Abstract
Introduction
Medical students who engage in clinical learning in healthcare settings can be potential methicillin-resistant Staphylococcus aureus (MRSA) carriers.
Methods
This is a descriptive cross-sectional study having a follow-up approach. Three batches of medical students who were studying at the Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka (1st, 3rd and 5th study years of MBBS course) were screened for nasal and axillary MRSA colonization. The first-year students were screened before and 6 months after clinical learning. The knowledge of the students about infection control was scored (percentage) using a questionnaire in the one week before and later one year after the hospital exposure. Data was compared using two-sample t test.
Results
The percentage of MRSA colonization was 6.36% (7/110) and 49.57% (59/119) before clinical exposure and after 2.5 years of exposure, respectively (p<0.012). The percentage of correct responses obtained by the students for the questionnaire about infection control was 28% and 66.9% one week before the exposure to the hospitals and one year after the exposure to the hospitals, consecutively.
Conclusions
MRSA carriage was significantly associated with the time duration of the clinical training of the medical students. The knowledge of students about infection control was significantly inadequate one week before the hospital exposure and they have gained the knowledge only after being exposed to the hospitals.
Keywords: Clinical learning activities, medical students, MRSA colonization
Introduction
Staphylococcus aureus is associated with both community and hospital-acquired infections.1 The spectrum of the diseases caused by S. aureus varies from mild skin infection to endocarditis or life-threatening septicemia.2 Methicillin-resistant S. aureus (MRSA), widely known as the superbug, was first reported in the early 1960s.3 Within 10-12 years, it rapidly spread in the community and healthcare settings.4 At present, MRSA is endemic in healthcare settings globally, which results in a heavy disease burden.4,5
MRSA can be found as a common skin colonizer in healthy individuals. In addition to that, MRSA colonizes the anterior nares, axilla, and perineum. The person who is colonized with MRSA could transmit the organism to others via skin to skin contact or contaminating the materials. The MRSA carriers can be transient, intermittent, or persistent.5 The colonization can persist for days to years, influenced by the host immune response, competitive inhibition from other microbes in residential flora, and selection pressure with the use of antimicrobials.6 Persistent carriers are more heavily colonized at multiple sites. Thus, persistent carriers are more likely to transmit the organism to others and are more likely to become infected themselves than transient carriers. In fact, MRSA carriage is a marker that reflects someone’s vulnerability to contain and transmit medically essential pathogens in the healthcare setting.7
A patient who acquires MRSA during a hospital stay has an increased risk for MRSA infections following discharge or during subsequent hospitalizations. MRSA carriers also serve as a reservoir for further transmission as they move through and across different units for specific treatments, especially in tertiary care hospitals.
MRSA screening and decolonization strategies are applied to inpatients waiting for cardiac, orthopedic, and prosthesis implantation surgeries.8 At present, screening and decolonization of clinical staff for MRSA are not routinely practiced.6 In healthcare settings, main MRSA carriers are the healthcare personnel, including doctors, nurses, and other support staff. Exposure to the hospital environment of a prolonged duration would have been the cause of the increased carriage of MRSA among these healthcare professionals.5-7 Another group belonging to this category is that of the medical students who conduct clinical examinations in relation to various clinical appointments as part of their training. The clinical training is initiated at the latter half of the second year and most of the time these medical students are not closely monitored for proper hygienic practices and inadequately educated about infection control prior to their clinical training. This fact raised our concern about assessing MRSA carriage among the medical students, who have been exposed to the hospital environment.
Methods
Study setting and participants
This is a cross-sectional study having a follow-up approach regarding a cohort of medical students. The study was conducted from January 2015 to January 2017. These students undergo their clinical training at Teaching Hospital Anuradhapura (THA), Sri Lanka. THA is the main teaching hospital affiliated to the Rajarata University of Sri Lanka. This hospital is an 1839 bedded tertiary care medical facility consisting of a university professorial teaching unit; it is located in Anuradhapura, North Central Province of Sri Lanka.9
Three batches of medical students (1st, 3rd, and 5th year of their MBBS degree course) who were studying at the Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka were screened for MRSA colonization. Moreover, the 1st year students were re-screened after 6 months of starting their clinical training at Teaching Hospital, Anuradhapura. Third year medical students were at the 5th month of exposure to healthcare settings while fifth year students were at the 30th month. The ethical clearance for this study was obtained from the Ethics and Research Committee (ERC), Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka.
