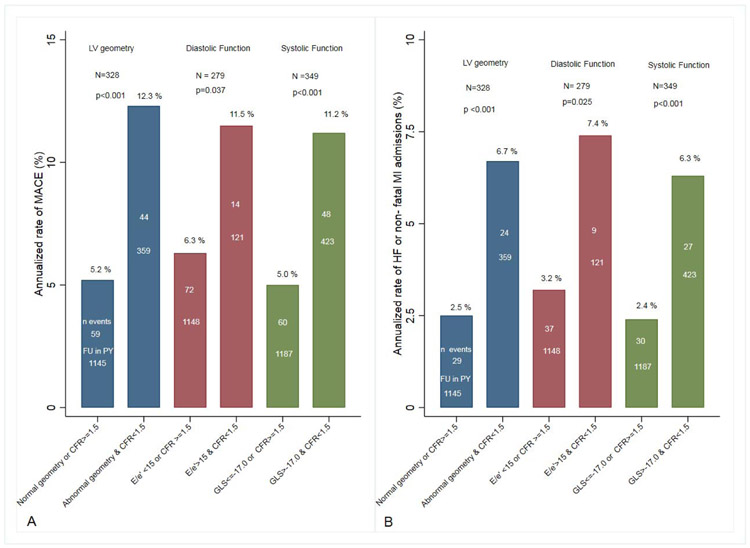
To explore the hypothesis that severe coronary microvascular dysfunction is an important determinant of the transition from adaptive to maladaptive LV remodeling in CKD, we next explored the prognostic value of abnormal CFR across categories of LV geometry, diastolic and systolic function. (Figure 4 A & B) summarizes the annualized rates of MACE and the composite of hospitalizations for heart failure and non-fatal MI by categories of CFR and LV structure and function. We observed a consistent higher rate of MACE and hospitalizations for heart failure and non-fatal MI when abnormalities in LV geometry, systolic and diastolic function coexisted with severe coronary microvascular dysfunction (all p <0.05).
Figure 4.
Annualized Rate of MACE by categories of LV structure/function by Coronary Flow Reserve (Panel A).
Annualized Rate of Heart Failure or non-fatal myocardial hospitalizations by categories of LV structure/Function by Coronary Flow Reserve (Panel B).
Higher rates of adverse events were seen when abnormalities in coronary microvascular dysfunction co-existed with abnormalities in LV geometry, systolic and diastolic function (all p<0.05). Plots and p-values were derived from Poisson regression models.
CFR= Coronary flow reserve, LV= left ventricle, GLS= peak global longitudinal strain, E = Early wave of mitral inflow, e’= early diastolic mitral annular velocity, MACE = major adverse cardiovascular event (composite of death, non-fatal myocardial infarction and heart failure).
(N= total population, n events, annualized rate and follow up in person years (FU in PY))
Mediation analysis to explore the link between renal dysfunction, impaired cardiac mechanics and adverse cardiovascular events
We assumed a biologically plausible path where CMD mediates the effect of impaired renal function on LV remodeling and adverse CV outcomes. In fully adjusted models, CMD was a significant mediator of the relationship between impaired renal function and LV diastolic dysfunction, accounting for 19–24% of the total effect; LV systolic dysfunction, accounting for 19–42% of the total effect; and LV wall stress, accounting for 7% of the total effect. Similarly, CMD was also a significant mediator of the relationship between impaired renal function and adverse cardiovascular events, accounting for 32% of the total effect in fully adjusted models (Table 4 and Supplemental Table 7).
Table 4.
