Abstract
Introduction
Gastroenteritis in children is responsible for high morbidity and mortality. Our aim was to determine the serum and fecal levels of interleukin-6 (IL-6) and interleukin-8 (IL-8) in children with acute gastroenteritis of viral and bacterial etiology to assess their utility as diagnostic biomarkers for these infections.
Methods
In this case-control study, the children were classified according to the pathogen recovered from the stool by bacterial culture or by direct viral antigen detection by enzyme immunoassay (EIA) into 50 children with acute bacterial gastroenteritis and 50 children with acute viral gastroenteritis. In addition, 50 apparently healthy children were included as a control group. Blood and stool samples were subjected to detection of IL-6 and IL-8.
Results
There were statistically significant elevations of total leucocytes counts, absolute neutrophils count, C-reactive protein, serum IL-6 and serum IL-8 in children with gastroenteritis compared to healthy children (p<0.001). CRP, serum IL-6 and IL-8 had significantly elevated levels in children with bacterial gastroenteritis compared to viral gastroenteritis. Fecal IL-6 and IL-8 had significantly elevated levels in children with acute gastroenteritis than in healthy control (p<0.001). The area under the curve (AUC) showed that CRP and serum IL-6 could be used as discriminative markers for acute bacterial gastroenteritis in children, in comparison to serum IL-8.
Conclusions
Elevated serum IL-6 and CRP can aid in differentiation between viral and bacterial gastroenteritis. Serum IL-8 had limited discrimination ability between viral and bacterial gastroenteritis. Stool levels of IL-6 and IL-8 were elevated in children with viral and bacterial gastroenteritis, however, their assessment by enzyme linked immunosorbent assay had technical limitations to be used as differentiation biomarkers.
Keywords: Fecal IL-6, IL-8, bacterial gastroenteritis, AUC
Introduction
Gastroenteritis in children is a common infectious disease that is associated with marked morbidity and mortality representing 20% of all pediatric deaths.1 The etiology of this infection can be viral such as rotavirus, norovirus, astrovirus and enteric adenovirus, bacterial such as Salmonella, Shigella, Escherichia coli, or protozoa, mainly due to fecal-oral route.2-4
The accurate and rapid diagnosis of the etiology of acute diarrhea in children is a must to prevent the improper use of antibiotic therapy. The differentiation between bacterial or viral etiology based upon clinical features is difficult due to the indifference of the clinical symptoms.5 Moreover, the laboratory diagnosis depending on presence of occult blood and leucocytes in the stool may not even give conclusive results. The gold standard diagnosis of bacterial gastroenteritis is the time-consuming stool culture.6
There are various cytokines that can be used as biomarkers of acute infections. A cascade of cytokines that involves interleukin-6 (IL-6), interleukin-8 (IL-8) and tumor necrosis factor-α is activated in response to the infection.5,7 Interleukin-6 is a multifunction cytokine produced by lymphoid and non-lymphoid cells and has a variety of functions including the regulation of immunity, the acute-phase response and hematopoiesis.8-10 Interleukin-8 is responsible for chemotaxis of the inflammatory cells like neutrophils and lymphocytes to the site of inflammation.11 Both cytokines have a vital role in immunity to the mucosal infections.12
The use of IL-6 and IL-8 in serum as diagnostic biomarkers for the etiology of gastroenteritis depends upon several factors such as the time of release and maintenance of the elevated levels and the type of samples that can be used for such assessment.8,10 There are limited studies about the use of IL-6 and IL-8 as diagnostic biomarkers for acute gastroenteritis in children.6,7
The present work aimed to detect the levels of IL-6 and IL-8 in the serum and stool of children with acute bacterial and viral gastroenteritis to assess their utilities as diagnostic biomarkers for these infections.
Methods
This study was a case-control study that included children complaining of acute diarrhea recruited from Mansoura University Children’s Hospital, Egypt from March 2018 until March 2019. The children were classified according to the pathogen recovered from the stool by the bacterial culture or by the direct viral antigen detection using enzyme immunoassay (EIA) into 50 children with acute bacterial gastroenteritis and 50 children with acute viral gastroenteritis.