MRSA and S. aureus isolation
The study procedure was explained to the medical students, and the informed consent was obtained following the ethical procedure as stipulated by the ERC of the Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka.
The samples from both anterior nares (using one swab) and both axillae (using another swab) were obtained from the medical students using sterile cotton swabs. The swabs were moistened using sterile normal saline. Swabs were labeled as nasal or axillary according to the body site. The swabs were dipped into the labeled tubes containing 7.5% NaCl in BHI broth and followed by overnight incubation at 37°C in ambient air incubator. All the swabs were sub-cultured in blood agar and MacConkey agar, simultaneously.10
Blood agar plates having growth were sub-cultured in Mannitol salt agar for further differentiation of Staphylococcus aureus from Staphylococcus epidermidis. The presence of S. aureus was confirmed using DNAse and tube coagulase tests. The selected and confirmed S. aureus cultures were used to detect methicillin resistance using oxacillin agar plate dilution method.11 The S. aureus strains having a minimal inhibitory concentration of oxacillin (MIC) ≥4 mg/L were taken as MRSA.12 According to CLSI-m100-s28 manual, the disc diffusion test was performed to assess the antimicrobial susceptibility against antibiotics other than oxacillin (doxycycline, sulfamethoxazole-trimethoprim (SXT), clindamycin, erythromycin, ciprofloxacin, and levofloxacin).
Data collection
Demographical data were collected using a questionnaire (Appendix), querying about any hospital stay, past medical/surgical history, and use of deodorants and topical application of anti-MRSA antibiotics over the past 6 months. The knowledge of the students about infection control was assessed, scored (as percentage) and compared using a questionnaire, 1 week before hospital exposure, and 1 year after hospital exposure.
Data analysis
The data were double-checked and transported to SAS 9.1 (2005 New Jersey) for statistical analysis.12 Continuous data were expressed in measures of central tendency. MRSA and S. aureus colonization rates were analyzed using a Chi-squared test and scores in the questionnaire were analyzed using two-sample T test. Further, Chi-squared test was performed to detect the association with a duration of clinical exposure and MRSA carriage.
Results
The mean age of participants was 22±2.1 years. The percentages of MRSA colonization according to the gender were: 31.5% in male students and 26.5% in female students.
Longitudinal evaluation of first year medical students
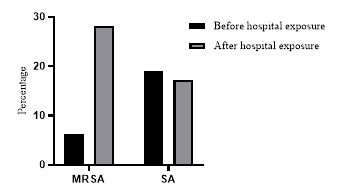
The percentage of MRSA colonization was 6.4% (7/110) in the batch in their 1st year of study before having been exposed to healthcare settings. Among MRSA colonized students, the nasal colonization was detected in all 7 positive students, and only 4 students had axillary colonization. Following 6 months of exposure to the healthcare settings, in the same batch, MRSA colonization was increased up to 28.1% (31/110). All of them had nasal colonization, while only 19 had axillary colonization. The MRSA and S. aureus acquisition following clinical exposure was significant. (p=0.011, Chi(2)=51.11). Following 6 months of their clinical training, the percentage of S. aureus colonization (17.2%) was significantly lower than the percentage of MRSA colonization (p=0.003, Chi(2)=26.11) - Figure 1.
Figure 1. Proportions of methicillin resistant Staphylococcus aureus (MRSA) and Staphylococcus aureus (SA) colonization among 1st year medical students before and after 6 months of exposure to healthcare settings.
Cross-sectional analysis of third year medical students
The 3rd year students who had 5 months of exposure to the healthcare settings showed 25.4% (29/114) MRSA colonization. This percentage was roughly similar to the above-described other group of students (follow-up screening of 1st year students) who had 6 months of exposure to healthcare settings. Nasal colonization was observed in all 29 (25.4%) MRSA positive students, and only 19 (16.6%) of them had axillary colonization.