Results for Mediation model for hypothesized pathway via microvascular dysfunction to LV dysfunction and adverse cardiovascular event in chronic kidney disease
| Diastolic indices | Standardized Regression coefficients for CFR in mediation model, p-value |
% effect mediated via CFR |
|---|---|---|
| Lateral E/e’ | −0.13, 0.027 | 19% |
| Septal E/e’ | −0.15, 0.005 | 24% |
| Systolic indices | ||
| GLS | −0.48, <0.001 | 42% |
| Radial | 0.27, <0.001 | 19% |
| GCS | −0.35, <0.001 | 34% |
| Markers of LV wall stress | ||
| Natural log NTproBNP | −0.20, 0.003 | 7% |
| Adverse cardiovascular event | ||
| Composite clinical endpoint (MACE) | −0.30, 0.026 | 32% |
Adjusted regression models included CFR=Coronary Flow Reserve, eGFR= estimated glomerular filtration rate using chronic kidney disease epidemiology collaboration (CKD-EPI) formula , Age, Gender, Race, Hypertension, Hyperlipidemia, Diabetes, Peripheral vascular disease, Stroke, Left ventricular mass indexed and resting left ventricular ejection fraction
LV= Left ventricle, E = Early wave of mitral inflow, e’= early diastolic mitral annular velocity, GLS= peak global longitudinal strain, GRS= peak global radial strain, GCS= peak global circumferential strain, NTproBNP = N-terminal pro B-type natriuretic peptide, MACE = major adverse cardiovascular event (composite of death, non-fatal myocardial infarction and heart failure).
Discussion
Our results show that in symptomatic patients with chronic renal impairment without overt obstructive CAD, the severity of coronary microvascular dysfunction is a significant predictor of abnormalities in left ventricular mechanics and adverse cardiovascular outcomes. The link between impaired renal function, myocardial dysfunction and cardiovascular disease events was substantially mediated by coronary microvascular dysfunction. These findings provide new and important mechanistic insights into the pathophysiology and associated clinical risk of CKD associated cardiomyopathy.
Chronic kidney dysfunction has been independently associated with a graded reduction in coronary microvascular function8, 9, 18, 28-34 and abnormal left ventricular structure and function even in the absence of obstructive coronary artery disease.5-7, 35, 36 Consequently, the current study was designed to investigate the inter-relationship between coronary microvascular dysfunction, abnormalities in cardiac structure and mechanics, and clinical outcomes. The findings in the current study suggest that coronary microvascular dysfunction is an important link between CKD, adverse left ventricular remodeling, and clinical risk. Exactly how coronary microvascular dysfunction may lead to impaired LV mechanics and increased risk cannot be determined from this study. However, there are several possible mechanisms that may help explain our findings. Chronic renal dysfunction has been associated with structural (arteriolar remodeling and capillary rarefaction) and functional (endothelial- and smooth muscle-cell dysfunction) abnormalities in the coronary microvasculature in animal models37, 38 and humans.39-41 In the setting of left ventricular hypertrophy, a very common feature in CKD-associated cardiomyopathy, abnormalities in microvascular structure and function lead to myocardial ischemia, subclinical injury and fibrosis.42, 43 The significant associations between severely impaired coronary flow reserve, measures of diastolic and systolic dysfunction independent of LV mass, and clinical outcomes in our study provide important new evidence that the development of severe microvascular dysfunction likely signals the transition from physiologic to pathologic LV remodeling that increases the risk of heart failure and death in patients with CKD. This is also supported by the fact that CFR, but not eGFR, predicted adverse LV mechanical dysfunction and clinical outcomes. In fact, the association between measures of LV mass, structure, diastolic and systolic dysfunction with severely impaired CFR identified patients at the highest risk for hospitalizations for heart failure and myocardial infarction.
To our knowledge, our study is first and largest to comprehensively explore these associations and suggest a possible pathway to development of uremic cardiomyopathy in human beings without overt ischemic heart disease. Our study is clinically important because it advances this understanding and suggests CMD as a target for novel therapeutic approaches to reduce cardiovascular disease risk in uremic cardiomyopathy. In addition, these data help validate intermediate endpoints may be helpful in design of future clinical trials in CKD patients.