The inclusion criteria were children with acute diarrhea with good nutritional status before the acute episode. Exclusion criteria were children with history of antibiotic therapy in the previous 72 hours, malnutrition, malignancy or acute diarrhea associated with systemic infections. In addition, 50 apparently healthy children with no history of antibiotic therapy in the previous 72 hours were included as a control group. The control children attended the hospital clinics for routine medical check and investigations.
The study was approved by Mansoura Faculty of Medicine Ethical Committee (R.19.8.576) and signed informed consent was obtained from the parents of each child including control children. Each participating child was subjected to complete history questionnaire and clinical examination.
Stool samples
Stool sample was obtained from each child in a clean container and transported to the laboratory within 30 minutes. The stool sample was subjected to microscopic examination for parasite infestation according to the standard technique and detection of occult blood by the slide test (Hema-Screen Lab Pack, EKF Diagnostics, Boerne, TX, USA). The negative slide test indicated no occult blood in stool, the positive slide test indicated + blood in stool and the apparent blood in the sample indicated +2 blood in stool.
Stool culture was performed according to the standard microbiological techniques by culture the stool on xylose lysine deoxycholate agar, blood agar, Clostridium difficile Selective Agar and Campy-BAP media (Becton Dickinson, Sparks, MD, USA).
Viral antigens detection for rotavirus, norovirus, adenovirus and astrovirus were carried out by specific enzyme immunoassay (Ridascreen; R-Biopharm AG, Darmstadt, Germany) according to the manufacturer’s guide.
Stool samples were subjected to an extraction process for further determination of IL-6 and IL-8. Briefly, the stool sample was diluted with phosphate buffer saline with phenylmethylsulfonyl fluoride (1 mg/mL; Wako Pure Chemical Industries Ltd, Osaka, Japan) and soy trypsin inhibitor (1mg/mL; Wako Pure Chemical Industries Ltd) then a centrifugation was performed for 15 minutes. The supernatant was filtered by a microbiological filter 0.45 μm (Minisart N; Sartorius, Gottingen, Germany) then stored at -80°C until the time of the assay.13
Blood samples
Five-milliliter blood samples were obtained from the children within the first three days of the acute diarrhea and divided into two aliquots. One aliquot was withdrawn over EDTA for determination of total leucocytes counts (WBCs) by Sysmex system (Sysmex Corporation, Kobe, Japan) and the absolute neutrophils count (ANCs) was calculated by the following formula: WBC count X (% bands + % neutrophils) X 0.01. Sera were separated from the second blood aliquots and subjected to determination of C-reactive protein (CRP) by the turbidimetry (Quantia-CRP US; Tulip Diagnostics, Alto Santa Cruz, Goa, India). Then sera were stored at-80°C for subsequent determination of IL-6 and IL-8 by ELISA according to the manufacturer’s guide (Quantikine ELISA, R&D Systems, Inc., Minneapolis, MN, USA).
Statistical analysis
The data were analyzed by SPSS 24 (Statistical Package of Social Sciences, Version 24, IBM Corp, 2016, Armonk, NY, USA). Quantitative data were expressed as mean± standard deviation (SD) and qualitative data were expressed as numbers and percentages. The comparison between categorical variables was performed by Chi-square test or Fisher’s exact test, as appropriate. The Mann-Whitney test was used to compare continuous variables between two groups. A p value less than 0.05 was considered statistically significant. To evaluate the accuracy of the diagnostic tests and level of serum cytokines, the receiver operating characteristic (ROC) curve was plotted and the areas under the curves (AUCs) were calculated for comparison.
Results
This study included 100 children with acute gastroenteritis: 50 children with acute bacterial gastroenteritis and 50 children with acute viral gastroenteritis, in addition to 50 children as a healthy control group. There was a significant difference in the age of the studied children, with younger age for children with acute viral gastroenteritis (3.4±1.2 years) and older age for children with acute bacterial gastroenteritis (6.2±2.2 years), Table 1.