Cross-sectional analysis of fifth year medical students
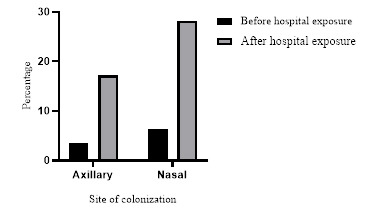
The 5th year students who were exposed to healthcare settings for 2 years and 6 months duration showed 49.6% (59/119) MRSA colonization; nasal colonization was observed in all 59 positive students, and only 46 had axillary colonization (Figure 2). Nasal colonization (34.6%) by MRSA was significantly higher than axillary colonization (24.5%) [p=0.002, Chi(2)=31.12].
Figure 2. Proportions of axillary and nasal methicillin resistant Staphylococcus aureus colonization in 1st year medical students before and after 6 months of exposure to healthcare settings.
MRSA colonization among medical students was significantly increased with the length of duration of their exposure to healthcare settings [p<0.001, Chi(2)=53.74] (Figure 3).
Figure 3. Proportion of methicillin resistant Staphylococcus aureus (MRSA) and Staphylococcus aureus (SA) among medical students following zero (1st year), 6 months (1st follow-up and 3rd year) and 30 months (5th year) of exposure to healthcare settings.

All MRSA isolates were susceptible to vancomycin, teicoplanin, and linezolid (Table 1). Based on the MIC of oxacillin, most MRSA isolates (71.4%) had low-level oxacillin resistance (MIC in the range of 4 to <16 mg/L) in first year medical students before the exposure to healthcare settings. In the same group after 5-months of healthcare exposure, 53.3% of isolates showed MIC of oxacillin in the range of 16 to <128 mg/L. In the fifth-year medical students following 30 months of healthcare exposure, 33.9% showed MIC >128 mg/L. Moreover, among MRSA isolates recovered from students before exposure to the healthcare settings, all were susceptible to doxycycline, sulfamethoxazole-trimethoprim (SXT), clindamycin, erythromycin, ciprofloxacin, and levofloxacin. Isolates recovered from students who had 30 months of clinical exposure had significant levels of antimicrobial resistance to all tested antibiotics except for SXT: doxycycline [p=0.012, Chi(2)=26.21]; clindamycin [p=0.011, Chi(2)=22.31]; erythromycin [p=0.012, Chi(2)=28.11]; ciprofloxacin [p=0.031, Chi(2)=18.31]; levofloxacin [p=0.042, Chi(2)=14.73]; gentamicin [p=0.011, Chi(2)=19.21]; chloramphenicol [p=0.021, Chi(2)=21.32] and fusidic acid [p=0.011, Chi(2)=32.74] (Table 1).
Table 1. Comparison of antimicrobial resistance patterns of MRSA isolates among medical students over the duration of clinical exposure.
| Antibiotic | Number and percentage (%) of resistant strains in 1st year students, first evaluation (n=112) | Number and percentage (%) of resistant strains in 1st year students, 6 months follow-up and 3rd year students with clinical exposure for 6 months (n=226) | Number and percentage (%) of resistant strains in 5th year students (30 months of clinical exposure) (n=119) | Statistical analysis |
|---|---|---|---|---|
| MRSA (n=7) | MRSA (n=60) | MRSA (n=59) | ||
| Oxacillin MIC range (mg/L)4 to <16 | 5 | 12 | 14 | p=0.081 |
| 16 to <128 | 2 | 32 | 25 | p=0.032, Chi(2)=18.74 |
| >128 | 0 | 16 | 20 | p=0.021, Chi(2)=24.34 |
| DOX | 0 (0%) | 12 (20%) | 39 (66.1%) | p=0.012, Chi(2)=26.21 |
| SXT | 0 (0%) | 6 (10%) | 12 (20%) | p=0.075 |
| DA | 0 (0%) | 6 (10%) | 24 (40.6%) | p=0.011, Chi(2)=22.31 |
| E | 0 (0%) | 6 (10%) | 26 (44.1%) | p=0.012, Chi(2)=28.11 |
| CIP | 0 (0%) | 18 (30%) | 44 (74.5%) | p=0.031, Chi(2)=18.31 |
| LEV | 0 (0%) | 12 (20%) | 24 (40.6%) | p=0.042, Chi(2)=14.73 |
| GEN | 2 (28.5%) | 11 (18.3%) | 32 (54.2%) | p=0.011, Chi(2)=19.21 |
| C | 1 (14.4%) | 4 (6.6 %) | 22 (37.2 %) | p=0.021, Chi(2)=21.32 |
| FA | 0 (0%) | 2 (3.3 %) | 18 (31%) | p=0.011, Chi(2)=32.74 |
C – chloramphenicol; CIP – ciprofloxacin; DA – clindamycin; DOX – doxycycline; E – erythromycin; FA – fusidic acid; GEN – gentamicin; LEV – levofloxacin; SXT – sulfamethoxazole-trimethoprim.