Study Limitations
Our study is a single-center observational study and, as such, has some inherent limitations. First, the study cohort was identified from a clinical database of symptomatic patients referred for evaluation of suspected ischemic heart disease, thus possibly limiting the generalizability of our findings to lower risk asymptomatic individuals. Second, the patients in the study had no overt obstructive CAD on the basis of a normal myocardial perfusion PET scan with preserved LV function. In addition, patients with known CAD, as defined by a history of prior revascularization and/or myocardial infarction were excluded. A visually normal rest/stress myocardial perfusion PET scan, as used in this study, has very high sensitivity and negative predictive value to exclude significant flow-limiting coronary artery disease.44 Diffuse quantitative flow abnormalities in the context of visually normal myocardial perfusion PET scans (i.e., no perfusion defects) largely represent diffuse atherosclerosis and microvascular dysfunction.45 However, we do acknowledge that although it is conceivable that some patients in this cohort may have had some flow-limiting CAD without perfusion abnormalities, our clinical experience and the available evidence with PET suggests this to be unlikely.46, 47 Third, a positive mediation analysis, as we report here, is consistent with, but not demonstrative of, causation—particularly given that the data are cross-sectional. The mediation analyses were exploratory to guide further longitudinal or interventional studies. Although the percent mediation effect is included as hypothesis-generating, we acknowledge the fact that the results are not as robust in analysis with relatively modest sample size.48, 49 Fourth, the data on etiology of CKD in those with eGFR<60 was not available limiting our ability to determine if the associations observed in this study are modified by the cause of CKD. The mediation analyses were exploratory, to infer causation, further longitudinal or interventional studies are needed. These limitations are substantially counterbalanced by several important innovations, including the unique, demonstration of a possible pathway for development of uremic cardiomyopathy and their associations with cardiovascular disease events in human beings.
In conclusion, our study shows for the first time, an association between impaired renal function, coronary microvascular dysfunction, adverse LV remodeling and myocardial dysfunction, and subsequent risk of adverse cardiovascular events. Furthermore, our study raises the possibility that efforts to attenuate microvascular disease could produce benefits on myocardial dysfunction and cardiovascular events. Future longitudinal studies are needed to validate our findings and provide insights into how to further reduce the burden of cardiovascular events in CKD associated cardiomyopathy.
Supplementary Material
Clinical Perspective.
What is new?
Among patients with chronic kidney disease without obstructive coronary artery disease, coronary microvascular disease is associated with impaired LV mechanics and cardiovascular risk
The link between impaired renal function, myocardial dysfunction and cardiovascular disease events is partially mediated by coronary microvascular dysfunction.
What are the clinical implications?
Presence of coronary microvascular dysfunction signals the transition from physiologic to pathologic LV remodeling that increases the risk of heart failure and death in patients with CKD
Coronary microvascular disease is a potential target for novel therapeutic approaches to reduce cardiovascular disease risk in uremic cardiomyopathy
Acknowledgements:
Sources of Funding: This work was supported in part by American College of Cardiology Presidential Career Development Award to Dr Bajaj, and grants from the National Institutes of Health including T32 HL094301 to Drs. Bajaj, Zhou, Gupta, Fujikura, Bravo and Divakaran; T32 HL076136 and KL2TR002542 to Dr. Osborne; R01HL132021 to Dr. Di Carli; and K23HL135438 to Dr. Taqueti. Dr Bajaj is also supported by Walter B. Frommeyer, Junior Fellowship in Investigative Medicine and National Center for Advancing Translational Research of the National Institutes of Health under award number UL1TR001417. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American College of Cardiology or National Institutes of Health.
Abbreviations:
- CV
cardiovascular
- CKD
chronic kidney disease
- CMD
coronary microvascular dysfunction
- CAD
coronary artery disease
- PET
positron emission tomography
- CFR
Coronary flow reserve
- GLS
global longitudinal
- GRS
Global radial strain
- GCS
Global circumferential strain
- eGFR
estimated glomerular filtration rate
- LV
left ventricle
- SCD
sudden cardiac death
- MI
myocardial infarction
- PET
Positron emission tomography
- 2-D
2-dimensional
- BMI
body mass index
- SSS
summed stress score
- CT
Computed tomography
- LVEF
left ventricular ejection fraction
- MBF
myocardial blood flow
- ASE
American Society of Echocardiography
- NTproBNP
N-terminal pro–B-type natriuretic peptide
- ECG
electrocardiogram
- LVMI
left ventricular mass index
- RWT
relative wall thickness
- MACE
major adverse cardiovascular event
Footnotes
Disclosure statement:
Dr Charytan: Personal Fees for Consulting or Advisory Boards-Fresenius Medical Care, Medtronic, Amgen, Eli Lilly-Boehringer Ingelheim, Fees for service on trial steering, safety and monitoring, or clinicals event committees-PLC medical, Zoll Medical, Janssen, Merck, Allena Pharmaceuticals, Astra Zeneca. Dr. Dorbala received consulting fees from General Electric Health Care. HJ Harms has a financial interest in MedTrace Pharma, Inc. Dr. Di Carli received consulting fees from Sanofi Aventis and GE Healthcare, and investigator-initiated research grants from Spectrum Dynamics and Gilead Sciences. Other authors reported no disclosures
References
- 1.National institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. December 2016. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed on August 8, 2017.