Table 1. Demographic, clinical and laboratory results of the studied groups with acute gastroenteritis.
| Bacterial gastroenteritis group (n=50) | Viral gastroenteritis group (n=50) | Control group (n=50) | P value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 28 (56%) | 25 (50%) | 28 (56%) | 0.973* |
| Female | 22 (44%) | 25 (50%) | 22 (44%) | |
| Age | 6.2±2.2 | 3.4±1.2 | 5.3±2.3 | p<0.001** |
| Abdominal pain | 19 (38%) | 19 (38%) | - | 0.582* |
| Fever | 29 (58%) | 23 (46%) | - | 0.580* |
| Blood in stool | 41 (82%) | 11 (22%) | - | p<0.001** |
| Pus in stool | 29 (58%) | 17 (34%) | - | p<0.001** |
| Bacterial pathogens | ||||
| E. coli | 22 (44%) | |||
| Salmonella Typhi | 13 (26%) | |||
| Campylobacter jejuni | 8 (16%) | |||
| Clostridioides difficile | 7 (14%) | |||
| Viral pathogens | ||||
| Rotavirus | 22 (44%) | |||
| Norovirus | 15 (30%) | |||
| Adenovirus | 10 (20%) | |||
| Astrovirus | 7 (14%) | |||
| Total leucocytes count ×103/cmm | 9.9±1.9 | 9.4±2.3 | 6.1±1.0 | p<0.001** p1=0.239** |
| Absolute neutrophil count ×103/cmm | 7.1±2.1 | 6.4±1.5 | 3.6±1.1 | p<0.001** p1=0.058** |
| CRP, mg/dL | 8.7±2.1 | 5.1±2.1 | 0.2±0.07 | p<0.001** p1<0.001** |
| Serum IL-6, pg/mL | 44.8±15.1 | 11.1±7.2 | 0.3±0.1 | p<0.001** p1<0.001** |
| Serum IL-8, pg/mL | 30.2±18.5 | 22.9±10.7 | 1.9±0.2 | p<0.001** p1=0.016** |
Data is presented as n (%) or mean±standard deviation.
Pearson Chi-square test.
ANOVA test.
p: Comparison between patients with bacterial gastroenteritis, viral gastroenteritis and controls.
p1: Comparison between patients with bacterial gastroenteritis and viral gastroenteritis.
The prevalence of blood (>+2) in stool was significantly higher in children with acute bacterial gastroenteritis (82%) in comparison to children with acute viral gastroenteritis (22%), p<0.001. Moreover, the prevalence of pus in the stool samples was significantly more prevalent in children with acute bacterial gastroenteritis (58%) than children with acute viral gastroenteritis (34%), p<0.001, Table 1.
The most frequent bacterial pathogens isolated from the stool culture were E. coli (44%), followed by Salmonella Typhi (26%), while the most frequent viral pathogens were rotavirus (44%) followed by norovirus (30%) as shown in Table 1 (some stool samples revealed mixed viral infections, while mixed viral and bacterial infections were excluded). There were statistically significant elevations of WBCs, ANC, CRP, serum IL-6 and serum IL-8 in children with gastroenteritis compared to the control group (p<0.001). The levels of CRP, IL-6 and IL-8 were significantly elevated in sera of children with bacterial gastroenteritis compared to children with viral gastroenteritis (Table 1).
Tables 2 and 3 summarize the values of IL-6 and IL8 in the stool of the studied children. Interleukin-6 was detected in the stool of 7 healthy children, 40 children with acute viral gastroenteritis and 34 children with acute bacterial gastroenteritis. The level of IL-6 in the stool of the children with acute gastroenteritis was significantly elevated in comparison to the healthy children, p<0.001. Interleukin-8 was detected in 11, 10 and 10 stool samples of the children from the control, acute viral gastroenteritis and acute bacterial gastroenteritis groups, respectively. The level of IL-8 in the stool of the children with acute gastroenteritis was significantly elevated compared to the children of the healthy control group, p<0.001.