P<0.05 considered as significant; all MRSA isolates were susceptible to vancomycin, teicoplanin, linezolid and tigecycline.
The knowledge of the students about infection control
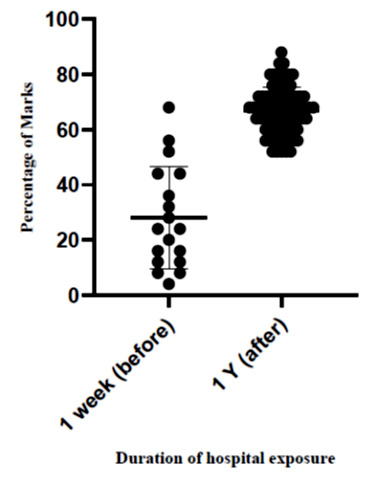
The percentage of correct responses obtained by the students for the questionnaire was 28% and 66.9% 1 week before the exposure to the hospitals and 1 year after exposure to the hospitals, consecutively (Figure 4). Following exposure to healthcare settings the knowledge improvement was significantly observed on common hospital acquired pathogens [p=0.001, t=33.21] MRSA prevalent sites [p=0.002, t=23.24] and infection control methods [p=0.001, t=19.34]. Hygienic practices among medical students were almost similar before and 1 year after exposure to the healthcare settings.
Figure 4. Knowledge about infection prevention among the medical students 1 week before hospital exposure and 1 year after exposure.
Discussion
The necessity of detecting the MRSA carriers among healthcare workers (HCWs) in hospitals, is debatable1,13 because decolonizing procedures are not routinely practiced.8,14 However, these individuals act as potential source of nosocomial infection for their patients and thereby, may cause an extended duration of patient stays in the hospital. The best methods for controlling the nosocomial transmission of MRSA infections is the adherence to hand hygiene.1,15
The reported prevalence of colonization with MRSA differs between institutions and geographic regions; this may in part be due to differences in terms of study design, sample size and methods used for MRSA detection.8,16
A review article by Albrich and Harbarth, considering the research publications between January 1980 and March 2006 and involving 127 investigations, reported that 4.6% of healthcare workers were infected or colonized with MRSA.17 When the South Asian region is considered, studies from India reported an MRSA carriage rate of 1.8% in Pondicherry,18 6.6% in Delhi19 and 2% in Madurai.20 An MRSA carriage of 2% was reported in Nepal.21 A high (38.9%) nasal carriage rate for MRSA was identified in Nigeria. In Ethiopia, it was 20.6%.22 In our study, the MRSA carriage was observed as 49% among the medical students, after they had been exposed to healthcare settings for two years and six months period. These students live in highly crowded university hostels, which would be another potential reason for this high percentage of MRSA carriage.
In this study, we compared MRSA carriage before and after exposure to the healthcare settings in the first-year medical students. The MRSA carriage after exposure to the healthcare settings was significantly increased from 6.3% to 28.1%. Moreover, a statistically significant association was observed between MRSA carriage and the time duration of the medical students’ training in healthcare settings. Indian and Ethiopian studies conducted in 2014 and 2013 consecutively show female predominance among MRSA carriage.23 However, in our study, the gender discrepancy was not evident; thus, both sexes had a roughly similar rate of MRSA carriage (31.5% and 26.5%).