- 2.United States Renal Data System. 2013 Atlas of CKD & ESRD. 2013. https://www.usrds.org/atlas13.aspx. Accessed on August 8,2017.
- 3.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J and Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN and Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc imaging. 2014;7:703–714. [DOI] [PubMed] [Google Scholar]
- 5.Parfrey PS and Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606–1615. [DOI] [PubMed] [Google Scholar]
- 6.Clyne N, Lins LE and Pehrsson SK. Occurrence and significance of heart disease in uraemia. An autopsy study. Scand J Urol Nephrol. 1986;20:307–311. [DOI] [PubMed] [Google Scholar]
- 7.Ikram H, Lynn KL, Bailey RR and Little PJ. Cardiovascular changes in chronic hemodialysis patients. Kidney Int. 1983;24:371–376. [DOI] [PubMed] [Google Scholar]
- 8.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Dorbala S, Charytan DM, Blankstein R and Di Carli MF. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging. 2012;5:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah NR, Charytan DM, Murthy VL, Skali Lami H, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Foster CR, Hainer J, Gaber M, Klein J, Dorbala S, Blankstein R and Di Carli MF. Prognostic Value of Coronary Flow Reserve in Patients with Dialysis-Dependent ESRD. J Am Soc Nephrol. 2016;27:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R and Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hensen LCR, Goossens K, Delgado V, Abou R, Rotmans JI, Jukema JW and Bax JJ. Prevalence of left ventricular systolic dysfunction in pre-dialysis and dialysis patients with preserved left ventricular ejection fraction. Eur J Heart Fail. 2018;20:560–568. [DOI] [PubMed] [Google Scholar]
- 12.Hensen LCR, Goossens K, Delgado V, Rotmans JI, Jukema JW and Bax JJ. Prognostic Implications of Left Ventricular Global Longitudinal Strain in Predialysis and Dialysis Patients. Am J Cardiol. 2017;120:500–504. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T and Coresh J. A new equation to estimate glomerular filtration rate. Ann Internal Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T and Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med. 2009;50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goudarzi B, Fukushima K, Bravo P, Merrill J and Bengel FM. Comparison of the myocardial blood flow response to regadenoson and dipyridamole: a quantitative analysis in patients referred for clinical 82Rb myocardial perfusion PET. Eur J Nucl Med Mol Imaging. 2011;38:1908–1916. [DOI] [PubMed] [Google Scholar]
- 16.Rossen JD, Quillen JE, Lopez AG, Stenberg RG, Talman CL and Winniford MD. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18:485–491. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, Harrington M, Hainer J, Rimoldi O, Dorbala S, Bhatt DL, Blankstein R, Camici PG and Di Carli MF. Integrated Noninvasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients With Stable Coronary Artery Disease. Circulation. 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ and Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossen JD, Quillen JE, Lopez JAG, Stenberg RG, Talman CL and Winniford MD. Comparison of coronary vasodilation with intravenous dipyridamole and adenosine. J Am Coll Cardiol. 1991;18:485–491. [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T and Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 21.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF and Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. J Nucl Med. 2005;46:1264–1271. [PubMed] [Google Scholar]
- 22.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B and Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 23.Pirat B, Khoury DS, Hartley CJ, Tiller L, Rao L, Schulz DG, Nagueh SF and Zoghbi WA. A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol. 2008;51:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU and Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS and Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N and Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–2100. [DOI] [PubMed] [Google Scholar]
- 27.Hicks R and Tingley D. Causal Mediation Analysis. Stata J. 2012;11:605–619. [Google Scholar]
- 28.Charytan DM, Shelbert HR, Di Carli MF. Coronary microvascular function in early chronic kidney disease. Circ Cardiovasc Imaging. 2010;3:663–671. [DOI] [PubMed] [Google Scholar]