Table 2. Comparison of the level of interleukin (IL)-6 in the stool of children of the control, acute bacterial gastroenteritis and acute viral gastroenteritis groups.
| Bacterial gastroenteritis group (n=34) | Viral gastroenteritis group (n=40) | Control group (n=7) | P value | |
|---|---|---|---|---|
| Stool IL-6 median (interquartile range), pg/mL | 0.3 (0.2-0.3) | 0.1 (0.03-2.0) | 0.1 (0.1-0.12) | p<0.001* |
Mann-Whitney U test.
Table 3. Comparison of the level of interleukin (IL)-8 in the stool of children of the control, acute bacterial gastroenteritis and acute viral gastroenteritis groups.
| Bacterial gastroenteritis group (n=10) | Viral gastroenteritis group (n=10) | Control group (n=11) | P value | |
|---|---|---|---|---|
| Stool-IL-8 interquartile range, pg/mL | 2.5-0.7 | 4.7-1.8 | 1.9-0.2 | p<0.001* |
Mann-Whitney U test.
The diagnostic values of serum markers such as CRP, IL-6 and IL-8 to discriminate between viral gastroenteritis and bacterial gastroenteritis were evaluated by the ROC curve analysis. There was a high discriminative value as determined by AUC for CRP and IL-6 (AUC 0.870 and 0.971, respectively) and a low discriminative value for IL-8 (AUC 0.600, Table 4). The optimal cut-off value for CRP was 15 mg/dL with sensitivity and specificity 96% and 88%, respectively. The optimal cut-off value for IL-6 was 10.5 pg/mL with sensitivity and specificity 100% and 58%, respectively, while the optimal cut-off value for IL-8 was 21 pg/mL with sensitivity 56% and specificity 44% (Figure 1).
Table 4. Area under the curves for CRP, IL-6 and IL-8 to differentiate acute bacterial gastroenteritis from acute viral gastroenteritis.
| Test Variables | AUC | Standard error | Asymptotic significance | 95% confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| CRP | 0.870 | 0.034 | <0.001 | 0.803 | 0.937 |
| IL-6 | 0.971 | 0.013 | <0.001 | 0.945 | 0.997 |
| IL-8 | 0.600 | 0.060 | 0.085 | 0.482 | 0.717 |
AUC – area under the curve; CRP – C-reactive protein; IL – interleukin.
Figure 1. ROC curve analysis for CRP, IL-6 and IL-8 to differentiate acute bacterial gastroenteritis from acute viral gastroenteritis.
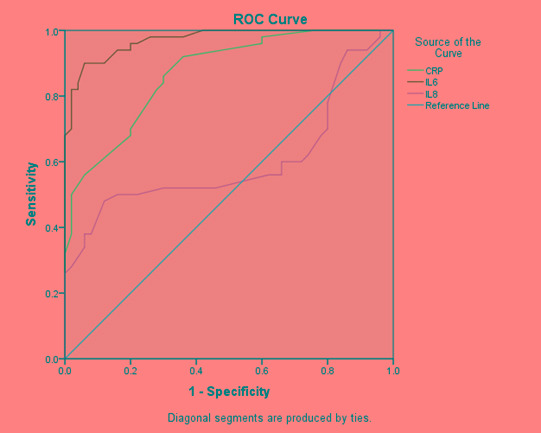
CRP – C-reactive protein; IL – interleukin; ROC – receiver operating characteristic curve
Discussion
The diagnosis of the etiology of acute diarrhea is mandatory for appropriate management and to avoid unnecessary antibiotics use. For rapid screening tests such as fecal blood and presence of leucocytes, although they are rapid, their results are contradictory and not specific. The role of cytokines such as IL-6 in the defense mechanisms in gastroenteritis is well-known. The pro-inflammatory cytokines stimulate macrophages and neutrophils to produce the reactive oxygen species to fight the infection. Certain cytokines such as IL-6 and IL-8 are released from the epithelium of the gastrointestinal tract to produce its inflammatory responses to infections locally and systemically.13
In the present study, E. coli and Salmonella Typhi were the most prevalent pathogens causing bacterial gastroenteritis, while rotavirus and norovirus were the main causes of acute viral gastroenteritis in accordance with many literatures.5-7,14 Blood and pus in stool were significantly higher in children with acute bacterial gastroenteritis in comparison to children with acute viral gastroenteritis in agreement with a previous study.7
The present work studied the use of IL-6 and IL-8 with other acute markers of inflammation as rapid distinguishing markers for the differential diagnosis of viral from bacterial gastroenteritis in children.