In our study, the nasal carriage of MRSA was 100% among the medical students who were colonized, and axillary carriage was 75%, which became significantly lower than nasal carriage. In 2008, a study among healthcare employees revealed 68% nasal and 44% axillary-groin MRSA colonization.24 In routine practice, nasal, axillary, and perineal swabbing is done for MRSA screening and considered positive if any of the swabs yield MRSA. We have not done the perineal MRSA screening, and this would slightly underestimate the percentage of MRSA carriage. However, the nasal carriage is predominant in most of the studies as resembled in our results, which would effectively minimize the perineal related underestimation.9,21-24 The low-level oxacillin resistance would be due to borderline oxacillin resistant S. aureus phenotype and to confirm that, beta-lactamase production needs to assessed.10,22-24 The difference in MIC values and antibiotic susceptibility patterns of MRSA isolates, between before exposure and after exposure indicate that the students would have acquired MRSA from healthcare settings. To confirm this, we need to analyze the antimicrobial susceptibility changes in different MRSA isolates in clinical settings over the study period. Unfortunately, such data is not available. A study conducted in 2012-2013 in THA found that MRSA bacteremia was detected in 9.3% of patients.11 Furthermore, the sequencing data of the PVL gene is required to differentiate the community-acquired and healthcare setting acquired MRSA strains, which exists as a potential drawback in this study.
The knowledge of medical students about the infection control after 1 year of their clinical study was satisfactory; however, the students who were immediately before the hospital exposure (1 week before) had poor knowledge (Figure 4). First, 5 months after exposure to the hospitals, a sharp rise of MRSA colonization is observed, which would reflect the initial knowledge gap of medical students in infection control (Figure 3 and Figure 4). The medical students are aware of good hygienic practices theoretically after 1 year of their clinical training (Figure 4); however, the application part of their theoretical knowledge is not closely monitored, which would make them more vulnerable to acquire MRSA. A recent study suggests that among first year medical students, knowledge on infection control measures was poor.25
Although the medical staff of THA is kept under the vigilance of infection control practices, the medical students’ adherence to the proper hygienic practices was not closely monitored and exempted from infection control programs confined to the hospital staff.
Conclusions
The MRSA carriage among medical students was significantly high following exposure to healthcare settings. Moreover, the MRSA carriage was significantly associated with the time duration of the clinical training of the medical students. Further, living in crowed hostels could have influenced MRSA colonization. Most of the MRSA isolates exhibited a high level of oxacillin resistance (MIC >128 mg/L) and resistance to other commonly prescribed antibiotics. This would signify the nosocomial acquisition of MRSA by medical students, which alarms of having proper knowledge on infection control when they are ready to be exposed to the hospitals, which is lacking in the student group of this study. Moreover, medical students are required to be closely monitored for their routine practice of the infection control knowledge in healthcare settings.
Acknowledgements
The authors would like to acknowledge study subjects and laboratory staff at the Department of Microbiology, Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka, Saliyapura, Sri Lanka, for the support.
Appendix.
Questionnaire:
-
Name 3 nosocomial pathogens which could be multidrug resistant
A ……………………………………………..
B ………………………………………………
C ………………………………………………
-
Mention a unit in-which methicillin resistant Staphylococcus aureus (MRSA) would be prevalent in THA (Teaching Hospital Anuradhapura)
………………………………………………
-
Name 3 places in the hospital where the medical student can contact MRSA
A …………………………………….
B ……………………………………..
C ………………………………………
MRSA screening done? Yes/ No, if yes when ……………………………………………MRSA? Positive / negative
Have you heard about five moment of hand hygiene? Yes No
If yes how did you hear about them
a). taught in a lecture b) taught by a clinician c) from a poster d) from a newspaper e) From the social media any other: ………………………………………………………………………………………….
-
Do you wash your hands in following occasions (dis=disinfectants)
-
With dis. Without dis NO
A. Before touching the patient ………… …………… ……
B. After touching the patient ………… …………… ……
C. Before doing an invasive procedure ………… …………… ……
D. After doing an invasive procedure ………… …………… ……
E. After coming to the faculty from the hospital ………… …………… ……
F. Going to the hostel/boarding place after hospital ………… …………… ……
-
-
What do you use to wash your hands?