- 29.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO and Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int.2006;69:266–271. [DOI] [PubMed] [Google Scholar]
- 30.Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ and Powers ER. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J. 147:1017–1023. [DOI] [PubMed] [Google Scholar]
- 31.Caliskan Y, Oflaz H, Demirturk M, Yazici H, Turkmen A, Cimen A, Elitok A and Yildiz A. Coronary flow reserve dysfunction in hemodialysis and kidney transplant patients. Clin Transplant. 2008;22:785–793. [DOI] [PubMed] [Google Scholar]
- 32.Bozbas H, Pirat B, Demirtas S, Simsek V, Yildirir A, Sade E, Sayin B, Sezer S, Karakayali H and Muderrisoglu H. Evaluation of coronary microvascular function in patients with end-stage renal disease, and renal allograft recipients. Atherosclerosis. 2009;202:498–504. [DOI] [PubMed] [Google Scholar]
- 33.Niizuma S, Takiuchi S, Okada S, Horio T, Kamide K, Nakata H, Yoshihara F, Nakamura S, Kawano Y, Nakahama H, Iwanaga Y and Nakatani S. Decreased coronary flow reserve in haemodialysis patients. Nephrol Dial Transplant. 2008;23:2324–2328. [DOI] [PubMed] [Google Scholar]
- 34.Bezante GP, Viazzi F, Leoncini G, Ratto E, Conti N, Balbi M, Agosti S, Deferrari L, Deferrari G and Pontremoli R. Coronary flow reserve is impaired in hypertensive patients with subclinical renal damage. Am J Hypertens.2009;22:191–196. [DOI] [PubMed] [Google Scholar]
- 35.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS and Shlipak MG. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambi RS, Gaur AK, Hotchandani R, Aggarwal KK, Kaur S, Gupta M, Jain S, Krishna CK, Chopra HK, Anand V, Srivastava S, Gupta R and Parashar SK. Patterns of left ventricular hypertrophy in chronic kidney disease: an echocardiographic evaluation. Indian Heart J. 2011;63:259–268. [PubMed] [Google Scholar]
- 37.Amann K, Neususs R, Ritz E, Irzyniec T, Wiest G and Mall G. Changes of vascular architecture independent of blood pressure in experimental uremia. Am J Hypertens. 1995;8:409–417. [DOI] [PubMed] [Google Scholar]
- 38.Amann K, Wiest G, Zimmer G, Gretz N, Ritz E and Mall G. Reduced capillary density in the myocardium of uremic rats--a stereological study. Kidney Int. 1992;42:1079–1085. [DOI] [PubMed] [Google Scholar]
- 39.Tatematsu S, Wakino S, Kanda T, Homma K, Yoshioka K, Hasegawa K, Sugano N, Kimoto M, Saruta T and Hayashi K. Role of nitric oxide-producing and -degrading pathways in coronary endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2007;18:741–749. [DOI] [PubMed] [Google Scholar]
- 40.Stenvinkel P Endothelial dysfunction and inflammation-is there a link? Nephrol Dial Transplant. 2001;16:1968–1971. [DOI] [PubMed] [Google Scholar]
- 41.Charytan D Is left ventricular hypertrophy a modifiable risk factor in end-stage renal disease. Curr Opin Nephrol Hypertens. 2014;23:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubin RF, Li Y, He J, Jaar BG, Kallem R, Lash JP, Makos G, Rosas SE, Soliman EZ, Townsend RR, Yang W, Go AS, Keane M, Defilippi C, Mishra R, Wolf M and Shlipak MG. Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. 2013;14:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigelman E, Cohen L, Aharon-Hananel G, Levy R, Rozenbaum Z, Saada A, Keren G and Entin-Meer M. Pathological presentation of cardiac mitochondria in a rat model for chronic kidney disease. PLoS One. 2018;13:e0198196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E, Hoffmann U and Leiner T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8:e002666. [DOI] [PubMed] [Google Scholar]
- 45.Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F and Di Carli MF. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nuc Med. 2014;55:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R and Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, Bhatt DL, Di Carli MF and Taqueti VR. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J Am Coll Cardiol. 2018;72:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackinnon DP, Warsi G and Dwyer JH. A Simulation Study of Mediated Effect Measures. Multivariate Behav Res. 1995;30:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman LS. Confidence intervals and statistical power of the ‘Validation’ ratio for surrogate or intermediate endpoints. J Stat Plan Inference. 2001;96:143–153. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.