In this work, the common acute phase reactant CRP, which is routinely measured in children with acute infections, had sensitivity and specificity 96% and 88%, respectively, with a cut-off value 15 mg/dL to differentiate between the acute bacterial and viral gastroenteritis. In another literature using the ROC curve analysis, the CRP cut-off value 2.0 mg/dL had sensitivity and specificity 91.3% and 81.8%, respectively,7 while using cut-off ≥ 2 mg/dL, the sensitivity and specificity of CRP were 83.3% and 76.2%, respectively, in a different work.5 On the other hand, a CRP cut-off >13 mg/L had sensitivity and specificity 54% and 72%, respectively.14 Some literatures had suggested that CRP alone could not differentiate between bacterial and viral gastroenteritis14 and its sensitivity as a discriminative marker increased when combined with another diagnostic marker such as IL-6 and IL-8.5
In the current study, serum IL-6 was significantly increased in all children with gastroenteritis in comparison to the healthy children and its level was found to be significantly elevated in bacterial gastroenteritis compared to viral gastroenteritis. These findings were in line with previous reports that had documented the value of IL-6 level measurement in differentiating bacterial gastroenteritis from other causes of gastroenteritis including viral gastroenteritis.5-7,14 The increase of IL-6 as a part of acute phase response with prolonged persistence in the circulation assists in the destruction or inhibition of microorganisms.14-15
The optimal cut-off value for serum IL-6 was 10.5 pg/mL with sensitivity and specificity 100% and 58%, respectively. This cut-off value was slightly higher than previous data with better sensitivity but with lower specificity. The discrepancy between the results of the present study and the former one can be attributed to the temporal release of cytokines after challenge by endotoxins with difference in the values according to the time of assessment, being higher within 36 to 72 hours.6,14
In the present work, the level of IL-6 in the stool was significantly elevated in a considerable number of children with gastroenteritis in relation to the control group. This finding reflects direct mucosal release of IL-6 during the infections. However, the release of IL-6 was not detected in all the studied children and this may be attributed to several factors such as degradation of the cytokines in the stool or dilution of the cytokine in the patients with diarrhea.7,16,17
The present study evaluated the value of serum IL-8 as a biomarker that can be used to differentiate between bacterial and viral gastroenteritis. Although the serum level was statistically significant increased in children with gastroenteritis compared to the control group, its discriminating value was poor as confirmed by the ROC curve analysis with the area under the curve being 0.6. On the other hand, serum IL-6 and CRP had a good discriminative ability between bacterial and viral gastroenteritis as reflected by the AUC. These findings were in line with previous reports that had stated that serum IL-6 and CRP were good markers while IL-8 was a less valuable tool in differentiation between bacterial and viral gastroenteritis.5,7
The level of IL-8 was significantly increased in stool samples of children with acute gastroenteritis than in the control children; however, it was detected in a low number of the studied children. This may be related to the low concentration of this cytokine in stool samples below the detection limit of the ELISA kit used for the detection.
Conclusions
The present study concludes that differentiation between viral gastroenteritis and bacterial gastroenteritis can rely upon the elevated serum IL-6 and CRP. Serum IL-8 had limited discrimination ability between viral and bacterial gastroenteritis. Stool levels of IL-6 and IL-8 were elevated in children with viral and bacterial gastroenteritis, however, their assessment by ELISA had technical limitations to be used as differentiation biomarkers.
Acknowledgements
The authors would like to thank all support staff and participating patients in this study.