A. Tap water
B. Sterile water
C. Surgical spirit
D. Hydrogen peroxide
E. Alcohol 70%
Have you ever used alcohol hand rub? Yes No
If yes what is the color of the liquid ………….. Cannot remember Yes/ No
Mention the most effective sanitizer which can be prepared in the lab to sanitize the hands and lab surface………………….
How often do you clean your clinical coat; times per semester …………………
Do you disinfect your clinical coat when cleaning Yes□ No□
Gloves are available enough in the hospital Yes□ No□
Do you use gloves in patient handling Yes□ No□
If yes are the gloves sterile? Yes/ No
Where do you discard used gloves: Just leave alone on place on floor or table or trolly / to the dustbin / sharp bin / septic waste disposer in the ward
Footnotes
Authors’ contributions statement: JAASJ and WK developed the methodology and performed sample collection and processing. JAASJ, SP and WK drafted the manuscript for final submission. JAASJ and WK did the statistical analysis. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: Rajarata University research grant under University Grant Commission Sri Lanka.
Erratum: Jayaweera JAAS, Pilapitiya S, Kumbukgolla W. The relationship between the exposure to healthcare settings and colonization with methicillin-resistant Staphylococcus aureus among medical students. GERMS 2020;10(1):34-43. doi: 10.18683/germs.2020.1183
The titles of author no. 3 have been corrected from “BSc, MPhil, PhD, Senior Lecturer” to “BSc, MPhil, Senior Lecturer”.
The corrected version of the article has been available online as of 01 September 2020.
References
- 1.Orlin I, Ronkey A, Onn A, Glikman D, Peretz A. Hospital clones of methicillin resistant Staphylococcus aureus are carried by medical students even before healthcare exposure. Antimicrob Resist Infect Control. 2017;6:15. doi: 10.1186/s13756-017-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CJ, Huang YC. Community-acquired methicillin-resistant Staphylococcus aureus in Taiwan. J Microbiol Immunol Infect. 2005;38:376–82. [PubMed] [Google Scholar]
- 3.Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8:289–301. doi: 10.1016/S1473-3099(08)70097-5. [DOI] [PubMed] [Google Scholar]
- 4.Hsueh PR, Liu CY, Luh KT. Current status of antimicrobial resistance in Taiwan. Emerg Infect Dis. 2002;8:132–7. doi: 10.3201/eid0802.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YC, Ho CF, Chen CJ, Su LH, Lin TY. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin Microbiol Infect. 2008;14:1167–72. doi: 10.1111/j.1469-0691.2008.02115.x. [DOI] [PubMed] [Google Scholar]
- 6.Eveillard M, Martin Y, Hidri N, Boussougant Y, Joly Guillou M. Carriage of methicillin resistant Staphylococcus aureus among hospital employees: prevalence, duration, and transmission to households. Infect Control Hosp Epidemiol. 2004;25:114–20. doi: 10.1086/502360. [DOI] [PubMed] [Google Scholar]
- 7.Weiser MC, Moucha CS. The current state of screening and decolonization for the prevention of Staphylococcus aureus surgical site infection after total hip and knee arthroplasty. J Bone Joint Surg Am. 2015;97:1449–58. doi: 10.2106/JBJS.N.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayaweera AS, Karunarathne M, Kumbukgolla WW, Thushari HL. Prevalence of methicillin resistant Staphylococcus aureus (MRSA) bacteremia at Teaching Hospital Anuradhapura, Sri Lanka. Ceylon Med J. 2017;62:110–1. doi: 10.4038/cmj.v62i2.8478. [DOI] [PubMed] [Google Scholar]
- 9.Negi B, Kumar D, Kumbukgolla W. Antibacterial activity of adamantyl substituted cyclohexane diamine derivatives against methicillin resistant Staphylococcus aureus and Mycobacterium tuberculosis. RSC Adv. 2014;4:11962–6. doi: 10.1039/c4ra00224e. [DOI] [Google Scholar]