Footnotes
Authors’ contributions statement: All authors have contributed equally to the study and manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
References
- 1.Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet. 2010;375:870–2. doi: 10.1016/S0140-6736(09)61798-0. [DOI] [PubMed] [Google Scholar]
- 2.Muhsen K, Kassem E, Rubenstein U, et al. No evidence of an increase in the incidence of norovirus gastroenteritis hospitalizations in young children after the introduction of universal rotavirus immunization in Israel. Hum Vaccin Immunother. 2019;15:1284–93. doi: 10.1080/21645515.2019.1599522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramani S, Kang G. Viruses causing childhood diarrhea in the developing world. Curr Opin Infect Dis. 2009;22:477–82. doi: 10.1097/QCO.0b013e328330662f. [DOI] [PubMed] [Google Scholar]
- 4.Dennehy PH. Viral gastroenteritis in children. Pediatr Infect Dis J. 2011;30:63–4. doi: 10.1097/INF.0b013e3182059102. [DOI] [PubMed] [Google Scholar]
- 5.Lin CH, Hsieh CC, Chen SJ, Wu TC, Chung RL, Tang RB. The diagnostic value of serum interleukins 6 and 8 in children with acute gastroenteritis. J Pediatr Gastroenterol Nutr. 2006;43:25–9. doi: 10.1097/01.mpg.0000235764.30743.5b. [DOI] [PubMed] [Google Scholar]
- 6.Herlina H, Manoppo J, Umboh A. Bacterial enteric pathogens and serum interleukin-6 levels in children with acute diarrhea. Paediatr Indones. 2016;56:144–8. doi: 10.14238/pi56.3.2016.144-8. [DOI] [Google Scholar]
- 7.Chen SM, Lin CP, Tsai JD, Chao YH, Sheu JN. The significance of serum and fecal levels of interleukin 6 and interleukin 8 in hospitalized children with acute rotavirus and norovirus gastroenteritis. Pediatr Neonatol. 2014;55:120–6. doi: 10.1016/j.pedneo.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Rankin JA. Biological mediators of acute inflammation. AACN Clin Issues. 2004;15:3–17. doi: 10.1097/00044067-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann L, Kagnoff MF. Cytokines in host defense against Salmonella. Microbes Infect. 2001;3:1191–200. doi: 10.1016/S1286-4579(01)01479-4. [DOI] [PubMed] [Google Scholar]
- 10.Song M, Kellum JA. Interleukin-6. Crit Care Med. 2005;33:S463–5. doi: 10.1097/01.CCM.0000186784.62662.A1. [DOI] [PubMed] [Google Scholar]
- 11.Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391–8. [PubMed] [Google Scholar]
- 12.Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defense. Curr Opin Microbiol. 1999;2:99–103. doi: 10.1016/S1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- 13.Saiki T, Mitsuyama K, Toyonaga A, Ishida H, Tanikawa K. Detection of pro- and anti-inflammatory cytokines in stools of patients with inflammatory bowel disease. Scand J Gastroenterol. 1998;33:616–22. doi: 10.1080/00365529850171891. [DOI] [PubMed] [Google Scholar]
- 14.Yeung CY, Lee HC, Lin SP, et al. Serum cytokines in differentiating between viral and bacterial enterocolitis. Ann Trop Paediatr. 2004;24:337–43. doi: 10.1179/027249304225019163. [DOI] [PubMed] [Google Scholar]
- 15.Vaisman N, Leibovitz E, Dagan R, Barak V. The involvement of IL-6 and IL-8 in acute invasive gastroenteritis of children. Cytokine. 2003;22:194–7. doi: 10.1016/S1043-4666(03)00177-7. [DOI] [PubMed] [Google Scholar]
- 16.Hamiel U, Bahat H, Kozer E, Hamiel Y, Ziv-Baran T, Goldman M. Diagnostic markers of acute infections in infants aged 1 week to 3 months: a retrospective cohort study. BMJ Open. 2018;8:e018092. doi: 10.1136/bmjopen-2017-018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko G, Jiang ZD, Okhuysen PC, DuPont HL. Fecal cytokines and markers of intestinal inflammation in international travelers with diarrhea due to noroviruses. J Med Virol. 2006;78:825–8. doi: 10.1002/jmv.20630. [DOI] [PubMed] [Google Scholar]