- 10.Negi B, Kumar D, Kumbukgolla W. Anti-methicillin resistant Staphylococcus aureus activity, synergism with oxacillin and molecular docking studies of metronidazole-triazole hybrids. Eur J Med Chem. 2016;115:426–37. doi: 10.1016/j.ejmech.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Jayaweera JAAS, Karunarathne M, Kumbukgolla WW. The importance of timely introduction of vancomycin therapy against methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and severity of MRSA bacteremia at Teaching Hospital, Anuradhapura, Sri Lanka. Int J One Health. 2017;3:7–11. doi: 10.14202/IJOH.2017.7-11. [DOI] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing, 28th ed. CLSI supplement M100-S28. Wayne, PA: CLSI; 2018. [Google Scholar]
- 13.SAS Institute Inc . Language reference: concepts. 3rd ed. Cary, NC, USA: SAS Institute Inc.; 2005. [Google Scholar]
- 14.Mathanraj S, Sujatha S, Sivasangeetha K, Parija SC. Screening for methicillin-resistant Staphylococcus aureus carriers among patients and health care workers of a tertiary care hospital in Southern India. Indian J Med Microbiol. 2009;27:62–4. [PubMed] [Google Scholar]
- 15.Jayaweera JAAS, Kumbukgolla WW. Antibiotic resistance patterns of methicillin-resistant Staphylococcus aureus (MRSA) isolated from livestock and associated farmers in Anuradhapura, Sri Lanka. Germs. 2017;7:132–9. doi: 10.18683/germs.2017.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakya B, Shrestha S, Mitra T. Nasal carriage rate of methicillin resistant Staphylococcus aureus among at National Medical College Teaching Hospital, Birgunj, Nepal. Nepal Med Coll J. 2010;12:26–9. [PubMed] [Google Scholar]
- 17.Mathanraj S, Sujatha S, Sivasangeetha K, Parija SC. Screening for methicillin-resistant Staphylococcus aureus carriers among patients and healthcare workers of a tertiary care hospital in Southern India. Indian J Med Microbiol. 2009;27:62–64. [PubMed] [Google Scholar]
- 18.Fadeyi A, Bolaji BO, Oyedepo OO, et al. Methicillin resistant Staphylococcus aureus carriage amongst healthcare workers of the critical care units in a Nigerian hospital. Am J Infect Dis. 2010;6:18–23. doi: 10.3844/ajidsp.2010.18.23. [DOI] [Google Scholar]
- 19.Radhakrishna M, D'Souza M, Kotigadde S, Saralaya VK, Kotian SM. Prevalence of methicillin resistant Staphylococcus aureus carriage amongst health care workers of critical care units in Kasturba Medical College Hospital, Mangalore, India. J Clin Diagn Res. 2013;7:2697–700. doi: 10.7860/JCDR/2013/5160.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaumburg F, Alabi AS, Peters G, Becker K. New epidemiology of Staphylococcus aureus infection from Africa. Clin Microbiol Infect. 2014;20:589–96. doi: 10.1111/1469-0691.12690. [DOI] [PubMed] [Google Scholar]
- 21.Gebreyesus A, Gebre-Selassie S, Mihert A. Nasal and hand carriage rate of methicillin resistant Staphylococcus aureus (MRSA) among health care workers in Mekelle Hospital, North Ethiopia. Ethiop Med J. 2013;51:41–7. [PubMed] [Google Scholar]
- 22.Omuse G, Kariuki S, Revathi G. Unexpected absence of meticillin-resistant Staphylococcus aureus nasal carriage by healthcare workers in a tertiary hospital in Kenya. J. Hosp Infect. 2012;80:71–3. doi: 10.1016/j.jhin.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Wijesekara PNK, Kumbukgolla WW, Jayaweera JAAS, Rawat DS. Review on usage of vancomycin in livestock and humans: Maintaining its efficacy, prevention of resistance and alternative therapy. Vet Sci. 2017;4:pii: E6. doi: 10.3390/vetsci4010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8:289–301. doi: 10.1016/S1473-3099(08)70097-5. [DOI] [PubMed] [Google Scholar]
- 25.Carmona-Torre F, Torrellas B, Rua M, Yuste JR. Staphylococcus aureusnasal carriage among medical students. Lancet Infect Dis. 2017;17:477–9. doi: 10.1016/S1473-3099(17)30188-3. [DOI] [PubMed] [Google Scholar]


